Abstract
Obstetric anesthesia has evolved over the course of its history to encompass comprehensive aspects of maternal care, ranging from cesarean delivery anesthesia and labor analgesia to maternal resuscitation and patient safety. Anesthesiologists are concerned with maternal and neonatal outcomes and with preventing and managing complications that may present during childbirth. The current review will focus on recent advances in obstetric anesthesia, including labor anesthesia and analgesia, cesarean delivery anesthesia and analgesia, the effects of maternal anesthesia on breastfeeding and fever, and maternal safety. The impact of these advances on maternal and neonatal outcomes is discussed. Past and future progress in this field will continue to have significant implications on the health of women and children.
Introduction
Obstetric anesthesiology has historically bridged multiple disciplines including obstetrics, maternal-fetal medicine, neonatology, general surgery, and anesthesiology. Virginia Apgar, a surgeon-turned-obstetric anesthesiologist, is best known for her namesake neonatal assessment scoring system. She is widely credited for early advances in neonatology. Her contributions exemplify how obstetric anesthesiologists sought answers to scientific questions about anesthetic effects on the mother fetus and neonate. Early investigations focused on the use of volatile agents for labor anesthesia, shifted to opioids and amnestics, and then to neuraxial techniques. Studies focused on the effects of these interventions on labor and the newborn.
The “birth” of obstetric anesthesia began with the introduction of ether labor analgesia by obstetrician James Young Simpson in 1847.1 While Simpson publicized this intervention as effective and innovative, he expressed reservations about its unknown effects on labor and the fetus. The medical community expressed concerns about safety and toxicity. Women’s rights to request and receive labor pain relief was controversial – religious mores of the nineteenth century viewed pain, including labor pain, as divine punishment, and interference was considered sinful.2 Ultimately, the clinical use of ether and chloroform for labor analgesia was not driven by the scientific community, but by a shift in the social attitudes of patients who demanded it, persuaded by public rhetoric from Suffragettes and other feminist advocates.2 In the early 20th century, “twilight sleep,” a combination of morphine and scopolamine, became common, but was ultimately abandoned due to its depressant effects on the neonate. In the mid-twentieth century, general anesthesia for cesarean delivery gave rise to airway complications, including failed tracheal intubations, maternal aspiration and Mendelsohn syndrome (aspiration pneumonitis).3 Anesthesiologists began focusing their efforts on reducing anesthesia-related adverse maternal and neonatal outcomes, including airway-associated morbidity and mortality. As a result, neuraxial labor anesthesia became increasingly used by the 1980s, although it was simultaneously feared to be a risk factor for cesarean delivery.4 Fortunately, most concerns were resolved by rigorous research, and by refining regional anesthesia approaches.5 Advances that led to reductions in anesthesia-related maternal morbidity and mortality included the use of an epidural test dose, incremental epidural injection of local anesthetic, elimination of bupivacaine 0.75% for epidural anesthesia, and lipid emulsion therapy for local anesthetic systemic toxicity. Past and ongoing research in obstetric anesthesiology has contributed to a substantial reduction of anesthesia-related maternal mortality.5
Obstetric anesthesiologists have contributed to interdisciplinary initiatives advancing maternal safety (Figure 1). Randomized control trials and impact studies improved understanding that neuraxial labor analgesia does not independently influence the risk for cesarean delivery. Postpartum pain management has improved, and multimodal strategies enhanced so analgesic efficacy is maximized while maternal and fetal side effects are minimized. Anesthesia effects on lactation, maternal fever, neonatal acid-base status, and cognitive development continue to be explored. Safer care systems emphasize low-dose neuraxial anesthesia, hemorrhage preparedness and management, and team crisis simulation. In this review, we focus on obstetric anesthesia advancements over the last two decades, with emphasis on the past decade. Continuing progress will have important consequences to obstetric medicine, anesthesiology, and perioperative patient care.
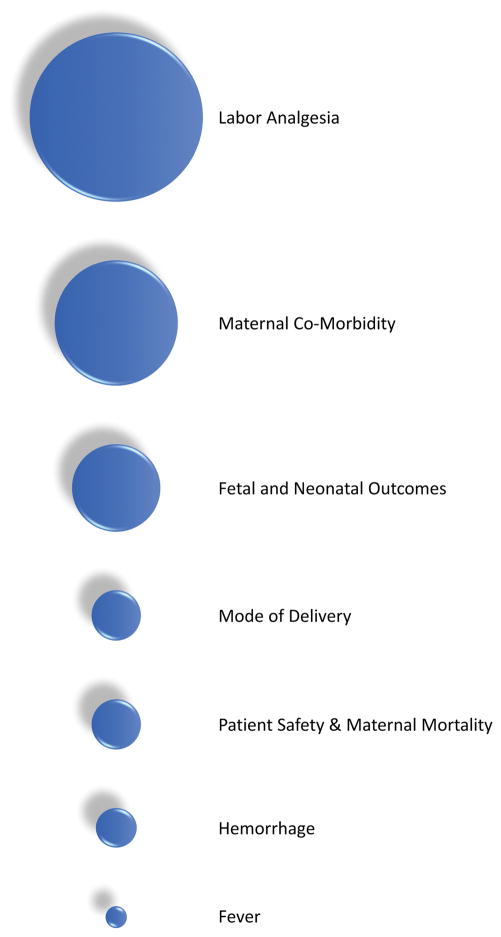
Figure 1.
Subject areas of obstetric anesthesiology research advancements on maternal and neonatal outcomes over the last decade. Bubble size indicates relative impact of each topic.
Labor Analgesia and Anesthesia
Methods of Labor Analgesia
Neuraxial analgesia: initiation and maintenance
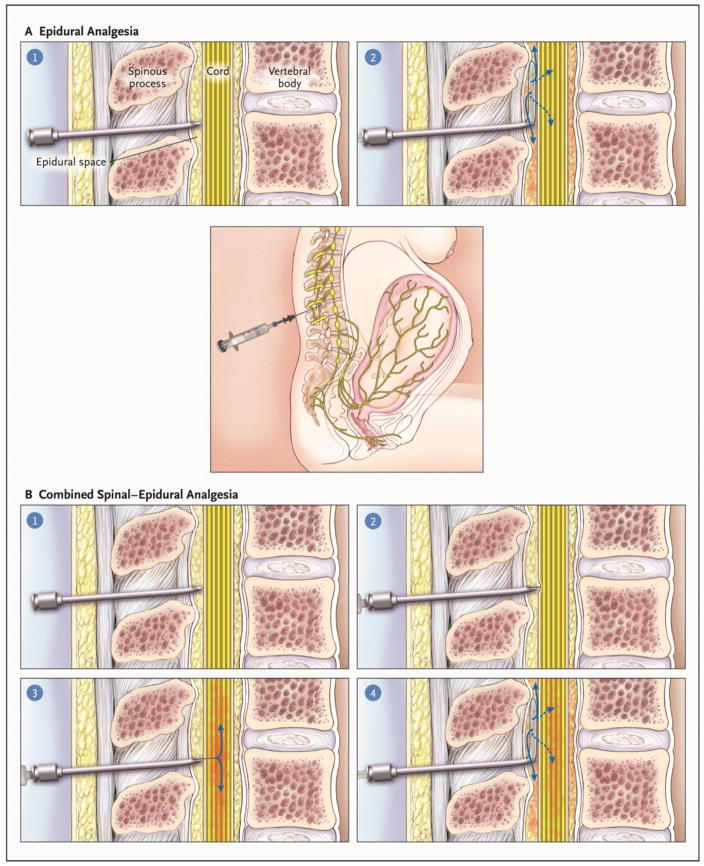
Labor neuraxial analgesia is usually initiated by one of two methods: epidural or combined spinal-epidural analgesia (Figure 2).6 Combined spinal-epidural analgesia is often used for initiation of analgesia in advanced labor because of rapid onset of effective analgesia.7,8 Combined spinal-epidural analgesia has faster onset (2 – 5 minutes) than epidural analgesia (15 – 20 minutes), greater uniformity in sensory blockade and improved sacral dermatome coverage.9 While some studies report greater satisfaction and sense of control associated with combined spinal-epidural analgesia, the meta-analyses do not support this observation.9 Some experts have argued that confirmation of correct epidural catheter placement is delayed following initiation of combined spinal-epidural analgesia; however, a 2016 study suggests that may not be the case and favors combined spinal-epidural analgesia for earlier detection of failed epidural analgesia.10 Other studies have shown that epidural catheters sited as part of a combined spinal-epidural technique fail less often, both during labor and for intrapartum cesarean delivery.11,12 A possible explanation for these findings is confirmation of correct placement of the tip of the epidural needle in the epidural space by virtue of cerebrospinal fluid visualization through the spinal needle. A 2014 meta-analysis did not find a definitive benefit of combined spinal-epidural analgesia for catheter replacement rates, supplemental epidural dosing, and epidural vein cannulation; although the meta-analysis was limited by significant between-study heterogeneity.13 A higher risk of uterine tachysystole after combined spinal-epidural analgesia than epidural analgesia has been reported and may be attributable to the rapid decrease in circulating catecholamines (which have a tocolytic effect) that accompanies rapid-onset of labor analgesia.8
Figure 2.
Epidural analgesia technique (A) vs. combined spinal epidural technique (B). In epidural analgesia, the epidural space is located using an epidural needle, by a loss-of-resistance technique. A 19–20 gauge epidural catheter is threaded into the space and used to dose medications. In combined spinal-epidural analgesia, the epidural space is located in the same fashion, and prior to threading the epidural catheter, a small 25–27 gauge spinal needle is introduced through the epidural needle to puncture the dura and to bolus a single dose of local anesthetic with or without opioid. The spinal needle is removed and a 19–20 gauge epidural catheter is threaded for subsequent dosing. Figure from Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med. 2003 23;348:319–32.6
A modification of the combined spinal-epidural technique is dural puncture epidural analgesia.14,15 In this technique, the epidural space is identified and the dura is punctured with a 25-gauge or smaller pencil-point spinal needle, but no intrathecal medication is injected; an epidural catheter is threaded in the routine manner. Dural puncture epidural analgesia may be associated with improved sacral analgesia compared to epidural analgesia, with less pruritus, hypotension, supplemental epidural doses, and uterine tachysystole than combined spinal-epidural analgesia.14,15 A likely mechanism is the dural hole acts as a conduit to enhance epidural medication translocation into the intrathecal space, allowing enhanced coverage of sacral nerve roots while avoiding the side effects associated with conventional combined spinal-epidural analgesia. Dural puncture epidural analgesia may be a viable technique for patients with a suspected difficult airway or failed epidural labor analgesia, for whom confirmation of correct epidural needle placement is critical, without incurring the side effects of spinal medication dosing.
Modern labor analgesia favors initiation and maintenance of analgesia with low-dose local anesthesia and opioid solutions to minimize risks of local anesthetic systemic toxicity (unintentional intravascular injection) or high- or total-spinal anesthesia (unintentional intrathecal injection). These low-dose strategies also minimize hemodynamic effects and placental drug transfer.16 Dilute local anesthetics reduce the risk for motor block which may contribute to instrumental delivery and postpartum nerve palsies.17 Initiation of contemporary labor epidural analgesia combines low-dose, long-acting amide local anesthetics, typically a bolus of 5–15 mL of bupivacaine 0.0625% – 0.125%, with a lipid soluble opioid, typically fentanyl 50–100 μg or sufentanil 5–10 μg.18 The drugs used to initiate combined spinal-epidural analgesia may vary based on the stage of labor. An opioid-only intrathecal dose (e.g., fentanyl 25 μg) is highly effective in treating pain associated with the first stage of labor, although it is accompanied by a high incidence of pruritus; a combination of intrathecal local anesthetic and lipid soluble opioid (e.g., bupivacaine 1.25–2.5 mg and fentanyl 15 μg) effectively treats somatic pain of the late first and second stages of labor.18 Epidural analgesia is usually maintained with an infusion of bupivacaine 0.05%–0.1% with fentanyl 1.5–3 μg/mL or sufentanil 0.2–0.33 μg/mL at a rate of 8–15 mL/hour into the epidural space.18 Combining local anesthetic with lipid soluble opioid allows for profound visceral and somatic analgesia. The synergy between opioid and local anesthetic medications allows dose-reduction of both drugs, minimizing side-effects.19
Continuous epidural infusion vs. programmed intermittent bolus
Prior to the advent of infusion pump technology, maintenance of labor analgesia occurred by manual intermittent boluses throughout labor. A major disadvantage of this maintenance strategy was that analgesia would eventually regress, leading to recurrence of pain, requiring another manual bolus; thus, analgesia was episodic. With the advent of infusion pumps, continuous epidural infusion techniques became popular. This technique resulted in more stable analgesia and reduced supplemental epidural dosing for breakthrough pain compared to manual intermittent bolus strategies.7 As technology improved, patient-administered bolusing (patient-controlled epidural analgesia) was introduced. Evidence from randomized trials support that analgesia is superior when patient-controlled epidural analgesia is used with a background infusion compared to without a background infusion.7,20,21 Patient-controlled epidural analgesia is preferable to fixed-rate continuous epidural infusion because of lower total local anesthetic dose consumption, and lower incidence of motor blockade, and need for anesthesia provider interventions.7 Settings for patient-controlled epidural analgesia are variable, but generally include a background infusion of bupivacaine 0.05%–0.1% with fentanyl 1.5–3 μg/mL or sufentanil 0.2–0.33 μg/mL at 5–8 mL/hour, a bolus of 5–10mL, and a lock-out interval of 10–20 minutes.16
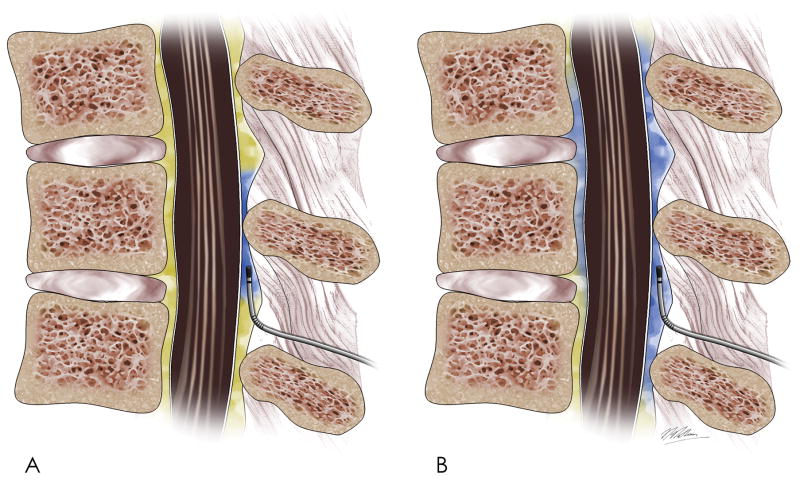
Programmed intermittent epidural bolus has been recently investigated for maintenance of labor epidural analgesia. Rather than administering the maintenance dose as a continuous infusion, with or without patient-controlled epidural analgesia, it is administered by the infusion pump programmed to deliver boluses of epidural solution at regular intervals. The likely mechanism of improved analgesia is greater medication spread in the epidural space (the epidural catheter is usually sited in a mid-lumbar epidural interspace, and satisfactory labor analgesia requires coverage of both low-thoracic and sacral dermatomes (Figure 3). One dosing strategy involves a solution of bupivacaine 0.625% with fentanyl 2 μg/mL with an intermittent epidural bolus of 6-mL every 30 minutes, in addition to patient-controlled epidural analgesia allowing a 5-mL bolus with 10-minute lockout.22 The programmed intermittent epidural bolus technique allows maintenance of analgesia with less local anesthetic without impairing maternal analgesia and satisfaction, is associated with fewer supplemental epidural doses (less breakthrough pain), and has reduced risk for motor block and instrumented delivery.22–25 In one trial, motor block occurred more frequently (odds ratio 21.2, 95% confidence interval 4.9 to 129.3, P < 0.001) and earlier in women randomized to receive continuous epidural infusion compared with a programmed intermittent epidural bolus to maintain analgesia. Instrumental delivery occurred more frequently in the continuous epidural infusion group (20% v. 7%, P = 0.03).23 A meta-analysis of 9 trials showed lower local anesthetic dose and higher satisfaction scores with programmed intermittent epidural bolus.25 Higher local anesthetic doses may be associated with reduced pelvic floor muscle tone, reduced mobility, impaired Valsalva maneuvers, and risk for instrumental delivery.26 Administration of local anesthetic by continuous infusion is inherently safer than bolus dosing. Bolus dosing by a human (anesthesia provider or patient) offers safety because the presence of pain suggests that the catheter is not malpositioned in the subarachnoid space. A potential disadvantage of programmed intermittent epidural bolus is unintentional high neuroblockade that may accompany catheter migration into the intrathecal space.27
Figure 3.
Maintenance of epidural analgesia by continuous epidural infusion vs. programmed intermittent epidural bolus. Differences in spread (blue pigment) of equivalent doses of local anesthetic over course of 1 hour in (A) continuous epidural infusion and in (B) programmed intermittent epidural bolus are depicted.
Newer equipment now enables use of programmed intermittent epidural bolus in clinical practice. The focus of current research is identifying optimal settings for epidural bolus volume and interval, bolus infusion rate, and local anesthetic concentration.28
Systemic opioids for labor analgesia
Systemic opioids are an alternative to women for whom neuraxial analgesia may be contraindicated, cannot be achieved (technical failure to place an epidural catheter), or who prefer an alternative method of labor analgesia. A common approach involves fentanyl patient-controlled intravenous analgesia, typically 25 μg every 10–15 minutes, with an hourly lockout of 100 μg.29 In the past decade, remifentanil patient-controlled intravenous analgesia has gained popularity due to its titratability and short latency (60–90 seconds). However, timing the self-administered bolus dose with the peak of uterine contractions is difficult; the peak analgesic effect typically occurs with the second contraction after the button is pushed, and contraction frequency may be irregular. Because remifentanil is rapidly metabolized by plasma esterases, it is appealing for reduced fetal placental transfer, and for rapid fetal clearance of drug. Remifentanil patient-controlled intravenous analgesia provides reasonable analgesia and maternal satisfaction, but maternal sedation, respiratory depression and apnea are well-described.30,31 In one trial, the risk for maternal oxygen desaturation was significantly higher in women receiving remifentanil compared to fentanyl.32 Monitoring of respiratory variables (respiratory rate, end-tidal carbon dioxide, pulse oximetry, heart rate, and pulmonary index) has low positive predictive values for surveillance of maternal apnea.33 Therefore, remifentanil patient-controlled intravenous analgesia should be accompanied by continuous respiratory monitoring; we believe this monitoring is ideally achieved by 1:1 provider observation (nurse, midwife, or anesthesia provider).34,35
Remifentanil patient-controlled intravenous analgesia is not superior to neuraxial labor analgesia techniques. A meta-analysis of 5 randomized trials found higher pain scores in women receiving remifentanil.36 However, one randomized trial noted that while pain scores reductions were greater with neuraxial analgesia, patient satisfaction scores were not different.30 These findings support the repeated observation that patient satisfaction for labor analgesia is not driven solely by reductions in pain intensity. In a 2014–2015 survey, only 36% (95% confidence interval 26 to 46) of academic obstetric units in the United States used remifentanil for labor analgesia, with most doing so less than 5 times a year.35
Compared to remifentanil, fentanyl patient-controlled intravenous analgesia for labor analgesia has a lower rate of maternal sedation and respiratory depression; however, it has a higher rate of neonatal respiratory depression requiring resuscitation at delivery.37 In one study, 59% of neonates whose mothers used fentanyl compared with 25% for remifentanil patient-controlled intravenous analgesia required resuscitation (odds ratio, 4.33; 95% CI: 1.75 to 10.76).37 Remifentanil may offer modest analgesic advantage over fentanyl (mean visual analog scale (VAS) score, remifentanil: 46 mm v. fentanyl 60 mm, P < 0.01).32
Nitrous Oxide
There is a renewed interest in the United States in nitrous oxide (N2O) for labor analgesia, although it has been integrated into labor analgesia in other parts of the world (e.g. Europe) for many years. Women who use N2O report improved maternal satisfaction and coping compared to no analgesia, although its analgesic efficacy is inferior to neuraxial labor analgesia.38 These findings are not surprising, given that maternal experience is known to be influenced by factors such as a sense of control and ability to participate in decision making, and is not solely influenced by the provision of effective labor analgesia.39
N2O for labor analgesia has a long history of safe maternal use, although rigorous study is lacking and questions remain regarding neonatal-childhood outcomes and occupational risks of exposure.29 In experimental models and in some clinical settings, N2O has been shown to be neurotoxic and genotoxic, with adverse effects on the hematologic and immunologic systems.40–43 Although several studies have reported no adverse neonatal events of this nature after maternal exposure to N2O for labor, these studies have been limited by flaws in study design, conduct, analysis, and reporting.38 N2O is a potent greenhouse gas, although some experts contend that medical use of N2O has little environmental impact.40 Occupational exposure (reproductive toxicity) may be a concern if N2O delivery does not employ robust scavenging equipment.40
N2O for labor analgesia and neuraxial analgesia result in similar degrees of maternal satisfaction. Its analgesic efficacy exhibits high inter-individual variability. However, interest in increasing women’s choices for labor analgesia and patient satisfaction in United States hospitals makes offering N2O during labor analgesia an attractive option.
Pharmacogenomics and pain genetics
Scientific advancements in genetic medicine will likely allow development of personalized pain management strategies in the future, but our current knowledge is still inadequate for precision labor analgesia. For example, a single nucleotide polymorphism (SNP) of the μ-opioid receptor gene (OPRM1, A118G) may be present in up to 30% of the obstetric population, and is linked to altered responsiveness to neuraxial opioids; the polymorphism increases binding and potency of β-endorphins.44 These properties are linked to later request for analgesia and lower neuraxial fentanyl and sufentanil dose requirements (ED50) in labor, compared to women with the wild-type alleles.44,45 In apparent contrast to these study results are the findings of a study from Asia; women who were homozygous for the A118G polymorphism had increased opioid dose requirements after cesarean delivery, and more breakthrough pain.46 A 2009 meta-analysis of studies of the effect of the OPRM1 A118G polymorphism on pain included studies from North America, Asia, and Europe and found no effect of the polymorphism on opioid dose requirement.47
The influence of genetic polymorphisms on labor progress has been investigated. Terkawi et al. found that polymorphisms in the β2-adrenergic receptor gene (ADRB2) were linked to labor pain; however, these polymorphisms explained less than 1% of the inter-subject variability.48 Similarly, catechol-O-methyltransferase (COMT) and oxytocin (OXTR) gene receptor polymorphisms were linked to slower transitions to active labor and slower latent phase of labor.49 While genetic factors will likely not entirely explain inter-individual differences in labor pain and labor progress, continuing advances in pain genetics and pharmacogenetics may contribute to our future ability to provide individualized therapies for labor pain and analgesia.
Effect of Labor Analgesia on Labor Progress and Mode of Delivery
Labor neuraxial analgesia and risk for instrumental delivery
Epidural labor analgesia has been linked to increased risk for instrumental vaginal delivery, although the nature of the relationship is controversial. Challenges to definitive investigations include obstetrician practice and the likelihood that instrumental delivery is attempted more often when effective neuraxial analgesia is present (Table 1). Understanding the relationship between neuraxial analgesia and operative delivery is important because modern obstetrical skills in instrumental vaginal delivery is declining;18,50 this trend may result in rising, indirect associations between labor neuraxial analgesia and increased rates of second stage cesarean deliveries.
Table 1.
Challenges to definitive investigations on labor neuraxial analgesia’s effect on risk for instrumental delivery.
| Factor/Confounder | Comment |
|---|---|
| Density of neuraxial block at second stage of labor | Dense analgesia may: (1) impair maternal expulsive efforts (motor block); (2) impede maternal coordination of expulsive effort with uterine contraction (dense sensory block); (3) excessively relax pelvic floor muscle tone and impair fetal head rotation |
| Obstetrician Practice | None of the trials are blinded, therefore, obstetricians who make the decision to perform an instrumental vaginal delivery are not blinded to group allocation. Obstetricians may be more likely to perform instrumented delivery in a woman with effective second stage analgesia Randomized control trials on this topic have been performed in academic centers, where an obligation to teach instrumental delivery exists |
| Practice Type | Randomized control trials from academic centers have shown an association between neuraxial analgesia and instrumental delivery Impact studies (pre-post studies carried out primarily at military medical centers or other non-training institutions) have failed to find an association between neuraxial analgesia and instrumental delivery |
| Factors influencing degree of neuraxial block | Higher local anesthetic concentrations and higher higher total doses are linked to higher risk for instrumental delivery; method of neuraxial analgesia maintenance (i.e. continuous infusion, programmed intermittent bolus) show variable results for rates of instrumental vaginal delivery, primarily driven by differences in concentration and motor block |
| Method of neuraxial labor analgesia initiation | Comparisons of combined spinal-epidural and epidural techniques for outcome of instrumental delivery have had conflicting results |
Table based on Wong CA: Epidural and Spinal Analgesia/Anesthesia for Labor and Vaginal Delivery, Obstetric Anesthesia: Principles and Practice. Edited by Chestnut DH, Mosby, 2014, pp 496.18
Meta-analyses of randomized trials comparing labor neuraxial analgesia to systemic opioids found that the mean duration of the first and second stages of labor were prolonged in neuraxial analgesia groups by 30 minutes and 15 minutes, respectively, and the rate of instrumental vaginal delivery was increased in women receiving neuraxial analgesia (relative risk, 1.42, 95% confidence interval 1.28 to 1.57, 23 trials, 7935 women).51 However, many of the trials that were included in the meta-analyses used epidural bupivacaine concentrations of 0.25%. This concentration is considered high, by modern standards. Addressing this concern, the COMET (Comparative Obstetric Mobile Epidural Trial) Study compared low-dose labor epidural techniques to a “traditional” or high-dose technique in a randomized controlled design.52 The high-dose group received epidural analgesia initiated with 10 mL bupivacaine 0.25% (25 mg), with subsequent boluses of 10-mL bupivacaine 0.25% (25 mg) on request (but no more than hourly). One low-dose group received epidural bupivacaine 0.1% with fentanyl 2 μg/mL; analgesia was maintained with an infusion. The second low-dose group had combined spinal-epidural initiation (spinal dose: bupivacaine 2.5 mg and fentanyl 25 μg) and maintenance analgesia by intermittent injections of 0.1% bupivacaine with fentanyl. The investigators found that high-dose epidural analgesia was associated with a reduced rate of normal spontaneous vaginal delivery. These differences were explained by reduced instrumental vaginal delivery rates in the low-dose groups.52 There was no difference in total dose of local anesthetic between groups, likely due to method of analgesia maintenance: the high-dose group had medication delivered by intermittent bolus, whereas the low-dose group had medication delivered by continuous infusion. Specific analgesic technique and drug combination/dose may be influential; a meta-analysis comparing combined spinal-epidural and epidural analgesia showed that instrumental deliveries were lower in combined spinal-epidural compared to “high-dose” epidural analgesia, but not compared to “low-dose” epidural analgesia.9 The true effect and impact of labor epidural analgesia on risk for instrumental delivery remains poorly understood.
More recently, an observational study of over 600,000 deliveries in the Netherlands did not demonstrate a change in instrumental delivery rates despite almost tripling the labor neuraxial analgesia rate from 7.7% to 21.9% over 10 years.53 A meta-analysis of 28,443 patients showed no effect of increasing availability of labor neuraxial analgesia on instrumental delivery rates.54 Concentration and motor function may be important; a meta-analysis of 11 randomized trials compared the instrumental delivery rate in high- versus low-concentration local anesthetic solution groups, and low-concentration strategies were linked to reduced risk for assisted vaginal delivery and motor block.17 Many studies have noted a relationship between total local anesthetic dose and motor blockade, but the association between motor blockade and instrumental delivery has been inconsistent.18 Although controversy persists, the available evidence suggests that functional labor analgesia is associated with risk for instrumental delivery, possibly by virtue of analgesic density and motor impairment.18 Instrumental vaginal delivery may increase risk for lacerations and other perineal injuries, neonatal facial or cranial injuries, and pelvic organ prolapse. Given these undesirable outcomes, the goal of modern labor epidural analgesia favors minimizing motor blockade by initiating and maintaining analgesia using low-concentration local anesthetic solutions.7 Nevertheless, minimizing risk for instrumental delivery while maximizing patient comfort requires skillful attention to individual patient needs and clinical circumstances.
Mode of delivery
Early observational studies identified an association between neuraxial labor analgesia and increased rates of cesarean delivery; however, the relationship is not surprising given that women requesting neuraxial analgesia are more likely to be experiencing more painful labor.18 Factors associated with more painful labor are themselves associated with an increased risk for cesarean delivery (e.g., fetal malrotation, fetal-pelvic disproportion, dysfunctional labor).18 Early trials were limited by methodologic concerns, including mixed populations of nulliparous and parous women, use of different types of neuraxial analgesia, inconsistent density of blockade, and high protocol violation and study group crossover rates.55–57 A study from Parkland Hospital in Dallas, TX, USA (where the patient population is primarily indigent and labor is managed by the same group of obstetricians and midwives) compared the cesarean delivery rate in women receiving epidural analgesia to women receiving systemic meperidine analgesia.55 A per protocol analysis suggested that the cesarean delivery rate was higher among women who used epidural analgesia (9% vs. 3.9%).55 However, the rate of crossover from meperidine to the epidural group was approximately 33%. After performing an intent-to-treat analysis, the cesarean delivery rate was not different (6%) between groups).58 In a subsequent study at the same hospital, there was no difference in cesarean delivery rates when intravenous patient-controlled analgesia was used as a control. Use of this methodology resulted in better analgesia in the control group; only 5 out of 357 patients crossed over.59
A 2011 systematic review of 38 randomized trials did not identify a link between labor epidural analgesia and risk for cesarean delivery.51 Impact studies (comparison of the institution’s cesarean delivery rate before and after the introduction of a neuraxial labor analgesia service) have shown no association between labor neuraxial analgesia and cesarean delivery.54,60–62 Altogether, although the debate persists, the evidence does not support that neuraxial labor analgesia increases the risk for cesarean delivery.7
“Early” labor epidural analgesia (i.e., epidural analgesia performed during the latent phase of labor) was historically believed to be a risk factor for cesarean delivery. Observational trials suggested that women who requested neuraxial analgesia early in labor (commonly defined as cervical dilation less than 4 cm) had a higher cesarean delivery rate.63 This translated into a common practice among obstetric practitioners in the 1990s, advising their patients to avoid epidural analgesia in early labor.
In contrast to observational trials, multiple randomized control trials comparing early to later initiation of labor neuraxial analgesia failed to find a link between early use and risk for cesarean delivery (Table 2).64–70 These trials compared early labor neuraxial analgesia and systemic opioid analgesia; women randomized to receive early systemic opioid analgesia received neuraxial analgesia later in labor. The trials were well controlled; and crossover rates were not excessive. In two separate trials, Chestnut et al. found early epidural analgesia among nulliparous women was not associated with increased risk for cesarean delivery in both spontaneous and oxytocin-induced or -augmented labor.65,66 These findings were important because they supported the provision of epidural analgesia during latent labor, whereas this practice was formerly thought to increase risk for cesarean delivery. Later, Wong et al. also found no difference in the rate of cesarean delivery among women who received combined spinal-epidural analgesia at less than 4 centimeters of cervical dilation compared with those who received early labor systemic opioid analgesia followed by epidural analgesia later in labor; onset and intensity of analgesia were superior in the combined spinal-epidural analgesia group.64 Ohel et al. found similar results; the rates of cesarean delivery in women who received early compared with late epidural analgesia were similar (13% v. 11%, P = 0.77).68
Table 2.
Summary of randomized, controlled trials investigating effect of early labor epidural analgesia on mode of delivery in nulliparous women.
| Study | Year | Comparison Groups | Patient Population | N | Early Neuraxial Analgesia | Late Neuraxial Analgesia |
|---|---|---|---|---|---|---|
| Chestnut65 | 1994 | Early Epidural | Spontaneous labor | 172 | 17/172 (10%) | 13/162 (8%) |
| Late Epidural | 162 | |||||
|
| ||||||
| Chestnut66 | 1994 | Early Epidural | Receiving oxytocin | 74 | 13/74 (18%) | 14/75 (19%) |
| Late Epidural | 75 | |||||
|
| ||||||
| Luxman67 | 1998 | Early Epidural | Spontaneous labor | 30 | 2/30 (6.6%) | 3/30 (10%) |
| Late Epidural | 30 | |||||
|
| ||||||
| Wong64 | 2005 | Early CSE | Spontaneous labor | 366 | 33/366 (18%) | 75/362 (21%) |
| Late Epidural | 362 | |||||
|
| ||||||
| Ohel68 | 2006 | Early Epidural | Spontaneous or induced labor | 221 | 28/221 (13%) | 25/228 (11%) |
| Late Epidural | 228 | |||||
|
| ||||||
| Wang69 | 2009 | Early Epidural | Spontaneous labor | 6394 | 1486/6394 (23%) | 1456/6399 (23%) |
| Late Epidural | 6399 | |||||
|
| ||||||
| Wong70 | 2009 | Early Epidural | Induction of labor | 406 | 134/406 (33%) | 126/400 (32%) |
| Late Epidural | 400 | |||||
N: number of subjects in the study
CSE, combined spinal-epidural
All studies were powered for the primary outcome of cesarean delivery. “Early” neuraxial in most studies was defined as neuraxial analgesia initiated at less than 4 centimeters’ cervical dilation, or at a cervical dilation of “at least” 1 centimeter.
Considering these findings, the data linking labor epidural analgesia to cesarean delivery may be better explained by the observation that women with more painful labors, especially early labor pain, are more likely to require cesarean deliveries due to obstetrical factors such as fetal macrosomia, malrotation, and dysfunctional labor.71–73 The practice of avoiding neuraxial labor analgesia in early labor for fear that it will adversely affect the mode of delivery should be completely abandoned.7
Progress of labor
While some studies have demonstrated a modest prolongation of the first stage of labor (mean approximately 30 minutes),74 others have shown neuraxial analgesia is associate with to faster labor. Wong et al. and Ohel et al. found early labor neuraxial analgesia resulted in faster labor compared to treating early labor pain with systemic opioids and initiating neuraxial analgesia later in labor.64,68 A 2017 meta-analysis did not find a relationship between low-concentration epidural analgesia and the duration of labor; however, studies were of low quality and the confidence intervals were wide.75
The reasons for the conflicting results are multifold. Methodologically, trials differ in how they define the onset of labor. Epidural analgesia may delay cervical examination due to effective analgesia (examinations establishing full cervical dilation are typically deferred until the parturient complains of rectal pressure). Epidural analgesia has been linked to both increased and decreased uterine activity.8,76–78 Decreased uterine activity may be explained by co-administration of intravenous fluid, reducing circulating antidiuretic hormone and reducing endogenous oxytocin (both hormones are produced by the posterior pituitary gland).77 Increased uterine activity may be explained by a rapid reduction in circulating catecholamines associated with initiation of analgesia;8,78 the withdrawal of β2-adrenergic activity (tocolytic) may result in frequent and more intense uterine contractions leading to uterine tachysystole. Heterogeneous effects of epidural analgesia on uterine activity and first stage of labor may also be explained by variability in neurophysiological responses to labor, pain, and analgesia.79
Effective epidural analgesia is associated with a prolonged second stage of labor, with an estimated mean difference of 15 minutes, which is not clinically meaningful.74 However, the duration of the second stage of labor at the 95th percentile may be prolonged up to 2 hours in both nulliparous and parous women with epidural analgesia.80,81 The impact of prolonged second stage of labor on maternal and neonatal outcomes deserves scrutiny. Older studies have not shown adverse maternal or neonatal outcomes associated with prolonged second stage of labor, provided that the fetal heart rate tracing remains reassuring and there is progressive fetal descent.82–84 However, in a large multicenter observational study, longer periods of active pushing were associated with an increased relative risk for neonatal complications, such as mechanical ventilation, sepsis, brachial plexus palsy, encephalopathy, and death, although the absolute risk was low.85 Other studies have shown an increased risk of adverse maternal outcomes (e.g., chorioamnionitis, high-degree lacerations, atony, hemorrhage, fever) for every additional hour spent in the second stage of labor.86,87 Given the association between prolonged second stage of labor and adverse maternal and neonatal outcomes, the effect that neuraxial analgesia may have on labor duration remains an important research question.
Neuraxial anesthesia for external cephalic version
External cephalic version is a procedure wherein a breech fetus at 36 to 39 weeks’ gestation is manually rotated to the vertex presentation, permitting a trial of labor and vaginal delivery. The procedure is an important strategy for prevention of primary cesarean delivery (17% of primary cesarean deliveries are due to fetal malpresentation).88 Prevention of primary cesarean delivery is an important public health concern given the high rates of cesarean delivery, maternal morbidities associated with cesarean delivery compared to vaginal delivery, and increasing health care costs and maternal risk in subsequent pregnancies after primary cesarean delivery. Neuraxial anesthesia for attempted external cephalic version is associated with a higher success rate.89
The findings of early studies of the role of neuraxial anesthesia in external cephalic version were equivocal.90,91 Some obstetricians are concerned that neuraxial analgesia will mask pain related to uterine rupture or placental abruption, rare but catastrophic complications of external cephalic version. A 2011 meta-analysis allays these concerns, showing no differences in the rates of placental abruption or uterine rupture in neuraxial anesthesia vs. control groups who received no analgesia or systemic opioid analgesia. 92 Risk for cesarean delivery for non-reassuring fetal heart rate was also not different between neuraxial anesthesia and control groups.
Meta-analyses of randomized control trials have identified a 13–50% increase in the rate of successful external cephalic version with neuraxial anesthesia; most women who have a successful external cephalic version have a successful vaginal delivery.89,92,93 The results of early meta-analyses suggested that the success rate may be dose-dependent: denser neuroblockade has a higher success rate.93 Surgical-level neuraxial anesthesia is postulated to enhance relaxation of abdominal wall musculature, assisting the manual efforts of the obstetrician. However, a 2017 study in which women were randomized to receive combined spinal-epidural analgesia with intrathecal fentanyl combined with varying doses of bupivacaine (2.5, 5, 7.5, and 10 mg) did not support a dose-response effect on external cephalic version success rate (50%, 52%, 52% and 49%, respectively; P = 0.99).94 There were no differences in obstetrician rating for abdominal relaxation. An advantage of neuraxial anesthesia for external cephalic version is the ability to convert to surgical anesthesia in the event of emergency cesarean delivery. Disadvantages of neuraxial analgesia/anesthesia for external cephalic version include hypotension and delayed hospital discharge, both of which may be dose-dependent. Hypotension is typically easily treated, but requires close monitoring. An economic analysis on the use of neuraxial anesthesia for external cephalic version found it to be cost-effective, assuming an improved success rate of at least 11% from a baseline of 38%.95 This finding is explained by the large differences in costs between vaginal delivery and cesarean delivery.
Oral Intake in Labor
Aspiration pneumonitis or solid gastric content asphyxiation was a leading cause of anesthesia-related maternal mortality.3 The stomach shifts cephalad, displacing the lower-esophageal sphincter into the thorax.96 Lower esophageal sphincter pressure declines by 50% during pregnancy.97 Reduced motilin produces slower intestinal transit times.98 While pregnancy does not increase gastric emptying time, endogenous or exogenous opioids prolong gastric emptying times.99,100
To address aspiration-related maternal mortality in the middle part of the 20th century, the following practices became the cornerstone of modern obstetric anesthesia practice: 1) widespread use of neuraxial anesthesia; 2) oral intake restrictions during labor; 3) pre-anesthetic antacid administration; 4) rapid-sequence induction for general anesthesia; 5) improvements in anesthesia training; and 6) improvements in advanced airway devices. These practices are reflected in current American Society of Anesthesiologists recommendations.7 Because of these practices, maternal mortality from aspiration has declined to extremely low levels (estimated case fatality rate, 6.5 per million anesthetics in the Unites States).5,101,102 Closed claims analysis shows a significant reduction in malpractice claims from aspiration.103 Because of the modern rarity of aspiration-related mortality, and with growing interest in limiting medical interventions during low-risk labor, liberalizing oral intake during labor is appealing.104 The World Health Organization advocates no interference with a woman’s desire to eat and drink during low-risk labor.105 Liberalizing oral intake might have advantages for patient satisfaction, and it seems intuitive that providing energy during a demanding metabolic period might improve outcomes. Nil per os practices in pregnancy have been linked to a state of “accelerated starvation” due to shifts to glycogenolytic and gluconeogenesis metabolic pathways.106
Early studies shed light on outcomes with liberalized oral intake strategies in labor.107–109 In one study, women were randomized to a light meal or to water; epidural analgesia with opioid-containing solutions was permitted.109 Women in the light diet group had lower plasma β-hydroxybutyrate and non-esterified fatty acids, indicating ketosis prevention. However, there were no differences in lactate, labor duration, Apgar scores, and umbilical cord blood gases. Light diet consumers were more likely to vomit, and vomited higher volumes of particulate matter, during labor. In another study, rates of vomiting were similar between water and sports drinks, while reduced markers of ketoacidosis without increases in gastric volumes were found in sports drink consumers.107 A large trial found no differences in the rate of vaginal delivery, duration of labor, cesarean delivery, or vomiting.108
Meta-analyses in low-risk deliveries show no effect of food intake on mode of delivery and neonatal well-being, although pooled data were insufficient to address the risk for aspiration.110,111 There are two possible interpretations of these data. First, given the contemporary rarity of aspiration, maternal wishes should take priority, and oral intake guidelines liberalized to allow maternal decision-making for light meals during low-risk labors. Alternatively, women seem to tolerate limited oral intake in labor without negative consequences, and considering the large decrease in maternal mortality since nil per os strategies were implemented, there is no need to liberalize oral intake restrictions. Current American Society of Anesthesiologists Guidelines allow clear liquid intake in uncomplicated labor and complete avoidance of particulate and solid food.7 Nil per os strategies for parturients undergoing elective surgery (e.g. scheduled cesarean delivery or postpartum tubal ligation) include fasting for 2 hours for clear liquids and 6 to 8 hours for solid food, depending upon fat content.7
Considering the historical context in which nil per os strategies developed, along with ethical and logistical challenges of conducting a trial addressing harm, we will likely continue seeing global and cultural discrepancies on oral intake during labor. Based on available data and history, our practice is to avoid solid food and particulate liquid ingestion in labor, particularly if parenteral or neuraxial opioids were administered, to allow glucose-containing clear liquids as tolerated, and to restrict oral intake in individuals after considering co-morbidities that may increase the risk for cesarean delivery or aspiration (e.g., obesity, diabetes mellitus, suspected difficult airway, and nonreassuring fetal heart rate tracing).
Anesthesia for Cesarean Delivery
Advances in spinal anesthesia for cesarean delivery
Single-shot spinal anesthesia is the most common technique for cesarean delivery due to its simplicity, quality of sensory blockade, and reliability. In contrast to epidural anesthesia, the total local anesthetic dose is lower; there is no risk for local anesthetic systemic toxicity and minimal fetal drug transfer.12,112 The effective dose for hyperbaric bupivacaine in 95% of patients (ED95) is 13 mg when administered with intrathecal fentanyl and morphine. Higher doses (e.g., 15 mg) are associated with longer duration, but also with higher sensory blockade to cervical dermatomes, and a higher incidence and degree of hypotension.113
Adding a lipid-soluble opioid (e.g., fentanyl, sufentanil) to local anesthesia enhances intraoperative anesthesia by reducing the total dose of local anesthetic, reducing hypotension, nausea, and vomiting.114 Enhanced anesthesia is associated with less stimulation upon surgical traction of the viscera, contributing to a lower rate of nausea, vomiting, and intraoperative supplemental analgesia.114 Adding morphine (a water-soluble opioid) confers postoperative analgesia of up to 36 hours.115 Epinephrine (0.1–0.2 mg) is often added in clinical practice, producing a 15% increase in block duration and improving the quality of intraoperative analgesia, while increasing block recovery time.116 Clonidine improves intraoperative analgesia and reduces shivering and hyperalgesia, but is associated with hypotension and sedation; its use in this setting is off-label.117
Conversion of epidural analgesia to surgical anesthesia
Epidural analgesia is converted to surgical anesthesia by administering high-concentration local anesthetic. 2% Lidocaine with epinephrine 1:200,000, 15 to 20 mL is commonly used. The addition of 8.4% sodium bicarbonate (1 mL for every 10-mL local anesthetic solution) alkalinizes the local anesthetic solution, which hastens onset of action. 3% 2-Chloroprocaine, 15 to 20 mL may be used for urgent deliveries because of its shorter latency. Successful conversion to epidural anesthesia is critical to avoid general anesthesia; emergency general anesthesia is linked to poor outcomes (postoperative pain and sedation, intraoperative awareness, postpartum hemorrhage, and morbidity and mortality from aspiration or failed tracheal intubation). The ability to successfully convert epidural analgesia to anesthesia for intrapartum cesarean delivery has been proposed as a quality metric; in the United Kingdom, the National Institute for Health and Care Excellence (NICE) guidelines state that general anesthesia should be used in <1% of all elective cesarean deliveries and <5% of all emergency cesarean deliveries.118
Several risk factors for failed conversion include delivery urgency, supplemental analgesia during labor, initiation by epidural rather than combined spinal-epidural technique, and anesthesia by generalist compared with obstetric anesthesiologists.11,12 In one study, generalist anesthesiologists had significantly increased risk for failed conversion of epidural analgesia to anesthesia for cesarean delivery (odds ratio 4.6, 95% confidence interval 1.8 to 11.5).11 Reasons for increased successful conversion by obstetric anesthesiologists may include increased likelihood to manipulate the catheter, active management of breakthrough labor pain, assessment of catheter functionality and analgesic quality throughout labor, integration of information on labor and maternal-fetal status into analgesia management, and enhanced team communication to anticipate intrapartum cesarean delivery.11
Intraoperative hypotension: the ideal vasopressor for cesarean delivery
Spinal anesthesia hypotension is caused by a decrease in systemic vascular resistance; cardiac output increases.119 The ideal vasopressor to maintain uterine perfusion has been an area of intense research for several decades. Uteroplacental blood flow lacks autoregulation, making it directly dependent on uterine perfusion pressure and inversely proportional to uterine vascular resistance. Pure α1-adrenergic receptor agonists (phenylephrine) were expected to reduce uterine blood flow and induce fetal acidosis, and ephedrine was found to be superior to α1-agonists in fetal animal studies. The first human trials comparing phenylephrine and ephedrine were conducted in the late 20th century. Neonatal outcomes (umbilical artery pH, base excess) were better in groups randomized to phenylephrine.120–122 No study found neonatal depression despite very large maternal doses of phenylephrine (in one study the 75th percentile dose was 2130 μg).120–123 Consistently, the incidence of nausea and vomiting is lower with phenylephrine infusion. While maternal bradycardia occurred with phenylephrine, patients were asymptomatic and no adverse events were noted.
Ephedrine is associated with fetal acidosis due to placental transfer and direct fetal metabolism activation, but not from uterine blood flow perturbation.124 Experts conclude the efficacy and safety of phenylephrine make it superior for systemic vascular resistance restoration after spinal anesthesia.125,126 Prophylactic phenylephrine infusions (vs. intermittent boluses) are effective in preventing hypotension and require fewer anesthesia provider interventions.127 The current evidence supports prophylactic phenylephrine, titrated to maintain blood pressure near baseline (the usual dose range is 25 to 100 μg/min).125–128
Notably, most research comparing vasopressor therapy for cesarean delivery has been in healthy women undergoing elective cesarean delivery. Investigations for neonatal outcomes in maternal-fetal dyads with compromised placental function (e.g, preeclampsia) have been lacking. In 2017, a randomized double-blind trial compared phenylephrine and ephedrine infusion strategies in women with preeclampsia presenting for cesarean delivery under spinal anesthesia.129 There were no differences in umbilical arterial pH between groups. Similarly, among women with preeclampsia with severe features who also had nonreassuring fetal status, a bolus dose of phenylephrine to treat spinal anesthesia-induced hypotension did not result in better fetal acid-base status compared with ephedrine.130 It appears that for pre-eclamptic patients undergoing cesarean delivery, fetal outcomes are not influenced by choice of phenylephrine or ephedrine for prevention or treatment of spinal-anesthesia induced hypotension.
Several investigators suggest norepinephrine has characteristics of the “ideal” vasopressor to prevent and treat hypotension, but current evidence is limited.128 In one trial, patients receiving norepinephrine had higher heart rate and cardiac output compared with phenylephrine.131 The incidence of nausea and vomiting did not differ. Norepinephrine use was associated with lower umbilical artery and vein plasma catecholamine concentration and higher umbilical venous pH and oxygen content, potentially indicating higher uteroplacental oxygen delivery; the absolute differences were small (oxygen content phenylephrine, 11.8 mL/dL; oxygen content norepinephrine, 12.7 mL/dL; P = 0.047).131 In a study on post-spinal anesthesia hypotension in cesarean delivery, norepinephrine 8 μg was equivalent to phenylephrine 100μg for the treatment of the first episode of hypotension.132 Considering the existence of a highly-effective standard (phenylephrine infusion), additional accumulation of evidence is necessary before norepinephrine becomes a new standard.128
Supplemental oxygen
While supplemental oxygen is often routinely applied during cesarean delivery, evidence supporting improvement in maternal and neonatal outcomes is lacking, and some suggest it may cause harm by promoting free-radical generation and lipid peroxidation.133,134 A trial of 80% vs. 30% oxygen during cesarean delivery did not prevent wound infections or endometritis.135 A meta-analysis of 11 trials of supplemental oxygen found no benefit for maternal desaturation and neonatal Apgar scores.136 No convincing evidence of harm was identified, although higher maternal and neonatal markers of free-radicals were measured when supplemental oxygen was administered; the clinical significance of these findings is not clear. Data are lacking on the benefits or harms of supplemental oxygen in women with comorbid conditions (e.g., preeclampsia, obesity, labor with nonreassuring fetal heart rate tracing) or in intrauterine resuscitation. Theoretically, these neonates may be at increased risk of harm with hyperoxia because of greater lipid peroxidation from ischemia-reperfusion injury. The available evidence suggests that routine supplemental oxygen for scheduled, healthy cesarean deliveries with neuraxial anesthesia is not beneficial,136 and its elimination may improve patient comfort.
Post-cesarean delivery pain and analgesia
Pain after cesarean delivery is heterogeneous in expression and intensity. The ability to predict the severity and chronicity of post-cesarean delivery pain has the potential to personalize anesthetic care by identifying the patients at highest risk for severe pain and debilitation. Recent work has focused on psychometric and psychophysical profiling. Expected postoperative pain, baseline anxiety, and baseline fear of pain are independent predictors for increased postoperative opioid use, accounting for 40% of variance in postoperative pain and opioid used.137 Pan et al. validated a three-item questionnaire predicting pain after cesarean delivery;138 a follow-up study applied the questionnaire to a tailored analgesia regimen targeted at women at high risk for severe post-cesarean delivery pain.139 This type of work is key to advancing individualized pain management strategies in obstetrics.
Multimodal analgesia is the gold standard for post-cesarean delivery analgesia.140 An common strategy uses neuraxial morphine, scheduled nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, and limits systemic opioids to the treatment of breakthrough pain. Neuraxial morphine is the most effective component of post-cesarean delivery analgesia.141,142 It is easy to administer, inexpensive, and provides superior and prolonged analgesia for both static and dynamic pain.142 Its dynamic pain advantage is important for functional mobility in this population. Neuraxial morphine-related side effects include pruritus, nausea, urinary retention, and respiratory depression, although the risk for the latter is significantly lower when morphine is administered neuraxially than systemically.143,144 Side effects are dose-dependent; high-dose intrathecal morphine (>100 μg) has lower-lasting analgesia (4.5 hours) compared with low-dose morphine (50 to100 μg), but is associated with a higher rate of pruritus and vomiting.145 Pain scores and supplemental systemic morphine consumption do not differ between the high- and low-doses.
NSAIDs such as ketorolac, diclofenac, and ibuprofen are essential components of multimodal post-cesarean delivery analgesia. Their use spares opioids by up to 50%, translating to a 30% reduction of opioid-related side effects such as vomiting and sedation.146 The package insert for ketorolac states that practitioners should “exercise caution when ketorolac is administered to a nursing woman.”147 The excretion of ketorolac in breast milk is minimal and the American Academy of Pediatrics lists ketorolac as, “usually compatible with breastfeeding.”147,148 Given the safety profile of ketorolac is unlikely to be different from ibuprofen, a NSAID widely used in the postpartum period, we routinely use ketorolac in our practice if contraindications are not present. Contraindications to NSAIDs include renal disease (e.g. renal dysfunction in preeclampsia), sustained hypertension after delivery in a patient with preeclampsia, and a history of Roux-en-Y gastric bypass surgery.
The use of acetaminophen also exhibits opioid-sparing effects by up to 20% and has an additive effect when administered concomitantly with NSAIDs.149 Scheduling NSAIDs and acetaminophen after cesarean delivery confers greater reductions in supplemental opioid use compared to pro re nata (p.r.n.) administration.150
Peripheral nerve blocks for post-cesarean delivery analgesia
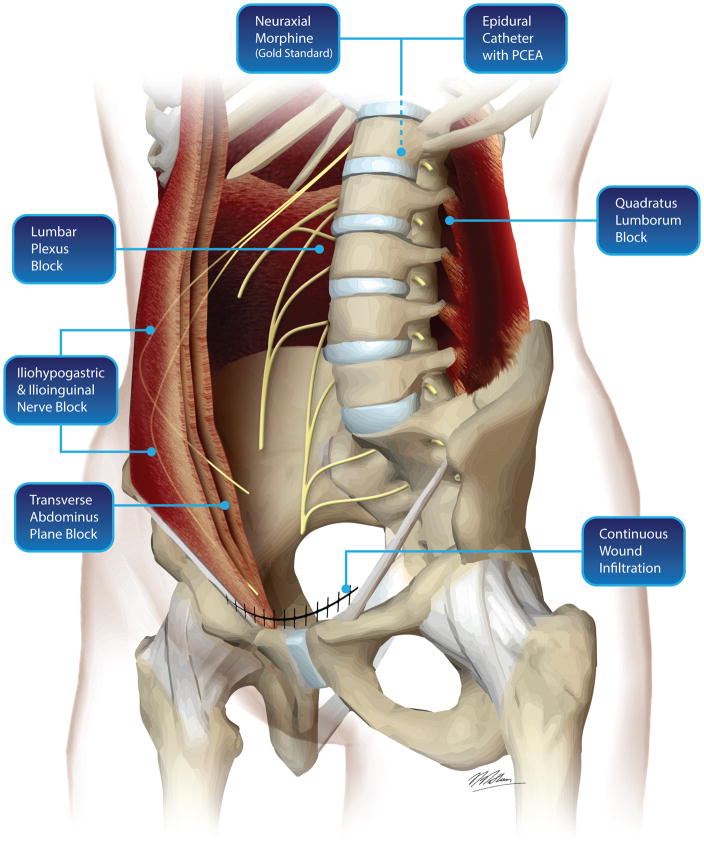
When other post-cesarean delivery pain management modalities are compared to neuraxial morphine, neuraxial morphine consistently performs best for analgesic quality (Figure 4). Nevertheless, alternative modes of postcesarean delivery analgesia have been proposed. Peripheral nerve blocks for Pfannenstiel and low-transverse incisional pain have been examined, including transversus abdominis plane, quadratus lumborum, and ilioinguinal-iliohypogastric blocks, and continuous wound infiltration. Transversus abdominis plane block is not superior to intrathecal morphine for post-cesarean delivery analgesia. In a comparison of intrathecal morphine combined with ropivacaine transversus abdominis plane block to intrathecal morphine combined with a sham block, there were no differences in pain with movement at 24 hours, and no differences in supplemental opioid dose.151 Two meta-analyses concluded that transversus abdominis plane block is not superior to intrathecal morphine, but transversus abdominis plane block may be useful when neuraxial morphine is not part of the pain management strategy (e.g., cesarean delivery with general anesthesia, contraindications to neuraxial morphine).152,153 The likely explanation for these findings is that transversus abdominis plane block is useful for treating incisional pain, but not visceral pain. A transversus abdominis plane block may be helpful for “rescue” analgesia for breakthrough pain after neuraxial morphine.154 Transversus abdominis plane block may be associated with subclinical signs of local anesthetic systemic toxicity, therefore, patients must be monitored closely after transversus abdominis plane block.155 Considering the evidence, the addition of transversus abdominis plane block to the gold standard (multimodal analgesia) is not routinely necessary for effective post-cesarean delivery analgesia.
Figure 4.
Post-cesarean delivery pain management options and anatomical locations of peripheral nerve blocks.
A quadratus lumborum block may have advantages over the transversus abdominis plane block because of its more superficial location (easier ultrasound visualization, theoretically improved safety). It involves deposition of local anesthetic into the fascial plane located between quadratus lumborum and erector spinae muscles; this space is continuous with the paravertebral space, thus enhancing medication spread to the include the sympathetic chain. In two randomized trials, quadratus lumborum block combined with spinal anesthesia was found to be superior to spinal anesthesia alone, and to transversus abdominis plane block with spinal anesthesia.156,157 A major limitation of these trials was the absence of comparison to intrathecal morphine (spinal anesthesia regimens did not have intrathecal morphine), therefore, no conclusions currently can be made about the superiority of the block to current standard of care.
Local anesthetic wound infiltration may be beneficial if cesarean delivery is performed under general anesthesia, but not under spinal anesthesia.158 Continuous wound infiltration improves pain on movement and reduces opioid use, but high infusion rates required to achieve this benefit lead to wound leakage and low patient and practitioner acceptability.158 Risk for surgical site infection is not increased, but these studies have not been powered for this outcome.159 Continuous wound infusion is less effective than parenteral morphine and NSAIDs.158 Most trials have not included neuraxial morphine comparisons, so no definitive comments can be made about superiority to neuraxial morphine. Similar to other nerve blocks, trials comparing ilioinguinal-iliohypogastric blocks to intrathecal morphine have not shown a benefit, but these blocks may have a role in rescue analgesia.160–163 Overall, while multimodal analgesia with neuraxial morphine, NSAIDs, and acetaminophen is the gold standard for post-cesarean delivery pain, supplemental analgesia using transversus abdominis plane, quadratus lumborum, ilioinguinal-iliohypogastric blocks, or wound infiltration may be useful in cases of breakthrough pain, or when the gold standard multimodal analgesia cannot be delivered (e.g. cesarean delivery under general anesthesia, contraindications to NSAID administration).
Obstetric Anesthesia Outcomes
Effects of labor analgesia on the fetus
Fetal bradycardia is occasionally observed after initiation of neuraxial labor analgesia. One trial found the incidence of fetal bradycardia was higher after combined spinal-epidural than epidural analgesia (32% v. 6%), although the study was limited by nonstandardized spinal dosing and monitoring for only 15 minutes after injection.8 One trial found fetal bradycardia was higher after intrathecal sufentanil 7.5 μg only compared with sufentanil 1.5 μg combined with epinephrine 2.5 μg and bupivacaine 2.5 mg. Although the authors concluded that the rate of fetal bradycardia was directly related to the intrathecal sufentanil dose, this conclusion requires further study; the low-dose sufentanil was administered in combination with other drugs (i.e., more than one variable was manipulated among groups). Importantly, there were no differences in neonatal outcomes (Apgar score, umbilical artery pH).78 A 2016 meta-analysis of 17 randomized trials found that fetal heart rate abnormalities are more likely to occur with combined spinal-epidural techniques; however, a sensitivity analysis including only studies that used low-concentration epidural bupivacaine was underpowered to determine whether a difference in fetal bradycardia exists.164 Whether the observed fetal heart rate abnormalities are tied to worse neonatal outcomes is unclear. The mechanism of analgesia-mediated bradycardia is thought to be rapid decrease in circulating epinephrine concentration with the onset of neuraxial analgesia. Epinephrine is a tocolytic, and its acute withdrawal may contribute to uterine tachysystole, reducing placental perfusion time (only occurs in uterine diastole). Reassuringly, studies have not found a difference between combined spinal-epidural and epidural techniques and emergency cesarean delivery.78,165 The usual measures of in utero fetal resuscitation (change in maternal position, intravenous fluid bolus, discontinuation of exogenous oxytocin) are usually successful in restoring fetal heart rate. Occasionally, administration of a tocolytic (nitroglycerin, terbutaline) is necessary.
Breastfeeding
Neuraxial analgesia’s effect on breastfeeding is controversial. Most studies are observational and results are conflicting; some have identified a negative association, some found no relationship, and some found a positive relationship.166 Studies lack control for multiple confounding variables (e.g., dosing and type of analgesia, intrapartum interventions, timing and method of breastfeeding measurements, social support, maternal return-to-work status) known to influence breastfeeding success. Factors likely more important than labor epidural analgesia include early maternal-infant bonding, skin-to-skin contact, and breastfeeding support.167 A randomized trial found that epidural infusion solutions containing fentanyl concentrations as high as 2 μg/mL for maintenance of labor analgesia did not impact rates of successful breastfeeding at six weeks postpartum.168
Breastfeeding outcomes after general vs. neuraxial anesthesia for cesarean delivery are also unclear. In one study, women receiving general and neuraxial anesthesia for cesarean delivery were similarly successful at breastfeeding in the immediate postpartum period (96% regional v. 89% general); however, at 6 months, fewer women who received general anesthesia were breastfeeding (39% vs. 71%).169 Results were similar from an observational trial in Turkey, where women self-select either general or neuraxial anesthesia for cesarean delivery.170 However, women who self-select general anesthesia likely differ in other factors known to affect breastfeeding success. Postoperative pain control is likely important; postoperative epidural analgesia is linked to successful breastfeeding and infant weight gain.171
Fever and neonatal sepsis workup
Labor neuraxial analgesia is associated with intrapartum fever of noninfectious inflammatory origin. Multiple studies support that labor epidural analgesia is linked to clinical fever (temperatures > 38.0° C).172 Study limitations include uncontrolled factors such as obstetric management, selection bias, crossover and dropout, and measurement error.172 Concerningly, maternal fever in general (not restricted to epidural-associated fever) is associated with poor neonatal outcomes, including assisted ventilation, low 1- and 5-min Apgar scores, seizures, and hypotonia.172 These outcomes occur more commonly in women who receive epidural analgesia and had a fever, but not among women who received epidural analgesia and remained afebrile.173
Neonatal sepsis evaluation and maternal and neonatal antibiotic exposure is significantly increased among mother-infant dyads with labor epidural-associated fever.174–176 Current evidence supports that maternal fever related to labor epidural analgesia is noninfectious and inflammatory in origin, mediated by cytokines. Among women receiving labor epidural analgesia, those with elevated IL-6 levels on admission are more likely to develop fever.172 Other proposed theories include local anesthetic agonism of the TRPV-1 (“capsaicin”) receptor, triggering the release of IL-6 and other inflammatory cytokines.172 Besides increased risk for neonatal sepsis evaluation and prophylactic treatment, it is not clear whether labor epidural-associated fever impacts short- or long-term adverse infant outcomes. Research is now focusing on the implications of noninfectious inflammation on neonatal outcomes. Future work should also emphasize diagnostic means to differentiate labor epidural-associated fever from fever caused by chorioamnionitis and funisitis (inflammation within the umbilical cord), as the latter are known to be linked to poor neonatal outcomes.
Infant and childhood neurocognitive outcomes
Some observational studies have linked intrapartum anesthetic exposure to autism spectrum disorders; others have failed to demonstrate this relationship.177–179 The challenges in conducting and interpreting these studies lie in the multiple confounders which independently impact risk for autism spectrum disorders (e.g. maternal conditions requiring anesthetic exposure, social environments dictating the same). An imperative exists to determine the effects of maternal anesthetic exposure on fetal, neonatal, and childhood neurocognitive outcomes,180 but currently there is little evidence that these considerations should change anesthetic clinical decision-making during labor and delivery.
Depression
Several studies suggest labor analgesia interventions may be associated with reduced postpartum depression risk.181,182 In 2014, Ding et al. found that epidural labor analgesia in Chinese women was associated with a reduced risk for postpartum depression (odds ratio 0.31, 95% confidence interval 0.12–0.82).181 There were several methodological limitations to the study. The cohort may not have been depression-free upon enrollment and there was a high loss-to-follow-up rate in the epidural analgesia group, possibly inflating the protective effect of epidural analgesia.
Nevertheless, an established relationship between pain and depression exists in the nonobstetric population,182 and given the dearth of data on this relationship in obstetrics, additional research is needed. The link between labor pain and postpartum depression may be biological; activation of neural networks in psychological pain overlap with physical pain neural networks.182 Pain catastrophizing is known to be linked to severity of the experienced physical pain.182 Other data suggest that analgesia may explain the protective relationship between the use of labor neuraxial analgesia and postpartum depression symptoms, although the relative influence of labor analgesia on postpartum depression may be less than other established risk factors such as baseline anxiety or depression, obesity, and genital tract trauma during delivery.183 An observational study noted a protective interaction effect for depression among women who planned and actually used labor epidural analgesia; women who planned to avoid labor epidural analgesia, but ultimately requested and used it, had higher risk for positive postpartum depression screening, but this relationship was thought mediated by difficult labor rather than unmet expectations.184 In view of the uncertainty in existing literature, coupled with plausible psychological and biological mechanisms explaining the relationship between labor pain and postpartum depression, additional research is clearly indicated to determine the true relationship between labor pain, labor analgesia, and postpartum depression; if a link is established, targeted approaches using preventative labor analgesic therapies for vulnerable women may prove to be protective for postpartum depression.
Anesthesiology Contributions to Maternal Safety
Mortality due to anesthesia
Anesthesia-related maternal mortality has decreased significantly over the last half-century. Maternal mortality ratios due to anesthesia in the United States are currently estimated at 1.0 per million live births – a 59% reduction from the period of 1979 – 1990.5 Morbidity and mortality associated with modern-day anesthesia care are often associated with complications of neuraxial anesthesia (e.g. high or total spinal anesthesia after failed epidural anesthesia and unrecognized spinal catheters).5,102,185 Importantly, anesthesiologists continue to play a key role in the prevention of nonanesthesia-related direct and indirect maternal deaths such as those caused by hemorrhage, hemodynamic instability, critical illness, and sepsis.5,102
Postpartum hemorrhage and patient blood management
Postpartum hemorrhage is a leading cause of maternal morbidity, cardiac arrest, and mortality worldwide. It accounts for approximately 12.5% of pregnancy-related deaths (1.8 deaths per 100,000 live births) in the United States.186 Most cases of hemorrhage-related maternal mortality are preventable.186 Protocolized approaches to postpartum hemorrhage have been developed, which have been shown to result in improved outcomes in many settings.187 The National Partnership for Maternal Safety is a multidisciplinary work group including anesthesiologists, maternal-fetal medicine specialists, obstetricians, nurses, and nurse-midwives. The group has provided a consensus bundle on best practices for obstetric hemorrhage.188 Despite the evidence showing improvement in outcomes, there appears to be limited adoption of these protocols; in 2014, only 67% of academic obstetric anesthesia units in the United States reported the use of a postpartum hemorrhage protocol, with greater use in hospitals with delivery volumes over 3,000 per year.189 Additional work to identify barriers to protocol adoption in low-volume centers will shed light on implementation strategies.
Maternal hematologic physiology differs from the nonpregnant state; severe obstetric hemorrhage is more likely to be associated with early hypofibrinogenemia.190,191 In the setting of postpartum hemorrhage, early assessment of fibrinogen levels should be undertaken; levels < 200 mg/dL should prompt aggressive monitoring and treatment. The American Society of Anesthesiologists guidelines specify that fibrinogen levels should be treated early in obstetric hemorrhage.192 Over-transfusion and under-resuscitation both carry risks. Efforts aimed at avoiding over-transfusion are likely in the best interest of the parturient as restrictive transfusion strategies are linked to lower risks for infections, cardiac events, and death.193,194 However, this goal must be balanced with risk of under-resuscitation, because maternal death from hemorrhage is often attributable to delayed recognition and under-resuscitation.102
Professional society guidelines for obstetric blood management differ from each other and from nonobstetric guidelines.191 The American College of Obstetricians and Gynecologists (ACOG) specifically recommends cell salvage for women with rare antibodies and if banked blood is not available, and for women who refuse allogeneic transfusion.195 Cell salvage may also limit allogeneic blood consumption and be cost-saving.196,197 Point-of-care testing has gained attention for its potential use in postpartum hemorrhage due to rapid results and detection of hyperfibrinolysis. Viscoelastic tests (thromboelastography) may be useful in assessing clot strength and thrombin generation.198 However, in major obstetric hemorrhage, laboratory testing performed better at detecting large aberrations in coagulation values, which correlated better with estimated blood loss, than thromboelastography.199 Point-of-care testing to guide component transfusion in obstetric hemorrhage may mitigate allogeneic transfusion, but whether laboratory-guided transfusion improves maternal outcomes has not been well studied.
The administration of antifibrinolytic agents (tranexamic acid) in obstetric hemorrhage has received recent attention. Its prophylactic use in planned cesarean deliveries leads to clinically insignificant bleeding differences.200 Thromboembolic complication data in this population have been lacking. In 2017, results were published from the World Maternal Antifibrinolytic (WOMAN) Trial, which compared tranexamic acid vs. placebo in 20,060 women with a clinical diagnosis of postpartum hemorrhage;201 198 hospitals in 21 countries were included, primarily low-resource settings with high rates of maternal hemorrhage deaths. Women randomly received tranexamic acid 1 gram or placebo. Death due to hemorrhage was significantly reduced in women who received tranexamic acid (1.5% vs. 1.9%; risk ratio 0.81, 95% confidence interval 0.65 to 1.00; P = 0.045). The need for laparotomy to control bleeding was reduced (risk ratio 0.64, 95% confidence interval 0.49 to 0.85; P=0.002). Importantly, maternal death was reduced by 31% if tranexamic acid was given within 3 hours of birth. Tranexamic acid was beneficial regardless of cause of hemorrhage (e.g., trauma, atony). The risk of hysterectomy and thromboembolic events were not different. The authors concluded that tranexamic acid should be given as soon as possible in postpartum hemorrhage regardless of cause, or after any bleeding associated with hemodynamic instability. This conclusion is consistent with our own clinical practice. Tranexamic acid is likely safe in obstetrics; whether the benefit of preventing death due to bleeding can be extrapolated to well-resourced countries is unknown.
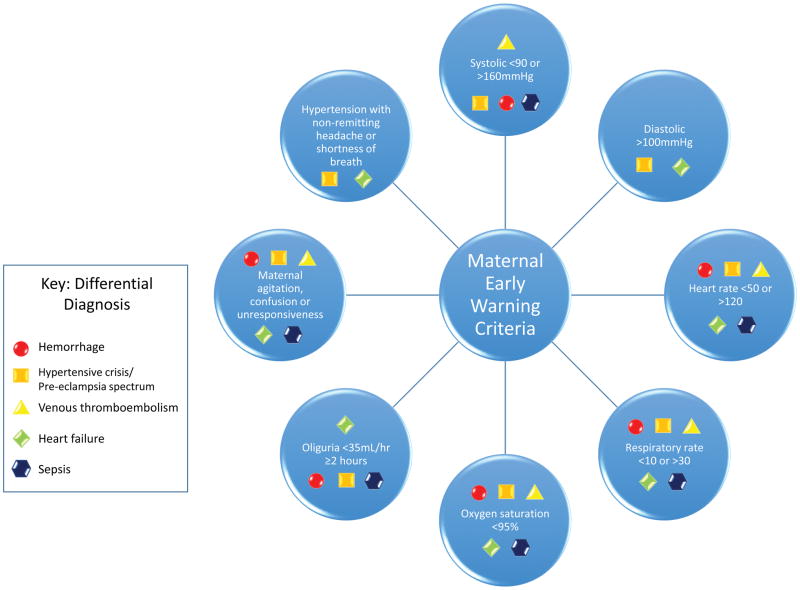
Early warning systems
The Modified Early Obstetric Warning System (MEOWS) was first described and recommended by the United Kingdom’s Confidential Enquiries into Maternal and Child Health, a national program that investigated all maternal deaths and other adverse outcomes.102 The group recognized that late recognition of maternal morbidity was contributing to poor outcomes and recommended a warning/screening system that included vital signs parameters (e.g., temperature, blood pressure, respirations, neurological response, and urine output). A study published in 2011 validated these parameters and established threshold for elevated morbidity.202 The parameters performed well as a screening tool, with a sensitivity of 89%, specificity of 79%, and negative predictive value of 98%. In the United States, modifications were proposed by the National Partnership for Maternal Safety, based on expert consensus from a multidisciplinary group of obstetricians, nurses, midwives, and anesthesiologists.203,204 The group recommend immediate action if any of the maternal early warning criteria in Figure 5 were met. Anesthesia providers are instrumental to early hemorrhage recognition, treatment, and implementation of Maternal Early Warning Systems and should actively participate in establishing these systems.
Figure 5.
Maternal Early Warning Criteria. The presence of any of these abnormal “triggers” should activate an immediate bedside evaluation by a physician or qualified clinician who can accelerate care toward prompt diagnosis and treatment of the underlying condition. Considerations for potential differential diagnoses are noted. Any nurse or clinician who is concerned about maternal status should feel empowered to raise concerns up the chain of command to achieve an appropriate response. Mechanisms for escalating notifications should be established. The triggers listed are not comprehensive for all possible obstetrical scenarios and are not intended to replace clinical judgement. Adapted from Mhyre JM, D’Oria R, Hameed AB, Lappen JR, Holley SL, Hunter SK, Jones RL, King JC, D’Alton ME: The maternal early warning criteria: a proposal from the national partnership for maternal safety. Obstet Gynecol 2014 124: 782–6.204
Oxytocin protocols
Active management of the third stage of labor reduces postpartum hemorrhage risk. Prophylactic uterotonic agents (oxytocin) are given and controlled umbilical cord traction for placenta delivery is performed. Studies published in the past decade, primarily by anesthesiologists, have identified safe methods for oxytocin administration for active management of the third stage of labor. The motivation to provide safe oxytocin doses stems from the uncommon but severe side effects associated with oxytocin, including dose-dependent cardiac conduction abnormalities, coronary vasospasm, and severe acute hyponatremia leading to seizures (oxytocin bears structural similarity to vasopressin).205 Furthermore, high doses of oxytocin are not necessary to achieve clinical gains for active management of the third stage of labor. A randomized trial compared oxytocin administration using a “rule-of-threes” algorithm to “wide open” infusion of oxytocin (30 units in 500 mL normal saline). In the “rule-of-threes” group, a 3-unit/3 mL oxytocin bolus was administered immediately after cesarean delivery, with optional repeat boluses of 3-unit/3 mL oxytocin at 3 minutes and at 6 minutes after delivery. This approach resulted in uterine tone at 3, 6, 9 and 12 minutes after delivery that was no less adequate than standard treatment. The control group received significantly more oxytocin, while there were no differences in blood loss or need for additional uterotonic agents.206
Oxytocin is often given as an infusion due to its short half-life of 1–5 minutes, thus a low-dose infusion protocol has been studied. George et al. estimated that the oxytocin infusion ED90 for satisfactory uterine tone in women undergoing elective cesarean delivery is 0.3 units per minute (18 units per hour).207 Pre-post studies following the institutional introduction of low-dose oxytocin infusion protocols have found reduced total dose of oxytocin with no impact on rates of postpartum hemorrhage, volume of estimated blood loss, or secondary uterotonic administration.208,209
Oxytocin receptor desensitization may explain the risk for postpartum hemorrhage from refractory atony in intrapartum cesarean delivery following oxytocin exposure during labor.210,211 In vitro tests involving human myometrial strips exposed to 2 hours of oxytocin pre-treatment vs. control demonstrated that the motility index (frequency × amplitude) of strips not exposed to oxytocin were significantly greater than those pre-treated with oxytocin.212,213 In vitro testing has not identified whether “resting periods” are effective in re-sensitizing myometrium. Therefore, giving more oxytocin in the setting of desensitization may not achieve the desired effect of increased uterine tone; in these cases, a different uterotonic agent that works by a different mechanism is indicated. In another study, the ED90 of oxytocin infusion for women with prior labor exposure to oxytocin was 44 units per hour, much higher than the ED90 for women without prior exposure to oxytocin.214 However, this higher dose is associated with more side effects, including nausea, vomiting, and ST segment depression. Further in vivo and in vitro investigations may elucidate the clinical significance of oxytocin desensitization, and may inform oxytocin protocols for women exposed to oxytocin during labor.
Safety bundles
The National Partnership for Maternal Safety goal is to reduce maternal morbidity and mortality in the United States. The United States is the only country in the developed world that has had increasing rates of maternal mortality since 1990. The maternal mortality ratio in the United States was 12.4 per 1000,000 live births (95% confidence interval 11.1 to 13.9) in 1990; by 2013, it increased to 18.5 (95% confidence interval 14.8–22.9).215 Maternal morbidity and mortality are frequently preventable, and guidance on best practices is instrumental in preventing maternal deaths.187 The National Partnership for Maternal Safety has developed safety “bundles” for maternal care in the areas of obstetric hemorrhage, hypertension in pregnancy, perinatal depression and anxiety, reduction of primary cesarean birth, support after a severe maternal event, and venous thromboembolism.216–218 Bundles are based on the best available evidence and are endorsed by multiple professional groups including the American College of Obstetricians and Gynecologists, American Society of Anesthesiologists, American College of Nurse-Midwives, Association of Women’s Health, Obstetric and Neonatal Nurses, among others. Each bundle is organized into five major areas: Readiness, Recognition, Response, and Reporting and Systems Learning. The resources are free and openly available to the public at www.safehealthcareforeverywoman.org. Given the anesthesia provider’s expertise in resuscitation and systems-based response, we are ideal participants in multidisciplinary shared leadership strategies to implement these bundles.
Conclusions
Advances in obstetric anesthesiology over the last decade have spanned multiple areas. Enhancements in neuraxial labor analgesic techniques, postpartum neuraxial pain management modalities, and prevention of intraoperative hypotension during cesarean delivery have contributed to improvements in care. Still more progress is needed in many areas, including questions about acute postpartum pain and its potential influence on chronic pain, the influence of labor pain on perinatal depression, labor epidural-associated fever, and the impact of labor analgesia on the duration of the second stage of labor and instrumental vaginal delivery. Current and future scientific work on individual physiologic characteristics of pain, labor progress, and other aspects of obstetric care may enhance clinicians’ ability to personalize obstetric anesthesia therapies and interventions. Comparative effectiveness studies on diagnostic and treatment modalities for pain during labor and the puerperium, the progress of labor, and obstetric hemorrhage, as well as the effects of these modalities on patient-centered outcomes, are necessary as our discipline advances further into the 21st century.
Summary Statement.
Modern obstetric anesthesia care emphasizes multidisciplinary, evidence-based practice. Basic, translational, and clinical scientific research propels further evolution. This review article highlights recent advances in obstetric anesthesia and their impact on maternal-fetal-neonatal outcomes.
Acknowledgments
Funding: Supported by the Department of Anesthesiology, University of Pittsburgh School of Medicine; Department of Anesthesiology, University of Iowa Carver College of Medicine and McGovern Medical School; Department of Anesthesiology, Northwestern University Feinberg School of Medicine; and by the Department of Obstetrics & Gynecology, University of Pittsburgh School of Medicine. Dr. Lim is supported by an award from the NIH/ORWH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH), NIH K12HD043441 and by the NIH Ruth Kirschstein National Service Award, NIH T32MG075770. Dr. Eltzschig is supported by National Institute of Health Grants R01 DK097075, R01- HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900. Dr. Facco is supported by National Institute of Health Grant R01-HL120354.
Footnotes
Conflicts of Interest: None
References
- 1.Caton D. John Snow’s practice of obstetric anesthesia. Anesthesiology. 2000;92:247–52. doi: 10.1097/00000542-200001000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Caton D. The influence of social values on obstetric anesthesia. AMA J Ethics. 2015;17:253–7. doi: 10.1001/journalofethics.2015.17.3.msoc1-1503. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 4.Thorp JA, Hu DH, Albin RM, McNitt J, Meyer BA, Cohen GR, Yeast JD. The effect of intrapartum epidural analgesia on nulliparous labor: a randomized, controlled, prospective trial. Am J Obstet Gynecol. 1993;169:851–8. doi: 10.1016/0002-9378(93)90015-b. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins JL, Chang J, Palmer SK, Gibbs CP, Callaghan WM. Anesthesia-related maternal mortality in the United States: 1979–2002. Obstet Gynecol. 2011;117:69–74. doi: 10.1097/AOG.0b013e31820093a9. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med. 2003;348:319–32. doi: 10.1056/NEJMra021276. [DOI] [PubMed] [Google Scholar]
- 7.Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016:270–300. doi: 10.1097/ALN.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 8.Abrao KC, Francisco RP, Miyadahira S, Cicarelli DD, Zugaib M. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:41–7. doi: 10.1097/AOG.0b013e31818f5eb6. [DOI] [PubMed] [Google Scholar]
- 9.Simmons SW, Taghizadeh N, Dennis AT, Hughes D, Cyna AM. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2012;10:CD003401. doi: 10.1002/14651858.CD003401.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth JM, Pan JC, Ross VH, Russell GB, Harris LC, Pan PH. Combined Spinal Epidural Technique for Labor Analgesia Does Not Delay Recognition of Epidural Catheter Failures: A Single-center Retrospective Cohort Survival Analysis. Anesthesiology. 2016;125:516–24. doi: 10.1097/ALN.0000000000001222. [DOI] [PubMed] [Google Scholar]
- 11.Bauer ME, Kountanis JA, Tsen LC, Greenfield ML, Mhyre JM. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth. 2012;21:294–309. doi: 10.1016/j.ijoa.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Riley ET, Cohen SE, Macario A, Desai JB, Ratner EF. Spinal versus epidural anesthesia for cesarean section: a comparison of time efficiency, costs, charges, and complications. Anesth Analg. 1995;80:709–12. doi: 10.1097/00000539-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Heesen M, Van de Velde M, Klohr S, Lehberger J, Rossaint R, Straube S. Meta-analysis of the success of block following combined spinal-epidural vs epidural analgesia during labour. Anaesthesia. 2014;69:64–71. doi: 10.1111/anae.12456. [DOI] [PubMed] [Google Scholar]
- 14.Cappiello E, O’Rourke N, Segal S, Tsen LC. A randomized trial of dural puncture epidural technique compared with the standard epidural technique for labor analgesia. Anesth Analg. 2008;107:1646–51. doi: 10.1213/ane.0b013e318184ec14. [DOI] [PubMed] [Google Scholar]
- 15.Chau A, Bibbo C, Huang CC, Elterman KG, Cappiello EC, Robinson JN, Tsen LC. Dural Puncture Epidural Technique Improves Labor Analgesia Quality With Fewer Side Effects Compared With Epidural and Combined Spinal Epidural Techniques: A Randomized Clinical Trial. Anesth Analg. 2017;124:560–569. doi: 10.1213/ANE.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 16.Wong CA. Advances in labor analgesia. Int J Womens Health. 2010;1:139–54. doi: 10.2147/ijwh.s4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sultan P, Murphy C, Halpern S, Carvalho B. The effect of low concentrations versus high concentrations of local anesthetics for labour analgesia on obstetric and anesthetic outcomes: a meta-analysis. Can J Anaesth. 2013;60:840–54. doi: 10.1007/s12630-013-9981-z. [DOI] [PubMed] [Google Scholar]
- 18.Wong CA. In: Epidural and Spinal Analgesia/Anesthesia for Labor and Vaginal Delivery, Obstetric Anesthesia: Principles and Practice. Chestnut DH, editor. Mosby: 2014. p. 490. [Google Scholar]
- 19.Ngan Kee WD, Khaw KS, Ng FF, Ng KK, So R, Lee A. Synergistic interaction between fentanyl and bupivacaine given intrathecally for labor analgesia. Anesthesiology. 2014;120:1126–36. doi: 10.1097/ALN.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 20.Bremerich DH, Waibel HJ, Mierdl S, Meininger D, Byhahn C, Zwissler BC, Ackermann HH. Comparison of continuous background infusion plus demand dose and demand-only parturient-controlled epidural analgesia (PCEA) using ropivacaine combined with sufentanil for labor and delivery. Int J Obstet Anesth. 2005;14:114–20. doi: 10.1016/j.ijoa.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Missant C, Teunkenst A, Vandermeersch E, Van de Velde M. Patient-controlled epidural analgesia following combined spinal-epidural analgesia in labour: the effects of adding a continuous epidural infusion. Anaesth Intensive Care. 2005;33:452–6. doi: 10.1177/0310057X0503300405. [DOI] [PubMed] [Google Scholar]
- 22.Wong CA, McCarthy RJ, Hewlett B. The effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analg. 2011;112:904–11. doi: 10.1213/ANE.0b013e31820e7c2f. [DOI] [PubMed] [Google Scholar]
- 23.Capogna G, Camorcia M, Stirparo S, Farcomeni A. Programmed intermittent epidural bolus versus continuous epidural infusion for labor analgesia: the effects on maternal motor function and labor outcome. A randomized double-blind study in nulliparous women. Anesth Analg. 2011;113:826–31. doi: 10.1213/ANE.0b013e31822827b8. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie CP, Cobb B, Riley ET, Carvalho B. Programmed intermittent epidural boluses for maintenance of labor analgesia: an impact study. Int J Obstet Anesth. 2016;26:32–8. doi: 10.1016/j.ijoa.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25.George RB, Allen TK, Habib AS. Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg. 2013;116:133–44. doi: 10.1213/ANE.0b013e3182713b26. [DOI] [PubMed] [Google Scholar]
- 26.Thornton JG, Capogna G. Reducing likelihood of instrumental delivery with epidural anaesthesia. Lancet. 2001;358:2. doi: 10.1016/s0140-6736(00)05295-8. [DOI] [PubMed] [Google Scholar]
- 27.Betti F, Carvalho B, Riley ET. Intrathecal Migration of an Epidural Catheter While Using a Programmed Intermittent Epidural Bolus Technique for Labor Analgesia Maintenance: A Case Report. A A Case Rep. 2017 doi: 10.1213/XAA.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho B, George RB, Cobb B, McKenzie C, Riley ET. Implementation of Programmed Intermittent Epidural Bolus for the Maintenance of Labor Analgesia. Anesth Analg. 2016;123:965–71. doi: 10.1213/ANE.0000000000001407. [DOI] [PubMed] [Google Scholar]
- 29.Markley JC, Rollins MD. Non-Neuraxial Labor Analgesia: Options. Clin Obstet Gynecol. 2017;60:350–364. doi: 10.1097/GRF.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 30.Stocki D, Matot I, Einav S, Eventov-Friedman S, Ginosar Y, Weiniger CF. A randomized controlled trial of the efficacy and respiratory effects of patient-controlled intravenous remifentanil analgesia and patient-controlled epidural analgesia in laboring women. Anesth Analg. 2014;118:589–97. doi: 10.1213/ANE.0b013e3182a7cd1b. [DOI] [PubMed] [Google Scholar]
- 31.Tveit TO, Halvorsen A, Seiler S, Rosland JH. Efficacy and side effects of intravenous remifentanil patient-controlled analgesia used in a stepwise approach for labour: an observational study. Int J Obstet Anesth. 2013;22:19–25. doi: 10.1016/j.ijoa.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Douma MR, Verwey RA, Kam-Endtz CE, van der Linden PD, Stienstra R. Obstetric analgesia: a comparison of patient-controlled meperidine, remifentanil, and fentanyl in labour. Br J Anaesth. 2010;104:209–15. doi: 10.1093/bja/aep359. [DOI] [PubMed] [Google Scholar]
- 33.Weiniger CF, Carvalho B, Stocki D, Einav S. Analysis of Physiological Respiratory Variable Alarm Alerts Among Laboring Women Receiving Remifentanil. Anesth Analg. 2017;124:1211–1218. doi: 10.1213/ANE.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 34.Saravanakumar K, Garstang JS, Hasan K. Intravenous patient-controlled analgesia for labour: a survey of UK practice. Int J Obstet Anesth. 2007;16:221–5. doi: 10.1016/j.ijoa.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Aaronson J, Abramovitz S, Smiley R, Tangel V, Landau R. A Survey of Intravenous Remifentanil Use for Labor Analgesia at Academic Medical Centers in the United States. Anesth Analg. 2017;124:1208–1210. doi: 10.1213/ANE.0000000000001622. [DOI] [PubMed] [Google Scholar]
- 36.Liu ZQ, Chen XB, Li HB, Qiu MT, Duan T. A comparison of remifentanil parturient-controlled intravenous analgesia with epidural analgesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2014;118:598–603. doi: 10.1213/ANE.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 37.Marwah R, Hassan S, Carvalho JC, Balki M. Remifentanil versus fentanyl for intravenous patient-controlled labour analgesia: an observational study. Can J Anaesth. 2012;59:246–54. doi: 10.1007/s12630-011-9625-0. [DOI] [PubMed] [Google Scholar]
- 38.Likis FE, Andrews JC, Collins MR, Lewis RM, Seroogy JJ, Starr SA, Walden RR, McPheeters ML. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118:153–67. doi: 10.1213/ANE.0b013e3182a7f73c. [DOI] [PubMed] [Google Scholar]
- 39.Attanasio L, Kozhimannil KB, Jou J, McPherson ME, Camann W. Women’s Experiences with Neuraxial Labor Analgesia in the Listening to Mothers II Survey: A Content Analysis of Open-Ended Responses. Anesth Analg. 2015;121:974–80. doi: 10.1213/ANE.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King TL, Wong CA. Nitrous oxide for labor pain: is it a laughing matter? Anesth Analg. 2014;118:12–4. doi: 10.1213/ANE.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 41.Collado V, Nicolas E, Faulks D, Hennequin M. A review of the safety of 50% nitrous oxide/oxygen in conscious sedation. Expert Opin Drug Saf. 2007;6:559–71. doi: 10.1517/14740338.6.5.559. [DOI] [PubMed] [Google Scholar]
- 42.Rooks JP. Safety and risks of nitrous oxide labor analgesia: a review. J Midwifery Womens Health. 2011;56:557–65. doi: 10.1111/j.1542-2011.2011.00122.x. [DOI] [PubMed] [Google Scholar]
- 43.Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109:707–22. doi: 10.1097/ALN.0b013e3181870a17. [DOI] [PubMed] [Google Scholar]
- 44.Landau R, Cahana A, Smiley RM, Antonarakis SE, Blouin JL. Genetic variability of mu-opioid receptor in an obstetric population. Anesthesiology. 2004;100:1030–3. doi: 10.1097/00000542-200404000-00042. [DOI] [PubMed] [Google Scholar]
- 45.Camorcia M, Capogna G, Stirparo S, Berritta C, Blouin JL, Landau R. Effect of mu-opioid receptor A118G polymorphism on the ED50 of epidural sufentanil for labor analgesia. Int J Obstet Anesth. 2012;21:40–4. doi: 10.1016/j.ijoa.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, Teo YY, Tan EC. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–6. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 47.Walter C, Lotsch J. Meta-analysis of the relevance of the OPRM1 118A>G genetic variant for pain treatment. Pain. 2009;146:270–5. doi: 10.1016/j.pain.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Terkawi AS, Jackson WM, Hansoti S, Tabassum R, Flood P. Polymorphism in the ADRB2 gene explains a small portion of intersubject variability in pain relative to cervical dilation in the first stage of labor. Anesthesiology. 2014;121:140–8. doi: 10.1097/ALN.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 49.Terkawi AS, Jackson WM, Thiet MP, Hansoti S, Tabassum R, Flood P. Oxytocin and catechol-O-methyltransferase receptor genotype predict the length of the first stage of labor. Am J Obstet Gynecol. 2012;207:184e1–8. doi: 10.1016/j.ajog.2012.06.079. [DOI] [PubMed] [Google Scholar]
- 50.Goetzinger KR, Macones GA. Operative vaginal delivery: current trends in obstetrics. Womens Health (Lond) 2008;4:281–90. doi: 10.2217/17455057.4.3.281. [DOI] [PubMed] [Google Scholar]
- 51.Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011:CD000331. doi: 10.1002/14651858.CD000331.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Comparative Obstetric Mobile Epidural Trial Study Group UK. Effect of low-dose mobile versus traditional epidural techniques on mode of delivery: a randomised controlled trial. Lancet. 2001;358:19–23. doi: 10.1016/S0140-6736(00)05251-X. [DOI] [PubMed] [Google Scholar]
- 53.Wassen MM, Hukkelhoven CW, Scheepers HC, Smits LJ, Nijhuis JG, Roumen FJ. Epidural analgesia and operative delivery: a ten-year population-based cohort study in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2014;183:125–31. doi: 10.1016/j.ejogrb.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 54.Segal S, Su M, Gilbert P. The effect of a rapid change in availability of epidural analgesia on the cesarean delivery rate: a meta-analysis. Am J Obstet Gynecol. 2000;183:974–8. doi: 10.1067/mob.2000.106677. [DOI] [PubMed] [Google Scholar]
- 55.Ramin SM, Gambling DR, Lucas MJ, Sharma SK, Sidawi JE, Leveno KJ. Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol. 1995;86:783–9. doi: 10.1016/0029-7844(95)00269-w. [DOI] [PubMed] [Google Scholar]
- 56.Bofill JA, Vincent RD, Ross EL, Martin RW, Norman PF, Werhan CF, Morrison JC. Nulliparous active labor, epidural analgesia, and cesarean delivery for dystocia. Am J Obstet Gynecol. 1997;177:1465–70. doi: 10.1016/s0002-9378(97)70092-9. [DOI] [PubMed] [Google Scholar]
- 57.Halpern SH, Leighton BL, Ohlsson A, Barrett JF, Rice A. Effect of epidural vs parenteral opioid analgesia on the progress of labor: a meta-analysis. JAMA. 1998;280:2105–10. doi: 10.1001/jama.280.24.2105. [DOI] [PubMed] [Google Scholar]
- 58.Sharma SK, Leveno KJ. Update: Epidural Analgesia does not increase csarean births. Curr Anesthesiol Rep. 2000;2:18–24. [Google Scholar]
- 59.Sharma SK, Alexander JM, Messick G, Bloom SL, McIntire DD, Wiley J, Leveno KJ. Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology. 2002;96:546–51. doi: 10.1097/00000542-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Yancey MK, Pierce B, Schweitzer D, Daniels D. Observations on labor epidural analgesia and operative delivery rates. Am J Obstet Gynecol. 1999;180:353–9. doi: 10.1016/s0002-9378(99)70213-9. [DOI] [PubMed] [Google Scholar]
- 61.Fogel ST, Shyken JM, Leighton BL, Mormol JS, Smeltzer JS. Epidural labor analgesia and the incidence of cesarean delivery for dystocia. Anesth Analg. 1998;87:119–23. doi: 10.1097/00000539-199807000-00026. [DOI] [PubMed] [Google Scholar]
- 62.Gribble RK, Meier PR. Effect of epidural analgesia on the primary cesarean rate. Obstet Gynecol. 1991;78:231–4. [PubMed] [Google Scholar]
- 63.Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol. 1999;94:600–7. doi: 10.1016/s0029-7844(99)00377-4. [DOI] [PubMed] [Google Scholar]
- 64.Wong CA, Scavone BM, Peaceman AM, McCarthy RJ, Sullivan JT, Diaz NT, Yaghmour E, Marcus RJ, Sherwani SS, Sproviero MT, Yilmaz M, Patel R, Robles C, Grouper S. The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. N Engl J Med. 2005;352:655–65. doi: 10.1056/NEJMoa042573. [DOI] [PubMed] [Google Scholar]
- 65.Chestnut DH, McGrath JM, Vincent RD, Jr, Penning DH, Choi WW, Bates JN, McFarlane C. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80:1201–8. doi: 10.1097/00000542-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Chestnut DH, Vincent RD, Jr, McGrath JM, Choi WW, Bates JN. Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin? Anesthesiology. 1994;80:1193–200. doi: 10.1097/00000542-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Luxman D, Wolman I, Groutz A, Cohen JR, Lottan M, Pauzner D, David MP. The effect of early epidural block administration on the progression and outcome of labor. Int J Obstet Anesth. 1998;7:161–4. doi: 10.1016/s0959-289x(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 68.Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006;194:600–5. doi: 10.1016/j.ajog.2005.10.821. [DOI] [PubMed] [Google Scholar]
- 69.Wang F, Shen X, Guo X, Peng Y, Gu X Labor Analgesia Examining G. Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a five-year randomized controlled trial. Anesthesiology. 2009;111:871–80. doi: 10.1097/ALN.0b013e3181b55e65. [DOI] [PubMed] [Google Scholar]
- 70.Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113:1066–74. doi: 10.1097/AOG.0b013e3181a1a9a8. [DOI] [PubMed] [Google Scholar]
- 71.Alexander JM, Sharma SK, McIntire DD, Wiley J, Leveno KJ. Intensity of labor pain and cesarean delivery. Anesth Analg. 2001;92:1524–8. doi: 10.1097/00000539-200106000-00034. [DOI] [PubMed] [Google Scholar]
- 72.Hess PE, Pratt SD, Soni AK, Sarna MC, Oriol NE. An association between severe labor pain and cesarean delivery. Anesth Analg. 2000;90:881–6. doi: 10.1097/00000539-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 73.Panni MK, Segal S. Local anesthetic requirements are greater in dystocia than in normal labor. Anesthesiology. 2003;98:957–63. doi: 10.1097/00000542-200304000-00024. [DOI] [PubMed] [Google Scholar]
- 74.Sharma SK, McIntire DD, Wiley J, Leveno KJ. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142–8. doi: 10.1097/00000542-200401000-00023. discussion 6A. [DOI] [PubMed] [Google Scholar]
- 75.Wang TT, Sun S, Huang SQ. Effects of Epidural Labor Analgesia With Low Concentrations of Local Anesthetics on Obstetric Outcomes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Anesth Analg. 2016 doi: 10.1213/ANE.0000000000001709. [DOI] [PubMed]
- 76.Cheek TG, Samuels P, Miller F, Tobin M, Gutsche BB. Normal saline i.v. fluid load decreases uterine activity in active labour. Br J Anaesth. 1996;77:632–5. doi: 10.1093/bja/77.5.632. [DOI] [PubMed] [Google Scholar]
- 77.Rahm VA, Hallgren A, Hogberg H, Hurtig I, Odlind V. Plasma oxytocin levels in women during labor with or without epidural analgesia: a prospective study. Acta Obstet Gynecol Scand. 2002;81:1033–9. doi: 10.1034/j.1600-0412.2002.811107.x. [DOI] [PubMed] [Google Scholar]
- 78.Van de Velde M, Teunkens A, Hanssens M, Vandermeersch E, Verhaeghe J. Intrathecal sufentanil and fetal heart rate abnormalities: a double-blind, double placebo-controlled trial comparing two forms of combined spinal epidural analgesia with epidural analgesia in labor. Anesth Analg. 2004;98:1153–9. doi: 10.1213/01.ANE.0000101980.34587.66. table of contents. [DOI] [PubMed] [Google Scholar]
- 79.Pan PH, Eisenach JC. In: The Pain of Childbirth and Its Effect on the Mother and the Fetus, Chestnut’s Obstetric Anesthesia: Principles and Practice. 5. Chestnut DH, editor. Philadelphia, PA: Elsevier Saunders; 2014. p. 421. [Google Scholar]
- 80.Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol. 2014;123:527–35. doi: 10.1097/AOG.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, Hatjis CG, Ramirez MM, Bailit JL, Gonzalez-Quintero VH, Hibbard JU, Hoffman MK, Kominiarek M, Learman LA, Van Veldhuisen P, Troendle J, Reddy UM Consortium on Safe L. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–7. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Derham RJ, Crowhurst J, Crowther C. The second stage of labour: durational dilemmas. Aust N Z J Obstet Gynaecol. 1991;31:31–6. doi: 10.1111/j.1479-828x.1991.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 83.Menticoglou SM, Manning F, Harman C, Morrison I. Perinatal outcome in relation to second-stage duration. Am J Obstet Gynecol. 1995;173:906–12. doi: 10.1016/0002-9378(95)90364-x. [DOI] [PubMed] [Google Scholar]
- 84.Saunders NS, Paterson CM, Wadsworth J. Neonatal and maternal morbidity in relation to the length of the second stage of labour. Br J Obstet Gynaecol. 1992;99:381–5. doi: 10.1111/j.1471-0528.1992.tb13753.x. [DOI] [PubMed] [Google Scholar]
- 85.Grobman WA, Bailit J, Lai Y, Reddy UM, Wapner RJ, Varner MW, Caritis SN, Prasad M, Tita AT, Saade G, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Association of the Duration of Active Pushing With Obstetric Outcomes. Obstet Gynecol. 2016;127:667–73. doi: 10.1097/AOG.0000000000001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rouse DJ, Weiner SJ, Bloom SL, Varner MW, Spong CY, Ramin SM, Caritis SN, Peaceman AM, Sorokin Y, Sciscione A, Carpenter MW, Mercer BM, Thorp JM, Jr, Malone FD, Harper M, Iams JD, Anderson GD Eunice Kennedy Shriver National Institute of Child H, Human Development Maternal-Fetal Medicine Units N. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357e1–7. doi: 10.1016/j.ajog.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Ray C, Audibert F, Goffinet F, Fraser W. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361e1–7. doi: 10.1016/j.ajog.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 88.American College of O, Gynecologists, Society for Maternal-Fetal M. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693–711. doi: 10.1097/01.AOG.0000444441.04111.1d. [DOI] [PubMed] [Google Scholar]
- 89.Magro-Malosso ER, Saccone G, Di Tommaso M, Mele M, Berghella V. Neuraxial analgesia to increase the success rate of external cephalic version: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215:276–86. doi: 10.1016/j.ajog.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 90.Hofmeyr GJ. Interventions to help external cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2004:CD000184. doi: 10.1002/14651858.CD000184.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Cluver C, Hofmeyr GJ, Gyte GM, Sinclair M. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2012;1:CD000184. doi: 10.1002/14651858.CD000184.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sultan P, Carvalho B. Neuraxial blockade for external cephalic version: a systematic review. Int J Obstet Anesth. 2011;20:299–306. doi: 10.1016/j.ijoa.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Lavoie A, Guay J. Anesthetic dose neuraxial blockade increases the success rate of external fetal version: a meta-analysis. Can J Anaesth. 2010;57:408–14. doi: 10.1007/s12630-010-9278-4. [DOI] [PubMed] [Google Scholar]
- 94.Chalifoux LA, Bauchat JR, Higgins N, Toledo P, Peralta FM, Farrer J, Gerber SE, McCarthy RJ, Sullivan JT. Effect of Intrathecal Bupivacaine Dose on the Success of External Cephalic Version for Breech Presentation: A Prospective, Randomized, Blinded Clinical Trial. Anesthesiology. 2017;127:625–632. doi: 10.1097/ALN.0000000000001796. [DOI] [PubMed] [Google Scholar]
- 95.Carvalho B, Tan JM, Macario A, El-Sayed YY, Sultan P. Brief report: a cost analysis of neuraxial anesthesia to facilitate external cephalic version for breech fetal presentation. Anesth Analg. 2013;117:155–9. doi: 10.1213/ANE.0b013e31828e5bc7. [DOI] [PubMed] [Google Scholar]
- 96.Van Thiel DH, Gavaler JS, Stremple J. Lower esophageal sphincter pressure in women using sequential oral contraceptives. Gastroenterology. 1976;71:232–4. [PubMed] [Google Scholar]
- 97.Fisher RS, Roberts GS, Grabowski CJ, Cohen S. Altered lower esophageal sphincter function during early pregnancy. Gastroenterology. 1978;74:1233–7. [PubMed] [Google Scholar]
- 98.Chiloiro M, Darconza G, Piccioli E, De Carne M, Clemente C, Riezzo G. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538–43. doi: 10.1007/s005350170056. [DOI] [PubMed] [Google Scholar]
- 99.Wong CA, Loffredi M, Ganchiff JN, Zhao J, Wang Z, Avram MJ. Gastric emptying of water in term pregnancy. Anesthesiology. 2002;96:1395–400. doi: 10.1097/00000542-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 100.Porter JS, Bonello E, Reynolds F. The influence of epidural administration of fentanyl infusion on gastric emptying in labour. Anaesthesia. 1997;52:1151–6. doi: 10.1111/j.1365-2044.1997.238-az0373.x. [DOI] [PubMed] [Google Scholar]
- 101.Lewis G. Saving Mothers’ Lives: the continuing benefits for maternal health from the United Kingdom (UK) Confidential Enquires into Maternal Deaths. Semin Perinatol. 2012;36:19–26. doi: 10.1053/j.semperi.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, Millward-Sadler H, Neilson J, Nelson-Piercy C, Norman J, O’Herlihy C, Oates M, Shakespeare J, de Swiet M, Williamson C, Beale V, Knight M, Lennox C, Miller A, Parmar D, Rogers J, Springett A. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 103.Davies JM, Posner KL, Lee LA, Cheney FW, Domino KB. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology. 2009;110:131–9. doi: 10.1097/ALN.0b013e318190e16a. [DOI] [PubMed] [Google Scholar]
- 104.Sperling JD, Dahlke JD, Sibai BM. Restriction of oral intake during labor: whither are we bound? Am J Obstet Gynecol. 2016;214:592–6. doi: 10.1016/j.ajog.2016.01.166. [DOI] [PubMed] [Google Scholar]
- 105.Technical Working Group. World Health Organization; Birth: 1997. Care in normal birth: a practical guide; pp. 121–3. [PubMed] [Google Scholar]
- 106.Metzger BE, Ravnikar V, Vileisis RA, Freinkel N. “Accelerated starvation” and the skipped breakfast in late normal pregnancy. Lancet. 1982;1:588–92. doi: 10.1016/s0140-6736(82)91750-0. [DOI] [PubMed] [Google Scholar]
- 107.Kubli M, Scrutton MJ, Seed PT, O’Sullivan G. An evaluation of isotonic “sport drinks” during labor. Anesth Analg. 2002;94:404–8. doi: 10.1097/00000539-200202000-00033. table of contents. [DOI] [PubMed] [Google Scholar]
- 108.O’Sullivan G, Liu B, Hart D, Seed P, Shennan A. Effect of food intake during labour on obstetric outcome: randomised controlled trial. BMJ. 2009;338:b784. doi: 10.1136/bmj.b784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scrutton MJ, Metcalfe GA, Lowy C, Seed PT, O’Sullivan G. Eating in labour. A randomised controlled trial assessing the risks and benefits. Anaesthesia. 1999;54:329–34. doi: 10.1046/j.1365-2044.1999.00750.x. [DOI] [PubMed] [Google Scholar]
- 110.Malin GL, Bugg GJ, Thornton J, Taylor MA, Grauwen N, Devlieger R, Kardel KR, Kubli M, Tranmer JE, Jones NW. Does oral carbohydrate supplementation improve labour outcome? A systematic review and individual patient data meta-analysis. BJOG. 2016;123:510–7. doi: 10.1111/1471-0528.13728. [DOI] [PubMed] [Google Scholar]
- 111.Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013:CD003930. doi: 10.1002/14651858.CD003930.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garry M, Davies S. Failure of regional blockade for caesarean section. Int J Obstet Anesth. 2002;11:9–12. doi: 10.1054/ijoa.2001.0903. [DOI] [PubMed] [Google Scholar]
- 113.Norris MC. Patient variables and the subarachnoid spread of hyperbaric bupivacaine in the term parturient. Anesthesiology. 1990;72:478–82. doi: 10.1097/00000542-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 114.Dahlgren G, Hultstrand C, Jakobsson J, Norman M, Eriksson EW, Martin H. Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for cesarean section. Anesth Analg. 1997;85:1288–93. doi: 10.1097/00000539-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 115.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–44. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 116.Abouleish EI. Epinephrine improves the quality of spinal hyperbaric bupivacaine for cesarean section. Anesth Analg. 1987;66:395–400. doi: 10.1213/00000539-198705000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Lavand’homme PM, Roelants F, Waterloos H, Collet V, De Kock MF. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–55. doi: 10.1213/ane.0b013e31817f1595. [DOI] [PubMed] [Google Scholar]
- 118.Purva M, Russell I, Kinsella M. In: 8.8 Caesarean section anaesthesia: technique and failure rate., Raising the Standard: A Compendium of Audit Recipes for Continuous Quality Improvement in Anaesthesia. 3. Colvin JR, Peden CJ, editors. London, Engalnd: 2012. pp. 220–221. [Google Scholar]
- 119.Dyer RA, Reed AR, van Dyk D, Arcache MJ, Hodges O, Lombard CJ, Greenwood J, James MF. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–65. doi: 10.1097/ALN.0b013e3181b437e0. [DOI] [PubMed] [Google Scholar]
- 120.Hall PA, Bennett A, Wilkes MP, Lewis M. Spinal anaesthesia for caesarean section: comparison of infusions of phenylephrine and ephedrine. Br J Anaesth. 1994;73:471–4. doi: 10.1093/bja/73.4.471. [DOI] [PubMed] [Google Scholar]
- 121.LaPorta RF, Arthur GR, Datta S. Phenylephrine in treating maternal hypotension due to spinal anaesthesia for caesarean delivery: effects on neonatal catecholamine concentrations, acid base status and Apgar scores. Acta Anaesthesiol Scand. 1995;39:901–5. doi: 10.1111/j.1399-6576.1995.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 122.Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–6. doi: 10.1097/00000539-200204000-00028. table of contents. [DOI] [PubMed] [Google Scholar]
- 123.Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–74. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 124.Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–12. doi: 10.1097/ALN.0b013e3181b160a3. [DOI] [PubMed] [Google Scholar]
- 125.Macarthur A, Riley ET. Obstetric anesthesia controversies: vasopressor choice for postspinal hypotension during cesarean delivery. Int Anesthesiol Clin. 2007;45:115–32. doi: 10.1097/AIA.0b013e31802b8d53. [DOI] [PubMed] [Google Scholar]
- 126.Habib AS. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–90. doi: 10.1213/ANE.0b013e3182373a3e. [DOI] [PubMed] [Google Scholar]
- 127.Ngan Kee WD. The use of vasopressors during spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2017 doi: 10.1097/ACO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 128.Smiley RM. More perfect? Int J Obstet Anesth. 2017 doi: 10.1016/j.ijoa.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 129.Higgins N, Fitzgerald PC, van Dyk D, Dyer RA, Rodriguez N, McCarthy RJ, Wong CA. The Effect of Prophylactic Phenylephrine and Ephedrine Infusions on Umbilical Artery Blood pH in Women With Preeclampsia Undergoing Cesarean Delivery With Spinal Anesthesia: A Randomized, Double-Blind Trial. Anesth Analg. 2017 doi: 10.1213/ANE.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 130.Dyer RA, Emmanuel A, Adams SC, Lombard CJ, Arcache MJ, Vorster A, Wong CA, Higgins N, Reed AR, James MF, Joolay Y, Schulein S, van Dyk D. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth. 2017 doi: 10.1016/j.ijoa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 131.Ngan Kee WD, Lee SW, Ng FF, Tan PE, Khaw KS. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–45. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 132.Ngan Kee WD. A Random-allocation Graded Dose-Response Study of Norepinephrine and Phenylephrine for Treating Hypotension during Spinal Anesthesia for Cesarean Delivery. Anesthesiology. 2017;127:934–941. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 133.Khaw KS, Wang CC, Ngan Kee WD, Pang CP, Rogers MS. Effects of high inspired oxygen fraction during elective caesarean section under spinal anaesthesia on maternal and fetal oxygenation and lipid peroxidation. Br J Anaesth. 2002;88:18–23. doi: 10.1093/bja/88.1.18. [DOI] [PubMed] [Google Scholar]
- 134.Solberg R, Andresen JH, Escrig R, Vento M, Saugstad OD. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr Res. 2007;62:559–63. doi: 10.1203/PDR.0b013e318156e8aa. [DOI] [PubMed] [Google Scholar]
- 135.Duggal N, Poddatoori V, Noroozkhani S, Siddik-Ahmad RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery: a randomized trial. Obstet Gynecol. 2013;122:79–84. doi: 10.1097/AOG.0b013e318297ec6c. [DOI] [PubMed] [Google Scholar]
- 136.Chatmongkolchart S, Prathep S. Supplemental oxygen for caesarean section during regional anaesthesia. Cochrane Database Syst Rev. 2016;3:CD006161. doi: 10.1002/14651858.CD006161.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carvalho B, Zheng M, Harter S, Sultan P. A Prospective Cohort Study Evaluating the Ability of Anticipated Pain, Perceived Analgesic Needs, and Psychological Traits to Predict Pain and Analgesic Usage following Cesarean Delivery. Anesthesiol Res Pract. 2016;2016:7948412. doi: 10.1155/2016/7948412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pan PH, Tonidandel AM, Aschenbrenner CA, Houle TT, Harris LC, Eisenach JC. Predicting acute pain after cesarean delivery using three simple questions. Anesthesiology. 2013;118:1170–9. doi: 10.1097/ALN.0b013e31828e156f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Booth JL, Harris LC, Eisenach JC, Pan PH. A Randomized Controlled Trial Comparing Two Multimodal Analgesic Techniques in Patients Predicted to Have Severe Pain After Cesarean Delivery. Anesth Analg. 2016;122:1114–9. doi: 10.1213/ANE.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sutton CD, Carvalho B. Optimal Pain Management After Cesarean Delivery. Anesthesiol Clin. 2017;35:107–124. doi: 10.1016/j.anclin.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 141.Cohen SE, Subak LL, Brose WG, Halpern J. Analgesia after cesarean delivery: patient evaluations and costs of five opioid techniques. Reg Anesth. 1991;16:141–9. [PubMed] [Google Scholar]
- 142.Lim Y, Jha S, Sia AT, Rawal N. Morphine for post-caesarean section analgesia: intrathecal, epidural or intravenous? Singapore Med J. 2005;46:392–6. [PubMed] [Google Scholar]
- 143.Carvalho B. Respiratory depression after neuraxial opioids in the obstetric setting. Anesth Analg. 2008;107:956–61. doi: 10.1213/ane.0b013e318168b443. [DOI] [PubMed] [Google Scholar]
- 144.Popping DM, Elia N, Marret E, Wenk M, Tramer MR. Opioids added to local anesthetics for single-shot intrathecal anesthesia in patients undergoing minor surgery: a meta-analysis of randomized trials. Pain. 2012;153:784–93. doi: 10.1016/j.pain.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 145.Sultan P, Halpern SH, Pushpanathan E, Patel S, Carvalho B. The Effect of Intrathecal Morphine Dose on Outcomes After Elective Cesarean Delivery: A Meta-Analysis. Anesth Analg. 2016;123:154–64. doi: 10.1213/ANE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 146.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–60. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 147.TORADOL(R), ketorolac tromethamine [package insert] Nutley, New Jersey: Roche Laboratories; 2013. pp. 1–27. Reference ID: 3281582. [Google Scholar]
- 148.American Academy of Pediatrics Committee on D. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–89. doi: 10.1542/peds.108.3.776. [DOI] [PubMed] [Google Scholar]
- 149.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–9. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 150.Valentine AR, Carvalho B, Lazo TA, Riley ET. Scheduled acetaminophen with as-needed opioids compared to as-needed acetaminophen plus opioids for post-cesarean pain management. Int J Obstet Anesth. 2015;24:210–6. doi: 10.1016/j.ijoa.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 151.Costello JF, Moore AR, Wieczorek PM, Macarthur AJ, Balki M, Carvalho JC. The transversus abdominis plane block, when used as part of a multimodal regimen inclusive of intrathecal morphine, does not improve analgesia after cesarean delivery. Reg Anesth Pain Med. 2009;34:586–9. doi: 10.1097/aap.0b013e3181b4c922. [DOI] [PubMed] [Google Scholar]
- 152.Abdallah FW, Halpern SH, Margarido CB. Transversus abdominis plane block for postoperative analgesia after Caesarean delivery performed under spinal anaesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012;109:679–87. doi: 10.1093/bja/aes279. [DOI] [PubMed] [Google Scholar]
- 153.Mishriky BM, George RB, Habib AS. Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review and meta-analysis. Can J Anaesth. 2012;59:766–78. doi: 10.1007/s12630-012-9729-1. [DOI] [PubMed] [Google Scholar]
- 154.Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following Cesarean delivery: a case series. Can J Anaesth. 2013;60:299–303. doi: 10.1007/s12630-012-9866-6. [DOI] [PubMed] [Google Scholar]
- 155.Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000. doi: 10.1093/bja/aet015. [DOI] [PubMed] [Google Scholar]
- 156.Blanco R, Ansari T, Girgis E. Quadratus lumborum block for postoperative pain after caesarean section: A randomised controlled trial. Eur J Anaesthesiol. 2015;32:812–8. doi: 10.1097/EJA.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 157.Blanco R, Ansari T, Riad W, Shetty N. Quadratus Lumborum Block Versus Transversus Abdominis Plane Block for Postoperative Pain After Cesarean Delivery: A Randomized Controlled Trial. Reg Anesth Pain Med. 2016;41:757–762. doi: 10.1097/AAP.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 158.Bamigboye AA, Hofmeyr GJ. Local anaesthetic wound infiltration and abdominal nerves block during caesarean section for postoperative pain relief. Cochrane Database Syst Rev. 2009:CD006954. doi: 10.1002/14651858.CD006954.pub2. [DOI] [PubMed] [Google Scholar]
- 159.Tharwat AA, Yehia AH, Wahba KA, Ali AE. Efficacy and safety of post-cesarean section incisional infiltration with lidocaine and epinephrine versus lidocaine alone in reducing postoperative pain: A randomized controlled double-blinded clinical trial. J Turk Ger Gynecol Assoc. 2016;17:1–5. doi: 10.5152/jtgga.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vallejo MC, Steen TL, Cobb BT, Phelps AL, Pomerantz JM, Orebaugh SL, Chelly JE. Efficacy of the bilateral ilioinguinal-iliohypogastric block with intrathecal morphine for postoperative cesarean delivery analgesia. ScientificWorldJournal. 2012;2012:107316. doi: 10.1100/2012/107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Coffman JC, Fiorini K, Small RH. Ilioinguinal-iliohypogastric block used to rescue ineffective transversus abdominis plane block after cesarean delivery. Int J Obstet Anesth. 2015;24:394–5. doi: 10.1016/j.ijoa.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 162.Kim ES, Kim HK, Baik JS, Ji YT. Continuous Ilioinguinal-iliohypogastric Nerve Block for Groin Pain in a Breast-feeding Patient after Cesarean Delivery. Korean J Pain. 2016;29:193–6. doi: 10.3344/kjp.2016.29.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Naghshineh E, Shiari S, Jabalameli M. Preventive effect of ilioinguinal nerve block on postoperative pain after cesarean section. Adv Biomed Res. 2015;4:229. doi: 10.4103/2277-9175.166652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hattler J, Klimek M, Rossaint R, Heesen M. The Effect of Combined Spinal-Epidural Versus Epidural Analgesia in Laboring Women on Nonreassuring Fetal Heart Rate Tracings: Systematic Review and Meta-analysis. Anesth Analg. 2016;123:955–64. doi: 10.1213/ANE.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 165.Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274–81. doi: 10.1111/j.1471-0528.2002.01380.x. [DOI] [PubMed] [Google Scholar]
- 166.French CA, Cong X, Chung KS. Labor Epidural Analgesia and Breastfeeding: A Systematic Review. J Hum Lact. 2016;32:507–20. doi: 10.1177/0890334415623779. [DOI] [PubMed] [Google Scholar]
- 167.Chang ZM, Heaman MI. Epidural analgesia during labor and delivery: effects on the initiation and continuation of effective breastfeeding. J Hum Lact. 2005;21:305–14. doi: 10.1177/0890334405277604. quiz 315–9, 326. [DOI] [PubMed] [Google Scholar]
- 168.Lee AI, McCarthy RJ, Toledo P, Jones MJ, White N, Wong CA. Epidural Labor Analgesia-Fentanyl Dose and Breastfeeding Success: A Randomized Clinical Trial. Anesthesiology. 2017;127:614–624. doi: 10.1097/ALN.0000000000001793. [DOI] [PubMed] [Google Scholar]
- 169.Lie B, Juul J. Effect of epidural vs. general anesthesia on breastfeeding. Acta Obstet Gynecol Scand. 1988;67:207–9. doi: 10.3109/00016348809004203. [DOI] [PubMed] [Google Scholar]
- 170.Kutlucan L, Seker IS, Demiraran Y, Ersoy O, Karagoz I, Sezen G, Kose SA. Effects of different anesthesia protocols on lactation in the postpartum period. J Turk Ger Gynecol Assoc. 2014;15:233–8. doi: 10.5152/jtgga.2014.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hirose M, Hara Y, Hosokawa T, Tanaka Y. The effect of postoperative analgesia with continuous epidural bupivacaine after cesarean section on the amount of breast feeding and infant weight gain. Anesth Analg. 1996;82:1166–9. doi: 10.1097/00000539-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 172.Arendt KW, Segal BS. The association between epidural labor analgesia and maternal fever. Clin Perinatol. 2013;40:385–98. doi: 10.1016/j.clp.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 173.Petrova A, Demissie K, Rhoads GG, Smulian JC, Marcella S, Ananth CV. Association of maternal fever during labor with neonatal and infant morbidity and mortality. Obstet Gynecol. 2001;98:20–7. doi: 10.1016/s0029-7844(01)01361-8. [DOI] [PubMed] [Google Scholar]
- 174.Kaul B, Vallejo M, Ramanathan S, Mandell G. Epidural labor analgesia and neonatal sepsis evaluation rate: a quality improvement study. Anesth Analg. 2001;93:986–90. doi: 10.1097/00000539-200110000-00038. [DOI] [PubMed] [Google Scholar]
- 175.Lieberman E, Lang JM, Frigoletto F, Jr, Richardson DK, Ringer SA, Cohen A. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics. 1997;99:415–9. doi: 10.1542/peds.99.3.415. [DOI] [PubMed] [Google Scholar]
- 176.Mayer DC, Chescheir NC, Spielman FJ. Increased intrapartum antibiotic administration associated with epidural analgesia in labor. Am J Perinatol. 1997;14:83–6. doi: 10.1055/s-2007-994103. [DOI] [PubMed] [Google Scholar]
- 177.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123:1293–300. doi: 10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- 178.Flick RP, Lee K, Hofer RE, Beinborn CW, Hambel EM, Klein MK, Gunn PW, Wilder RT, Katusic SK, Schroeder DR, Warner DO, Sprung J. Neuraxial labor analgesia for vaginal delivery and its effects on childhood learning disabilities. Anesth Analg. 2011;112:1424–31. doi: 10.1213/ANE.0b013e3181f2ecdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hattori R, Desimaru M, Nagayama I, Inoue K. Autistic and developmental disorders after general anaesthetic delivery. Lancet. 1991;337:1357–8. doi: 10.1016/0140-6736(91)93045-b. [DOI] [PubMed] [Google Scholar]
- 180.American Society of Anesthesiologists. ASA Response to the FDA Med Watch Warning. 2016 https://www.asahq.org/advocacy/fda-and-washington-alerts/washington-alerts/2016/12/asa-response-to-the-fda-med-watch.
- 181.Ding T, Wang DX, Qu Y, Chen Q, Zhu SN. Epidural labor analgesia is associated with a decreased risk of postpartum depression: a prospective cohort study. Anesth Analg. 2014;119:383–92. doi: 10.1213/ANE.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 182.Wisner KL, Stika CS, Clark CT. Double duty: does epidural labor analgesia reduce both pain and postpartum depression? Anesth Analg. 2014;119:219–21. doi: 10.1213/ANE.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lim G, Facco F, Farrell L, Gold M, Wasan A. Intrapartum Pain Improvement as a Predictor for Postpartum Depression. Pittsburgh Journal of Anesthesiology. 2017:182. [Google Scholar]
- 184.Orbach-Zinger S, Landau R, Harousch AB, Ovad O, Caspi L, Kornilov E, Ioscovich A, Bracco D, Davis A, Fireman S, Hoshen M, Eidelman LA. The Relationship Between Women’s Intention to Request a Labor Epidural Analgesia, Actually Delivering With Labor Epidural Analgesia, and Postpartum Depression at 6 Weeks: A Prospective Observational Study. Anesth Analg. 2017 doi: 10.1213/ANE.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 185.D’Angelo R, Smiley RM, Riley ET, Segal S. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505–12. doi: 10.1097/ALN.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 186.De La Rosa K, Mhyre J, Anderson FW. Maternal Mortality From Hemorrhage in Michigan 1998–2011 [8] Obstet Gynecol. 2016;127(Suppl 1):3S. [Google Scholar]
- 187.Main EK, Cape V, Abreo A, Vasher J, Woods A, Carpenter A, Gould JB. Reduction of Severe Maternal Morbidity from Hemorrhage Using A State Perinatal Quality Collaborative. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 188.Main EK, Goffman D, Scavone BM, Low LK, Bingham D, Fontaine PL, Gorlin JB, Lagrew DC, Levy BS National Parternship for Maternal S, Council for Patient Safety in Women’s Health C. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Anesth Analg. 2015;121:142–8. doi: 10.1097/AOG.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 189.Kacmar RM, Mhyre JM, Scavone BM, Fuller AJ, Toledo P. The use of postpartum hemorrhage protocols in United States academic obstetric anesthesia units. Anesth Analg. 2014;119:906–10. doi: 10.1213/ANE.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 190.Charbit B, Mandelbrot L, Samain E, Baron G, Haddaoui B, Keita H, Sibony O, Mahieu-Caputo D, Hurtaud-Roux MF, Huisse MG, Denninger MH, de Prost D, Group PPHS. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–73. doi: 10.1111/j.1538-7836.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 191.Shaylor R, Weiniger CF, Austin N, Tzabazis A, Shander A, Goodnough LT, Butwick AJ. National and International Guidelines for Patient Blood Management in Obstetrics: A Qualitative Review. Anesth Analg. 2017;124:216–232. doi: 10.1213/ANE.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.American Society of Anesthesiologists Task Force on Perioperative Blood M. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122:241–75. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 193.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127:124–131. e3. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 194.Shaz BH, Hillyer CD, Waters JH. Patient blood management: key for accountable care organizations. JAMA Surg. 2013;148:491–2. doi: 10.1001/jamasurg.2013.69. [DOI] [PubMed] [Google Scholar]
- 195.American College of Obstetricians Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–47. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 196.Albright CM, Rouse DJ, Werner EF. Cost savings of red cell salvage during cesarean delivery. Obstet Gynecol. 2014;124:690–6. doi: 10.1097/AOG.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 197.Waters JH, Lee JS, Karafa MT. A mathematical model of cell salvage compared and combined with normovolemic hemodilution. Transfusion. 2004;44:1412–6. doi: 10.1111/j.1537-2995.2004.04050.x. [DOI] [PubMed] [Google Scholar]
- 198.Butwick A, Ting V, Ralls LA, Harter S, Riley E. The association between thromboelastographic parameters and total estimated blood loss in patients undergoing elective cesarean delivery. Anesth Analg. 2011;112:1041–7. doi: 10.1213/ANE.0b013e318210fc64. [DOI] [PubMed] [Google Scholar]
- 199.Karlsson O, Jeppsson A, Hellgren M. Major obstetric haemorrhage: monitoring with thromboelastography, laboratory analyses or both? Int J Obstet Anesth. 2014;23:10–7. doi: 10.1016/j.ijoa.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 200.Novikova N, Hofmeyr GJ, Cluver C. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev. 2015:CD007872. doi: 10.1002/14651858.CD007872.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Collaborators TWT. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, nrandomised, double-blind, placebo-controlled trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)30638-4. E-pub ahead of print.: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Singh S, McGlennan A, England A, Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS) Anaesthesia. 2012;67:12–8. doi: 10.1111/j.1365-2044.2011.06896.x. [DOI] [PubMed] [Google Scholar]
- 203.Maternal Early Warning Criteria. National Partnership for Maternal Safety; 2017. [Google Scholar]
- 204.Mhyre JM, D’Oria R, Hameed AB, Lappen JR, Holley SL, Hunter SK, Jones RL, King JC, D’Alton ME. The maternal early warning criteria: a proposal from the national partnership for maternal safety. Obstet Gynecol. 2014;124:782–6. doi: 10.1097/AOG.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 205.Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG. 2010;117:76–83. doi: 10.1111/j.1471-0528.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 206.Kovacheva VP, Soens MA, Tsen LC. A Randomized, Double-blinded Trial of a “Rule of Threes” Algorithm versus Continuous Infusion of Oxytocin during Elective Cesarean Delivery. Anesthesiology. 2015;123:92–100. doi: 10.1097/ALN.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 207.George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth. 2010;57:578–82. doi: 10.1007/s12630-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 208.Dagraca J, Malladi V, Nunes K, Scavone B. Outcomes after institution of a new oxytocin infusion protocol during the third stage of labor and immediate postpartum period. Int J Obstet Anesth. 2013;22:194–9. doi: 10.1016/j.ijoa.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 209.Lee AI, Wong CA, Healy L, Toledo P. Impact of a third stage of labor oxytocin protocol on cesarean delivery outcomes. Int J Obstet Anesth. 2014;23:18–22. doi: 10.1016/j.ijoa.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 210.Grotegut CA, Paglia MJ, Johnson LN, Thames B, James AH. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204:56e1–6. doi: 10.1016/j.ajog.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Phaneuf S, Rodriguez Linares B, TambyRaja RL, MacKenzie IZ, Lopez Bernal A. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil. 2000;120:91–7. doi: 10.1530/jrf.0.1200091. [DOI] [PubMed] [Google Scholar]
- 212.Balki M, Ramachandran N, Lee S, Talati C. The Recovery Time of Myometrial Responsiveness After Oxytocin-Induced Desensitization in Human Myometrium In Vitro. Anesth Analg. 2016;122:1508–15. doi: 10.1213/ANE.0000000000001268. [DOI] [PubMed] [Google Scholar]
- 213.Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pretreatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119:552–61. doi: 10.1097/ALN.0b013e318297d347. [DOI] [PubMed] [Google Scholar]
- 214.Lavoie A, McCarthy RJ, Wong CA. The ED90 of prophylactic oxytocin infusion after delivery of the placenta during cesarean delivery in laboring compared with nonlaboring women: an up-down sequential allocation dose-response study. Anesth Analg. 2015;121:159–64. doi: 10.1213/ANE.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 215.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber R, Huynh C, Dicker D, Templin T, Wolock TM, Ozgoren AA, Abd-Allah F, Abera SF, Abubakar I, Achoki T, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Agardh EE, Akena D, Alasfoor D, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Al Kahbouri MJ, Alla F, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Antonio CA, Anwari P, Arnlov J, Arsenijevic VS, Artaman A, Asad MM, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Basu A, Basu S, Beardsley J, Bedi N, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhutta Z, Bin Abdulhak A, Blore JD, Basara BB, Bose D, Breitborde N, Cardenas R, Castaneda-Orjuela CA, Castro RE, Catala-Lopez F, Cavlin A, Chang JC, Che X, Christophi CA, Chugh SS, Cirillo M, Colquhoun SM, Cooper LT, Cooper C, da Costa Leite I, Dandona L, Dandona R, Davis A, Dayama A, Degenhardt L, De Leo D, del Pozo-Cruz B, Deribe K, Dessalegn M, deVeber GA, Dharmaratne SD, Dilmen U, Ding EL, Dorrington RE, Driscoll TR, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Felicio MM, Fereshtehnejad SM, de Lima GM, Forouzanfar MH, Franca EB, Gaffikin L, Gambashidze K, Gankpe FG, Garcia AC, Geleijnse JM, Gibney KB, Giroud M, Glaser EL, Goginashvili K, Gona P, Gonzalez-Castell D, Goto A, Gouda HN, Gugnani HC, Gupta R, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Havmoeller R, Hay SI, Pi IB, Hoek HW, Hosgood HD, Hoy DG, Husseini A, Idrisov BT, Innos K, Inoue M, Jacobsen KH, Jahangir E, Jee SH, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kabagambe EK, Kan H, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazanjan K, Kazi DS, Kemp AH, Kengne AP, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni C, Kulkarni VS, Kumar GA, Kumar K, Kumar RB, Kwan G, Lai T, Lalloo R, Lam H, Lansingh VC, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li X, Li Y, Li Y, Liang J, Liang X, Lim SS, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, London SJ, Lotufo PA, Ma J, Ma S, Machado VM, Mainoo NK, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Mason-Jones AJ, Mehndiratta MM, Mejia-Rodriguez F, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mokdad AH, Mola GL, Monasta L, de la Cruz Monis J, Hernandez JC, Moore AR, Moradi-Lakeh M, Mori R, Mueller UO, Mukaigawara M, Naheed A, Naidoo KS, Nand D, Nangia V, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nieuwenhuijsen MJ, Nisar MI, Nolte S, Norheim OF, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Park JH, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pesudovs K, Petzold M, Poenaru D, Polanczyk GV, Polinder S, Pope D, Pourmalek F, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, ur Rahman S, Raju M, Rana SM, Refaat A, Ronfani L, Roy N, Pimienta TG, Sahraian MA, Salomon JA, Sampson U, Santos IS, Sawhney M, Sayinzoga F, Schneider IJ, Schumacher A, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shakh-Nazarova M, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Sigfusdottir ID, Silberberg DH, Silva AP, Singh JA, Skirbekk V, Sliwa K, Soshnikov SS, Sposato LA, Sreeramareddy CT, Stroumpoulis K, Sturua L, Sykes BL, Tabb KM, Talongwa RT, Tan F, Teixeira CM, Tenkorang EY, Terkawi AS, Thorne-Lyman AL, Tirschwell DL, Towbin JA, Tran BX, Tsilimbaris M, Uchendu US, Ukwaja KN, Undurraga EA, Uzun SB, Vallely AJ, van Gool CH, Vasankari TJ, Vavilala MS, Venketasubramanian N, Villalpando S, Violante FS, Vlassov VV, Vos T, Waller S, Wang H, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, Wilkinson JD, Woldeyohannes SM, Wong JQ, Wordofa MA, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Naghavi M, Murray CJ, Lozano R. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.D’Alton ME, Friedman AM, Smiley RM, Montgomery DM, Paidas MJ, D’Oria R, Frost JL, Hameed AB, Karsnitz D, Levy BS, Clark SL. National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Obstet Gynecol. 2016;128:688–98. doi: 10.1097/AOG.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 217.Kendig S, Keats JP, Hoffman MC, Kay LB, Miller ES, Moore Simas TA, Frieder A, Hackley B, Indman P, Raines C, Semenuk K, Wisner KL, Lemieux LA. Consensus Bundle on Maternal Mental Health: Perinatal Depression and Anxiety. Obstet Gynecol. 2017;129:422–430. doi: 10.1097/AOG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rosenbaum T, Mhyre JM. The Anesthesiologist’s Role in the National Partnership for Maternal Safety’s Hemorrhage Bundle: A Review Article. Clin Obstet Gynecol. 2017 doi: 10.1097/GRF.0000000000000278. [DOI] [PubMed] [Google Scholar]