Introduction
Accurate and precise summary measures of pain intensity over a period of time are essential for understanding the pathophysiological, behavioral, and emotional processes associated with chronic pain, and for developing effective treatment approaches [16,21]. The measurement of pain intensity has traditionally focused on patients’ average or usual pain levels over a given time period [16,39]. However, a focus on average pain misses temporal dynamics of pain intensity that experts believe are essential indicators of disease activity [14,21]. As stated by Dworkin and colleagues, “the temporal aspects of pain—including variability in intensity; … durability of pain relief; and frequency, duration, and intensity of pain episodes—have not received adequate attention in pain research” [16, p.13].
Methodological advances using ecological momentary assessments (EMA), which captures the ebb and flow of immediate experiences with high resolution, present the opportunity to study dynamic processes in pain and how they unfold over time [52,55]. EMA research has allowed exploration of fundamental dynamics of pain experiences in patients’ natural daily environments, including the variability [2,30,53], context and activity dependence [45,47], and diurnal cyclicity [8,33,62] of pain intensity.
A promising new framework for assessing unexplored temporal patterns in momentary pain is offered by regime-switching models – an approach to analyzing time series data [28,29]. Using regime-switching models, we test the possibility that patients experience recurrent shifts between elevated and reduced pain states (called “regimes”), which can vary in levels and duration. Regime-switching models originated in econometrics several decades ago [28,29], but they have only recently been applied in health sciences; for example, to characterize mood swings in patients with depressive and bipolar disorder [26,27] and to examine shifting incidence rates of infectious diseases [40,42].
In this article, we use regime-switching models to develop new metrics summarizing an individual’s pain over time based on repeated assessments of momentary pain intensity. To our knowledge, this will be the first application of regime-switching models to chronic pain research. Three new measures emerge from such analyses: the amplitude of shifts between elevated and reduced pain, how long pain states tend to persist over time, and the extent to which periods of elevated pain or pain relief are short-lived or enduring. We determine whether or not the new measures meaningfully enhance the information obtained from momentary pain assessments by determining their incremental utility in predicting patient health outcomes after accounting for a measure of the average pain level. We contrast the new information with average pain intensity, because parsimony suggests that we only adopt new measures if they contribute to existing ones (and the average is the most common metric). Specifically, we examine cross-sectional and longitudinal associations of the regime-switching pain measures with patients’ physical functioning and emotional health outcomes. We also examine whether or not the new measures contribute to understanding patients’ retrospective judgments of their pain and to their global impressions of change over time.
Methods
Participants
The present report used from an existing study of rheumatology patients in community care [60,63]. The data were collected between January 2004 and November 2004. Patients were followed over a 3-month period, where some of the patients enrolled in the study were about to start a change in their pain treatment (i.e., starting a new treatment, adding a new treatment to the current regimen, switching to a different treatment, or increasing the current treatment dose) after the baseline assessment. The reason for recruiting a heterogeneous sample with some patients undergoing changes in treatment was to ensure that the sample showed meaningful variation in changes in pain and functioning over time for the purposes of longitudinal analyses. The protocol was reviewed and approved by all institutions involved.
Eligible participants had a diagnosis of at least 1 of the following chronic pain disorders: fibromyalgia, rheumatoid arthritis, and osteoarthritis. Confirmation of diagnosis was provided by the participants’ rheumatologist. Additional eligibility criteria included feeling pain for more than 6 months; pain for ≥ 3 days per week; pain ≥ 3 hours per day; average level of pain >3 (on a 0–10 rating scale with 0 = no pain and 10 = excruciating pain); between the ages of 18 and 80 years; having no sight or hearing problems; being fluent in English; having no difficulty holding a pen or writing; waking up by 10:00 AM and going to bed no earlier than 7:00 PM; having no serious psychiatric impairment, no alcohol and/or drug problem, not planning any major surgery while participating in the study, and not having participated in an EMA study within the last 5 years.
Recruitment for this study was conducted at two community rheumatology offices. Of 729 patients approached, 339 (47%) patients agreed to participate in a telephone screen, 294 (40%) were successfully contacted and completed the interview, and 220 (30%) were eligible. Of those who were eligible, 129 (59%) came to the research office to begin the protocol, and 116 (53%) completed the study.
Procedure
At an initial visit, patients completed an informed consent, a medical release for confirmation of their diagnosis, and baseline questionnaires of emotional and physical functioning. After that, participants engaged in two 1-week measurement periods that were separated by a break of approximately 3 months (mean=86.1 days, SD=6.0; 227 min=73; max=112). At the Month-1 and Month-3 measurement periods, participants provided EMA data of momentary pain intensity, pain interference, and affect for one week (additional questions about location and current activities were also administered, but are not considered here). In addition to the collection of EMA data, patients were telephoned by a research assistant at 3 evenings to complete assessments of daily activities and physical functioning. The phone calls were scheduled on alternating evenings with two calls during the week and one on the weekend. At the conclusion of each measurement week, patients returned to the research office to complete a 7-day recall pain questionnaire. At their final office visit, patients completed a global assessment of change rating. A $100 reimbursement was paid to those who completed the protocol.
EMA data was collected with a Sony Clie computer (Model PEG610C) with proprietary software (invivodata, inc., Scotts Valley, CA). Patients were trained in the use of the electronic diary via a 60-minute presentation and practice session. The electronic diary was programmed to deliver 8–9 prompts daily (assuming a 16 hour day) using a stratified random sampling scheme that generated a prompt randomly between 35 and 177 minutes after the previous prompt. Sampling in this manner yields an average prompt interval of 107 minutes (1.8 hours). EMA questions were presented for completion via a touch screen and entries were electronically time and date stamped. The diary issued an audible alarm to prompt patients for momentary pain entries and alerted them if they missed an assessment. The software also included provisions to help patients incorporate the diary into their daily routine, such as allowing them to suspend prompting for defined periods of sleep or other uninterruptible activities. The average number of random prompts (delivered or suspended) was 54.0 in Month 1 (7.7 per day) and 53.7 in Month 3 (7.7 per day). Compliance with the EMA protocol (calculated based on the number of completed prompts divided by the sum of completed, missed, and suspended prompts) was comparable across Month 1 (mean = 87%; SD=8.4) and Month 3 (mean = 85%; SD=9.6).
Measures
Momentary assessments of pain intensity
Momentary pain was assessed with the following pair of questions: “Before Prompt: Were you in any pain?” and if yes, “How much pain did you feel?” (rated on a 0–100 visual analog scale with endpoints of “No Pain” and “Extreme Pain”). The responses to the two questions were combined into a single pain intensity score using the visual analogue rating if a patient indicated being in pain and recording a score of 0 if a patient indicated that s/he was in no pain in the first question. Scores were transformed onto a 0–10 scale (i.e., divided by 10, to avoid model convergence problems resulting from large sample variances) for data analysis purposes. Intraclass correlations (ICCs), which represent the amount of between-person variance relative to the total (between- and within-person) variance, were ICC = .45 for the Month-1 and ICC = .56 for the Month-3 assessment periods. Thus, about half of the variance in momentary pain intensity ratings was attributable to within-person fluctuations.
Cross-sectional health outcomes measures
Questionnaires assessing depression, anxiety, and general mental and physical health were administered at the first office visit at the beginning of the study.
Beck Depression Inventory-II (BDI)
The 21-item self-report instrument measures cognitive, affective, and somatic aspects of depressed mood [7]. The BDI has demonstrated validity in distinguishing depression severity levels and has been found to be sensitive to treatment change [6]. Higher scores reflect more severe depression levels. Cronbach’s alpha was .88 in the present sample.
Spielberger State-Trait Anxiety Scale (STAI)
The 20-item trait version of the instrument is a widely used measure assessing dispositional anxiety [58]. The instrument has demonstrated adequate test-retest reliability and convergent validity, and has been found useful as a screening tool of anxiety in rheumatology patients [65]. Higher STAI scores imply greater anxiety levels. Cronbach’s alpha was .91 in this sample.
SF-36 health survey version 2 (SF-36)
The SF-36 is a 36-item generic measure of physical and mental health that is widely accepted for use in general and disease-specific populations [68]. Aggregate component scores for physical health and mental health were calculated using the respective norm-based factor score coefficients for each of the eight subscales within the SF-36v2 [43]. Cronbach’s alphas for the subscales ranged from .75 to .91 (median of .87). Higher scores reflect better physical and mental health.
Longitudinal health outcomes measures
Measures of momentary negative affect and pain interference were administered as part of EMA data collection during the Month-1 and Month-3 measurement weeks. Measures of daily physical functioning limitations and difficulties with activities of daily living (ADL) were administered with evening phone calls during each of the two weeks.
Negative affect
Four adjective-checklist items derived from the Positive and Negative Affect Schedule [PANAS; 69] and adapted for momentary reports were used to measure negative affect. Participants rated how much they felt the following four emotions at the time of the prompt: depressed, angry, frustrated, and worried, using a 0–100 visual analogue scale anchored “not at all” to “extremely”. The four items were averaged into a composite negative affect score for analysis. ICCs representing the variance attributable to reliable between-person differences within each measurement period were .65 (Month 1) and .74 (Month 3).
Pain interference
Participants provided momentary ratings in response to the item “before prompt, how much was your pain limiting your activities?”, using a 4-point response scale (0=none to 3 = a lot). ICCs were .51 at the Month-1 and .57 at the Month-3 assessment period.
Physical functioning limitations
Daily functioning limitations were assessed with the 10-item physical functioning subscale from the SF-36 [68], adapted for daily reports. Patients were asked to indicate limitations of 10 physical activities today (e.g., vigorous activities such as running, lifting heavy objects, or strenuous sports), using a 3-point response scale (0=not limited at all; 2=limited a lot). ICCs were .82 at the Month-1 and .83 at the Month-3 assessment period.
Activities of daily living (ADL) difficulties
Using 10 items from the Functional Status Assessment Instrument [34] adapted for daily reporting, patients were asked to rate difficulties with putting on clothes, buttoning a shirt/blouse, washing their body, writing, opening a container, dialing a phone, vacuuming, reaching into low cabinets, doing laundry, and doing yardwork, using a 4-point response scale (0=no difficulty; 3=severe difficulty). ICCs were .83 at the Month-1 and .87 at the Month-3 assessment period.
Retrospective summary judgments of pain
Retrospective measures (7-day recall and global impressions of change) were included in this study because they are very commonly used for treatment evaluation in chronic pain management and clinical trials [39,57]; further, patients’ global impressions of change represent an integral part of the anchor-based approach to determining clinically meaningful change [17,20].
Pain 7-day recall
Single-item recall measures of usual pain intensity and pain unpleasantness were administered at the end of each week of momentary assessments (Month 1 and Month 3). Patients were asked to indicate the “level of your usual pain over the last 7 days” and the “unpleasantness of your pain over the last 7 days.” Responses were recorded on a 0–100 visual analogue scale with endpoints “None” and “Extreme,” and were transformed onto a 0–10 scale for the present analyses.
Global impression of change
Patients’ global impression of change (GIC) was assessed at the end of the study (Month 3) with a single-item measure that asked patients to rate their current pain compared to their pain “about 3 months ago, when you first participated in our study”. Responses were made on a 7-point categorical scale with response option −3=very much worse, −2=much worse, −1=minimally worse, 0=unchanged, 1=minimally better, 2=much better, 3=very much better.
Data analysis
Overview of Markov regime-switching dynamic regression model
Regime-switching models are used to understand temporal dynamics inherent in repeated observations (here EMA data) that are assumed to alternate between discrete states of different levels. The states are referred to as “regimes”. Several variants of regime-switching models of varying complexity have been developed (for detailed introductions, see [28,29]). Whereas more complex models are useful for more precise modeling of temporal dynamics in a single (n = 1) time series with a large number of repeated observations (e.g., behavior of the stock market), in the present application we examine data from multiple (n = 116) patients to evaluate individual differences in pain dynamics. We therefore consider a relatively simple regime-switching model with few parameters, the Markov-switching dynamic regression model [28,29]. Consistent with previous applications [e.g., 26,27], we assume that regime-switching occurs between two states.
Given a time series yt, where pain ratings y are assessed at times t = 1, 2, … , T, the basic model can be expressed as
| (1) |
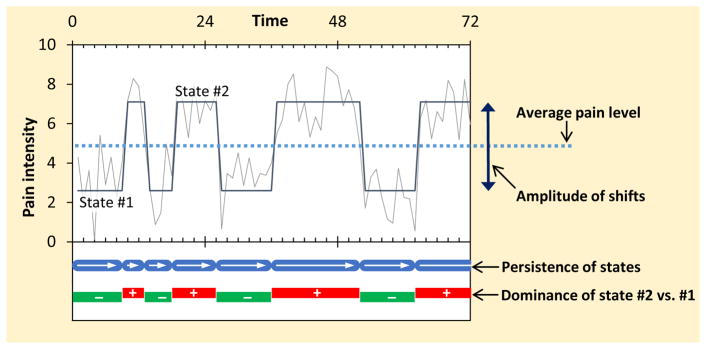
where μst is a state-dependent intercept term that takes on different values for state 1 (μ1) and state 2 (μ2), and εt is an error term. Applied to EMA pain intensity data, the two states represent lower (i.e., reduced) and higher (i.e., elevated) periods of pain. At every measurement occasion, the person is assumed to be in one of the states, and transitions between the states occur according to a Markov process in which the current state is related to the person’s most recent previous state. Moving from one time point t to the next time point t+1, the person can either remain in the same state or transition to the other state (see Figure 1).
Figure 1.
Schematic illustration of measures derived from regime switching model.
Estimation and inference in the regime switching framework is not a trivial task due to the fact that the states are unobserved (i.e., latent). In other words, which state a given pain rating belongs to is not known or determined based on a priori cutoffs, but needs to be inferred from the data (for details, see [28,29]). Given that the states are not directly observed, it can never be fully determined which of the states is realized for a person at a given point in time. However, the person’s mean level in each state and the person-specific transition probabilities of staying in a given state versus switching states across consecutive time points can be estimated. Further, from the transition probabilities, the length of time the person typically remains in the same state can be estimated as duration = d/(1-πi) hours, where πi is the probability of staying in state i if the person is in that state and d is the time distance between the momentary assessments, in hours.
Definition of model parameters
The parameters obtained from the Markov-switching dynamic regression model can be expressed in different ways, and we selected a parameterization for this study with the intention to capture maximally distinctive aspects of the pain experience. Figure 1 provides a visual description of the regime-switching measures derived for the study, and Table 1 provides an explanation of each measure and its interpretation.
Table 1.
Definition of regime switching measures
| Description | Meaning | |
|---|---|---|
| Average pain level | Mean of lower (state 1) and higher (state 2) pain intensity levels | Higher values indicate worse pain intensity on average across time |
| Amplitude of shifts | Difference between higher (state 2) and lower (state 1) pain intensity levels | Higher values indicate larger shifts in pain levels, such that the levels of higher and lower pain states are more distinctly different from each other |
| Persistence of states | Log odds of maintaining a pain state across consecutive time points, regardless of the level of states (i.e., mean of the log odds of maintaining state 1 and state 2) | Higher values indicate less frequent shifts in pain levels, such that a patient tends to remain in the same pain state over a longer period of time |
| Dominance of higher states | Difference between the log odds of maintaining a higher (state 2) versus maintaining a lower (state 1) pain state across consecutive time points | Positive values indicate that higher pain states tend to persist longer than lower pain states |
The Average pain level is the arithmetic mean of the two latent intercepts for higher and lower pain states for each person; it represents the general level of pain intensity experienced over time. The Amplitude of shifts is the distance between the pain levels in the two states (i.e., the difference between the latent intercepts of higher and lower pain states); it represents the magnitude of changes between states of reduced and elevated pain intensity experienced by the patient. The Persistence of states is the probability of staying in the same state from one momentary assessment to the next momentary assessment, reflecting how long a given pain state is expected to last. Finally, the Dominance of higher pain states refers to imbalance in the duration of higher versus lower pain states: whereas the Persistence measure reflects how enduring a patient’s pain states are in general, the Dominance measure reflects the extent to which higher pain states are more (or less) enduring than lower pain states. For analysis purposes, measures of Average pain and the Amplitude of shifts are expressed on the metric of the 0–10 pain scale, and the Persistence and Dominance measures are expressed in log odds (rather than proportions or odds ratios; to normalize the distribution of individual differences in these measures).
Time series data preparation
In preparing the individuals’ time series data for Markov-switching dynamic regression models, two issues require special consideration. First, applying the model requires that the time intervals between two consecutive measurements are approximately equal. This assumption is violated for the EMA data used in this study, which were collected at stratified random time points (with unequal intervals) during participants’ waking hours. To address this problem, we discretized the timing of momentary assessments so that the continuous EMA time scale was approximated by equally spaced integer values [4]. Specifically, the time variable was rescaled by forming consecutive bins of 2-hour time intervals and the observed assessment time points were sorted into these equally spaced bins. The time grid and regime-switching models were limited to waking hours (determined individually for each participant and day by the time the wake up alarm on the electronic diary was set to the time the diary was turned off at night) because a night is a relatively large time interval without data entry, and the physiological processes and experiences during sleep are qualitatively different from daytime experiences [37]. If two assessments were located in the same bin of the discretized time grid and a neighboring bin was empty, the assessment most proximal to the empty bin was moved into that bin. If three assessments were in the same bin and both neighboring bins were empty, the two assessments most proximal to the empty bins were moved [4]. In the few instances in which more than one assessment remained in the same bin after this sorting procedure (5.0% of the bins had 2 ratings, 0.1% had 3 ratings), EMA pain ratings in the same bin were averaged.
A second issue that needed to be considered was that the implementation of the Markov-switching dynamic regression model does not allow for gaps in the time series. For all time bins with no momentary assessment, missing values had to be assumed (either because no EMA prompt was administered during that time bin or because a prompt was missed by the participant). An average of 15.7% (SD = 6.8, range = 4.2%-37.7% in Month 1) and 17.3% (SD = 7.9, range = 3.1%-42.3% in Month 3) of the time bins did not have a momentary assessment. Data for empty time bins were imputed using the R software package imputeTS [44]. Imputed values were generated using Kalman filtering applied to a state-space model representation of the data, a recommended procedure for univariate time series data [15]. Given that the imputation procedure may give biased results if too many (especially sequential) data points are missing [15], sleep times were excluded from the time series and individuals with >30% missing values or with 4 or more sequentially missing values were eliminated from data analysis (n = 11 in Month 1, n = 13 in Month 3). In addition, 3 participants provided pain ratings of 0 for all momentary assessments at Month 3; these time series were also eliminated from analysis because regime-switching models are not defined if a person has no variance in the ratings. Thus, time-series data were available for n = 105 (Month 1) and n = 100 (Month 3) participants (none of the 116 participants had ineligible data for both assessment periods). Using the imputed discretized time-series data, Markov-switching dynamic regression models were estimated separately for each participant and assessment period using the mswitch command in Stata 14 [59].
Validity testing of regime-switching measures
The primary purpose of the analyses was to investigate whether the regime-switching measures meaningfully enhance the information obtained from EMA pain intensity data above the information inherent in the average pain level (i.e., above the most commonly considered pain characteristic). This was evaluated in two ways.
First, we examined whether momentary pain can be characterized by switches between distinct states for participants. If most patients’ pain levels are fairly stable and unchanging from moment to moment, there may not be a reasonable basis for considering multiple temporal states. To test this, we examined the proportion of time series for which a 2-state regime-switching model provided a better fit to the data than a statistical model that assumes a single pain intensity state. A single-state model is equivalent to an individual’s average pain level over the assessment period. We compared the fit of the 2-state versus single-state models using the corresponding asymptotic distribution of the likelihood-ratio statistic for regime-switching models tabulated in Garcia [24], separately for each individual and assessment week. Because a multitude (n = 205) of significance tests were conducted, we used the Benjamini-Hochberg procedure to control the false discovery rate and to avoid Type 1 errors from multiple comparisons [9].
Second, we evaluated the cross-sectional and longitudinal relationships between the regime-switching measures and several clinical health outcomes relevant for pain patients (emotional functioning, physical functioning, recall of pain, and global impressions of change). The question was whether the regime-switching measures involving the Amplitude of shifts, Persistence of states, and/or Dominance of higher pain states provided incremental validity [31] in predicting the health outcomes over what was predicted by patients’ Average pain. Hierarchical multiple regression procedures were used for this purpose. For each outcome variable, a first analysis step entered the Average pain measure as a predictor variable, and a second step simultaneously entered the regime-switching measures of Amplitude, Persistence, and Dominance to determine which ones, if any, uniquely explained additional variance in the outcome variable.
Cross-sectional relationships were examined by regressing the Month-1 outcomes on concurrent Month-1 regime-switching measures. Longitudinal relationships were examined using latent change scores estimated with structural equation models in which the Month-1 outcomes were regressed on Month-1 regime-switching measures, and within-person changes from Month-1 to Month-3 (i.e., effects of time) in the health outcomes were regressed on concurrent changes in the regime-switching measures [11]. For diary measures that were assessed at several (momentary or daily) time points during each assessment period, we accounted for the “nesting” of assessments in individuals by estimating latent growth models in which the set of person specific “true” means for each assessment period (i.e., Month-1 and Month-3) were treated as a random factor (i.e., latent variable) in the growth models [50]. It is important to note that we do not assume a causal (or “Granger-causal”) interpretation of the cross-sectional or longitudinal relationships. Rather, we are interested in “parallel processes” between changes in the regime-switching models and changes in the health outcomes. The multiple regression and growth curve analyses were conducted in Mplus version 7.4 [46]. Missing regime-switching measures were accommodated with maximum likelihood parameter estimation, such that all models were conducted using the available data for all 116 patients.
Results
Participants had a mean age of 57 years (range 19 to 80, SD = 13.12), and were predominantly female (84%), white (96%), and married (75%). Most were high school graduates (98%), with 75% having completed some college. About 14% of the sample received disability benefits. Physician-confirmed diagnoses were 84% osteoarthritis, 21% rheumatoid arthritis, and 36% fibromyalgia (see Table 2).
Table 2.
Participant characteristics (n=116)
| Age, mean ± SD (range) | 57.37 ± 13.12 (19 – 80) |
| Sex, n (%) | |
| Female | 98 (84.5%) |
| Male | 18 (15.5%) |
| Marital status, n (%) | |
| Never married | 6 (5.2%) |
| Married/ | 87 (75.0%) |
| Living with partner | 2 (1.7%) |
| Separated/divorced | 13 (11.2%) |
| Widowed | 8 (6.9%) |
| Education, n (%) | |
| Up to 11th grade | 2 (1.7%) |
| High school graduate | 27 (23.3%) |
| Some college | 34 (29.3%) |
| College graduate | 28 (24.1%) |
| Master’s/doctoral degree | 25 (21.6%) |
| Income, n (%) | |
| Less than $35,000 | 10 (8.6%) |
| $35,000 to $49,999 | 16 (13.8%) |
| $50,000 to $75,000 | 34 (29.3%) |
| Greater than $75,000 | 49 (42.2%) |
| Race, n (%) | |
| White | 111 (95.7%) |
| Not White | 5 (4.3%) |
| Ethnicity, n (%) | |
| Hispanic | 4 (3.5%) |
| Not Hispanic | 112 (96.6%) |
| Currently employed, n (%) | |
| No | 46 (39.7%) |
| Yes | 70 (60.3%) |
| Currently on disability, n (%) | |
| No | 100 (86.2%) |
| Yes | 16 (13.8%) |
| Chronic pain diagnosis, n (%) a | |
| Rheumatoid arthritis | 24 (20.7%) |
| Osteoarthritis | 98 (84.5%) |
| Fibromyalgia | 42 (36.2%) |
Note:
Chronic pain diagnoses are not mutually exclusive.
Characteristics of regime-switching measures
Descriptive characteristics and correlations among regime-switching measures at the Month-1 and Month-3 assessment periods are shown in Table 3. The mean of Patients’ Average pain levels was 3.84 (at Month 1) and 3.39 (at Month 3) on the 0–10 scale. The mean Amplitude of pain shifts was 3.47 (Month 1) and 3.32 (Month 3) scale points, which is approximately one-third of the full scale range. The mean Persistence of pain states (derived from the transition probabilities of pain states during the waking hours) was 10.3 hours (Month 1) and 9.9 hours (Month 3). For the Dominance of pain states, lower pain states tended to be more enduring than higher pain states: in Month 1, higher states were on average 1.44 hours shorter than lower pain states (higher states: 9.58 hours vs. lower states: 11.02 hours, OR = .84); in Month 3, higher pain states were on average 4.85 hours shorter than lower pain states (higher pain states: 7.86 hours vs. lower pain states: 12.71 hours, OR = .55).
Table 3.
Descriptive statistics and correlations between regime switching measures at Month 1 (above diagonal) and Month 3 (below diagonal)
| Average pain level | Amplitude of shifts | Persistence of states a | Dominance of higher states a | |
|---|---|---|---|---|
| Average pain level | .72*** | −.18 | .38*** | .09 |
| Amplitude of shifts | −.11 | .75*** | −.36*** | .13 |
| Persistence of states | .26** | −.37*** | .23* | .10 |
| Dominance of higher states | .11 | −.12 | .04 | .47*** |
|
| ||||
| Month 1 mean (SD) | 3.84 (1.62) | 3.47 (1.69) | 1.42 (0.92) | −0.17 (1.43) |
| Month 1 range | 0.94 – 8.58 | 0.50 – 7.61 | −0.11 – 3.91 | −3.36 – 3.66 |
| Month 3 mean (SD) | 3.39 (1.70) | 3.32 (1.85) | 1.38 (1.11) | −0.60 (1.81) |
| Month 3 range | 0.61 – 8.15 | 0.22 – 7.92 | −0.70 – 3.86 | −4.12 – 3.82 |
Note: Test-retest correlations are underlined on the main diagonal.
Values for means (SDs) and ranges are log odds.
p < .05;
p < .01;
p < .001.
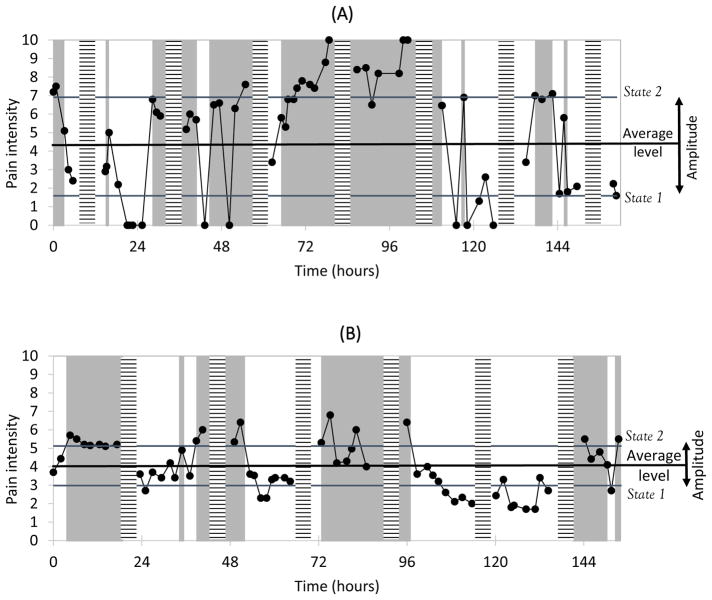
There were considerable individual differences in the scores for each of the regime-switching measures, as indicated by the standard deviations and ranges in Table 3. Figure 2 illustrates the momentary pain ratings and regime-switching measures of two selected patients in the sample. The two patients have comparable average pain levels, but patient A has a higher amplitude (shown by the difference between states 1 and 2), a lower persistence (shown by the average length of unshaded and shaded time periods), and a greater dominance of higher relative to lower pain states (shown by the relative length of shaded versus unshaded time periods) than patient B. In terms of correlations among the regime-switching measures, the dominance of higher states was not significantly correlated with any other measure (see Table 3). However, we observed moderate positive associations between patients’ Average pain level and the Persistence of states at each assessment period (Month 1: r = .38, Month 3: r = .26, ps < .01), suggesting that patients with overall higher pain levels experienced more enduring pain states. Moreover, the Persistence and Amplitude measures were negatively correlated at each assessment period (Month 1: r = −.36, Month 3: r = −.37, ps < .001), suggesting that patients with more enduring pain states tended to experience less pronounced pain shifts. Test-retest correlations showed moderate to high stability in Average pain (r = .72) and Amplitude (r = .75) measures, and low to moderate stability in Persistence (r = .23) and Dominance (r = .47) measures over the 3-month period.
Figure 2.
Data examples of two selected study participants. Observed pain intensity ratings are shown as connected dots. Unshaded areas represent model predicted time periods in state 1 (lower pain state) and gray areas represent model predicted time periods in state 2 (higher pain state). Horizontally striped bars represent day breaks. Regime-switching measures for patient in Panel A: Average pain level: 4.25; Amplitude of shifts between states: 5.32; Persistence of states: 8.19 hours; Dominance of higher states: 10.82 hours (state 2) vs. 6.35 hours (state 1), OR=2.03. Panel B: Average pain level: 4.05; Amplitude of shifts between states: 2.14; Persistence of states: 12.24 hours; Dominance of higher states: 11.21 hours (state 2) vs. 13.40 hours (state 1), OR=0.81.
Few demographic and medical correlates of the regime-switching measures were found: older patients tended to have lower Average pain levels (ps =.41 and .02 at Months 1 and 3, respectively). Being on disability was associated with higher Average pain levels at both months (p < .01). Patients with fibromyalgia tended to have a higher Persistence of pain states (ps =.02 and .33 at Months 1 and 3, respectively) than those (exclusively) diagnosed with osteoarthritis and rheumatoid arthritis.
Comparison of fit between single-state and 2-state models
We next examined whether a model that assumes regime-switches between two states fits the data better than a model that assumes a general average pain state. This was the case for the large majority of patients: likelihood ratio tests comparing the goodness of fit between 2-state and single-state models were significant for 83.8% of the patients (Benjamini-Hochberg corrected p < .001 for 57.1%, p < .01 for 16.2%, p < .05 for 10.5%) at Month-1 and for 85.0% of the patients (p < .001 for 68.0%, p < .01 for 15.0%, and p < .05 for 2.0%) at the Month-3 assessment week. Values of the likelihood ratio for the differences in model fit were significantly associated with the Amplitude of pain states at Month 1 (r = .30, p < .01) and Month 3 (r = .37, p < .001), but not with the other regime-switching measures (ps > .07). This indicates that a 2-state model outperformed a single-state model to the extent that the two states are clearly separated with a wide amplitude. Because we did not want to restrict the range of scores for the regime-switching measures in the subsequent validity analyses, the full sample of patients (regardless of model fit) was retained for the analyses described below.
Incremental validity of regime-switching measures
In these analyses we evaluated evidence for unique associations between the regime-switching measures and clinical outcomes involving patients’ emotional and physical functioning, recall of their pain, and their global impressions of change.
Cross-sectional associations with baseline health outcomes
Depression, anxiety, and general mental and physical health were assessed at the initial visit. The mean BDI depression score in the sample was 10.67 (SD = 7.09), with 73.3% of the participants showing minimal (scores 0 to 13), 12.9% mild (scores 14 to 19), 12.1% moderate (scores 20 to 28), and 1.7% severe (scores 29+) depression. The mean STAI trait anxiety score was 37.33 (SD = 9.64), which is the 65th percentile of the normal middle-aged adult population. The mean SF-36v2 physical component score was 34.81 (SD = 8.93) and the mean mental component score 47.98 (SD = 9.82), where both component scores have mean = 50 and SD = 10 in the general U.S. population.
Results from hierarchical multiple regression analyses regressing each of the health outcomes on the combination of Month-1 regime-switching measures are shown in Table 4. When entered at Step 1, higher Average pain levels were significantly associated with higher depression and anxiety, as well as with lower mental and physical health scores, explaining between 9.3% and 13.9% of the variance. Entered at Step 2, the Persistence of pain states was uniquely associated with each of the emotional functioning outcomes: depression (β = .24, p < .05), anxiety (β = .21, p < .05), and general mental health (β = −.27, p < .01), with the dynamic pain measures explaining an additional 3.6% to 5.2% of the variance in these outcomes. For physical health scores, none of the dynamic pain measures showed unique associations after controlling for Average pain (see Table 4).
Table 4.
Results for multiple regressions relating Month 1 health outcomes to Month 1 regime switching measures
| Depression | Anxiety | SF-36 Mental health | SF-36 Physical health | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| b (SE) | β | b (SE) | β | b (SE) | β | b (SE) | β | |
| Step 1 (R2) | (.097) | (.139) | (.093) | (.072) | ||||
| Average pain level | .97 (.42) | .22* | 1.88 (.56) | .32*** | −1.30 ( .58) | −.21* | −1.46 ( .53) | −.27** |
| Step 2 (ΔR2) | (.052) | (.036) | (.056) | (.019) | ||||
| Amplitude of shifts | .12 (.41) | .03 | .54 (.55) | .09 | −.21 ( .56) | −.04 | −.64 ( .55) | −.12 |
| Persistence of states | 1.87 (.78) | .24* | 2.24 (1.05) | .21* | −2.83 (1.08) | −.27** | −.14 (1.04) | −.01 |
| Dominance of higher state | .28 (.45) | .06 | −.34 (.60) | −.05 | −.03 ( .62) | .00 | −.45 (.59) | −.07 |
Note:
p < .05;
p < .01;
p < .001.
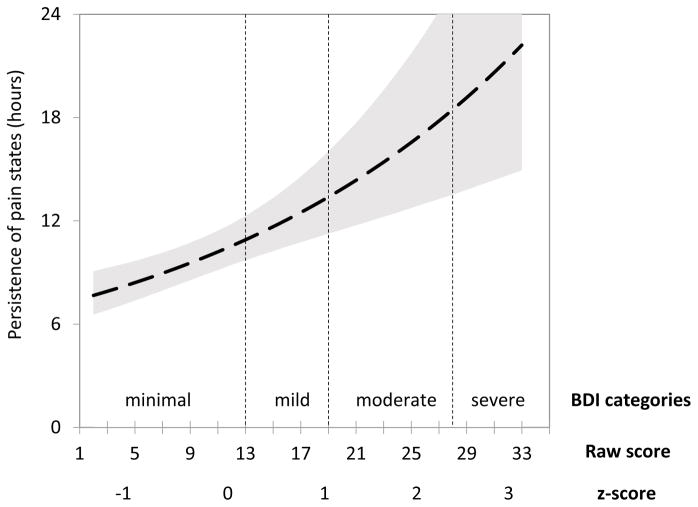
To illustrate the magnitude of the observed relationships between the Persistence of pain and emotional functioning, Figure 3 shows the expected duration of pain states (in hours) across different BDI depression levels. For patients with depression scores in the minimal range (BDI scores ≤13), a given pain state lasted on average between 7.7 and 10.9 hours before transitioning to the other pain state, whereas for patients with moderate depression (BDI scores 20–28), the pain states lasted on average between 13.9 and 18.5 hours.
Figure 3.
Persistence of pain states as a function of Beck Depression Inventory (BDI-II) depression scores. Shaded area represents 90% confidence interval.
Longitudinal associations with health outcomes
The study’s repeated diary assessments of negative affect, pain interference, physical functioning limitations, and ADL difficulties afforded the opportunity to examine longitudinal relationships between the regime-switching measures and these health outcomes. As shown in Table 5, the results for the regression analyses were very consistent across regressions predicting cross-sectional differences (between-person differences at Month-1, upper part of Table 5) and those predicting within-person changes (lower part of Table 5) in the health outcomes. For this reason, we describe only the results for within-person change.
Table 5.
Results for the prediction of initial status and change in health outcomes from regime-switching measures
| Negative affect | Pain interference | Phys. functioning limitations | ADL difficulties | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Est (SE) | β | Est (SE) | β | Est (SE) | β | Est (SE) | β | |
| Regression parameters | ||||||||
| Month-1 status | ||||||||
| Step 1 (R2) | (.168) | (.435) | (.123) | (.153) | ||||
| Average pain level | 3.23 ( .91) | .33*** | .28 (.03) | .61*** | .12 (.03) | .34*** | .12(.03) | .39*** |
| Step 2 (ΔR2) | (.055) | (.055) | (.035) | (.030) | ||||
| Amplitude of shifts | .80 ( .87) | .08 | −.10 (.03) | −.23*** | .01 (.03) | .02 | −.01 (.03) | −.03 |
| Persistence of states | 3.58 (1.68) | .21* | −.02 (.06) | −.03 | −.04 (.06) | −.06 | −.05 (.06) | −.10 |
| Dominance of higher state | 1.50 ( .95) | .14 | .08 (.03) | .16** | .07 (.03) | .16* | .06 (.03) | .18* |
| Rate of change | ||||||||
| Step 1 (R2) | (.232) | (.320) | (.163) | (.117) | ||||
| Change in average pain | 2.90 (.65) | .43*** | .21 (.03) | .59*** | .08 (.02) | .43*** | .08 (.02) | .39*** |
| Step 2 (ΔR2) | (.044) | (.142) | (.116) | (.133) | ||||
| Change in amplitude | .41 (.64) | .06 | .00 (.02) | .01 | .01 (.02) | .03 | −.02 (.02) | −.11 |
| Change in persistence | 1.08 (.49) | .22* | .02 (.03) | .07 | .00 (.02) | −.01 | −.02 (.02) | −.09 |
| Change in dominance | .04 (.44) | .01 | .09 (.02) | .36*** | .04 (.01) | .35*** | .04 (.02) | .33** |
| Intercepts | ||||||||
| Month-1 status | 19.08 (1.32) | .98 (.05) | .99 (.06) | .84 (.04) | ||||
| Rate of change | .48 ( .73) | −.21 (.04) | −.09 (.03) | −.10 (.03) | ||||
| Residual variance components | ||||||||
| Month-1 status | 192.2 (25.9) | .28 (.04) | .28 (.05) | .21 (.03) | ||||
| Rate of change | 52.0 ( 7.9) | .11 (.02) | .04 (.01) | .04 (.01) | ||||
| Residual Month 1 | 132.4 ( 2.6) | .48 (.01) | .05 (.01) | .05 (.01) | ||||
| Residual Month 3 | 99.7 ( 1.9) | .35 (.01) | .05 (.01) | .04 (.01) | ||||
Note: All predictor variables were grand mean centered. ADL = Activities of daily living.
p < .05;
p < .01;
p < .001.
Increases in Average pain were significantly associated with worsening in each of the health outcomes, accounting for 16% to 32% when entered at Step 1 in regression analyses. When entered at Step 2, the regime-switching measures entered at Step 2 uniquely explained between 4% and 15% of the variance. For negative affect, increases in the Persistence of pain states were uniquely associated with increases in negative emotional states (β = .22, p < .05). By contrast, increases in the Dominance of higher pain states were uniquely associated with increases in pain interference (β = .36, p < .001), physical functioning limitations (β = .35, p < .001), and ADL difficulties (β = .34, p < .001).
Associations with 7-day recall pain
In the next set of analyses, we examined whether the dynamic regime-switching measures contributed to patients’ retrospective summary judgments, that is, their recall of pain over the 7-day period. As shown in Table 6, the results for the regression analyses were again highly consistent across regressions predicting between-person differences (Month-1 recall, upper part of Table 6) and those predicting within-person changes (lower part of Table 6) in recall. We therefore elaborate only on the results for Month-1 to Month-3 changes in recall.
Table 6.
Results for the prediction of initial status and change in 7-day recall pain ratings from regime switching measures
| Recall of usual pain | Recall of pain unpleasantness | |||
|---|---|---|---|---|
|
|
|
|||
| b (SE) | β | b (SE) | β | |
| Regression parameters | ||||
| Month-1 pain recall | ||||
| Step 1 (R2) | (.464) | (.370) | ||
| Average pain level | .92 (.06) | .70*** | 1.01 (.09) | .64*** |
| Step 2 (ΔR2) | (.147) | (.193) | ||
| Amplitude of shifts | .34 (.09) | .27*** | .54 (.08) | .36*** |
| Persistence of states | −.16 (.17) | −.07 | −.08 (.23) | −.03 |
| Dominance of higher state | .37 (.07) | .25*** | .40 (.09) | .23*** |
| Change in pain recall | ||||
| Step 1 (R2) | (.470) | (.368) | ||
| Change in average pain level | 1.12 (.08) | .71*** | 1.31 (.15) | .63*** |
| Step 2 (ΔR2) | (.054) | (.101) | ||
| Change in amplitude | .21 (.09) | .13* | .56 (.16) | .27*** |
| Change in persistence | −.05 (.08) | −.04 | −.09 (.14) | −.05 |
| Change in dominance | .26 (.09) | .24** | .32 (.12) | .22** |
| Intercepts | ||||
| Month-1 status | 5.26 (.13) | 5.89 (.16) | ||
| Rate of change | −.74 (.14) | −1.07 (.19) | ||
| Residual variances | ||||
| Month-1 status | 1.84 (.27) | 2.80 (.40) | ||
| Rate of change | 1.90 (.37) | 3.78 (.71) | ||
Note: All predictor variables were grand mean centered.
p < .05;
p < .01;
p < .001.
As would be expected, changes in Average pain levels were substantially associated with concomitant changes in the 7-day pain recall measures (ps< .001), explaining 47% of the variance in the recall of “usual” pain and 37% of the variance in the recall of pain “unpleasantness”. Entered at Step 2, two regime-switching measures consistently augmented the prediction of change in recall pain, explaining between 5% and 10% of the variance: the Amplitude of pain states (β = .13, p < .05 for “usual” pain; β = .27, p < .001 for pain “unpleasantness”) and the Dominance of higher pain states (β = .24, p < .01 for “usual” pain; β = .22, p < .01 for pain “unpleasantness”).
Inspection of the unstandardized regression parameters in the final models shows that as the Amplitude of pain shifts increases by one scale point, recall of “usual” pain intensity during the same period is predicted to increase by .21 scale points and recall of pain “unpleasantness” by .56 scale points, above what would be expected based on changes in the Average pain level. Similarly, for the Dominance of higher pain states, each 1-unit increase in log-odds (for example, an increase in the duration of higher pain states from 5 to 10 hours, while the duration of lower pain states remains unchanged at 5 hours) is predicted to increase recall of “usual” pain by .26 scale points, and recall of pain “unpleasantness” by .32 scale points, above what would be expected based on changes in the Average pain level.
Associations with global impressions of change (GIC)
The final analyses examined whether regime-switching measures contribute to understanding patients’ GIC ratings, assessed at the end of the study. About half of the patients reported that their pain had improved over the 3-month period (very much better = 5.2%, much better = 21.6%, minimally better = 26.7%), 24.1% reported their pain as unchanged, and about one fourth reported worsening (minimally worse = 14.7%, much worse = 5.1%, very much worse = 2.6%). Because the GIC ratings were on an ordered categorical scale, ordinal logistic regressions were used, regressing the GIC category ratings on change scores for each of the regime-switching measures.
As shown in Table 7, changes in the Average pain level significantly predicted GIC ratings (Step 1: R2pseudo = .30). Entered at Step 2, changes in the Dominance of higher pain states provided a unique contribution to the prediction of the GIC ratings (Step 2: ΔR2pseudo = .06). To illustrate these results, we conducted follow-up analyses to describe changes in the Average pain and Dominance measures for groups of patients who indicated at least minimal improvement (minimally better, much better, or very much better; n = 62) and those indicating no improvement or worsening on the GIC item (n = 54). For patients reporting improvement, Average pain decreased by 0.92 scale points (p < .001) from 3.89 (Month 1) to 2.97 (Month 3), with reductions in the mean levels of both higher pain states (from 5.52 at Month 1 to 4.58 at Month 3) and lower pain states (from 2.27 at Month 1 to 1.36 at Month 3). With respect to changes in the Dominance of higher pain states, patients reporting improvement on the GIC item showed a decrease in the duration of higher pain states (from 10.7 hours at Month 1 to 6.5 hours at Month 3) coupled with a slight increase in the duration of lower pain states (from 10.8 hours at Month 1 to 11.7 hours at Month 3), together reflecting a decrease in the ratio of higher relative to lower pain states of OR = 0.47 (95% CI = 0.25 to 0.90). In comparison, patients reporting no improvement or worsening showed only minimal changes in the average pain level from an average of 3.75 (Month 1) to 3.80 (Month 3), as well as in the duration of higher (from 8.8 hours to 9.9 hours) and lower (from 11.5 hours to 11.2 hours) pain states, OR = 1.04 (95% CI = 0.71 to 1.53).
Table 7.
Results for ordinal logistic regressions predicting patients’ global impressions of change from changes in regime-switching measures
| b (SE) | OR | (OR 95% CI) | |
|---|---|---|---|
| Step 1 (R2pseudo = .298) | |||
| Change in average pain level | −1.01 (.19) | .36*** | (.25 – .53) |
| Step 2 (ΔR2pseudo = .062) | |||
| Change in amplitude of shifts | .15 (.18) | 1.16 | (.82 – 1.63) |
| Change in persistence of states | .02 (.16) | 1.02 | (.75 – 1.40) |
| Change in dominance of higher state | −.29 (.11) | .75** | (.61 – .93) |
Note: OR = odds ratio. CI = confidence interval. ΔR2pseudo = change in McKelvey & Zavoina’s R2 for ordered categorical outcomes.
p < .01;
p < .001.
Discussion
Advances in the measurement of pain intensity using EMA offer novel opportunities for understanding the temporal dynamics of pain in patients’ natural daily environment [5,10,63]. The goal of this study was to examine whether regime-switching models applied to EMA pain intensity ratings provide a useful tool for capturing pain characteristics that are uniquely associated with clinically relevant variables. We believe that the analyses yield an affirmative answer to this question, and several results are noteworthy.
For the large majority of patients, the pattern of pain intensity over the waking hours was significantly better described by a sequence of alternating states representing distinctly different pain levels than by a single constant pain state. This corresponds with prior results showing considerable momentary and daily pain variability in patients with rheumatoid arthritis [62], osteoarthritis [2,53], and fibromyalgia [30]. Indeed, patients in the present sample on average experienced an Amplitude of pain shifts of more than 3 scale points on a 0–10 pain scale. Pain states on average persisted for about 10 hours, with states of reduced pain lasting on average between 1.4 and 4.9 hours longer than elevated pain states. However, there was substantial variation in the Amplitude, Persistence, and Dominance of higher pain states across patients.
Of particular importance, each of the regime-switching measures showed unique associations with a number of clinically relevant outcomes after statistically controlling for patients’ Average pain: a greater Persistence of pain states consistently related to higher emotional distress, whereas a Dominance of higher pain states related to greater physical functioning limitations and impact of pain on daily activities. Furthermore, the Amplitude and Dominance measures uniquely contributed to 7-day recall pain, and the Dominance measure to patients’ global impressions of change. The findings were remarkably consistent cross-sectionally and longitudinally, suggesting that regime-switching measures are sensitive to capturing naturally occurring between-patient variation as well as systematic within-patient changes in temporal dynamics of pain.
The finding that patients with elevated emotional distress (depression, anxiety, negative affect, lower mental health) showed more Persistence of pain states is in line with research suggesting that emotional distress may affect the duration of pain episodes through maladaptive pain management strategies and a depletion of self-regulatory resources [35,41]. In accordance with the fear-avoidance model [66,67], emotionally distressed patients may be more likely to engage in a pattern of excessive self-pacing in an effort to maintain pain at low levels, but may also experience prolonged states of worsened symptoms due to a lack of adaptive coping resources [41]. As such, the Persistence measure may be linked to emotional distress because considerable effort is spent on behavioral avoidance to prevent or regulate pain exacerbations. The findings are also consistent with a daily diary (though not EMA) study in rheumatology patients showing that depression tended to be associated with more perseverance (a higher autocorrelation) of pain levels over consecutive days [1]. Similarly, research on temporal dynamics of emotions has shown that clinically depressed patients experience more prolonged negative affective states [32,48]. We found some evidence for more persistent pain states in patients with fibromyalgia compared to patients with rheumatoid arthritis and osteoarthritis, in line with research showing greater psychological comorbidities in these patients [70].
The Dominance of high pain states emerged as a predictor of limitations in physical functioning, impaired activities of daily living, and greater interference of pain with day-to-day activities. These findings may be of clinical interest because many studies have shown pain intensity and physical functioning to be only modestly associated [16]. Prolonged episodes of heightened pain intensity are a major debilitating and physically disabling aspect of rheumatologic diseases [49]. Thus, the finding that the Dominance of elevated pain states uniquely impacts patients’ daily performance has high face validity. Patterns of prolonged elevated pain with relatively brief periods of pain relief are also consistent with a dysregulation in descending pain modulation (such as a lack of habituation and abnormal sensitization) that characterize many chronic pain conditions [22,38,56]. Thus, a Dominance in the duration of higher pain states may capture processes associated with greater disease activity.
The regime-switching measures also had significant relationships with patients’ retrospective judgments of their pain: patients’ recall ratings of the usual level and unpleasantness of pain over the past 7 days were best predicted by a weighted sum of the Average pain, Amplitude of shifts, and Dominance of high pain states experienced over the recall period. A growing body of evidence has documented that cognitive heuristics play an important role in recall ratings of pain [18,54,61]. Specifically, the peak (or salience) memory heuristic predicts that symptoms of high intensity (e.g., exacerbations or flare-ups) are especially salient in memory and that people tend to overly attend to these peaks when recalling their past symptoms [23,54,61]. The present findings are in accordance with this prediction. That is, holding the Average pain level constant, patients recalled their usual pain as higher and as more unpleasant to the extent that they had experienced elevated pain states that were distinctly higher (Amplitude) and longer lasting (Dominance) than low pain states; this temporal pattern is likely characterized by distinctively painful episodes that are especially salient in memory [see 36,64]. This finding also has possible implications for intervention research: if different treatments have the same effect on patients’ average pain level, but reduce the amplitude of shifts in pain and the durability of pain relief at varying extents, this may likely affect the comparability of results based on clinical trials that rely on recall measures of pain [64].
Finally, this study found that patients’ GIC ratings were predicted by a change in the Dominance of higher pain states in addition to changes in Average pain. Changes in Amplitude did not significantly affect GIC, which speaks to the possibility that cognitive heuristics operate differentially in recall of the past 7 days (a judgment of the overall state over a moderately long period of time) compared to impressions of change over the past 3 months (a judgment of a longer-term trajectory) [12]. Nevertheless, our findings suggest that having longer lasting episodes of low pain may be especially desirable for patients and this may affect how they judge improvements in their overall pain status. In addition, retrospective and summative impressions are clinically important because they affect how patients evaluate the efficacy of treatment and how they may behave in the future [51]. Thus, patients who continue to experience a Dominance in the duration of higher pain states may feel less satisfied with treatment and less inclined to continue a therapeutic regimen even if it reduces their Average pain level.
Study limitations
There are several important limitations to this study, as well as directions for future research. The sample was predominantly female (as would be expected for rheumatology patients), White, well educated, and predominantly diagnosed with osteoarthritis; hence, we cannot say whether the results generalize to other demographic and diagnostic groups. In future research, it could be informative to examine whether regime-switching methods can contribute to accurate pain classification by comparing a broader range of chronic pain conditions for which different patterns of pain states would be a priori expected.
Furthermore, the observational study design does not allow conclusions as to whether regime-switching measures are causally implicated in patient functioning. Additional research is needed to investigate the sensitivity of regime-switching measures to the detection of changes in pain in randomized clinical trials, as well as their potential role as moderators of assay sensitivity [cf. 19].
The study was also limited to the simplest regime-switching model with 2 states. Various extensions of the model with more parameters (e.g., switching autoregressive parameters) have been developed [28,29] that could be potentially useful to extract additional temporal pain characteristics. Time varying covariates could be incorporated in the model to examine, for example, which social contexts or activities predict shifts between pain states or the maintenance of pain at lower (or higher) levels [26]. Moreover, models with more than 2 states might provide an even richer (but also more complex) characterization of temporal pain processes. It needs to be kept in mind, however, that more complex regime-switching models likely require a large number of repeated observations from each patient. In this study with a 1-week monitoring period and 7–8 assessments per day, we found moderate to high test-retest stabilities over a 3-month period for some but not all regime-switching measures. Using longer or more intense time-series would be preferable to increase the reliability and precision of regime-switching measurement [27].
Applications of regime-switching models to momentary experience data pose particular data analytic challenges because time intervals between EMA measurements have varying lengths. We took the approach of discretizing and imputing gaps in the time series, but we expect that future statistical advances will contribute to improved implementation of regime-switching models with EMA data [3,13,25]. Future research may also wish to examine whether regime-switching measures derived from EMA ratings contribute to the prediction of outcomes beyond end of day ratings of least, worst, and average pain. This is important to consider given that EMA ratings are more burdensome for study participants and less practical in clinical settings than once a day pain ratings.
Finally, while the present investigation was focused on the assessment of pain intensity, the presented techniques are potentially equally relevant for many other types of symptoms (e.g., fatigue, negative affect), coping strategies (e.g., catastrophizing), and behaviors (e.g., physical activity) that may be characterized by recurrent shifts in intensity over time.
Conclusion
Temporal characteristics of pain captured with regime-switching models have the potential to help better understand the relationships between pain intensity and patients’ emotional functioning and physical limitations, and to determine how retrospective summary judgments of pain are formed. Further studies are warranted to replicate the present findings and to delineate the potential role of regime-switching methods to support accurate pain classification, and to improve assay sensitivity and the detection of clinically meaningful intervention effects in randomized clinical trials.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR066200; A.A.S. and S.S., principal investigators) and the National Cancer Institute (R01 CA085819; A.A.S., principal investigator). We thank Alan T. Kaell, Daniel Arnold, and Steve Choi for their help in collecting the data.
Footnotes
Conflict of Interest
A.A.S. is a Senior Scientist with the Gallup Organization and a consultant with IQVIA and Adelphi Values, inc.
References
- 1.Affleck G, Tennen H, Urrows S, Higgins P. Individual differences in the day-to-day experience of chronic pain: A prospective daily study of rheumatoid arthritis patients. Health Psychology. 1991;10:419–426. doi: 10.1037//0278-6133.10.6.419. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD. The value of measuring variability in osteoarthritis pain. J Rheumatol. 2007;34:2132–2133. [PubMed] [Google Scholar]
- 3.Asparouhov T, Hamaker EL, Muthén B. Dynamic latent class analysis. Structural Equation Modeling: A Multidisciplinary Journal. 2017;24:257–269. [Google Scholar]
- 4.Asparouhov T, Hamaker EL, Muthén B. Dynamic Structural Equation Models. 2017 doi: 10.1080/00273171.2018.1446819. Retrieved from https://www.statmodel.com/download/DSEM.pdf. [DOI] [PubMed]
- 5.Bakshi N, Smith ME, Ross D, Krishnamurti L. Novel Metrics in the Longitudinal Evaluation of Pain Data in Sickle Cell Disease. The Clinical journal of pain. 2017;33:517–527. doi: 10.1097/AJP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 6.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 7.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 8.Bellamy N, Sothern R, Campbell J, Buchanan W. Rhythmic variations in pain, stiffness, and manual dexterity in hand osteoarthritis. Annals of the rheumatic diseases. 2002;61:1075–1080. doi: 10.1136/ard.61.12.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 10.Carpenter RW, Wycoff AM, Trull TJ. Ambulatory assessment: New adventures in characterizing dynamic processes. Assessment. 2016;23:414–424. doi: 10.1177/1073191116632341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coman E, Picho K, McArdle J, Villagra V, Dierker L, Iordache E. The paired t-test as a simple latent change score model. Frontiers in Psychology. 2013:4. doi: 10.3389/fpsyg.2013.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conner TS, Barrett LF. Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosomatic medicine. 2012;74:327–337. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Haan-Rietdijk S, Kuppens P, Bergeman C, Sheeber L, Allen N, Hamaker E. On the Use of Mixed Markov Models for Intensive Longitudinal Data. Multivariate behavioral research. 2017;52:747–767. doi: 10.1080/00273171.2017.1370364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nature Reviews Rheumatology. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durbin J, Koopman SJ. Time series analysis by state space methods. Vol. 38. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 16.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Erskine A, Morley S, Pearce S. Memory for pain: a review. Pain. 1990;41:255–265. doi: 10.1016/0304-3959(90)90002-U. [DOI] [PubMed] [Google Scholar]
- 19.Farrar JT, Troxel AB, Haynes K, Gilron I, Kerns RD, Katz NP, Rappaport BA, Rowbotham MC, Tierney AM, Turk DC. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: an ACTTION study. PAIN®. 2014;155:1622–1631. doi: 10.1016/j.pain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. The Journal of Pain. 2016;17:T10–T20. doi: 10.1016/j.jpain.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neuroscience letters. 2004;361:147–150. doi: 10.1016/j.neulet.2003.12.064. [DOI] [PubMed] [Google Scholar]
- 23.Fredrickson BL. Extracting meaning from past affective experiences: The importance of peaks, ends, and specific emotions. Cognition & Emotion. 2000;14:577–606. [Google Scholar]
- 24.Garcia R. Asymptotic null distribution of the likelihood ratio test in Markov switching models. International Economic Review. 1998:763–788. [Google Scholar]
- 25.Hamaker E, Wichers M. No Time Like the Present: Discovering the Hidden Dynamics in Intensive Longitudinal Data. Current Directions in Psychological Science. 2017;26:10–15. [Google Scholar]
- 26.Hamaker EL, Grasman RP, Kamphuis JH. Modeling BAS dysregulation in bipolar disorder: Illustrating the potential of time series analysis. Assessment. 2016;23:436–446. doi: 10.1177/1073191116632339. [DOI] [PubMed] [Google Scholar]
- 27.Hamaker EL, Grasman RPPP, Kamphuis JH. Regime-switching models to study psychological processes. In: Molenaar PC, Newell KM, editors. Individual Pathways of Change: Statistical Models for Analyzing Learning and Development. Washington, DC: American Psychological Association; 2010. pp. 155–168. [Google Scholar]
- 28.Hamilton JD. Estimation, inference and forecasting of time series subject to changes in regime. In: Maddala GS, Rao CR, Vinod HD, editors. Handbook of Statistics 11: Econometrics. San Diego, CA: Elsevier; 1993. pp. 231–260. [Google Scholar]
- 29.Hamilton JD. Time series analysis. Princeton: Princeton University Press; 1994. [Google Scholar]
- 30.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 2005;52:3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 31.Haynes SN, Lench HC. Incremental validity of new clinical assessment measures. Psychological assessment. 2003;15:456–466. doi: 10.1037/1040-3590.15.4.456. [DOI] [PubMed] [Google Scholar]
- 32.Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological Bulletin. 2015;141:901–930. doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- 33.Jamison RN, Brown GK. Validation of hourly pain intensity profiles with chronic pain patients. Pain. 1991;45:123–128. doi: 10.1016/0304-3959(91)90176-X. [DOI] [PubMed] [Google Scholar]
- 34.Jette A, Deniston O. Inter-observer reliability of a functional status assessment instrument. Journal of Chronic Disease. 1978;31:573–580. doi: 10.1016/0021-9681(78)90017-6. [DOI] [PubMed] [Google Scholar]
- 35.Keefe FJ, Lumley M, Anderson T, Lynch T, Carson KL. Pain and emotion: new research directions. Journal of clinical psychology. 2001;57:587–607. doi: 10.1002/jclp.1030. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi H, Yoshiuchi K, Miyasaka N, Ohashi K, Yamamoto Y, Kumano H, Kuboki T, Akabayashi A. Reliability of recalled self-report on headache intensity: investigation using ecological momentary assessment technique. Cephalalgia. 2006;26:1335–1343. doi: 10.1111/j.1468-2982.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 37.Lavie P. Sleep-wake as a biological rhythm. Annual review of psychology. 2001;52:277–303. doi: 10.1146/annurev.psych.52.1.277. [DOI] [PubMed] [Google Scholar]
- 38.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis research & therapy. 2011;13:211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litcher-Kelly L, Martino SA, Broderick JE, Stone AA. A systematic review of measures used to assess chronic musculoskeletal pain in clinical and randomized controlled clinical trials. The Journal of Pain. 2007;8:906–913. doi: 10.1016/j.jpain.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu HM, Zeng D, Chen H. Prospective infectious disease outbreak detection using Markov switching models. IEEE Transactions on Knowledge and Data Engineering. 2010;22:565–577. [Google Scholar]
- 41.Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. Journal of clinical psychology. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Beneito MA, Conesa D, López-Quilez A, López-Maside A. Bayesian Markov switching models for the early detection of influenza epidemics. Statistics in Medicine. 2008;27:4455–4468. doi: 10.1002/sim.3320. [DOI] [PubMed] [Google Scholar]
- 43.Maruish M, Kosinski M, Bjorner JB, Gandek B, Turner-Bowker DM, Ware JE. User’s Manual for the SF-36v2 Health Survey. Lincoln, RI: QualityMetric, Inc; 2011. [Google Scholar]
- 44.Moritz S. imputeTS: Time Series Missing Value Imputation. R package version 1.9. 2017 URL: https://CRAN.R-project.org/package=imputeTS.
- 45.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Care & Research. 2008;59:849–856. doi: 10.1002/art.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthén LK, Muthén BOM. plus user’s guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 47.Parrish BP, Zautra AJ, Davis MC. The role of positive and negative interpersonal events on daily fatigue in women with fibromyalgia, rheumatoid arthritis, and osteoarthritis. Health Psychology. 2008;27:694–702. doi: 10.1037/0278-6133.27.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. [DOI] [PubMed] [Google Scholar]
- 49.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis & Rheumatology. 2013;65:291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent Growth Curve Modeling. Thousand Oaks, CA: Sage; 2008. [Google Scholar]
- 51.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: a randomized trial. Pain. 2003;104:187–194. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 52.Salaffi F, Sarzi-Puttini P, Atzeni F. How to measure chronic pain: new concepts. Best Practice & Research Clinical Rheumatology. 2015;29:164–186. doi: 10.1016/j.berh.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE. Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: Associations with psychological variables. Pain. 2012;153:813–822. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider S, Stone AA, Schwartz JE, Broderick JE. Peak and end effects in patients’ daily recall of pain and fatigue: a within-subjects analysis. The Journal of Pain. 2011;12:228–235. doi: 10.1016/j.jpain.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 56.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain. 2008;140:420–428. doi: 10.1016/j.pain.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Smith SM, Hunsinger M, McKeown A, Parkhurst M, Allen R, Kopko S, Lu Y, Wilson HD, Burke LB, Desjardins P. Quality of pain intensity assessment reporting: ACTTION systematic review and recommendations. The Journal of Pain. 2015;16:299–305. doi: 10.1016/j.jpain.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 59.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 60.Stone AA, Broderick JE, Kaell AT. Single momentary assessments are not reliable outcomes for clinical trials. Contemporary clinical trials. 2010;31:466–472. doi: 10.1016/j.cct.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone AA, Broderick JE, Kaell AT, DelesPaul PA, Porter LE. Does the peak-end phenomenon observed in laboratory pain studies apply to real-world pain in rheumatoid arthritics? The Journal of Pain. 2000;1:212–217. doi: 10.1054/jpai.2000.7568. [DOI] [PubMed] [Google Scholar]
- 62.Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: examining momentary reports and correlates over one week. Arthritis Care Res. 1997;10:185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- 63.Stone AA, Broderick JE, Schneider S, Schwartz JE. Expanding options for developing outcome measures from momentary assessment data. Psychosomatic medicine. 2012;74:387–397. doi: 10.1097/PSY.0b013e3182571faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone AA, Schwartz JE, Broderick JE, Shiffman SS. Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) memory heuristic. Personality and Social Psychology Bulletin. 2005;31:1340–1346. doi: 10.1177/0146167205275615. [DOI] [PubMed] [Google Scholar]
- 65.VanDyke MM, Parker JC, Smarr KL, Hewett JE, Johnson GE, Slaughter JR, Walker SE. Anxiety in rheumatoid arthritis. Arthritis & Rheumatism. 2004;51:408–412. doi: 10.1002/art.20474. [DOI] [PubMed] [Google Scholar]
- 66.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 67.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 68.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical care. 1992:473–483. [PubMed] [Google Scholar]
- 69.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 70.Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. JCR: Journal of Clinical Rheumatology. 2006;12:124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.