Abstract
Objective
A pilot study to longitudinally quantify effect of onabotulinum toxin A (BoNT-A) on passive muscle properties in children with cerebral palsy (CP) using ultrasound shear wave elastography (SWE).
Design
Prospective longitudinal cohort study
Results
Between 1 and 3 months post-BoNT-A, a significant improvement in the shear modulus of the lateral gastrocnemius was found at 10° plantar flexion (PF) (−7.57 [−10.98, −5.07], p=0.02) and 0° PF (−14.74 [−18.21, −9.38], p=0.03). There was a notable, but non-significant difference in shear modulus at 20° PF, 10° PF, 0° PF between pre-BoNT-A and 1 month post-BoNT-A. Pre-BoNT-A shear modulus was not significantly different than 3 months post-BoNT-A at all foot positions. No significant differences in ankle passive range of motion or spasticity were found.
Conclusion
Despite no significant change in ankle range of motion or spasticity, SWE was able to detect a difference in lateral gastrocnemius passive muscle properties in children with CP following BoNT-A injections. The difference in passive muscle properties resolved by 3 months post-BoNT-A.
Keywords: Cerebral Palsy, Ultrasound Imaging, Elastography, Muscles
INTRODUCTION
In individuals with cerebral palsy (CP), spasticity is a common target for therapeutic intervention. Spasticity, defined in 1979, is a “motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (‘muscle tone’) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex.”1 Spasticity has a short-latency, phasic component (hyperreflexia) and a late, sustained activity component (hypertonia).2,3 The mechanism in which spasticity results in abnormal muscle stiffness is not clear, but likely contributes to increased active stiffness through the sustained hypertonia, leading to maladaptation of muscle architecture (i.e. sarcomere muscle fascicle lengths4 and increased collagen content of the extracellular matrix of the muscle5) affecting the passive muscle stiffness. Recently, using a noninvasive ultrasound technology, ultrasound shear wave elastography (SWE), it has been shown that passive muscle stiffness is significantly greater in children with CP as compared to age- and sex-matched typically developing children.6 This increased passive muscle stiffness in children with CP is thought to contribute to the development of muscle contractures and boney deformity.
Intramuscular botulinum toxin injections are commonly used to facilitate focal and temporary spasticity reduction in children with CP. Botulinum toxin injections are considered effective for spasticity reduction in children with CP, and when used in combination with physical and occupational therapy, may improve ambulation and hand function.7 Botulinum toxin injections have also been shown to improve passive range of motion (ROM).8 However, even with repeated injections, this improvement may only be temporary.9 Optimizing passive muscle stiffness and preventing contracture development is important as improved passive ROM has been associated with improved physical functioning.10 Thus, furthering our understanding of the effect of botulinum toxin on passive muscle stiffness through direct measurement of passive muscle stiffness in children with CP is needed.
This study has not been possible until recently, as previous methods for evaluation of passive muscle stiffness have been limited by inability to isolate individual muscles. Clinical measures of spasticity, including Modified Tardieu and Ashworth scales have subjectivity in the interpretation of the limb movement and limited reliability in repeated measures, particularly when used in young children with CP.11,12 Other tests including dynamometry, Ramp and Hold Test, and the Pendulum test can be challenging to perform and do not isolate passive muscle stiffness from joint or tendon stiffness. In contradistinction to these measures and tests, SWE is an ultrasound technique that can be used to quantify passive muscle stiffness in children and adults.6,13,14
Thus, this study is a longitudinal pilot study involving children with spastic CP who were planning to undergo onabotulinum toxin A (henceforth BoNT-A will refer to onabotulinum toxin A) injections at our institution. Clinically, BoNT-A has a plateau of its peak effect on spasticity reduction by approximately one to two months post-injection and loss of clinical effectiveness by 3 months post injection.15 However, it’s not clear if spasticity reduction coincides to changes in passive muscle properties. Therefore, this pilot study aimed to use SWE to quantify the effect and the duration of effect of BoNT-A on passive muscle stiffness in children with CP. This is a step towards creating a clinically feasible tool for monitoring effect and duration of effect of BoNT-A on passive muscle stiffness in order to provide evidence-based and individualized treatment.
METHODS
Participants
Ten children aged 2 to 12 years with spastic cerebral palsy who were undergoing BoNT-A injections to the gastrocnemius muscle for clinical reasons were recruited from Mayo Clinic’s Cerebral Palsy Clinic. Children were excluded if they had 1) any surgery to the tibia or fibula, gastrocnemius/soleus, or Achilles tendon, 2) any other orthopedic surgery to the lower limb in the previous 6 months, 3) any phenol injection to the limb of interest in the previous 6 months, 4) botulinum neurotoxin injection to the lower limb of interest in the previous 3 months, 5) planned active titration of systemic anti-spasticity medications during the study period, 6) serial casting of the lower limb of interest in the previous 6 months, 7) or inability to tolerate positioning for the study. Children could be undergoing BoNT-A injections to other muscles during the same session as the gastrocnemius injection session. Mayo Clinic Institutional Review Board approval was obtained before initiating the study. Written informed consent was obtained from a parent or guardian of each child, with written assent obtained from children if older than 7 years and cognitively able to provide assent.
Study Methods
This study consisted of a total of three study visits for each child. These visits were performed at time points of up to one month prior to BoNT-A injections, one month following BoNT-A injections, and 3 months following BoNT-A injections. The follow up time points corresponded with clinically expected timing of plateauing of the peak effect and wearing off of effect.15 Demographic information, physical exam, and SWE measurements were obtained at each visit as outlined below. With regard to the BoNT-A injections, BoNT-A was dosed and injected per the typical prescribing and injecting technique of the treating physician, representing the usual clinical practice of the physicians. At our institution, BoNT-A injection amounts are divided equally between the medial and lateral heads of the gastrocnemius muscle. Injection technique was per the injecting physician. For all children in this study anatomic identification (area of greatest muscle bulk on the posterior calf) and electrical stimulation were utilized for optimizing placement of injections.
For each child, demographic information collected included date of birth, weight, height, sex, medical and surgical history, medications, and previous botulinum toxin or phenol injections. Physical examination measurements collected were spasticity using the Modified Ashworth Scale (MAS), ankle passive ROM, Gross Motor Function Classification Scale (GMFCS) level, and type of CP. MAS testing for the ankle were performed with the child either seated or supine, depending on child’s ability and preference. While the reliability of the MAS for measuring spasticity has come into question, the Modified Tardieu Scale has been shown to have similar inter- and intra-rater reliability as the MAS, though in a subsequent version by Gracies et. al. training does appear to improve reliability of the Tardieu Scale measurements.12,16,17 Maximum passive joint ROM of the ankle for foot dorsiflexion (DF) and plantar flexion (PF) was performed using a goniometer with the child in supine position. When supine, the child’s hip was positioned at 0° extension and knee was placed in full extension (0° flexion). If the child did not have the ROM at the hip or knee to accommodate this positioning, the nearest position that the child could comfortably tolerate was used. Maximum passive ankle ROM was achieved when either the child demonstrated discomfort, either verbally or through other indications or a solid end point was reached with stretch.
Following the physical exam measurements, measurements of lateral gastrocnemius stiffness of the most affected leg were performed using SWE. The most affected leg was the side with greater gastrocnemius spasticity as determined by MAS score and/or lesser ankle dorsiflexion passive ROM. An Aixplorer ultrasonic scanner (SuperSonic Imagine, Aix-en-Provence, France) with a linear array transducer (SL15-4; SuperSonic Imagine, Aix-en-Provence, France) was used with musculoskeletal preset. The physics and mathematical approach for these measurements are described elsewhere.18–20 Briefly, the ultrasound probe generates transient shear waves in muscle by transmitting ultrasound push beams.19 The same probe then detects the shear waves travelling along the muscle fiber, the speed of which can be used to calculate the Young’s modulus.18,21 Young’s modulus is the output of the Aixplorer.
SWE measurements were obtained as previously described.6,13 Briefly, each child was assisted into a prone position with feet over the edge of the examination table. With a tape measure, circumferential measurements of the limb of interest were obtained, and the area of greatest muscle bulk was marked on the skin. The ultrasound transducer was positioned on the lateral gastrocnemius muscle over the area of greatest muscle bulk. The transducer was aligned with the direction of the muscle fiber. The distance from the fibular head to the proximal end of the ultrasound probe was measured and recorded to maintain positioning of the ultrasound transducer for the repeated measures. B-mode imaging was used to confirm positioning and alignment of the transducer. Appropriate transducer alignment was achieved when several fascicles of the lateral gastrocnemius could be traced (Figure 1). The transducer was held in place with minimal pressure on skin by one of the examiners. Surface electromyography (U-Control; Thought Technology Ltd, Québec, Canada) was used to ensure muscle relaxation. The Ucontrol was set at lowest scale range (X1, upper threshold 3.0) for detecting muscle activation. Electrodes were placed over the posterior calf near the area of the SWE measurement. A goniometer was used to ensure placement of the foot in 20° PF, 10° PF, and 0° PF. The goniometer was aligned with the shaft of the tibia or fibula and the calcaneus. The examiner then applied pressure along the plantar surface of the foot, using calcaneal movement as the dorsiflexion measurement and observing for inadvertent foot rotation or inversion/eversion. When correct foot position and sufficient muscle relaxation were present, SWE measurement was performed. Measures were obtained at three positions and then repeated twice. The three measures for each foot position were averaged to generate a single value for further analysis.
Figure 1.
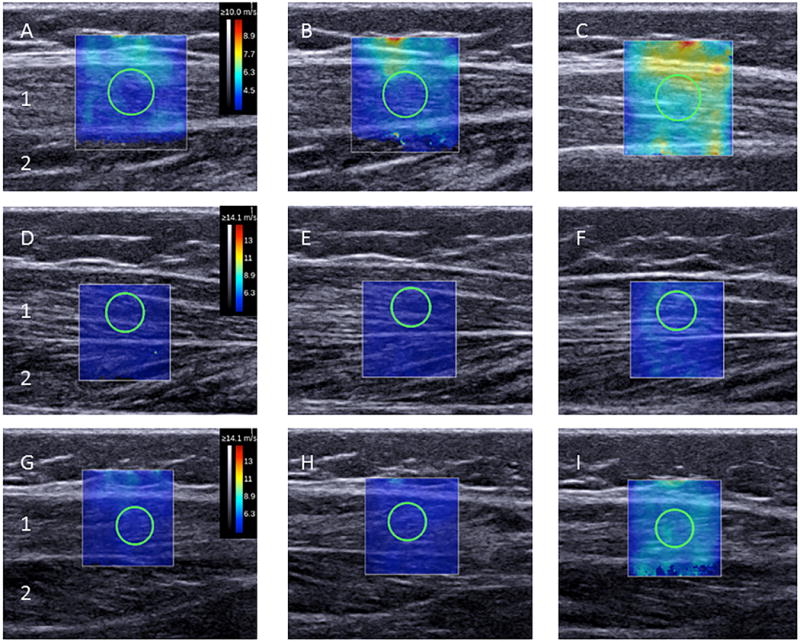
Representative B-mode Ultrasound Image with Super-Imposed 2-Dimensional (2-D) Elastograms from Right Lateral Gastrocnemius of a Child with Cerebral Palsy. Figure shows ultrasound images prior to BoNT-A injections (A-C), 1 month after BoNT-A injections (D-F), and 3 months after BoNT-A injections (G-I). A,D and,G are 20° plantar flexion; B,E, and H are 10° plantar flexion; and C,F, and I are 0° plantar flexion. The shear wave capture area (i.e. 2-D Elastogram) is the colored box located over the B-mode image of the lateral gastrocnemius muscle (1). The shear wave measurement area, also known as the region of interest, is circle inside the box. The soleus muscle (2) is also shown. The 2-D Elastogram colors indicated by the color bar on the right (A, D, G) are a visual representation of shear-wave speed. The tissues with high shear-wave speed (more stiff tissue) are yellow-red and tissues with low shear-wave speed (softer tissue) are purple-blue color.
The output for each SWE measurement is a 2-dimensional elastogram (Figure 1). On this elastogram, a region of interest over the lateral gastrocnemius muscle was selected such that muscle fascial borders, tendon, and blood vessels were excluded. As previously described,6,13,14 we used an open-source imaging plug-in for the DICOM reader (OsiriX Imaging Software, Geneva, Switzerland) to measure the mean Young’s modulus for the circular region of interest (mean diameter: 4.8 to 5.0 mm) within the SWE elastogram. The mathematical conversion from the shear wave speed to the Young’s and shear modulus assumes that the material is purely elastic, locally homogeneous, and isotropic. These assumptions do not apply to muscle. However, we have found strong correlations between the muscle shear modulus measurement from shear wave elastography and Young’s modulus measurements from traditional material testing.21 These correlations indicate that shear modulus measurements from shear wave elastography may be used to represent the true Young modulus of the muscle. Therefore, Young’s modulus was converted to shear modulus by the formula E = 3μ, where E is the Young’s modulus, and μ is the shear modulus. As shear modulus is the accepted measurement for reporting on anisotropic tissues such as muscle, we report the shear modulus as our SWE measurement.6,13,20,21
Statistical Analysis
Differences between lateral gastrocnemius shear modulus before, 1 month after BoNT-A, and 3 months after BoNT-A at each foot position for the most affected leg in children with CP was of primary interest. Categorical variables (i.e. Sex, GMFCS, MAS, CP type) were summarized using frequency and percentages. Continuous variables (i.e. Age, BMI, calf circumference, maximal ankle DF, shear modulus) were summarized using median, interquartile range (Q1, Q3) and range. Paired t-tests were used to compare differences overtime with respect to shear modulus, MAS, calf circumference, and ankle dorsiflexion passive ROM. Spearman Rank Correlations were used to explore the relationship between pairs of continuous variables (shear modulus and BoNT-A dose, shear modulus and ankle dorsiflexion passive ROM). Do to the limited sample size, Kruskal Wallis tests were used when comparing across three groups (GMFCS, CP Type, and history of any previous botulinum toxin injections). The level for significance for any p-value was set at less than 0.05. All analyses were conducted using SAS for Unix (version 9; SAS Institute Inc).
RESULTS
A total of ten children with CP were recruited for the study. One child was excluded following testing as he was unable to achieve muscle relaxation, despite multiple attempts at alternative positioning to facilitate comfort and relaxation. Therefore, nine children were used for analysis. Demographics, physical exam characteristics, and amount of BoNT-A injected are described in Table 1. The children ranged in age from two to nine years. All children were ambulatory (GMFCS I-III). Just over half of the children were boys (5 (55.6%)), had bilateral CP (5 (55.6%)), and had a history of previous BoNT-A injections to the gastrocnemius muscle (5 (55.6%)). Of those who had a previous BoNT-A injection to the gastrocnemius muscle, 6 months was the shortest period from previous injection. Some children with CP had a maximal ankle dorsiflexion of less than 0° during their initial goniometric testing (which was always performed before SWE). All these children were able to achieve 0° dorsiflexion during SWE testing.
Table 1.
Demographic Characteristics of Nine Children with Cerebral Palsy
| Characteristica | Median (Q1,Q3) |
|---|---|
| Age, mo | 60 (35, 92) |
| Age range, mo | 25–105 |
| Female, n (%) | 4 (44.4) |
| BMI, kg/m2 | 16.7 (15.9, 17.9) |
| BMI range, kg/m2 | 15.3–18.6 |
| GMFCS level*, n (%) | |
| I | 4 (44.4) |
| II | 3 (33.3) |
| III | 2 (22.2) |
| Cerebral palsy type, n (%) | |
| Bilateral | 5 (55.6) |
| Unilateral | 4 (44.4) |
| Previous BoNT-A to Gastrocnemius, n (%) | |
| Yes | 5 (55.6) |
| No | 4 (44.4) |
| Duration of time from previous BoNT-A**, months | 14 (9, 24) |
| Total BoNT-A Dose (units/kg) | 10.9 (8.6, 12.4) |
| Total BoNT-A Dose to Gastrocnemius (units/kg) | 3.3 (2.5, 3.9) |
| Calf circumference***, cm | 21.3 (20.4, 25.4) |
| Calf circumference range, cm Range | 20.0–27.2 |
| Maximal ankle DF, degrees | 3 (0, 5) |
| Maximal ankle DF range, degrees | −12–20 |
| Modified Ashworth Scale | 3 (3,3) |
| Modified Ashworth Scale range**** | 1–3 |
Abbreviations: BMI, body mass index; GMFCS, Gross Motor Function Classification System; BoNT-A, onabotulinum toxin A; cm, centimeter; DF, dorsiflexion.
Values are presented as median and 1rst and 3rd interquartile range (Q1, Q3) unless specified otherwise.
GMFCS ranges from I-V. However no there were no children in the study in IV and V.
n=5
n=8
Modified Ashworth Scale ranges from 1 to 4. However no there were no children in the study in level 4.
We found a significant change in shear modulus of the lateral gastrocnemius muscle at 10° PF between 1 and 3 months post BoNT-A and at 0° PF between 1 and 3 months post BoNT-A (Table 2, Figure 2). There was a notable, but non-significant difference in shear modulus of the lateral gastrocnemius muscle at 20° PF, 10° PF, 0° PF between pre-BoNT-A and 1 month post-BoNT-A (Table 2, Figure 2). Pre-BoNT-A shear modulus of the lateral gastrocnemius muscle was not significantly different than 3 months post-BoNT-A at all foot positions.
Table 2.
Difference in Physical Measurements and Shear Modulus of Children with Cerebral Palsy from pre-BoNT-A and Between 1 and 3 Months post-BoNT-A
| Measurementsa, median (Q1,Q3) | P Value b | |
|---|---|---|
| Calf circumference, cm | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A* | 0.20 (−0.10, 0.50) | 0.41 |
| Pre-BoNT-A – 3 Months Post-BoNT-A** | 0.25 (−0.25, 0.55) | 0.66 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A** | −0.05 (−0.30, 0.25) | 1.00 |
| Maximal ankle DF, degrees | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A | −1.00 (−5.00, 2.00) | 0.70 |
| Pre-BoNT-A – 3 Months Post-BoNT-A | 3.00 (−2.00, 6.00) | 0.48 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A | 4.00 (1.00, 8.00) | 0.11 |
| Modified Ashworth Scale | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A | 0.44 (0.00, 1.00) | 0.10 |
| Pre-BoNT-A – 3 Months Post-BoNT-A | 0.11 (0.00, 0.00 | 0.73 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A | −0.33 (−1.00,0.00) | 0.08 |
| Shear Modulus, kPa at 20° PF | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A | 3.89 (0.84, 8.68) | 0.06 |
| Pre-BoNT-A – 3 Months Post-BoNT-A | 1.75 (−3.94, 7.70) | 0.39 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A | −3.72 (−4.78, −0.98) | 0.13 |
| Shear Modulus, kPa at 10° PF | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A | 8.07 (2.11, 17.13) | 0.09 |
| Pre-BoNT-A – 3 Months Post-BoNT-A | 3.00 (−8.20, 10.05) | 0.73 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A | −7.57 (−10.98, −5.07) | 0.02 |
| Shear Modulus, kPa at 0° PF | ||
| Pre-BoNT-A – 1 Month Post-BoNT-A | 13.45 (−1.28, 42.39) | 0.08 |
| Pre-BoNT-A – 3 Months Post-BoNT-A | 4.77 (−17.81, 24.18) | 0.42 |
| 1 Month Post-BoNT-A – 3 Months Post-BoNT-A | −14.74 (−18.21, −9.38) | 0.03 |
Abbreviations: BoNT-A, onabotulinum toxin A; cm, centimeter; DF, dorsiflexion; kPa, kilopascal; PF, plantar flexion.
Values are presented as median and 1rst and 3rd interquartile range (Q1, Q3) unless specified otherwise.
p-values are from paired t-tests.
n=7
n=8
Figure 2.
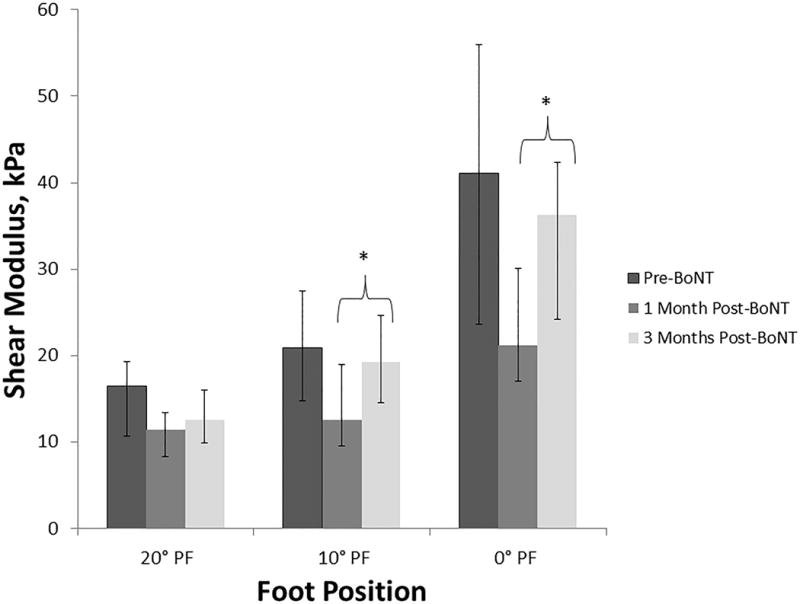
Stiffness of Lateral Gastrocnemius Muscle in Children with Cerebral Palsy pre-BoNT-A, 1 month post-BoNT-A, and 3 months post-BoNT-A. Abbreviations: kPa, kilopascals; PF, plantar flexion. * indicates that values differed significantly (P<0.05). The bars indicate Q1 and Q3 from median value for each foot position. Foot position is in degrees of PF, with 0° PF being the neutral position.
In addition, 3 (33.3%) of the children had improved MAS scores at 1 month-post-BoNT-A, with 2 children having a 1 point improvement and 1 child having a 2 point improvement. None had worsening of the MAS at 1 month post-BoNT-A. Two (22.2%) children sustained this improvement at 3 months post-BoNT-A. However, the overall changes in MAS were not statistically significant at the time points evaluated. With respect to maximal ankle dorsiflexion, five (55.6%) children had improvement at 1 month post-BoNT-A, with 4 (44.4%) children showing a reduction in maximal ankle dorsiflexion. Two (22.2%) children maintained the improvement in maximal ankle dorsiflexion at 3 months post-BoNT-A and 6 (66.7%) children showed a reduction in maximal ankle dorsiflexion from the initial visit at 3 months. Despite these changes, maximal ankle dorsiflexion did not significantly change across time points. Calf circumference was also not statistically different across the time points with all but one child having a calf circumference within 1 cm of the initial visit measurement at 1 and 3 months post-BoNT-A. (Summarized in Table 1)
DISCUSSION
BoNT-A (not FDA approved for the treatment of spasticity in children) appears to provide temporary improvement in passive muscle stiffness of the lateral gastrocnemius. Improvements in passive muscle stiffness were greatest at 1 month post-BoNT-A (at anticipated peak of effectiveness) with the largest difference at the maximum stretch we measured (0° PF). These improvements were statistically significant between 1 month post-BoNT-A and 3 months post-BoNT-A at 10° PF and 0° PF. We also found notable changes in passive muscle stiffness from pre-BoNT-A and 1 month-post BoNT-A at all foot positions, but these did not reach statistical significance, likely contributing factors include the number of children in our pilot study and the large standard deviations in SWE measurements of the lateral gastrocnemius muscle pre-BoNT-A.
Interestingly, while passive muscle stiffness of the lateral gastrocnemius did temporarily improve with BoNT-A injections, in this study, we did not find a significant change in ankle passive ROM. Thus, the improvement in passive stiffness may make it easier to stretch the gastrocnemius within the passive ROM the child had prior to the injections but does not result in greater passive ROM. This has some correlation to earlier work by Alhusaini et al, who looked at effect on BoNT-A on gastrocnemius hysteresis.22 While the authors did not find a significant difference in hysteresis, the hysteresis curve was shifted downward following BoNT-A injections, suggesting less torque (implying muscles were less stiff) to perform the passive ROM after BoNT-A.22 The children with CP in this study were all high level ambulators (GMFCS I and II) and had greater ankle passive ROM than the children in our study, which suggests the children in the previous study had less passive stiffness than the children in our study and/or the children in our study may have developed fixed muscle contracture. Fixed muscle contracture occurs despite use of BoNT-A, as shown in a retrospective study evaluating long-term effects of BoNT-A.9 One potential reason for a lack of joint contracture prevention with BoNT-A is a chronic maladaptation of sarcomeres in the muscle of children with CP. Specifically; in vivo studies have shown that muscle fibers of children with CP have fewer sarcomeres in series with these sarcomeres being longer.5,23 Thus, there may be less ability of the muscle to adapt or respond to passive stretching, even when spasticity is reduced. In addition, there may be fewer satellite cells in muscles of children with CP, thus reducing the regenerative ability of the muscles fibers (i.e. ability to generate more sarcomeres in series).24 These studies suggest that by time a child with CP develops maladaptive changes in joint passive ROM or increasing difficulties with physical functioning, the muscle may have limited capability to adapt, even with spasticity reducing interventions, due to intrinsic changes in the muscle.
While BoNT-A is intended to reduce spasticity, in this study, we did not find a significant reduction in spasticity in the group of children we studied. A lack of significant spasticity reduction may be multifactorial, including the numbers of children in this pilot study, resistance to BoNT-A for those previously injected, dose used in the gastrocnemius, possibility of inadvertent soleus injection, and the lack of sensitivity of the MAS.25–27 In future studies, we would like to investigate the correlation of the Dynamic Muscle Length portion of the Modified Tardieu Scale to SWE measurements of passive muscle stiffness changes with interventions to reduce spasticity.28 The rationale for using the Modified Tardieu Scale is that it has two measurements of particular interest.28 The first is the measurement of the joint angle at which the spastic catch is felt (designated as R1). The second is the measurement of the full passive range of motion (designated as R2). The difference between R2 and R1 is termed the dynamic component. Given our findings of a reduction in passive muscle stiffness of the lateral gastrocnemius at 1 month post-BoNT-A, without a significant change in joint passive ROM, we anticipate that BoNT-A has its greatest effect on reducing the dynamic component (i.e reduces the difference between R2 and R1). This reduction in the dynamic component may facilitate more functional ROM, by delaying the range in which the spastic catch is generated.
LIMITATIONS
There are a few limitations to this study. First, this is a pilot study. We identified statistical improvement of passive muscle properties with BoNT-A and evaluated a few associations, but due to sample size, were not able to explore additional correlations or draw more definitive conclusions on the associated factors we investigated. With optimization of our protocol, future studies with larger number of participants can be done to investigate other factors, such as dose of BoNT-A, effect of repeated BoNT-A injections, on passive muscle stiffness and correlations with clinical spasticity measures including the Dynamic Muscle Length component of the Modified Tardieu Scale. We would also like to investigate duration of effect of differing BoNTs on passive muscle stiffness, and other spasticity reducing techniques (selective dorsal rhizotomy, intrathecal baclofen pump, and oral medications) on passive muscle stiffness. Finally, we plan to investigate the relationship of passive gastrocnemius muscle stiffness and spasticity reducing interventions with ankle kinematics, kinetics, and temporal distance factors (i.e. step length and stride length) during walking in future studies.
Another limitation is a lack of children of more severe impairments (GMFCS levels IV and V). We had difficulty recruiting children with greater functional impairments as the parents felt their child would not tolerate positioning, would not be able to cooperate with testing (primarily due to cognition), or could not come back for the testing due to difficulty in transporting their child. The one child we recruited of GMFCS IV had great difficulty with positioning and relaxation. However, a short time after completion of the study, he was diagnoses with autism, which may have contributed to his difficulties with participation. In a previous study, we noted that overall children with CP had greater difficulty with muscle relaxation (as monitored with surface EMG) than typically developing children requiring longer study visits and occasional breaks during testing.6 We did not time study visits to see if this changed over the course of participation in the study (i.e. either due to BoNT-A facilitating relaxation or due to having an understanding of the study procedures). But this could be done in future studies. To maintain the clinical applicability and clinical translation of this study we chose to measure ankle passive ROM using a goniometer. The goniometer method has an approximately 5° to 14° variability in children with CP.29,30 This may be why some children with CP who had maximal ankle dorsiflexion of less than 0° during their initial goniometric testing were able to achieve 0° dorsiflexion following this initial testing. This difference in initial passive ROM was either due to response to initial stretch for goniometric measurement (which was always performed before SWE), inherent variability in goniometric measurements, or both. This range of variability in this measurement may have also contributed to the lack of significant difference in the passive ROM outcomes. In future studies, adding a measure of torque required for performing passive ROM (via biomechanics laboratory) and goniometer testing would provide additional information in this area.
Lastly, the dosing of BoNT-A was not controlled, but rather done based on clinical judgement of the treating pediatric rehabilitation physician. Thus, many children received BoNT-A injections to multiple muscles as was clinically indicated. For many children, this limited the dose of BoNT-A which could be injected to the gastrocnemius muscle, with the median dose in our study being 3.3 units/kg. This dose is on the lower end of the recommended dosing for a large muscle in the leg, but consistent with clinical practice in which more than one muscle is injected.27
CONCLUSIONS
In this pilot study, BoNT-A injections provide temporary, but statistically significant improvement in passive gastrocnemius muscle stiffness. We did not see statistically significant improvement in joint passive ROM or spasticity, possibly due to limitations of the study, thus the clinical significance of the change in passive muscle stiffness remains unclear. Continued work to advance the understanding of the mechanisms underlying the development of the abnormal passive muscle stiffness, along with identifying clinically meaningful changes in passive muscle stiffness, is critical for targeting prevention of the development of abnormal passive muscle stiffness and identifying interventions to provide long-term improvement.
Acknowledgments
The authors would like to thank Shirley Kingsley-Berg for her expertise in consenting participants, coordinating the study visits, and in contacting the participants. The authors would also like to thank the participants and their families for their time and participation in this study.
We would like to disclose that National Institutes of Health (NIH) (grants KL2TR000136, K12HD00109, F30 AG044075) and the Mayo Clinic Department of Physical Medicine and Rehabilitation for supported this research. In addition, this publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Footnotes
We would also like to disclose that there may be a perceived conflict of interest for author Pengfei Song, PhD, due to patents and licensing in the field of ultrasound elastography.
A portion of this work was presented at the Association of Academic Physiatrists Annual Meeting in Sacramento, California on February 20th, 2016 as part of the Rehabilitation Medicine Scientist Training Program Presentations.
References
- 1.Lance JW. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 1980. [Google Scholar]
- 2.Ibrahim IK, Berger W, Trippel M, Dietz V. Stretch-induced electromyographic activity and torque in spastic elbow muscles. Differential modulation of reflex activity in passive and active motor tasks. Brain. 1993;116:971–989. doi: 10.1093/brain/116.4.971. [DOI] [PubMed] [Google Scholar]
- 3.Thilmann AF, Fellows SJ, Garms E. The Mechanism of Spastic Muscle Hypertonus - Variation in Reflex Gain over the Time Course of Spasticity. Brain. 1991;114:233–244. [PubMed] [Google Scholar]
- 4.Lieber RL, Friden J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve. 2002;25:265–270. doi: 10.1002/mus.10036. [DOI] [PubMed] [Google Scholar]
- 5.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589:2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg JE, Eby SF, Song P, et al. Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev Med Child Neurol. 2016;58:1288–1294. doi: 10.1111/dmcn.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novak I, McIntyre S, Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 8.Koman LA, Brashear A, Rosenfeld S, et al. Botulinum toxin type a neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics. 2001;108:1062–1071. doi: 10.1542/peds.108.5.1062. [DOI] [PubMed] [Google Scholar]
- 9.Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009;51:120–127. doi: 10.1111/j.1469-8749.2008.03189.x. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DH, Kaufman KR, Wyatt MP, Chambers HG, Mubarak SJ. Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy. Gait Posture. 1999;10:1–9. doi: 10.1016/s0966-6362(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 11.Fosang AL, Galea MP, McCoy AT, Reddihough DS, Story I. Measures of muscle and joint performance in the lower limb of children with cerebral palsy. Dev Med Child Neurol. 2003;45:664–670. doi: 10.1017/s0012162203001245. [DOI] [PubMed] [Google Scholar]
- 12.Yam WKL, Leung MSM. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006;21:1031–1035. doi: 10.1177/7010.2006.00222. [DOI] [PubMed] [Google Scholar]
- 13.Brandenburg JE, Eby SF, Song P, et al. Feasibility and reliability of quantifying passive muscle stiffness in young children by using shear wave ultrasound elastography. J Ultras Med. 2015;34:663–670. doi: 10.7863/ultra.34.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eby SF, Cloud BA, Brandenburg JE, et al. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech. 2015;30:22–27. doi: 10.1016/j.clinbiomech.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilton AH. Injectable neuromuscular blockade in the treatment of spasticity and movement disorders. J Child Neurol. 2003;18(Suppl 1):S50–66. doi: 10.1177/0883073803018001S0701. [DOI] [PubMed] [Google Scholar]
- 16.Mutlu A, Livanelioglu A, Gunel MK. Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsy. BMC Musculoskelet Disord. 2008;9:44. doi: 10.1186/1471-2474-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracies JM, Burke K, Clegg NJ, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil. 2010;91:421–428. doi: 10.1016/j.apmr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg JE, Eby SF, Song P, et al. Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehab. 2014;95:2207–2219. doi: 10.1016/j.apmr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacourpaille L, Hug F, Bouillard K, Hogrel J-Y, Nordez A. Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol Meas. 2012;33:N19–28. doi: 10.1088/0967-3334/33/3/N19. [DOI] [PubMed] [Google Scholar]
- 21.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhusaini AAA, Crosbie J, Shepherd RB, Dean CM, Scheinberg A. No change in calf muscle passive stiffness after botulinum toxin injection in children with cerebral palsy. Dev Med Child Neurol. 2011;53:553–558. doi: 10.1111/j.1469-8749.2011.03930.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathewson MA, Ward SR, Chambers HG, Lieber RL. High resolution muscle measurements provide insights into equinus contractures in patients with cerebral palsy. J Orthop Res. 2015;33:33–39. doi: 10.1002/jor.22728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayanidhi S, Dykstra PB, Lyubasyuk V, McKay BR, Chambers HG, Lieber RL. Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy. J Orthop Res. 2015;33:1039–1045. doi: 10.1002/jor.22860. [DOI] [PubMed] [Google Scholar]
- 25.Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF. What does the Ashworth scale really measure and are instrumented measures more valid and precise? Dev Med Child Neurol. 2002;44:112–118. doi: 10.1017/s0012162201001761. [DOI] [PubMed] [Google Scholar]
- 26.Brandenburg JE, Krach LE, Gormley ME., Jr Use of rimabotulinum toxin for focal hypertonicity management in children with cerebral palsy with nonresponse to onabotulinum toxin. Am J Phys Med Rehabil. 2013;92:898–904. doi: 10.1097/PHM.0b013e31829231fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham HK, Aoki KR, Autti-Ramo I, et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11:67–79. doi: 10.1016/s0966-6362(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 28.Boyd RN, Graham HK. Objective measurement of clinical findings in the use of botulinum toxin type A for the management of children with cerebral palsy. Eur J Neurol. 1999;6:S23–S35. [Google Scholar]
- 29.Allington NJ, Leroy N, Doneux C. Ankle joint range of motion measurements in spastic cerebral palsy children: intraobserver and interobserver reliability and reproducibility of goniometry and visual estimation. J Pediatr Orthop B. 2002;11:236–239. doi: 10.1097/00009957-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 30.McDowell BC, Hewitt V, Nurse A, Weston T, Baker R. The variability of goniometric measurements in ambulatory children with spastic cerebral palsy. Gait Posture. 2000;12:114–121. doi: 10.1016/s0966-6362(00)00068-0. [DOI] [PubMed] [Google Scholar]


