Abstract
Ferritin subunits of heavy and light polypeptide chains self-assemble into a spherical nanocage that serves as a natural transport vehicle for metals but can include diverse cargoes. Ferritin nanoparticles are characterized by remarkable stability, small and uniform size. Chemical modifications and molecular re-engineering of ferritin yield a versatile platform of nanocarriers capable of delivering a broad range of therapeutic and imaging agents. Targeting moieties conjugated to the ferritin external surface provide multivalent anchoring of biological targets. Here, we highlight some of the current work on ferritin as well as examine potential strategies that could be used to functionalize ferritin via chemical and genetic means to enable its utility in vascular drug delivery.
Graphical abstract
1. Introduction
Medical goals for drug targeting are extraordinarily diverse. Potential utility of drug delivery systems transcends all medical specialties. Pathophysiological context and biological factors defining specifications for a suitable drug delivery system (DDS) are unique in every pathological condition and patient. Multitudes of natural, synthetic, and hybrid DDS using different principles and materials are needed for these purposes[1,2].
Among the key features are amenable routes of administration and delivery, therapeutic target, DDS and drug cargo and their features – pharmacokinetics, size, biocompatibility, durability, etc. In addition to these investigational parameters, important translational parameters to consider in designing an efficient targeted drug delivery system include amenability to scale-up, quality control and cost of production.
Natural carriers include biomolecules and their assemblies, as well as cells and their fragments. Biological nanoparticles assembled from natural or modified biomolecules exhibit various unique architectures and functional properties that render them strong contenders for targeted drug delivery. Numerous self-assembled biological nanoparticles exist in nature such as ferritin, virus-like particles (VLPs), heat-shock protein cages, chaperones, carboxysomes, and enzyme complexes[3–9]. These natural nanoparticles each provides a unique set of characteristics that could be applied in biotherapeutics.
Here we consider ferritin nanoparticles as carriers for vascular drug delivery. In this review we highlight some of the progress in the area of ferritin-based nanocarriers and their applications in therapeutics and imaging. Their potential as modular and stimuli-responsive drug delivery platform, as well as strategies for conjugation by genetic and chemical conjugation means will be discussed. Finally, we outline methods for pulmonary targeting, advancements in targeted pulmonary drug delivery systems, and the potential use for ferritin in pulmonary therapeutics and imaging.
2. Ferritin nanoparticles
Ferritin is a major iron-storage protein in the body consisting of 24 subunits that self-assemble to form spherical nanocages of around 12 nm in diameter with an interior cavity of 8 nm[10,11]. Two types of subunits make up the ferritin nanocages, heavy (21 kDa) and light (19 kDa) chains[12,13]. The proportion of these subunits varies in different tissues[14]. The heavy chain has catalytic ferroxidase activity that oxidizes Fe(II) to Fe(III) which is insoluble and nucleates at the core[15,16]. Ferritin nanocages can load up to 4500 iron atoms in their interior cavity[12,13]. Endogenous ferritin is a non-enzymatic antioxidant that plays a cytoprotective role inside the cells by sequestering iron and preventing its harmful pro-oxidative effects.[17]
Elevated serum ferritin levels have been observed during inflammatory conditions as well as in some cancers[18]. The primary role for circulatory ferritin is unclear, however it has been reported to bind to and be internalized by a range of cells. Human ferritin binding occurs preferentially via its heavy chain rather than light chain[19]. The ferritin heavy chain interacts with the human transferrin receptor 1 (TfR1) leading to its endocytosis. This natural TfR1 targeting capability has been used to target TfR1-overexpressing cancer cells as a nanoparticle-assisted anti-cancer drug delivery strategy[19,20]. Other ferritin receptors have been discovered in mice, such as T cell immunoglobulin and mucin domain protein-2 (TIM-2) which binds to heavy chain ferritin, and scavenger receptor class A, member 5 (SCARA5) which binds selectively to light chain ferritin. However, in humans, TfR1 remains as the primary ferritin receptor[19,21,22].
Ferritin has quite a broad range of applications that span from therapeutics to imaging, diagnostics, bioelectronics, water purification, and even bioactuators for potential development as artificial muscle. The natural capacity of ferritin to encapsulate metals provides a platform that can be easily modified to give it unique functional characteristics. Biomineralization of ferritin with metals such as Co, Pt, Mn, Ni, and others have been used in bioelectronics because of their good electron transfer and storage capacity for development bionanobattery, biofuel cells, biosensors, and others[23–25].
The iron-loading capacity of ferritin can also be used for complexation with oxyanions such as orthophosphates. The Dutch company BiAqua (www.biaqua.nl) has developed an innovative water treatment strategy using hyperthermophilic ferritin to adsorb oxyanion (phosphate and arsenic) water contaminants. This strategy circumvents the low affinity and biofouling issues that face other water purification strategies. Ferritin has even been proposed as a delivery platform in the nutritional field. Li M. et al.[26] developed calcium-loaded soybeen seed phytoferritin nanocages for use as edible calcium supplement. Encapsulation inside ferritin was to protect calcium ions from dietary absorption inhibitors such as tannic acid, oxalic acid, and zinc ions. Aside metal and mineral loading, ferritin has been incorporated into hydrogels for use as bioactuators with potential application in artificial muscle development. Nanocomposite hydrogel actuators were developed by embedding ferritin in poly(vinyl alcohol) (PVA) nanofibers. The ferritin acted to reinforce the nanofibers resulting in 230% increase in the elastic modulus compared to PVA hydrogels alone. Reversible bioactuation was observed by switching the pH, with contraction at pH 4 and expansion at pH 9[27].
2.1 Ferritin as modular drug delivery platform
Numerous groups have utilized the ferritin as a drug delivery platform by loading it with small molecule therapeutics and incorporating targeting moieties on the surface either chemically or genetically. Ferritin forms a natural self-assembled oligomeric protein complex with uniform size and structure amenable to chemical and genetic modification for attachment of various cargos and targeting moieties.
Some characteristics that make ferritin a promising drug delivery candidate include remarkable thermal stability (withstanding temperatures up to 80-100 °C) and pH stability (pH 3-10), monodispersity, small uniform size, biocompatibility, biodegradability, low cost large-scale production, hollow cavity (nanocage) with reversible assembly and disassembly for encapsulation of drugs and imaging agents, ease of conjugation by chemical procedures (ample surface area with reactive moieties for conjugation) and genetic means (reported genetic sequence available for modification by classic recombinant cloning strategies).
A number of groups have reported on ferritin as carrier for drug delivery. Ferritin-based nanotherapeutics have been developed by loading ferritin nanocages with small molecules such as doxorubicin[28,29], cisplatin[30–32], curcumin[33], carotenoids[34], and cerium oxide[35]. Ferritin also provides robust strategy for metal nanoparticle encapsulation[36–38]. Strategies used drug loading include pH- or salt-induced disassembly/reassembly of ferritin, diffusion-based encapsulation, and direct conjugation of drug to ferritin surface (Fig. 1a).
Fig. 1.
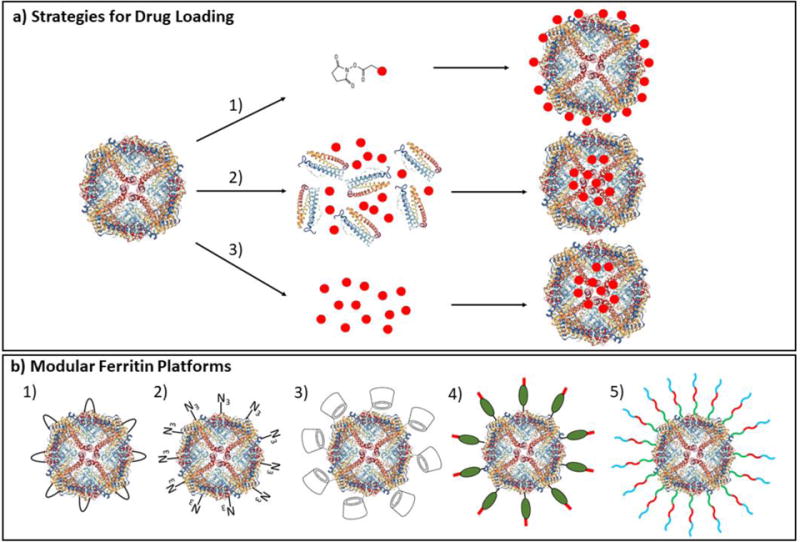
Illustration of drug loading strategies and modular ferritin platforms. a) Different routes for ferritin drug loading such as 1) chemical conjugation of drugs to ferritin surface, 2) pH- or salt-based assembly and disassembly of drugs inside ferritin, and 3) diffusion of small molecules through the surface pores of ferritin. b) Examples of modular ferritin platforms such as 1) ferritin incorporating Fc-binding peptide for binding targeting antibodies[39], 2) incorporation of unnatural azide-bearing amino acid in ferritin for click coupling to targeting ligands or antibodies (unpublished internal study), 3) ferritin surface conjugated with β-cyclodextrins for rapid drug loading[40], 4) ferritin incorporating protein G and 6X-His tag for antibody or cargo binding[41], and 5) engineered ferritin displaying functional peptides for siRNA delivery[42].
Some have incorporated certain functionalities into ferritin to make it more modular and hence a simpler modifiable platform (Fig. 1b). In order to avoid complex chemical conjugation strategies, Kang H.J. et al.[39] incorporated Fc-binding peptide between the D and E helices of ferritin from hyperthermophilic archaeon, as modular ferritin platform for binding Fc-region of antibodies in a non-covalent and orientation-specific manner. The platform was used to complex with anti-HER2 trastuzumab antibody and an antibody against folate receptor, demonstrating cell-specific in vitro binding. Another modular apoferritin platform strategy developed by Hwang M.P. et al.[41] was to genetically incorporate His tag and protein G in apoferritin for detection of cancer biomarker. Apoferritin was combined with antibodies that interact with their Fc region to protein G, as well as nanoconstructs (such as quantum dots, gold nanoparticles, or magnetic nanoparticles) that were surface-functionalized with Ni-NTA derivatives for interaction with His-tags on apoferritin. Lee E.J. et al.[42] developed a ferritin-based siRNA delivery system called Proteinticles. Proteinticles were designed by genetically engineering human ferritin to display peptides on its surface such as cationic peptide (CAP) for binding siRNA, tumor targeting peptide, cell penetrating peptides, and an enzymatic cleavage site for releasing siRNA inside tumor cells. The poly-siRNA-proteinticle complexes demonstrated effective gene silencing in tumor cells. The modular apoferritin platform provides a simple strategy to switch between various cargo probes and antibodies.
Most ferritin drug delivery reports encapsulate the therapeutic agents which requires the breakdown of ferritin for drug release. In order to allow for rapid drug release from ferritin, Kwon C. et al.[40] site-specifically conjugated 24 β-cyclodextrins (β-CD) molecules per ferritin for potential loading of small hydrophobic molecules. Fluorescein isothiocyanate-adamantane (FITC-AD) was loaded onto a ferritin-coated β-CD molecule. The study revealed drug release half-life of 3 hours, suitable for rapid drug delivery purposes, nonetheless release time could be increased with further fine-tuning.
Modifying ferritin to become stimuli-responsive adds greatly to its multifunctionality and controlled drug delivery capability. One group genetically modified ferritin to dissociate at pH 6 and reassemble at neutral pH. To do so, repeats of the GALA cell penetrating peptide were incorporated into E-helix truncated ferritin. The reversible transition of GALA from random coil to α-helix at acidic pH results in ferritin disassembly[43]. This may have applications in drug delivery, for inducible drug release at acidic pH as well as a method for drug loading in milder weak acid rather than the usual disassembly in a more damaging strong acid solution of pH 2.
For development of a multifunctional stimulus-responsive delivery system, Kang Y.J. et al.[44] incorporated thrombin cleavable peptide between the D and E helices of ferritin subunit. Thrombin cleavage induces the release of helix E and formation of 1.5 nm holes in the 4-fold axis of ferritin. A fluorophore was conjugated to the C-terminal genetically incorporated cysteine and NHS-PEG4-biotin was conjugated to the amine groups on the surface of ferritin for use as a targeting ligand for delivery to target cells over-expressing biotin receptors such as cancer cells. The fluorescent probe was released upon administration of thrombin. Various small molecules can be incorporated inside ferritin or genetically incorporated with peptides such as cytotoxic and apoptotic peptides for controlled release at target site.
3. Medical applications of ferritin
Ferritin has found wide-ranging utility in both therapeutics and imaging. The therapeutic applications of ferritin have primarily been focused on cancer therapy and vaccines.
3.1 Ferritin in cancer therapy
There are numerous studies on the use of ferritin for cancer therapy. Some have used the natural transferrin receptor targeting capability of ferritin to target transferrin-overexpressing cancer cells, while others have attached more cancer-specific targeting ligands. Falvo E. et al.[31] demonstrated chemical conjugation of PEG-linked EP1 monoclonal antibodies onto ferritin for retargeting it to melanoma cells. The ferritin was loaded with cisplatin (~50/Ft particle) using pH-based disassembly/reassembly strategy, followed by conjugation with PEG-antibody. The antibody conjugation led to 25-fold increase in specificity towards melanoma cells.
Another group genetically modified nanocages with RGD4C peptide and loaded them with doxorubicin for targeting αvβ3 integrin overexpressing tumor. Pre-complexation of doxorubicin with Cu(II) helped to increase the loading efficiency to up to 73 wt%. Increased circulation half-life, enhanced tumor growth inhibition, and decreased cardiotoxicity were observed compared to free doxorubicin[28].
In a recent report, Huang X. et al.[45] developed a ferritin-based lung inhalation delivery system capable of rapidly penetrating lung mucus and targeting lung tumor tissue (Fig. 2). Human ferritin was functionalized with different sized PEGs. FTn/FTn–PEG2k particles that contained both PEGylated ferritin subunits for mucus penetration and unPEGylated subunits for targeting tumor transferrin receptors demonstrated around 78% distribution on lung tracheal surfaces and was retained higher in upper airways 10 min after intratracheal administration. The FTn/FTn–PEG2k particles were then surface conjugated with doxorubicin molecules, which led to 60% survival after 60 days in a mouse lung cancer model as compared to untreated or free doxorubicin that led to median survival of 18 days.
Fig. 2.

Distribution and anti-tumor efficacy of FTn/FTn–PEG particles in mouse lung and proximal lung cancer model. a) in vivo distribution of FTn or FTn/FTn–PEGx (PEG sizes = 2, 5, and 10 kDa) labeled with Cy5 in large airways. Red (particles) DAPI (cell nuclei), b) quantification of labeled nanoparticles. Mice were administered intratracheally with particles and harvested at 10 minutes, and c) quantification of total fluorescence intensity throughout mouse trachea. FTn/FTn–PEG2k/DOX were administered intratracheally in an orthotopic mouse model of proximal lung cancer. d) IVIS imaging of 3LL lung tumor tissue bioluminescence signal over time, e) quantification of bioluminescence signal, and f) survival study of mice treated with and without treatment. Reprinted with permission from reference[45].
In order to improve on the drug encapsulation and prevent leakage from ferritin, Luo Y. et al.[46] introduced negatively charged poly-L-aspartic acid (PLAA) to absorb positively charged anti-cancer drug daunomycin (DN). Encapsulation of the PLAA/DN was carried out at pH 5 to induce swelling of the apoferritin hydrophobic channels. The drug loaded apoferritin was coated with hyaluronic acid to target hyaluronic acid receptor CD44 overexpressing lung cancer cells. In a recent study Zhang L. et al.[47] observed an active NLS-independent nuclear delivery of anticancer drug doxorubicin via H-chain apoferritin. Rapid nuclear translocation was observed within 15 minutes. This has great significance for nuclear anticancer drug delivery, primarily its potential for evading drug resistance mechanisms of the cell such as enzymatic deactivation and drug efflux.
3.2 Ferritin in vaccines
There are a number of protein particle-based vaccines on the market mainly utilizing virus-like particles. Ferritin with its multimeric nature, ease of genetic modification, easy handing, and large-scale production, certainly has potential in vaccine development.
The self-assembling nature of ferritin was used to induce trimerization of influenza heamagglutinin (HA) viral proteins on surface of ferritin for development as an influenza vaccine. The ferritin used in this case was a non-haem ferritin from Helicobacter pylori. Immunization with the HA-ferritin nanoparticles resulted in tenfold increased antibody titer production compared to the trivalent inactivated influenza vaccine. Some advantages of these novel vaccines include the utility of simple recombinant protein production strategy that removes the need to express hazardous viruses in cell culture or eggs[48].
Han J.A. et al.[49] utilized ferritin as an antigen delivery platform to dendritic cells for vaccine development. Ovalbumin-derived antigenic peptides OT-1 (CD8+ T cell epitope) or OT-2 (CD4+ T cell epitope) were incorporated at the C-terminus of ferritin. The OT-1 antigen-presentation on DC surface induced generation of antigen-specific cytotoxic effector T cells. The OT-2 antigen-presentation resulted in differentiation of CD4+ Th1 cells, which produced IFN-γ/IL-2 cytokines and CD4+ Th1 cells that produced IL-10/IL-13 cytokines. Incorporation of DC-targeting ligands and adjuvants is also being explored to further examine the ferritin-based antigen-delivery vaccine platform.
3.3 Ferritin in bioimaging
One of the biggest applications of ferritin has been in the field of bioimaging. Multifunctional ferritin nanocages have been developed for fluorescence and MRI imaging of tumor cells. One example is the work by Li K. et al.[50] which genetically fused RGD targeting moiety and GFP fluorescent protein to the N-terminus of human heavy chain ferritin. The self-assembled fluorescent ferritin nanocages were loaded with iron oxide by incubation with iron and H2O2 as oxidant. The iron oxide mineralized ferritin fusions were used to image αvβ3 integrin overexpressing tumor cells.
In order to make it a more multifunctional imaging system, Lin X. et al.[51] developed hybrid ferritin nanoparticles with activatable near-infrared fluorescence (NIRF) probes for tumor imaging. Two sets of ferritin were developed, one with Cy5.5 conjugated to matrix metalloproteinase (MMP) cleavable peptide, and the other with BHQ-3 quencher. The two sets of modified ferritin were disassembled in acidic pH and reassembled by bringing up the pH to form the hybrid ferritin nanoparticles. Upon administration of MMP-13, Cy5.5 was released and fluorescence was restored.
Hybrid ferritin nanoparticles were also developed incorporating RGD targeting moiety. In vivo animal study on tumor xenograft model demonstrated successful NIRF tumor imaging. Dual imaging of tumors has also been demonstrated using both NIRF and MRI imaging. Cy5.5 was conjugated to human heavy chain ferritin and loaded with iron oxide. The natural targeting capability of human ferritin to transferrin receptor 1 (TfR1) was used to target cancers overexpressing TfR1[52]. The RGD targeting moiety has been incorporated on ferritin for imaging vascular inflammation and angiogenesis. Here the RGD was aimed to target vascular macrophages as well as angiogenic endothelial cells, which overexpress αvβ3 integrins. Therefore, NIRF imaging with Cy5.5 labeled RGD-ferritin could be applied to image macrophage-rich carotid lesions, as well as macrophages and angiogenic endothelial cells in abdominal aortic aneurysm lesions[53].
In an innovative multimodal imaging system, Wang Z. et al.[54] developed a technique for loading radioisotopes inside ferritin for cancer theranostics (Fig. 3). The copper sulfide ferritin nanocages were formed by incubating radioactive 64CuCl2 with ferritin, followed by addition of Na2S solution to form stable copper sulfide nanoparticles inside ferritin. The CuS–ferritin nanocages demonstrated excellent PET and photoacoustic imaging as well as high photothermal conversion for cancer photothermal therapy.
Fig. 3.
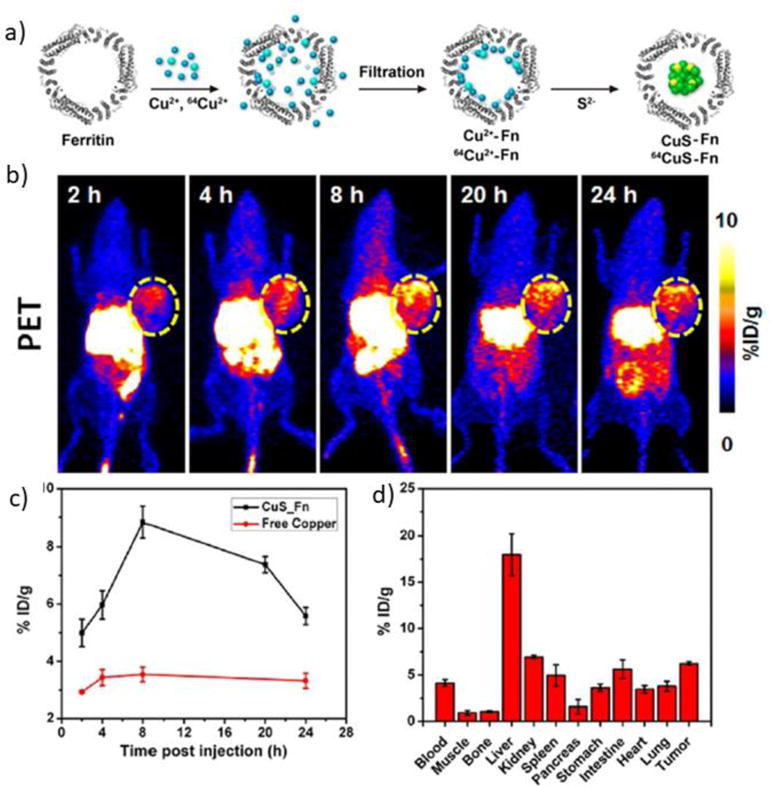
Copper sulfide-ferritin nanocages in cancer theranostics a) Schematic for synthesis of CuS–ferritin nanocarriers. b) Positron-emission tomography (PET) imaging of tumor-bearing mice I.V. injected with of 50 μCi 64CuS–ferritin nanocages at 2, 4, 8, 20, and 24 h. c) Tumor targeting of 64CuS–ferritin nanocages vs. free copper. d) Tissue biodistribution of 64CuS–ferritin nanocarriers 24 h after injection. Reprinted with permission from reference[54].
A ferritin-based theranostic agent has been developed for treatment of hepatocellular damage in thioacetamide-induced acute hepatitis animal model. MRI contrast agent GdHPDO3A and curcumin known for its anti-inflammatory, antioxidant, and antineoplastic activities were encapsulated inside apoferritin. MRI was used to assess the drug delivery efficiency[33]. Other MRI contrast agents such as gadolinium have also been encapsulated inside ferritin. Aime S. et al.[55] used pH-based disassembly/reassembly to encapsulate Gd(III) chelate, GdHPDO3A. Around ten GdHPDO3A chelates were encapsulated inside apoferritin. Malino A. et al.[56] developed a more efficient encapsulation strategy by utilizing cationic gadolinium chelate Gd-Me2DO2A, which increased the number of gadolinium chelates encapsulated to 36.
An advanced ferritin-based MRI imaging strategy was developed by Shapiro M.G. et al.[57] who designed ferritin-based sensors for detection of protein kinase A (PKA) activity using MRI. Two sets of genetically modified ferritins were designed, one incorporating kinase-inducible domain (KID) and the other KIX domain from the activator protein CBP. In presence of the PKA enzyme, Ft-KID was phosphorylated and in turn bound to the Ft-KIX. This interaction resulted in clustering of the iron-loaded modified ferritin nanoparticles and enhancement of the T2 relaxation in MRI. These “smart” ferritin-based sensors have applications both in vitro and in vivo for monitoring enzymatic activity.
4. Conjugation strategies
4.1 Genetically encodable linkers
The most common strategy for protein-protein conjugation involves attachment of cross-linkers to the exposed amino groups on the protein, however there is not much control over the number of cross-linkers attached or the orientation of the conjugated protein. This lack of control over conjugation can lead to formation of cross-linked aggregates, a reduction or loss of function of the protein, or low-efficiency labeling. Genetic incorporation of linkers into proteins can lead to more effective development of bioconjugate therapeutics by enabling site-specific conjugation in proper orientation.
Numerous bioconjugation strategies (Fig. 4) have been developed utilizing genetically encodable peptide or protein tags. Some examples of encodable tags include His Tag (interacts with Ni-NTA), HaloTag (interacts with chloroalkane-linked moieties), SNAP-tag (interacts with benzylguanine derivatives), Clip-tag (interacts with benzylcytosine derivatives), SpyTag/SpyCatcher, sortase tag, biotin acceptor peptide (for enzymatic conjugation of biotin-linked moieties), and heterodimeric peptides[58–61]. One simple bioconjugation strategy is the utilization of heterodimeric peptides that dimerize different proteins or moieties together. An example is the genetic tagging of scFvs and adenoviruses with leucine zipper coiled-coil heterodimeric peptides for attaching scFv antibody fragments onto adenoviruses in the same cellular supernantant from which they are released. Upon expression and release from the cell, the heterodimerization zipper domains induce the complexation of the scFvs moieties onto the adenovirus surface[62]. This strategy is especially suitable for adenoviruses, because their route of production and cellular release is different than the scFvs which require post-translational modification.
Fig. 4.

Illustration of various potential chemical and bioconjugation strategies available for attachment of cargo or targeting moieties to ferritin.
Homo- and hetero-dimerization peptides can also be used to design complex nanostructures. Gradisar H. et al.[63] formed tetrahedron-shaped structures out of a single polypeptide consisting of 12 coiled-coil peptides. 3 homo- and 3 hetero-dimeric coiled-coil peptide pairs self-assembled to form the tetrahedron-shaped structure. Other more complex polyhedral structures could also be developed using such polypeptide origami.
Proteins can also be oligomerized using coiled-coil peptide motifs. Some examples of coiled-coil peptides include GCN4 leucine zippers (dimers), fibritin (trimers), tetrabrachion (teramers), and cartilage oligomerization matrix protein (pentamers)[64–67]. In one case, Holler N. et al.[68] fused extracellular domains from receptors such as Fas and CD40 onto COMP pentamerization domain. The pentamerization resulted in greater solubility, high avidity, and enhanced ability to block FasL and CD40L. Another example is the development of bispecific decavalent single domain antibodies by fusing one set of single domain antibody to C-terminus of verotoxin B (VTB) pentamerization domain and the other set of single domain antibody to the N-terminus of VTB. These decabodies were designed to increase the avidity of the bispecific antibodies. Ferritin with its 24 subunits could potentially be used to provide a generous multimerization scaffold for single domain antibodies, to enhance the avidity and biological efficacy.
One promising site-specific conjugation is the use of bacterial-derived transpeptidase enzyme sortase to catalyze conjugation between proteins tagged with LPXTG motif and triglycine containing moieties. Chen Q. et al.[69] utilized the sortase-mediated conjugation to functionalize E2 protein cages. E2 protein subunits derived from the pyruvate dehydrogenase enzyme complex of Bacillus stearothermophilus self-assemble into 60mer protein cages of around 24 nm in size. A triglycine tag was incorporated at the N-terminus of E2 proteins which when fully assembled displayed 60 GGG tags available for sortase-mediated conjugation. LPTEG containing β-galactosidase or endogluconase enzymes and thermoresponsive elastin like polypeptides (ELP) were conjugated using sortase. The thermoresponsive ELPs were used as a simple temperature-based purification strategy.
A notable novel conjugation strategy is the SpyTag (peptide) and SpyCatcher (protein) based conjugation mediated by the SpyLigase enzyme leading to irreversible isopeptide bond formation. Fierer J.O. et al.[70] used the SpyTag/SpyCatcher strategy to develop a linearized polymer of affibodies to enhance the capture of circulating tumor cells. A SpyTag and a KTag (derived from SpyCatcher) in presence of SpyLigase polymerized end to end forming a polymer of more than 20 affibodies. Magnetic beads coated with the multivalent polymeric affibodies increased the capture of circulating cancer cells displaying low number of tumor antigens. Building on the the SpyTag/SpyCatcher technology, Fairhead et al.[71] developed chimeric SpyAvidin hubs to create modular protein nanoassemblies. The strategy combined SpyTag or SpyCatcher with streptavidin components to create SpyAvidin octamer or eicosamer protein architecture. The eicosamer nanoassembly was used to cluster MHC-peptide complexes for T cell stimulation, and was found to be superior to the conventional MHC tetramers.
Other bioconjugation strategies include incorporation of split-inteins, biotin acceptor peptides, and monovalent streptavidin. Intein-mediated conjugation is a process whereby N- and C-terminal halves of intein-containing proteins or molecules can be fused together by a process of protein splicing where the split inteins interact and self-excise, conjugating the two target molecules together[72,73]. Monovalent streptavidin is another recently introduced tagging moiety that can allow for more controlled site-specific conjugation rather than the original tetrameric streptavidin that is prone to aggregation as a result of its multivalent nature[74]. Genetic incorporation of biotin acceptor peptide (AP) in the protein of interest has also been tried for site-specific conjugation. The biotin ligase (BirA) enzyme derived from Escherichia coli has been used to biotinylate the acceptor peptide tagged proteins. One example is the incorporation of AP peptide on AMPA receptors on neurons for biotinylation and targeting with streptavidin-coated quantum dots for visualizing the localization of the receptors in live neuron synapses[75].
4.2 Chemical conjugation
The functional groups present on proteins can be used for conjugation of various moieties (Fig. 4). Some of the amino acid side-chain groups used for conjugation include amino group on lysine, thiol on cysteine, carboxylic acid on aspartic acids and glutamic acids, and hydroxyl moiety on tyrosine. Numerous heterobifunctional crosslinkers have been developed for protein conjugation. Primary amines on proteins can be conjugated to carboxylic acids on another protein using 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide (EDC) crosslinkers typically in combination with N-hydroxysuccinimide (NHS) that reacts with the carboxyl group and creates a more stable intermediate that then reacts with the primary amines[76–78].
Utilizing the crosslinker EDC along with N-hydroxysuccinamide (NHS), Andrade S. et al.[79] conjugated ghrelin to virus-like particles (VLPs) derived from Bluetongue virus (BTV). The anti-ghrelin vaccine was developed as strategy for treatment of obesity. Sulfhydryl functional groups can be introduced by heterobifunctional crosslinkers, such as N-succinimidyl S-acetylthioacetate (SATA) that react with primary amine on proteins[80,81]. Following the NHS conjugation to amines, the thioacetate is deprotected to display sulfhydryl group for reaction with thiol reactive compounds such as maleimides. The SATA reaction is typically coupled with corresponding heterobifunctional crosslinker sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC) that displays maleimides on target protein that is being conjugated. The SATA and SMCC is a common and simple crosslinking reaction for conjugating proteins together. This strategy has been used by us and others to conjugate various antibody and antibody fragments onto the ferritin surface[82–86].
For a more site-specific introduction of sulfhydryl groups, cysteines can be genetically introduced into the protein for site-selective conjugation. Such strategy can be used to introduce cysteines selectively on the exterior or interior of ferritin for attaching cargo or targeting moieties on the surface or interior of ferritin. VLPs have been also been modified using similar strategy. Peacey M. et al.[87] modified VLPs with sulfo-SMCC followed by conjugation to thiol containing peptides or to SATA treated proteins. Cytokine and antibody conjugations have also been carried out with the SATA and SMCC strategy. Li J. et al.[88] chemically conjugated recombinant human interleukin 2 protein with a monoclonal antibody. Functional IL-2 cytokine activity and the binding capacity of the antibody were both preserved using this conjugation strategy.
“Click” coupling reactions between azides and alkyne functional groups provide a simple and efficient bioconjugation strategy compatible with a broad range of temperatures, pH values, and solvents. One of the most popular click reactions is the Huisgen 1,3 dipolar-cycloaddition in presence of Cu(I) catalyst[89–91]. Azide and alkynes can be chemically incorporated on the protein of interest or introduced genetically via incorporation of unnatural amino acids. Patel K.G. et al.[92] incorporated unnatural methionine analogues azidohomoalanine and homoproparglyglycine into bacteriophage MS2 and Qb VLPs. The unnatural amino acids were then conjugated to azide and alkyne containing proteins such as scFv antibody fragments, granulocyte-macrophage colony stimulating factor (GM-CSF), immunostimulatory CpG DNA, and PEG. The methionine residues were globally replaced with the unnatural amino acids in a cell-free system rather than the methionine auxotrophic E. coli, demonstrating high yield and efficient incorporation of the unnatural amino acids.
Another interesting strategy that has been used to increase the cargo loading capacity of protein cages is the site-specific initiation of atom-transfer radical polymerization to form amine rich polymer inside the protein cage. The primary amine groups inside the cage provide conjugation sites for cargo attachment. Lucon J. et al.[93] used this strategy to enable high density loading of a gadolinium MRI reagent inside P22 virus-like particles.
5. Ferritin carriers for drug delivery to the pulmonary vasculature
5.1 Pulmonary targeting
Pulmonary vasculature is an important therapeutic target in many disease conditions, yet targeting ferritin to the pulmonary endothelium has not been explored. In our lab, we have addressed this novel aspect, using targeting strategy designed by us and others, i.e., conjugating carriers with antibodies and antibody fragments binding to endothelial surface determinants (“vascular immunotargeting”).
Examples of such ligands (Table 1) include antibodies to platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), angiotensin-converting enzyme (ACE), aminopeptidase P (APP), and plasmalemma vesicle protein-1 (PV1)[94]. Platelet-endothelial adhesion cell molecule-1, (PECAM-1/CD31) is constitutively expressed by the endothelium at 0.2–2 × 106 copies per cell[95]. It is mainly localized on the endothelial intercellular junctions. Anti-PECAM-1 antibody conjugated nanocarriers have been shown to accumulate in the lung and inhibit the endothelial inflammatory response[96].
Table 1.
Examples of endothelial determinants for targeting nanocarriers to the pulmonary vasculature
| Endothelial determinant | Vascular location | Regulation |
|---|---|---|
| PECAM-1 (CD31) | Throughout vasculature. | Stably expressed |
| ICAM-1 (CD54) | Throughout vasculature. | Increased expression during inflammation. |
| VCAM-1 (CD106) | Expressed in inflamed vascular endothelium | Increased expression during inflammation. |
| E-selectin (CD62E) | Expressed in inflamed vascular endothelium | Increased expression during inflammation. |
| P-selectin (CD62P) | Expressed in inflamed vascular endothelium | Activation by agonists can results in rapid transport to cell surface. |
| APP | Differentially expressed in vasculature. Increased expression in pulmonary vascular endothelium. Also expressed in heart, liver, and kidney. | Not known. |
| PV1 (PLVAP) | Expression in majority of tissues. | Increased expression by VEGF in vitro. |
| ACE (CD143) | Throughout vasculature. Increased in the lung capillaries. | ACE activity enhanced by chloride. |
| TM (CD141) | Throughout vasculature. Endothelial specific. | Downregulated by factors such as TNF, oxidative stress, hypoxia, C-reactive protein, cAMP, phorbolesters, and oxidized LDL. Upregulated by thrombin, retinoic acid, VEGF, and heat shock. |
Abbreviations: PECAM-1, platelet endothelial cell adhesion molecule 1; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule; APP, aminopeptidase P; PV1, Plasmalemmal vesicle associated protein; ACE, angiotensin-converting enzyme; TM, thrombomodulin.
Intercellular adhesion molecule-1, CD54 (ICAM-1) is constitutively expressed on the apical endothelial plasmalemma and is upregulated upon inflammation. On the other hand, vascular cell adhesion molecule-1 (VCAM-1) is expressed primarily only upon inflammation. Another interesting target is PV1 which has been reported to be enriched in pulmonary endothelial cells[97–99].
In addition to cell-specific delivery, subcellular targeting could be used to enhance the effectiveness of therapeutics. Incorporation of a subcellular targeting mechanism into the nanocarrier system could be used to direct the therapeutic to caveolar endosomes, to potentially more efficiently suppress the redox-active endosomes or the signaling endosomes which contain NADPH oxidases involved in inflammation and vascular oxidative stress. From our studies on pulmonary targeted drug delivery systems, a comparison of pulmonary targeted ICAM-1 and PECAM-1 nanoparticles demonstrated the superiority of the ICAM-1 targeted ferritin nanoparticles in lung targeting (Fig. 5). The anti-ICAM Ab/FNPs demonstrated a remarkable 160.9 ± 6.5 % ID/g lung targeting, which is superior to the other tested nanocarriers.
Fig. 5.
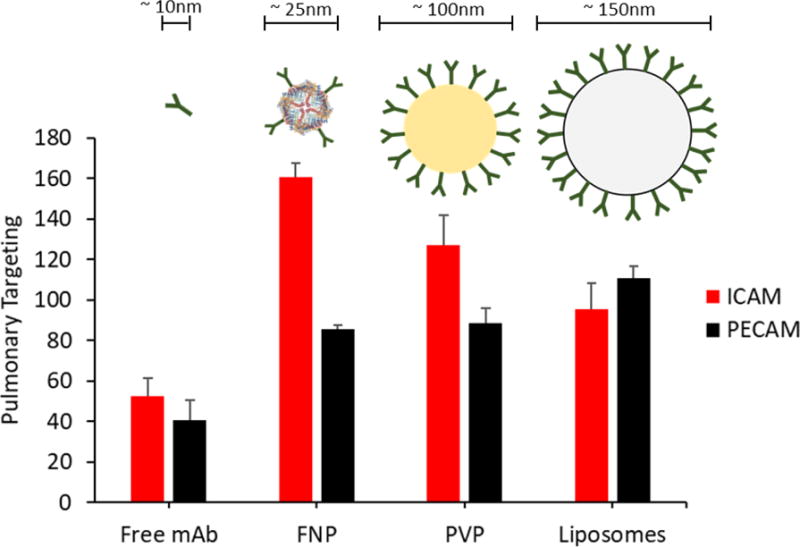
Pulmonary targeting of ICAM-1 and PECAM-1 directed nanoparticles. Targeting efficiency indicates % injected dose per gram tissue. mAb - isolated monoclonal antibody, FNP - ferritin nanoparticles, PVP - 100-nm polyvinylphenol particles, liposomes – 150-nm PEGylated immunoliposomes[100–103].
5.2 Potential application in pulmonary medicine
Ferritin nanoparticles, with their uniform size, stability, and biocompatibility, show promise as a therapeutic delivery platform with potential for translation to the clinical domain. Numerous small molecule anti-inflammatory, antioxidant, or other therapeutic agents can be potentially encapsulated inside ferritin or attached on the ferritin surface (Table 2). Targeting ferritin-based therapeutics to pulmonary endothelium could have great potential for treatment of various oxidative and inflammatory pulmonary conditions. The pulmonary vasculature is an important target for therapeutic delivery. Elevated reactive oxygen species (ROS) generated by enzymes such as NADPH oxidases in pulmonary endothelial cells has been found to be involved in vascular oxidative stress and the proinflammatory cascade that manifests into pathological conditions such as acute respiratory distress syndrome (ARDS)[104,105].
Table 2.
Anti-inflammatory agents that could benefit from targeting by nanocarriers
| Agent | Drawbacks | Examples |
|---|---|---|
| Antioxidants | Antioxidant enzymes are expensive to manufacture, poor pharmacokinetics, potential immunogenicity | SOD, Catalase, GSH, Cerium Oxide, NAC, EUK-134 |
| NO donors | Vasodilation, Headaches, Hypotension, Fainting | NONOates, NCX-1015 (21-NO-prednisolone), NO-donor salicylic acid co-drug |
| Anti-inflammatory cytokines or antagonists | Systemic side-effects, pleiotropic effects on immune system | IL-10, IL-1 receptor antagonist, anti-p55 TNF receptor antibody |
| siRNAs | Poor stability and pharmacokinetics, immunogenicity | siRNA targeting TNF, NF-κB p65, COX-2, ICAM-1, NADPH oxidase |
| Glucocorticoids | Systemic side-effects, immunodeficiency, steroid-induced osteoperosis, hyperglycemia, prone to infections | Dexamethasone, Beclomethasone, Budesonide, Prednisone |
| NSAIDs | Ulcers, bleeding, and kidney failure from long-term use. | Aspirin, Ibuprofen, Naproxen |
Abbreviations: SOD, superoxide dismutase; GSH, glutathione; NAC, N-acetylcysteine, NO, nitric oxide; NONOate, diazeniumdiolate; siRNA, small interfering RNA; NSAID, nonsteroidal anti-inflammatory drug.
Targeted delivery of therapeutics such as ROS-detoxifying interventions to the pulmonary endothelium could protect from oxidative damage and suppress pulmonary inflammation. Antioxidant enzymes have been conjugated to endothelial-specific antibodies as ROS-detoxifying intervention[96,106–111].
Platelet-endothelial adhesion cell molecule-1, CD31 (PECAM-1) antibody conjugated SOD or catalase nanoparticles have led to endothelial protection against ROS-induced injury such as ischemia/reperfusion, lung transplantation or inflammation[106–108]. Anti-PECAM-1 antibody conjugated nanocarriers have been shown to accumulate in the lung, and exhibit inhibition of endothelial inflammatory response. Kozower B.D. et al.[109] demonstrated PECAM-targeted delivery of catalase enzyme to pulmonary vasculature and retention of catalase activity during cold storage and transplantation in an in vivo lung transplantation model.
Exploring an inhalation delivery system, Yen C.C. et al.[112] delivered aerosolized recombinant human extracellular superoxide dismutase enzyme in prevention of hyperoxia-induced lung injury. The study demonstrated increased survival rate, decreased lung edema, and greater protection from systemic oxidative stress. The aerosolized SOD delivery strategy was proposed as an effective method of delivering SOD to critically ill patients in intensive care units for protection against oxygen toxicity, with therapeutic applications for acute respiratory distress syndrome.
Endothelial targeted immunoliposomes have been used for therapeutic delivery of small molecule and proteins therapeutics to pulmonary vasculature. Hood E.D. et al.[113] loaded PECAM-1 antibody-coated PEG-liposomes with MJ33, an NOX inhibitor. MJ33 acts by inhibiting cytosolic phospholipase 2 (PLA2), suppressing production of lyso-phospholipids and free fatty acids involved in NOX activation. The immunoliposomes inhibited expression of proinflammatory marker VCAM, decreased vascular permeability, and reduced alveolar edema by around 50%. These immunoliposomes have also been used to deliver EUK-134, a SOD/catalase mimetic. Suppression of inflammation and protection greater than 60% against pulmonary edema was observed in LPS-challenged mice[101]. Other examples of small molecule anti-inflammatory agents used with immunoliposome mediated delivery to lung, include delivery of dexamethasone[114] and prostaglandin E1[115].
Nitric oxide (NO) with its anti-inflammatory and vasodilator properties has been used for treatment acute lung injury, primarily in inhaled form. Gaseous nitric oxide has been reported to alleviate acute lung injury by decreasing inflammation, increasing pulmonary vasodilation, and enhancing gas exchange, however it can also lead to unfavorable byproduct formation such as nitrites, nitrates, peroxynitrates, and other reactive oxygen species that can be damaging to the lung[116,117]. In order to prevent the damaging effects of inhaled NO, NO-releasing prodrugs have been developed such as NO/nucleophile adducts (NONOates). Kirov M.Y. et al.[118] developed biodegradable linear polyethyleneimine-based NONOates (L-PEI-NONO). Aerosolized L-PEI-NONO was found to decrease pulmonary hypertension and lung edema, as well as increase gas exchange in endotoxin-induced acute lung injury model. Moreover, NONOate was shown to attenuate anti-inflammatory effects of PECAM-targeted SOD[119].
Pulmonary delivery of anti-inflammatory cytokines or inhibitors of inflammatory cytokines also has therapeutic potential in treatment of ARDS. Exogenous delivery of IL-10 cytokine has been found to decrease hyperoxia-induced lung injury. Decreased levels of myeloperoxidase, IL-6, TNF-α, MIP-2, iNOS, as well as decreased lung permeability and increased survival in mice were observed[120]. Pro-inflammatory cytokines such as TNFα and IL-1 play a major role in endothelial activation and vascular dysfunction. Alveolar macrophages have been reported to be a major source for IL-1 in ARDS. Antagonists against pro-inflammatory cytokines could help in treatment of ALI. Frank J.A. et al.[121] demonstrated protective effect of IL-1 receptor antagonist (Il-1Ra) in ventilator induced lung injury (VILI) mouse model, showing decreased pulmonary edema and reduced neutrophil recruitment. Attenuation of VILI has also been demonstrated by intratracheal delivery of single domain antibody against p55 TNF receptor[122].
Numerous therapeutic agents are available for pulmonary targeted-ferritin based delivery. The potential of this delivery system lies with the amount of therapeutic agent that can be encapsulated or conjugated to the surface without jeopardizing the targeting efficiency. Some groups have reported dense loading of therapeutic or imaging agents[28,93]. If the same high drug loading can be achieved with pulmonary therapeutic agents while maintaining efficient targeting, then this would open the door for translation of ferritin-based therapeutics into pulmonary medicine.
6. Challenges associated with ferritin
Notwithstanding emerging opportunities for ferritin-based drug delivery, we must pay attention to potentially problematic issues. The challenges as with most other nanocarriers include achieving optimal homogeneity, reproducibility, cargo loading capacity, targeting efficiency, pharmacokinetics, biocompatibility, ease of manufacturability, and cost-effectiveness. With the advances in genetic engineering and site-specific controlled conjugation strategies, there are numerous options available for incorporation of cargoes and targeting ligands in a more site-specific and orientation-specific manner, and at higher yield. The challenge that is critical for development ferritin formulations with high therapeutic efficacy is how to enhance the number of targeting ligands and cargoes in a manner such that they do not interfere with the loading of each other. This has been investigated by us, where increasing the surface conjugation of therapeutic protein (superoxide dismutase) resulted in decreased conjugation of antibodies and reduced targeting efficacy, as compared to encapsulation of the therapeutic protein which preserved the high targeting efficiency. In addition, the small size of ferritin has its advantages in certain targeting applications, however it also limits the number of antibodies or cargoes that can be loaded. Genetic modifications could be made to potentially alter the ferritin size to accommodate more targeting ligands and cargoes.
As with any drug delivery carrier, the biological challenges include adverse and unintended effects associated with impairment of the target and clearing cells and tissues, abnormal host defense reactions, derangement of reactive cascades of coagulation and complement in blood and other abnormalities. These undesirable reactions may ignite side effects. Further, drug delivery is likely to be intended for use in patients, not healthy people. Pathological factors typical of the disease may aggravate the response to ferritin carriers. For example, cytokines and other mediators of ongoing inflammation may aggravate the immune reaction to ferritin.
One concern general to biotherapeutics drugs and biological carriers is immune reactions. Both immunological theory and clinical practice imply that administration of biological materials in experimental mammalian animals and human patients may elicit immunological responses including activation of subsets of lymphocytes and production of antibodies and that this reaction is expected to be aggravated by using heterologous biomolecules from a different species. Human-anti-mouse IgG response provides a well-known example of this immunological reaction. Of note, immune reactions to non-biological carriers have also been reported, such as antibodies to PEG in humans[123–126]. Furthermore, use of “human” agents such as recombinant proteins is complicated with inevitable denaturing of a fraction of these reagents during the production, leading to exposure of normally hidden residues and epitopes. In addition, there is an issue of undetectable yet potentially active contaminants from the producing systems (not limited to endotoxin). Therefore, unintended immune responses represent common serious concerns for all drug delivery strategies. There are following avenues to address these concerns.
First is the use of human ferritin platforms or strategies to “humanize” ferritin from other species, such as by replacing most immunogenic residues and epitopes by human sequences and scaffolds, or total, achieved by replacing a heterologous component by human analogues. Second, increase in efficacy and specificity of targeted delivery will lead to dose reduction, hence alleviation of immune response. Third, studies by Hubbell and other labs suggest that some versions of molecular configuration of immunogenic molecules in a drug delivery system confers tolerance[127,128]. Fourth, in life-threatening conditions, treatment with potentially immunogenic yet needed drug delivery systems can be accompanied by immunosuppressive agents. Finally, in many acute conditions, the envisioned mode and regimen of administration i.e., single injection or infusion avoiding repetitive injections, will likely minimize immune response.
7. Conclusion
Ferritin is a biological “ready-to-use” nanoparticle with unique properties that could be exploited for use in imaging and therapeutics of pulmonary diseases. The protein exhibits natural capacity to encapsulate a range of metals and minerals, as well as imaging agents and therapeutics. Ferritin is highly versatile and amenable to various genetic and chemical modifications that can be used for incorporation of targeting moieties or therapeutic cargo. Numerous modifications can be carried out to make ferritin more modular and stimuli-responsive to simplify the construct assembly and enhance its therapeutic efficacy.
Ferritin has strong potential to make an efficient targeted drug delivery and imaging platform for clinical translation. One main factor that needs to be evaluated more thoroughly to determine its clinical practicality is its biocompatibility. Our recent in vivo animal biodistribution studies of pulmonary vascular immunotargeting have shown high pulmonary endothelial targeting with no noticeable undesired effects. These natural bio-nanoparticles could be easily modified and functionalized to treat various oxidative and inflammatory pulmonary conditions. The next phase of pulmonary therapeutic evaluation will determine its true place in clinical pulmonary drug delivery.
Acknowledgments
This work is supported by National Institute of Health (NIH) and National Heart, Lung and Blood Institute (NHLBI) HL125462, HL128398, and HL126874 grants to VRM, as well as by NSF grant (PD 09-6885, CHE-1508318) to IJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park K. The Controlled Drug Delivery Systems: Past Forward and Future Back. J Control Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhen Z, Tang W, Todd T, Xie J. Ferritins as nanoplatforms for imaging and drug delivery. Expert Opin Drug Deliv. 2014;11:1913–22. doi: 10.1517/17425247.2014.941354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgens C, Weyermann J, Zimmer A. Recombinant virus like particles as drug delivery system. Curr Pharm Biotechnol. 2005;6:49–55. doi: 10.2174/1389201053167202. [DOI] [PubMed] [Google Scholar]
- 5.Lee LA, Wang Q. Adaptations of nanoscale viruses and other protein cages for medical applications. Nanomedicine Nanotechnology, Biol Med. 2006;2:137–149. doi: 10.1016/j.nano.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Kramer RM, Li C, Carter DC, Stone MO, Naik RR. Engineered protein cages for nanomaterial synthesis. J Am Chem Soc. 2004;126:13282–13286. doi: 10.1021/ja046735b. [DOI] [PubMed] [Google Scholar]
- 7.Flenniken ML, Willits DA, Brumfield S, Young MJ, Douglas T. The Small Heat Shock Protein Cage from Methanococcus jannaschii is a Versatile Nanoscale Platform for Genetic and Chemical Modification. Nano Lett. 2003;3:1573–1576. [Google Scholar]
- 8.Kerfeld CA, Sawaya MR, Tanaka S, Nguyen CV, Phillips M, Beeby M, Yeates TO. Protein structures forming the shell of primitive bacterial organelles. Science. 2005;309:936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 9.Corchero JL, Cedano J. Self-assembling, protein-based intracellular bacterial organelles: emerging vehicles for encapsulating, targeting and delivering therapeutical cargoes. Microb Cell Fact. 2011;10:92. doi: 10.1186/1475-2859-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiancone E, Ceci P, Ilari A, Ribacchi F, Stefanini S. Iron and proteins for iron storage and detoxification. Biometals. 2004;17:197–202. doi: 10.1023/b:biom.0000027692.24395.76. [DOI] [PubMed] [Google Scholar]
- 11.Watt RK. The many faces of the octahedral ferritin protein. Biometals. 2011;24:489–500. doi: 10.1007/s10534-011-9415-8. [DOI] [PubMed] [Google Scholar]
- 12.Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 13.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta - Bioenerg. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 14.Arosio P, Adelman T, Drysdale J. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978;131:210–6. [PubMed] [Google Scholar]
- 15.Lawson DM, Treffry A, Artymiuk PJ, Harrison PM, Yewdall SJ, Luzzago A, Cesareni G, Levi S, Arosio P. Identification of the ferroxidase centre in ferritin. FEBS Lett. 1989;254:207–210. doi: 10.1016/0014-5793(89)81040-3. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Chasteen ND. Ferroxidase activity of ferritin: effects of pH, buffer and Fe(II) and Fe(III) concentrations on Fe(II) autoxidation and ferroxidation. Biochem J. 1999;338(Pt 3):615–618. [PMC free article] [PubMed] [Google Scholar]
- 17.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: A cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 18.Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol. 2007;293:C641–9. doi: 10.1152/ajpcell.00599.2006. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang M, Fan K, Zhou M, Duan D, Zheng J, Yang D, Feng J, Yan X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc Natl Acad Sci. 2014;111:14900–14905. doi: 10.1073/pnas.1407808111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Seaman WE, Di X, Wang W, Willingham M, Torti FM, Torti SV. Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0023800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Drexler IR, Chen X, Sanna-cherchi S, Mohammed F, Lin CS, Schmidt-ott KM, Andrews NC. Scara5 is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev Cell. 2010;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JW, Choi SH, Lillehei PT, King GC, Elliott JR, Chu SH, Park Y, Watt GD. Biologically Derived Nanoparticle Arrays via a Site-Specific Reconstitution of Ferritin and their Electrochemistry. Proc - Electrochem Soc. 2004 [Google Scholar]
- 24.Yamashita I, Iwahori K, Kumagai S. Ferritin in the field of nanodevices. Biochim Biophys Acta. 2010;1800:846–57. doi: 10.1016/j.bbagen.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Qiu H, Dong X, Sana B, Peng T, Paramelle D, Chen P, Lim S. Ferritin-templated synthesis and self-assembly of Pt nanoparticles on a monolithic porous graphene network for electrocatalysis in fuel cells. ACS Appl Mater Interfaces. 2013;5:782–7. doi: 10.1021/am3022366. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Zhang T, Yang H, Zhao G, Xu C. A novel calcium supplement prepared by phytoferritin nanocages protects against absorption inhibitors through a unique pathway. Bone. 2014;64:115–23. doi: 10.1016/j.bone.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Shin MK, Spinks GM, Shin SR, Kim SI, Kim SJ. Nanocomposite hydrogel with high toughness for bioactuators. Adv Mater. 2009;21:1712–1715. [Google Scholar]
- 28.Zhen Z, Tang W, Chen H, Lin X, Todd T, Wang G, Cowger T, Chen X, Xie J. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano. 2013;7:4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilic MA, Ozlu E, Calis S. A novel protein-based anticancer drug encapsulating nanosphere: apoferritin-doxorubicin complex. J Biomed Nanotechnol. 2012;8:508–14. doi: 10.1166/jbn.2012.1406. [DOI] [PubMed] [Google Scholar]
- 30.Ji XT, Huang L, Huang HQ. Construction of nanometer cisplatin core-ferritin (NCC-F) and proteomic analysis of gastric cancer cell apoptosis induced with cisplatin released from the NCC-F. J Proteomics. 2012;75:3145–57. doi: 10.1016/j.jprot.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Falvo E, Tremante E, Fraioli R, Leonetti C, Zamparelli C, Boffi A, Morea V, Ceci P, Giacomini P. Antibody-drug conjugates: targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale. 2013;5:12278–85. doi: 10.1039/c3nr04268e. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Wang X, Diao H, Zhang J, Li H, Sun H, Guo Z. Encapsulation of platinum anticancer drugs by apoferritin. Chem Commun (Camb) 2007;7345:3453–3455. doi: 10.1039/b705326f. [DOI] [PubMed] [Google Scholar]
- 33.Cutrin JC, Crich SG, Burghelea D, Dastrù W, Aime S. Curcumin/Gd loaded apoferritin: A novel “theranostic” agent to prevent hepatocellular damage in toxic induced acute hepatitis. Mol Pharm. 2013;10:2079–2085. doi: 10.1021/mp3006177. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Bai G, Yang R, Zang J, Zhou T, Zhao G. Encapsulation of β-carotene within ferritin nanocages greatly increases its water-solubility and thermal stability. Food Chem. 2014;149:307–312. doi: 10.1016/j.foodchem.2013.10.115. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Wei W, Yuan Q, Zhang X, Li N, Du Y, Ma G, Yan C, Ma D. Apoferritin–CeO2 nano-truffle that has excellent artificial redox enzyme activity. Chem Commun. 2012;48:3155. doi: 10.1039/c1cc15815e. [DOI] [PubMed] [Google Scholar]
- 36.Ping J, Pulsipher KW, Vishnubhotla R, Villegas JA, Hicks TL, Honig S, Saven JG, Dmochowski IJ, Johnson ATC. Structural-functional analysis of engineered protein-nanoparticle assemblies using graphene microelectrodes. Chem Sci. 2017;8:5329–5334. doi: 10.1039/c7sc01565h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulsipher KW, Villegas JA, Roose BW, Hicks TL, Yoon J, Saven JG, Dmochowski IJ. Thermophilic Ferritin 24mer Assembly and Nanoparticle Encapsulation Modulated by Interdimer Electrostatic Repulsion. Biochemistry. 2017;56:3596–3606. doi: 10.1021/acs.biochem.7b00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulsipher KW, Honig S, Deng S, Dmochowski IJ. Controlling gold nanoparticle seeded growth in thermophilic ferritin protein templates. J Inorg Biochem. 2017;174:169–176. doi: 10.1016/j.jinorgbio.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Kang HJ, Kang YJ, Lee YM, Shin HH, Chung SJ, Kang S. Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials. 2012;33:5423–5430. doi: 10.1016/j.biomaterials.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 40.Kwon C, Kang YJ, Jeon S, Jung S, Hong SY, Kang S. Development of Protein-Cage-Based Delivery Nanoplatforms by Polyvalently Displaying β-Cyclodextrins on the Surface of Ferritins Through Copper(I)-Catalyzed Azide/Alkyne Cycloaddition. Macromol Biosci. 2012;12:1452–1458. doi: 10.1002/mabi.201200178. [DOI] [PubMed] [Google Scholar]
- 41.Hwang MP, Lee JW, Lee KE, Lee KH. Think modular: a simple apoferritin-based platform for the multifaceted detection of pancreatic cancer. ACS Nano. 2013;7:8167–74. doi: 10.1021/nn403465a. [DOI] [PubMed] [Google Scholar]
- 42.Lee EJ, Lee SJ, Kang YS, Ryu JH, Kwon KC, Jo E, Yhee JY, Kwon IC, Kim K, Lee J. Engineered proteinticles for targeted delivery of siRNA to cancer cells. Adv Funct Mater. 2015;25:1279–1286. [Google Scholar]
- 43.Choi S-H, Choi K, Chan Kwon I, Ahn HJ. The incorporation of GALA peptide into a protein cage for an acid-inducible molecular switch. Biomaterials. 2010;31:5191–8. doi: 10.1016/j.biomaterials.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Kang YJ, Park DC, Shin HH, Park J, Kang S. Incorporation of thrombin cleavage peptide into a protein cage for constructing a protease-responsive multifunctional delivery nanoplatform. Biomacromolecules. 2012;13:4057–4064. doi: 10.1021/bm301339s. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Chisholm J, Zhuang J, Xiao Y, Duncan G, Chen X, Suk JS, Hanes J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci. 2017;114:E6595–E6602. doi: 10.1073/pnas.1705407114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y, Wang X, Du D, Lin Y. Hyaluronic acid-conjugated apoferritin nanocages for lung cancer targeted drug delivery. Biomater Sci. 2015;3:1386–94. doi: 10.1039/c5bm00067j. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Li L, Di Penta A, Carmona U, Yang F, Schöps R, Brandsch M, Zugaza JL, Knez M. H-Chain Ferritin: A Natural Nuclei Targeting and Bioactive Delivery Nanovector. Adv Healthc Mater. 2015;4:1305–10. doi: 10.1002/adhm.201500226. [DOI] [PubMed] [Google Scholar]
- 48.Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JRR, Rao SS, Kong WP, Wang L, Nabel GJ. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–6. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han JA, Kang YJ, Shin C, Ra JS, Shin HH, Hong SY, Do Y, Kang S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine Nanotechnology, Biol Med. 2014;10:561–569. doi: 10.1016/j.nano.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Li K, Zhang ZP, Luo M, Yu X, Han Y, Wei HP, Cui ZQ, Zhang XE. Multifunctional ferritin cage nanostructures for fluorescence and MR imaging of tumor cells. Nanoscale. 2012;4:188. doi: 10.1039/c1nr11132a. [DOI] [PubMed] [Google Scholar]
- 51.Lin X, Xie J, Zhu L, Lee S, Niu G, Ma Y, Kim K, Chen X. Hybrid ferritin nanoparticles as activatable probes for tumor imaging. Angew Chem Int Ed Engl. 2011;50:1569–72. doi: 10.1002/anie.201006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao C, Wang X, Cai Y, Sun L, Tian L, Wu H, He X, Lei H, Liu W, Chen G, Zhu R, Pan Y. Targeted in vivo imaging of microscopic tumors with ferritin-based nanoprobes across biological barriers. Adv Mater. 2014;26:2566–2571. doi: 10.1002/adma.201304544. [DOI] [PubMed] [Google Scholar]
- 53.Kitagawa T, Kosuge H, Uchida M, Dua MM, Iida Y, Dalman RL, Douglas T, McConnell MV. RGD-conjugated human ferritin nanoparticles for imaging vascular inflammation and angiogenesis in experimental carotid and aortic disease. Mol Imaging Biol. 2012;14:315–24. doi: 10.1007/s11307-011-0495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, Lu N, Zhang H, Tian R, Niu G, Liu G, Chen X, Diagnostics M, Medicine T, Imaging U, States U. Biomineralization-Inspired Synthesis of Copper Sulfide-Ferritin Nanocages as Cancer Theranostics. ACS Nano. 2017;10:3453–3460. doi: 10.1021/acsnano.5b07521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma CCR, Katz JK, Haik Y. Molecular Targeted Functional, Cellular and Molecular Imaging of Atherosclerosis With Antibody-Conjugated Superparamagnetic Particles Using Magnetic Resonance. NSTI-Nanotech. 2006:464–466. [Google Scholar]
- 56.Makino A, Kimura S. Preparation of peptide- and protein-based molecular assemblies and their utilizations as nanocarriers for tumor imaging. React Funct Polym. 2011;71:272–279. [Google Scholar]
- 57.Shapiro MG, Szablowski JO, Langer R, Jasanoff A. Protein nanoparticles engineered to sense kinase activity in MRI. J Am Chem Soc. 2009;131:2484–2486. doi: 10.1021/ja8086938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meredith GD, Wu HY, Allbritton NL. Targeted protein functionalization using Histags. Bioconjug Chem. 2004;15:969–82. doi: 10.1021/bc0498929. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, So MK, Loening AM, Yao H, Gambhir SS, Rao J. HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots. Angew Chem Int Ed Engl. 2006;45:4936–40. doi: 10.1002/anie.200601197. [DOI] [PubMed] [Google Scholar]
- 60.Hussain AF, Amoury M, Barth S. SNAP-tag technology: a powerful tool for site specific conjugation of therapeutic and imaging agents. Curr Pharm Des. 2013;19:5437–42. doi: 10.2174/1381612811319300014. [DOI] [PubMed] [Google Scholar]
- 61.Gu GJ, Friedman M, Jost C, Johnsson K, Kamali-Moghaddam M, Plückthun A, Landegren U, Söderberg O. Protein tag-mediated conjugation of oligonucleotides to recombinant affinity binders for proximity ligation. N Biotechnol. 2013;30:144–52. doi: 10.1016/j.nbt.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Glasgow JN, Mikheeva G, Krasnykh V, Curiel DT. A strategy for adenovirus vector targeting with a secreted single chain antibody. PLoS One. 2009;4:e8355. doi: 10.1371/journal.pone.0008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gradišar H, Božič S, Doles T, Vengust D, Hafner-Bratkovič I, Mertelj A, Webb B, Šali A, Klavžar S, Jerala R. Design of a single-chain polypeptide tetrahedron assembled from coiled-coil segments. Nat Chem Biol. 2013;9:362–6. doi: 10.1038/nchembio.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–44. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 65.Tao Y, Strelkov SV, Mesyanzhinov VV, Rossmann MG. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure. 1997;5:789–98. doi: 10.1016/s0969-2126(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 66.Stetefeld J, Jenny M, Schulthess T, Landwehr R, Engel J, Kammerer RA. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nat Struct Biol. 2000;7:772–6. doi: 10.1038/79006. [DOI] [PubMed] [Google Scholar]
- 67.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274:761–5. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Tanha J, Hirama T, Khieu NH, To R, Tong-Sevinc H, Stone E, Brisson JR, MacKenzie CR. Pentamerization of single-domain antibodies from phage libraries: A novel strategy for the rapid generation of high-avidity antibody reagents. J Mol Biol. 2004;335:49–56. doi: 10.1016/j.jmb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 69.Chen Q, Sun Q, Molino NM, Wang SW, Boder ET, Chen W. Sortase A-mediated multi-functionalization of protein nanoparticles. Chem Commun (Camb) 2015;51:12107–10. doi: 10.1039/c5cc03769g. [DOI] [PubMed] [Google Scholar]
- 70.Fierer JO, Veggiani G, Howarth M. SpyLigase peptide-peptide ligation polymerizes affibodies to enhance magnetic cancer cell capture. Proc Natl Acad Sci U S A. 2014;111:E1176–81. doi: 10.1073/pnas.1315776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fairhead M, Veggiani G, Lever M, Yan J, Mesner D, Robinson CV, Dushek O, van der Merwe PA, Howarth M. SpyAvidin hubs enable precise and ultrastable orthogonal nanoassembly. J Am Chem Soc. 2014;136:12355–63. doi: 10.1021/ja505584f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charalambous A, Antoniades I, Christodoulou N, Skourides PA. Split-Inteins for Simultaneous, site-specific conjugation of Quantum Dots to multiple protein targets In vivo. J Nanobiotechnology. 2011;9:37. doi: 10.1186/1477-3155-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiel IV, Volkmann G, Pietrokovski S, Mootz HD. An atypical naturally split intein engineered for highly efficient protein labeling. Angew Chem Int Ed Engl. 2014;53:1306–10. doi: 10.1002/anie.201307969. [DOI] [PubMed] [Google Scholar]
- 74.Lim KH, Huang H, Pralle A, Park S. Stable, high-affinity streptavidin monomer for protein labeling and monovalent biotin detection. Biotechnol Bioeng. 2013;110:57–67. doi: 10.1002/bit.24605. [DOI] [PubMed] [Google Scholar]
- 75.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci U S A. 2005;102:7583–8. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Staros JV, Wright RW, Swingle DM. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986;156:220–2. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 77.Bloemen M, Van Stappen T, Willot P, Lammertyn J, Koeckelberghs G, Geukens N, Gils A, Verbiest T. Heterobifunctional PEG Ligands for Bioconjugation Reactions on Iron Oxide Nanoparticles. PLoS One. 2014;9:e109475. doi: 10.1371/journal.pone.0109475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartczak D, Kanaras AG. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/sulfo-NHS coupling. Langmuir. 2011;27:10119–23. doi: 10.1021/la2022177. [DOI] [PubMed] [Google Scholar]
- 79.Andrade S, Pinho F, Ribeiro AM, Carreira M, Casanueva FF, Roy P, Monteiro MP. Immunization against active ghrelin using virus-like particles for obesity treatment. Curr Pharm Des. 2013;19:6551–6558. doi: 10.2174/13816128113199990506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mattson G, Conklin E, Desai S, Nielander G, Savage MD, Morgensen S. A practical approach to crosslinking. Mol Biol Rep. 1993;17:167–83. doi: 10.1007/BF00986726. [DOI] [PubMed] [Google Scholar]
- 81.Ishikawa E, Imagawa M, Hashida S, Yoshitake S, Hamaguchi Y, Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4:209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- 82.Shuvaev VV, Tliba S, Pick J, Arguiri E, Christofidou-Solomidou M, Albelda SM, Muzykantov VR. Modulation of endothelial targeting by size of antibody–antioxidant enzyme conjugates. J Control Release. 2011;149:236–241. doi: 10.1016/j.jconrel.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Howard MD, Hood ED, Greineder CF, Alferiev IS, Chorny M, Muzykantov V. Targeting to Endothelial Cells Augments the Protective Effect of Novel Dual Bioactive Antioxidant/Anti-Inflammatory Nanoparticles. Mol Pharm. 2014;11:2262–2270. doi: 10.1021/mp400677y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hnasko RM. Bioconjugation of Antibodies to Horseradish Peroxidase (HRP) Methods Mol Biol. 2015;1318:43–50. doi: 10.1007/978-1-4939-2742-5_4. [DOI] [PubMed] [Google Scholar]
- 85.Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simone E, Dziubla T, Shuvaev V, Muzykantov VR. Synthesis and characterization of polymer nanocarriers for the targeted delivery of therapeutic enzymes. Methods Mol Biol. 2010;610:145–64. doi: 10.1007/978-1-60327-029-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peacey M, Wilson S, Baird MA, Ward VK. Versatile RHDV virus-like particles: Incorporation of antigens by genetic modification and chemical conjugation. Biotechnol Bioeng. 2007;98:968–977. doi: 10.1002/bit.21518. [DOI] [PubMed] [Google Scholar]
- 88.Li J, Lee SE, Belciug M, Ring DB, Kwok CS. Chemical conjugation of a novel antibody-interleukin 2 immunoconjugate against c-erbB-2 product. Chin Med J (Engl) 2000;113:151–153. [PubMed] [Google Scholar]
- 89.Hein CD, Liu XM, Wang D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm Res. 2008;25:2216–30. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. Click chemistry for drug delivery nanosystems. Pharm Res. 2012;29:1–34. doi: 10.1007/s11095-011-0568-5. [DOI] [PubMed] [Google Scholar]
- 91.Frisch B, Hassane FS, Schuber F. Conjugation of ligands to the surface of preformed liposomes by click chemistry. Methods Mol Biol. 2010;605:267–77. doi: 10.1007/978-1-60327-360-2_18. [DOI] [PubMed] [Google Scholar]
- 92.Patel KG, Swartz JR. Surface functionalization of virus-like particles by direct conjugation using azide-alkyne click chemistry. Bioconjug Chem. 2011;22:376–387. doi: 10.1021/bc100367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lucon J, Qazi S, Uchida M, Bedwell GJ, LaFrance B, Prevelige PE, Douglas T. Use of the interior cavity of the P22 capsid for site-specific initiation of atom-transfer radical polymerization with high-density cargo loading. Nat Chem. 2012;4:781–8. doi: 10.1038/nchem.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howard M, Zern BJ, Anselmo AC, Shuvaev VV, Mitragotri S, Muzykantov V. Vascular targeting of nanocarriers: Perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2014;8:4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4:195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 96.Shuvaev VV, Han J, Yu KJ, Huang S, Hawkins BJ, Madesh M, Nakada M, Muzykantov VR. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J. 2011;25:348–57. doi: 10.1096/fj.10-169789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tkachenko E, Tse D, Sideleva O, Deharvengt SJ, Luciano MR, Xu Y, McGarry CL, Chidlow J, Pilch PF, Sessa WC, Toomre DK, Stan RV. Caveolae, fenestrae and transendothelial channels retain PV1 on the surface of endothelial cells. PLoS One. 2012;7:e32655. doi: 10.1371/journal.pone.0032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, Kobayashi T, Shipman SL, Moodie KL, Daghlian CP, Ernst PA, Lee HK, Suriawinata AA, Schned AR, Longnecker DS, Fiering SN, Noelle RJ, Gimi B, Shworak NW, Carrière C. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–18. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hnasko R, Ben-Jonathan N. Developmental regulation of PV-1 in rat lung: association with the nuclear envelope and limited colocalization with Cav-1. Am J Physiol Lung Cell Mol Physiol. 2005;288:L275–84. doi: 10.1152/ajplung.00236.2004. [DOI] [PubMed] [Google Scholar]
- 100.Khoshnejad M, Shuvaev VV, Pulsipher KW, Dai C, Hood ED, Arguiri E, Christofidou-Solomidou M, Dmochowski IJ, Greineder CF, Muzykantov VR. Vascular Accessibility of Endothelial Targeted Ferritin Nanoparticles. Bioconjug Chem. 2016;27:628–637. doi: 10.1021/acs.bioconjchem.5b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howard MD, Greineder CF, Hood ED, Muzykantov VR. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J Control Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simone EA, Zern BJ, Chacko AM, Mikitsh JL, Blankemeyer ER, Muro S, Stan RV, Muzykantov VR. Endothelial targeting of polymeric nanoparticles stably labeled with the PET imaging radioisotope iodine-124. Biomaterials. 2012;33:5406–5413. doi: 10.1016/j.biomaterials.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zern BJ, Chacko AM, Liu J, Greineder CF, Blankemeyer ER, Radhakrishnan R, Muzykantov V. Reduction of nanoparticle avidity enhances the selectivity of vascular targeting and PET detection of pulmonary inflammation. ACS Nano. 2013;7:2461–2469. doi: 10.1021/nn305773f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 105.Krause KH, Bedard K. NOX enzymes in immuno-inflammatory pathologies. Semin Immunopathol. 2008;30:193–4. doi: 10.1007/s00281-008-0127-2. [DOI] [PubMed] [Google Scholar]
- 106.Shuvaev VV, Muzykantov VR. Targeted modulation of reactive oxygen species in the vascular endothelium. J Control Release. 2011;153:56–63. doi: 10.1016/j.jconrel.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nowak K, Weih S, Metzger R, Albrecht RF, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293:L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 108.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331:404–11. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21:392–8. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 110.Preissler G, Loehe F, Huff IV, Ebersberger U, Shuvaev VV, Bittmann I, Hermanns I, Kirkpatrick JC, Fischer K, Eichhorn ME, Winter H, Jauch KW, Albelda SM, Muzykantov VR, Wiewrodt R. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation. 2011;92:380–7. doi: 10.1097/TP.0b013e318226bc6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Howard MD, Hood ED, Zern B, Shuvaev VV, Grosser T, Muzykantov VR. Nanocarriers for Vascular Delivery of Anti-Inflammatory Agents. Annu Rev Pharmacol Toxicol. 2014;54:205–226. doi: 10.1146/annurev-pharmtox-011613-140002.Nanocarriers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yen CC, Lai YW, Chen HL, Lai CW, Lin CY, Chen W, Kuan YP, Hsu WH, Chen CM. Aerosolized human extracellular superoxide dismutase prevents hyperoxia-induced lung injury. PLoS One. 2011;6:e26870. doi: 10.1371/journal.pone.0026870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release. 2012;163:161–9. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen XY, Wang SM, Li N, Hu Y, Zhang Y, Xu JF, Li X, Ren J, Su B, Yuan WZ, Teng XR, Zhang RX, Jiang DH, Mulet X, Li HP. Creation of lung-targeted dexamethasone immunoliposome and its therapeutic effect on bleomycin-induced lung injury in rats. PLoS One. 2013;8:e58275. doi: 10.1371/journal.pone.0058275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abraham E, Baughman R, Fletcher E, Heard S, Lamberti J, Levy H, Nelson L, Rumbak M, Steingrub J, Taylor J, Park YC, Hynds JM, Freitag J. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med. 1999;27:1478–1485. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 116.Siobal MS, Hess DR. Are inhaled vasodilators useful in acute lung injury and acute respiratory distress syndrome? Respir Care. 2010;55:144–57-61. [PubMed] [Google Scholar]
- 117.Afshari A, Brok J, Møller A, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg. 2011;112:1411–1421. doi: 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 118.Kirov MY, Evgenov OV, Kuklin VN, Virag L, Pacher P, Southan GJ, Salzman AL, Szabo C, Bjertnaes LJ. Aerosolized linear polyethylenimine-nitric oxide/nucleophile adduct attenuates endotoxin-induced lung injury in sheep. Am J Respir Crit Care Med. 2002;166:1436–1442. doi: 10.1164/rccm.2202021. [DOI] [PubMed] [Google Scholar]
- 119.Shuvaev VV, Han J, Tliba S, Arguiri E, Christofidou-Solomidou M, Ramirez SH, Dykstra H, Persidsky Y, Atochin DN, Huang PL, Muzykantov VR. Anti-Inflammatory Effect of Targeted Delivery of SOD to Endothelium: Mechanism, Synergism with NO Donors and Protective Effects In Vitro and In Vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li HD, Zhang QX, Mao Z, Xu XJ, Li NY, Zhang H. Exogenous interleukin-10 attenuates hyperoxia-induced acute lung injury in mice. Exp Physiol. 2015;100:331–40. doi: 10.1113/expphysiol.2014.083337. [DOI] [PubMed] [Google Scholar]
- 121.Frank JA, Pittet J-F, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63:147–53. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 122.Bertok S, Wilson MR, Morley PJ, de Wildt R, Bayliffe A, Takata M. Selective inhibition of intra-alveolar p55 TNF receptor attenuates ventilator-induced lung injury. Thorax. 2012;67:244–51. doi: 10.1136/thoraxjnl-2011-200590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 124.Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H. Intravenous Administration of Polyethylene Glycol-Coated (PEGylated) Proteins and PEGylated Adenovirus Elicits an Anti-PEG Immunoglobulin M Response. Biol Pharm Bull. 2012;35:1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 125.Verhoef JJF, Carpenter JF, Anchordoquy TJ, Schellekens H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today. 2014;19:1945–1952. doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 126.Verhoef JJF, Anchordoquy TJ. Questioning the use of PEGylation for drug delivery. Drug Deliv Transl Res. 2013;3:499–503. doi: 10.1007/s13346-013-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci Adv. 2015;1:e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maldonado RA, LaMothe RA, Ferrari JD, Zhang A-H, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, von Andrian UH, Kishimoto TK. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]


