Abstract
Macrophages with the M2b phenotype (Pheno2b-Mφ) in bacterial translocation sites have been described as cells to be responsible for the increased susceptibility of mice with gastrointestinal acute radiation syndrome to sepsis caused by gut bacteria. In this study, we tried to reduce the mortality of mice exposed to 7-10 Gy of γ-rays by controlling Pheno2b-Mφ polarization in bacterial translocation sites. MicroRNA-222 was induced in association with γ-irradiation. Pheno2b-Mφ polarization was promoted and maintained in γ-irradiated mice through the reduction of a long non-coding RNA growth arrest-specific 5 (a CCL1 gene silencer) influenced by this microRNA. Therefore, the host resistance of 7-9 Gy γ-irradiated mice to sepsis caused by bacterial translocation was improved after treatment with CCL1 antisense oligodeoxynucleotide. However, the mortality of 10 Gy γ-irradiated mice was not alleviated by this treatment. The crypts and villi in the ileum of 10 Gy γ-irradiated mice were severely damaged, but these were markedly improved after transplantation of intestinal lineage cells differentiated from murine embryonic stem cells. All 10 Gy γ-irradiated mice given both of the oligodeoxynucleotide and intestinal lineage cells survived, while all of the same mice given either of them died. These results indicate that high mortality rates of mice irradiated with 7-10 Gy of γ-rays are reducible by depleting CCL1 in combination with the intestinal lineage cell transplantation. These findings support the novel therapeutic possibility of victims who have gastrointestinal acute radiation syndrome for the reduction of their high mortality rates.
Keywords: Rodent, monocytes/macrophages, gene regulation
Introduction
Whole body γ-irradiation induces severe damages to the gastrointestinal tract (1-4). In our previous studies in a model of sepsis caused by bacterial translocation (5), mice exposed to a non-lethal dose (5 Gy) of whole body γ-irradiation (5 Gy WBI-mice) were shown to be highly susceptible to sepsis stemming from bacterial translocation. Gut bacteria-associated sepsis, however, does not develop in healthy mice (5, 6), because pathogens invaded from intestinal tracts are rapidly eliminated by host defense effector cells at the translocation sites (mesenteric lymph nodes, MLNs; and lamina propria, LP) (3). A major host antibacterial effector cell against bacterial translocation has been characterized as IL-12+CD38+iNOS+ F4/80+ cell (Pheno1-Mφ) (5, 6), and sepsis stemming from Enterococcus faecalis oral infection does not develop in healthy mice with interchangeable Mφ in the bacterial translocation sites (5, 6). Interchangeable Mφ easily switch to Pheno1-Mφ under bacterial antigen stimulation (5-7). However, the pathogens that translocate from the intestinal tracts are not eliminated by the Mφ distributed in the LP and MLNs of 5 Gy WBI-mice (5), although Mφ are highly resistant against γ-irradiation (8). Mφ isolated from the MLNs of 5 Gy WBI-mice are characterized as CCL1+IL-10+miR-27a+ F4/80+ cells (Pheno2b-Mφ) that inhibit the Mφ switching to Pheno1-Mφ (5). The increased susceptibility of 5 Gy WBI-mice to E. faecalis oral infection has been completely improved after elimination of Pheno2b-Mφ by CCL1 antisense oligodeoxynucleotide (ODN), an inhibitor of CCL1 (5) which is an essential chemokine on the prolongation of Pheno2b-Mφ (9).
In the present study, we tried to reduce the mortality of mice with gastrointestinal acute radiation syndrome (GIARS-mice, mice exposed to 7-10 Gy whole body γ-irradiation) through controlling Pheno2b-Mφ polarization. Similar to 5 Gy WBI-mice, Pheno2b-Mφ were isolated from the LP/MLNs of 7-10 Gy GIARS-mice. Different from 5 Gy WBI-mice, 7 Gy GIARS- mice died within 3 weeks of irradiation without any infections of external pathogens. These mice did not die after decontamination with an antibiotic mixture, but they were shown to be very susceptible to enterococcal oral infection, a model used for gut bacteria-associated sepsis. This indicates that the mortality of 7 Gy GIARS-mice, not exposed to external pathogens, is associated with bacterial translocation. Therefore, 7-9 Gy GIARS-mice survived for a month after γ-irradiation when Pheno2b-Mφ were eliminated from these mice by treatment with CCL1 antisense ODN. However, the ODN treatments did not prevent the deaths of 10 Gy GIARS-mice. Severe gastrointestinal damage (measured through the decreased crypt number and crypt regeneration in the ileum) was observed in 10 Gy GIARS-mice, and the damage was markedly improved after the transplantation of intestinal lineage cells differentiated from murine embryonic stem cells (ES-ICs). ES-ILCs were a mixture of enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. The 10 Gy GIARS-mice subjected to ES-IC transplantation and the ODN treatment in combination did not die for a month or more. These results indicate that high mortality rates of 7-10 Gy GIARS-mice can be reducible by the CCL1 antisense ODN treatment, with higher Gy levels needing an ES-IC transplantation in addition.
Materials and Methods
Mice and irradiation
BALB/c mice (9- to 12-week-old pathogen-free male and female) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were exposed to 5 Gy or 7-10 Gy of whole body γ-irradiation with a 137Cs-ray (0.662 MeV) irradiator (Mark I Model 30, J.L. Shepherd & Associates, San Fernando, CA) at a dose rate of 1.05 Gy/min, which was reduced from the 5.08 Gy/min via lead attenuators. Mice exposed to 5 Gy of γ-rays were abbreviated as 5 Gy WBI-mice, and mice exposed 7-10 Gy of γ-rays were abbreviated as GIARS-mice. Sepsis does not develop in 5 Gy WBI-mice without any infections of external pathogens, while it develops in GIARS-mice. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston (IACUC Approval Number: 0906044).
Reagents, cells, bacteria, and media
Streptavidin particles plus-DM, Cytofix/Cytoperm™ solution, IMag™ buffer, and PE-conjugated anti-IL-10, FITC-conjugated anti-CCL1, and isotype control mAbs were purchased from BD Biosciences (San Jose, CA). Biotin-conjugated anti-mouse F4/80 mAb was obtained from eBioscience (San Diego, CA). TRIzol reagent, Ambion mirVana miRNA isolation kit, a cell extraction buffer, and knockout serum replacement was perchased from Thermo Fisher Scientific (Waltham, MA, USA). Phosphorothioated CCL1 antisense ODN (5′-GAAGCCCGAGAACAT CAT-3′) and scrambled ODN (5′-CATCACAAATGCGACAGG-3′) were synthesized by Sigma-Proligo (Woodlands, TX). Activin A and FGF2 were purchased from PeproTech (Rocky Hill, NJ). Anti-E-cadherin, anti-Cxcr4, anti-Cdx2, anti-Muc2, anti-Lyz1, and anti-Chga antibodies were purchased from Abcam (Cambridge, MA). Protease inhibitor cocktail, bromoindirubin-3′-oxime (BIO), N-[(3,5-difluorophenyl) acetyl]-L-alanyl-2-phenylglycine-1,1-dimethylethyl ester (DAPT), mitomycin C, and M15 feeder cells (a source of mesoderm-derived growth factors) were purchased from Sigma-Aldrich (St. Louis, MO). Murine embryonic stem cells (day 3.5 blastocysts from BALB/cJ mice) were obtained from Jackson laboratory. E. faecalis (29212 strain), purchased from the American Type Culture Collection (Manassas, VA), was cultured in tryptic soy broth for the infection experiments (5, 6, 10). The cultures were centrifuged at 2,000×g for 15 min, and the bacterial pellet was suspended in PBS. The number of bacteria in the suspension was counted using a hemocytometer and adjusted to give the approximate desired inocula. The inocula were verified by serial 10-fold dilutions of the bacterial suspensions and plated on tryptic soy agar. Heat-killed E. faecalis was prepared by heating bacteria at 65°C for 30 min (5, 6, 10).
Preparation of intestinal lineage cells derived from murine embryonic stem cells (ES-ICs)
The differentiation of murine embryonic stem cells to definitive endoderm (E-cadherin+Cxcr4+) was induced by activin A and FGF2. Briefly, murine embryonic stem cells were plated at 1 × 106 cells/ml in 100-mm cell culture dish precoated with an M15 cell feeder layer (3 days of cultivation at 7 × 104 cells/ml) which was previously treated with mitomycin C (10 μg/ml for 4 h) and washed with PBS 3 times. The cells were cultured in DMEM containing 20 ng/ml of activin A, 50 ng/ml of FGF2, 10% FBS, and 4,500 mg/ml glucose for 5 days, as previously described (11). For differentiation to intestinal lineage cells, E-cadherin+Cxcr4+ cells sorted from these cells were further cultured on the same feeder cells in DMEM containing 5 μM of BIO (a Wnt/β-catenin activator), 10 μM of DAPT (a Notch signaling pathway inhibitor), 10% knockout serum replacement, and 2,000 mg/ml glucose for 10 days. Using flow cytometry, obtained cells were determined as ES-ICs consisting of enterocytes (Cdx2+), goblet cells (Muc2+), enteroendocrine cells (Chga+), and Paneth cells (Lyz1+) (11). Obtained ES-ICs were suspended in PBS at a concentration of 1 × 107 cells/ml, then these cells (0.2 ml) were transplanted i.v. to 10 Gy GIARS-mice 2 and 4 days post-irradiation.
Bacterial translocation and subsequent sepsis
The severity of infectious complications caused by spontaneous bacterial translocation in GIARS-mice was evaluated by (i) growth of bacteria in the blood, MLNs, liver, and kidneys, and (ii) the mortality rates of the test group mice in comparison with those of the control group mice. The quantity of bacteria in organ specimens was measured as previously described (5, 6). In some experiments, sepsis was induced by E. faecalis oral infection in GIARS-mice after antibiotic decontamination. Thus, GIARS-mice were decontaminated for 12 days (7 days before to 5 days after γ-irradiation) by an antibiotic cocktail (4 mg/ml of penicillin, streptomycin and bacitracin) in the drinking water (5, 6, 12, 13). When GIARS-mice were orally treated with the antibiotic cocktail, the number of microbiota was dramatically reduced (102 or 4 × 104 CFU/gram ileum of bacteria in mice 2 or 7 days after the end of the antibiotic treatment, Supplemental Fig. 1). In GIARS-mice without the antibiotic decontamination, 1010 CFU/gram ileum or more of bacteria were detected 7 days post-irradiation. For the stabilization of E. faecalis oral infection, decontaminated mice were injected orally with lansoprazole (a proton-pump inhibitor, 0.5 mg/mouse) on the day of the final antibiotic treatment (5, 6, 10). Two days after lansoprazole treatment, these mice were infected orally with 106 CFU/mouse of E. faecalis. Bacterial translocation and subsequent sepsis in decontaminated GIARS-mice (dGIARS-mice) were confirmed by using green fluorescent protein (GFP)-labeled E. faecalis. For the tracking of orally infected bacteria, pMV158-GFP plasmid construction was established in E. coli STBL3 and then transferred to E. faecalis by electrotransformation, as previously described (14). E. faecalis expressing GFP was detected by electroporation with pMV158-GFP plasmid using a Bio-Rad Gene Pulser Electroporator (Bio-Rad Laboratories Richmond, CA). The transformed E. faecalis was selected on tryptic soy agar supplemented with 4 μg/ml of tetracycline for 16 h. The transformed cells from single colony were grown in tryptic soy broth for 5-10 h, then GFP gene expression was induced by 2% maltose for 6 h (GFP-labeled E. faecalis). Two days after infection with GFP-labeled E. faecalis or non-labeled E. faecalis (1 × 106 CFU/mouse), the liver and kidneys were removed and a half of each organ was treated with collagenase IV (1 mg/ml) for 20 min at 37°C. E. faecalis in the cell suspension was detected by flow cytometry. Also, the remaining half was embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Torrance, CA). Frozen tissue sections (5 μm) were prepared using Leica CM350 S cryostat (Leica Biosystems, Wetzlar, Germany) and analyzed by Olympus BX51 fluorescence microscope (Olympus, Melville, NY).
Characterization of MLN-Mφ isolated from 7-10 Gy GIARS-mice
As a Mφ population, F4/80+ cells were prepared from the MLNs of dNormal mice, 5 Gy dWBI-mice, and 7-10 Gy dGIARS-mice, as previously described (5, 6, 15). In the majority of experiments, NLM-Mφ were isolated 7 Gy dGIARS-mice 7 days post-irradiation. As Pheno2b-Mφ, the numbers of CCL1+IL-10+ cells in the MLN-Mφ population were determined by flow cytometry, as previously described (5, 6). Flow cytometry data were analyzed in FlowJo 10.2 software (Tree Star, Ashland, OR, USA).
Gene expression analyses
For gene expression analysis, total RNAs were extracted from MLN-Mφ with TRIzol reagent according to the manufacturer's instructions. The cDNAs were synthesized with SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific). Quantitative real-time PCR (real-time PCR) was performed using an iTaq™ Universal SYBR® Green Supermix (Bio-Rad) on a ViiA 7 Real-Time PCR System (Thermo Fisher Scientific) with the following primers (forward/reverse): GAS5, 5′-CACCTCAAGTGAAGGCACTGC-3′ and 5′-CACCTCAGAAACAAAGGTGCAG3′; TNFSF14 (LIGHT), 5′-GATACGTCAAGCCCCTCAAG-3′ and 5′-CTGCATCAACGTCT TGGAGA-3′; CCL1, 5′-CAAGAGCATGCTTACGG-3′ and 5′-ATGACTGAGGTCTGTGA-3′; GAPDH, 5′-TGCACCACCAACTGCTTAG-3′ and 5′-GGATGCAGGGATGATGTTC-3′. The expression level was normalized to that of housekeeping gene GAPDH. In some experiments, total RNAs were extracted from the same cells using Ambion mirVana miRNA isolation kit according to the manufacturer's instruction. The expression levels of miR-27a, miR-222, and miR-361 were quantified using Taqman miRNA assay (Applied Biosystems) according to the manufacturer's instruction. The kit uses gene-specific stem-loop reverse transcription primers and Taqman probes to detect mature miRNA transcripts. PCR reaction was carried out on the ViiA 7 Real-Time PCR System. The expression level was normalized to that of miR-361.
To determine the role of GAS5 in Pheno2b-Mφ polarization, MLN-Mφ from 7 Gy dGIARS-mice 7 days post-irradiation were transduced with GAS5 gene via a lentiviral vector, as previously described (16). Murine GAS5 cDNA was amplified from pCMV-Sport6-GAS5 plasmid and cloned into pLenti7.3/V5-TOPO vector (pLenti7.3-GAS5) and lentiviruses were prepared using HEK293FT cells as described in the manufacturer's protocol. Seventy-two hours after transfection, supernatants were filtered (0.45-μm filter), and the virus suspension was titrated onto HEK293FT cells by flow cytometry using GFP expression from pLenti7.3/V5-TOPO vector. Mock viruses were generated by the same procedure using otherwise identical vector lacking GAS5 cDNA and served as a control lentivirus. In experiments, MLN-Mφ from 7 Gy dGIARS-mice were exposed to GAS5 lentivirus or control lentivirus, and cultured for 2 days. Cells obtained were stimulated with 100 ng/ml of LPS in culture plates that were previously coated with 100 μg/ml of murine IgG (16). Five days after stimulation, cells were analyzed for Pheno2b-Mφ (CCL1+IL-10+ cells) by flow cytometry.
Western blotting analysis
For the preparation of whole cell extract, MLN-Mφ were homogenized in a cell extraction buffer (Thermo Fisher Scientific) supplemented with a protease inhibitor cocktail (Sigma-Aldrich), according to the manufacturer's protocols. After incubating on ice for 15 min for cell disruption, the cell extracts were centrifuged at 12,000 × g for 10 min at 4°C. The obtained supernatants were determined for their protein concentrations using a Pierce BCA protein assay (Thermo Fisher Scientific). Twenty μg of proteins were separated on the SDS-PAGE gel (BioRad) and electrically transferred onto PVDF membranes. The membranes were incubated with monoclonal rabbit anti-UPF1 and anti-phosphorylated UPF1 (S1100) antibodies in TBS-T containing 1% (w/v) BSA at 4°C overnight. After washing three times, the membranes were incubated for 1 h with secondary antibodies conjugated to HRP. Antibody binding was detected using SuperSignal West Pico Chemiluminescent Substrate and X-ray film.
Recover from GI damage
The cross sections of ileum were obtained from 10 Gy GIARS-mice at 7 days post-irradiation, paraffin-embedded, sectioned, and stained with H&E. Stained sections were analyzed under LEICA DMLB optical microscope (Leica, Wetzlar, Germany) at magnification 100× (for counting the number of crypts) and 200× (for measuring the length of the villi) in three different filed. The images were captured by a CoolSNAP-Pro digital camera (Media Cybernetics, MD). All measurements were performed with the program AnalySIS Docu (Soft Imaging System GmgH, Munchen, Germany). The number of crypts per circumference was counted in well-oriented transverse cross sections of the ilea from three different mice. The length of the villi was measured from its basal region, which coincided with the top of the crypts. A line was drown from point on the base toward the point at the apex of the villus. The length of the line provided by the image analyzer was taken as the length of the villus.
Statistical analysis
Data are presented as mean ± SE. Results were statistically analyzed by a Student's t test. Kaplan-Meier curves were constructed, and a log-rank test was used to compare the survival of the groups. The results were considered to be significant if the p-value was lower than 0.05.
Results
Sepsis and Pheno2b-Mφ detected in 7 Gy GIARS-mice with (dGIARS-mice) or without (GIARS-mice) antibiotic decontamination
In our previous studies (5), mice exposed to 5 Gy of whole body Γ-irradiation (5 Gy WBI-mice) have been shown to be very susceptible to various infections. However, these mice did not die without any infections of external pathogens. On the other hand, all 7 Gy GIARS-mice died without any infections of external pathogens. Therefore, a role of infectious complications on the mortality of 7 Gy GIARS-mice was examined using decontaminated 7 Gy GIARS-mice (dGIARS-mice). The decontamination of these mice was performed by the administration of an antibiotic mixture by drinking water, as previously described (5, 6, 10). In the results, all 7 Gy GIARS-mice died within 24 days of the γ-irradiation, while the same mice decontaminated with an antibiotic mixture survived (Fig. 1A). Progressive growth of bacteria was seen in the MLNs and liver of 7 Gy GIARS-mice 6 to 12 days after the irradiation, while the bacterial growth in organs was not seen in 7 Gy dGIARS-mice (Fig. 1B). After E. faecalis oral infection, however, all of these mice died within 12 days of the infection (Fig. 1C). E. faecalis grew progressively in the MLNs and liver of these mice (Fig. 1D). Bacterial translocation and subsequent sepsis were confirmed in 7 Gy dGIARS-mice after oral infection with GFP-labeled E. faecalis. The labeled pathogen was detected in the liver and kidneys of these mice flow cytometrically and fluorescence microscopically (Supplemental Fig. 2A and B). The results shown in Fig. 1A-D indicate that the mortality of 7 Gy GIARS-mice is associated with sepsis caused by bacterial translocation.
Figure 1.
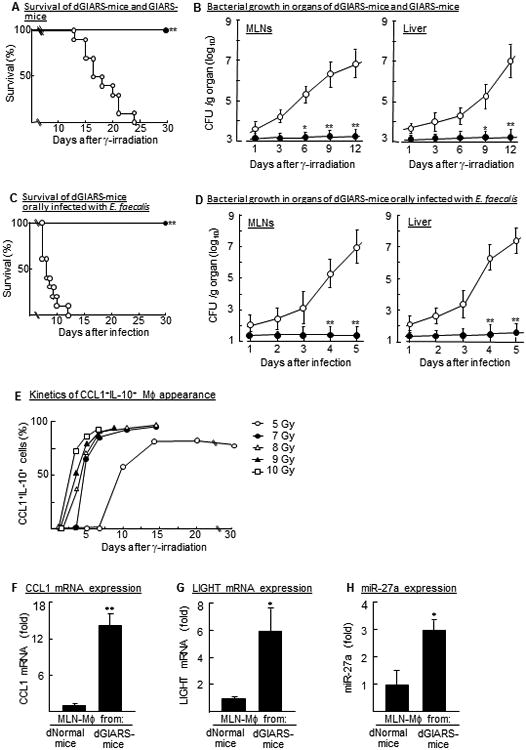
Sepsis and Pheno2b-Mφ demonstrated in 7 Gy GIARS-mice with or without antibiotic decontamination. (A) Mortality rates of 7 Gy GIARS-mice with (dGIARS-mice, ●) or without (GIARS-mice, ○) an antibiotic decontamination. **p <0.001 (log-rank test). (B) Bacterial growth in the MLNs and liver of dGIARS-mice (●) and GIARS-mice (○) 1 to 12 days post-irradiation. Data are displayed by the mean ± SE. *p <0.01, **p <0.001 (Student's t test). (C) Mortality rates of dGIARS-mice orally infected with (▼) or without (ɟ) E. faecalis. Seven days after γ-irradiation, dGIARS-mice were orally infected with 105 CFU/mouse of E. faecalis. **p <0.001 (log-rank test). (D) Bacterial growth in organs of dGIARS-mice infected with (○) or without (●) E. faecalis. Data are displayed by the mean ± SE. *p <0.01, **p <0.001 (Student's t test). (E) Appearance of Pheno2b-Mφ in the MLNs of 5 Gy WBI-mice and 7-10 Gy GIARS-mice. MLN-Mφ, isolated from various groups of mice 1 to 30 days post-irradiation, were analyzed for CCL1+IL-10+ cells by flow cytometry. (F-H) MLN-Mφ, isolated from dNormal mice and 7 Gy dGIARS-mice 7 days post-irradiation were analyzed for the expression of typical Pheno2b-Mφ biomarkers by real-time PCR; CCL1 mRNA (F), LIGHT mRNA (G), and miR-27a (H). Data are displayed as mean ± SE from three independent experiments. *p <0.01, **p <0.001 versus dNormal mouse MLN-Mφ (Student's t test). Data are representative of two (A-D) or three (E-H) independent experiments. Three (E-H), four to five (B, D), or ten (A, C) mice per group were used in each independent experiment.
In our previous studies (5, 6, 10), Pheno1-Mφ have been characterized as host antibacterial effector cells against sepsis stemming from bacterial translocation, and Pheno2b- Mφ are shown to be inhibitor cells on the Pheno1-Mφ polarization (5, 17). Therefore, we tried to detect Pheno2b-Mφ) in the MLNs of 7-10 Gy GIARS-mice. All of these experiments were performed in mice decontaminated with an antibiotic mixture (dGIARS-mice). The time course of the appearance of CCL1+IL-10+ cells (Pheno2b-Mφ) in the MLN-Mφ of 5 Gy WBI-mice was shown as a control (5). As shown in Fig. 1E, Pheno2b-Mφ were detected in the MLNs of 7-10 Gy dGIARS-mice 2 to 4 days post-irradiation, when these Mφ first appeared in the MLNs of 5 Gy WBI-mice 10 days post-irradiation. MLN-Mφ isolated from the MLNs of 7 Gy dGIARS-mice 7 days post-irradiation were identified as Pheno2b-Mφ by their biomarkers (Fig. 1F, CCL1 mRNA expression; 1G, LIGHT mRNA expression; 1H, miR-27a expression).
Pheno2b-Mφ polarization in 7 Gy dGIARS-mice
We examined how normal Mφ are switched to Pheno2b-Mφ after the γ-irradiation. miR-222, a promoter of DNA damage repairing (18, 19), has been described as an inhibitor of GAS5 expression (20). GAS5 is known as a silencer of CCL1 (21), which is an essential chemokine for the Pheno2b-Mφ polarization (9). A group of miRNAs drives the RNA degradation through the activation of NMD pathway (the UPF1 phosphorylation) (22, 23). Therefore, the expression of miR-222 and GAS5 RNA in MLN-Mφ from 7 Gy dGIARS-mice was investigated. In the results, the expression of miR-222 was shown to be minimal in the MLN-Mφ of dNormal mice, while it was markedly increased (4-fold, Fig. 2A) in the MLN-Mφ of 7 Gy dGIARS-mice. Similar increased expression of miR-222 was seen in MLN-Mφ from mice 8-10 Gy dGIARS-mice (data not shown). As shown in Fig. 2B, the expression of GAS5 RNA was greatly reduced in the MLN-Mφ of 7 Gy dGIARS-mice. GAS5 RNA expression is reduced through the UPF1 phosphorylation of the NMD pathway (16). Therefore, the phosphorylation of UPF1 in the total cellular proteins, derived from MLN-Mφ from 7 Gy dGIARS-mice, were analyzed by Western blotting (Fig. 2C, left panel) and quantified by densitometric analysis (Fig. 2C, right panel). In the results, phosphorylated UPF1 increased (6.5-fold) in MLN-Mφ from 7 Gy dGIARS-mice, as compared to those of dNormal mice. In the next experiments, a role of GAS5 on the prolongation of Pheno2b-Mφ was investigated using Pheno2b-Mφ from the MLNs of 7 Gy GIARS-mice after transduction with GAS5 gene. GAS5 gene transduction to Pheno2b-Mφ was performed in cultures of Mφ with GAS5 gene-encoded lentivirus for 2 days (16). In the results, Pheno2b-Mφ switched to non-Pheno2b-Mφ (IL-10−CL−Mφ after transduction with GAS5 RNA (Fig. 2D, left panel). Also, these Pheno2b-Mφ transduced with GAS5 RNA did not switch back to Pheno2b-Mφ, even when they were stimulated with a typical inducer of Pheno2b-Mφ (Fig. 2D, right panel). These results indicate that the Pheno2b-Mφ polarization is maintained through GAS5 RNA reduction influenced by miR-222, which is expressed in association with γ-irradiation.
Figure 2.
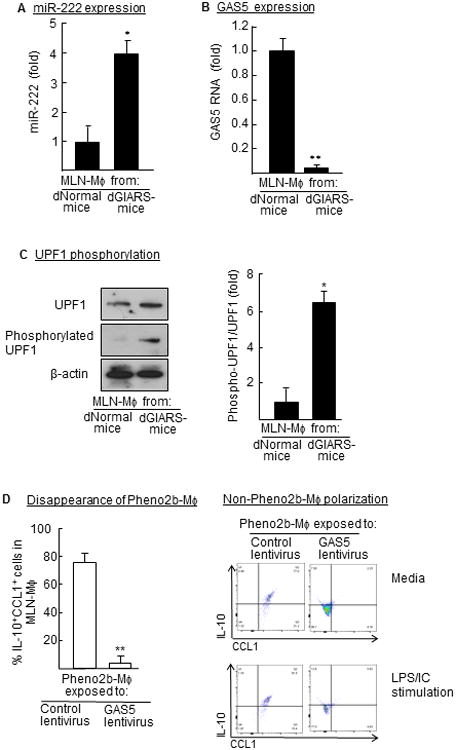
Pheno2b-Mφ polarization in 7 Gy dGIARS-mice. Mφ from the MLNs of dNormal mice and 7 Gy dGIARS-mice 7 days post-irradiation were tested for the expression of miR-222 (A, real-time PCR), GAS5 RNA (B, real-time PCR), and UPF1 phosphorylation (C, Western blotting, left panel). Phosphorylated UPF1 level was quantified by densitometric analysis (C, right panel). Data are displayed as the mean ± SE. *p <0.01, **p <0.001 versus dNormal mouse MLN-Mφ (Student's t test). Pheno2b-Mφ (MLN-Mφ from 7 Gy dGIARS-mice) were exposed to GAS5 lentivirus or control lentivirus, and cultured for 2 days (D, left panel). Cells obtained were stimulated by a mixture of LPS and immune complex (LPS/IC) for 5 days for the Pheno2b-Mφ polarization (D, right panel). Cells obtained were analyzed for CCL1+IL-10+ cells by flow cytometry. (D, left panel) Data are displayed as the mean ± SE. **p <0.001 versus Pheno2b-Mφ exposed to control virus (Student's t test). (A-D) Data are representative of three independent experiments. Three mice per group were used in each independent experiment.
Survival rates and bacterial growth in the organs of 7-9 Gy GIARS-mice treated with CCL1 antisense ODN
In our previous studies (5), MLN-Mφ from 5 Gy WBI-mice have been switched to non-Pheno2b-Mφ after treatment with CCL1 antisense ODN, and the resistance of 5 Gy WBI-mice to E. faecalis oral infection improved markedly after treatment with the ODN. Therefore, we examined the effect of the ODN on the survival of 7-9 Gy GIARS-mice. Additionally, bacterial growth in the blood, MLNs, liver, and kidneys of the ODN-treated mice 10 to 12 days after the γ-irradiation was measured. In these experiments, various GIARS-mice were treated with CCL1 antisense ODN or scrambled ODN (control) twice daily until end of the experiments starting 3 days after irradiation, according to the results in the previous studies (5). Although all 7-9 Gy GIARS-mice treated with scrambled ODN died, but all of the 7 Gy and 8 Gy GIARS-mice and 70% of the 9 Gy GIARS-mice treated with CCL1 antisense ODN survived (Fig. 3A-C, left panels). A large number of bacteria was detected in the blood and organs of 7 to 9 Gy GIARS-mice treated with scrambled ODN, while decreased bacterial growth in the blood and organs was seen in 7-9 Gy GIARS-mice treated with the ODN (Fig. 3A-C, middle and right panels). These results indicate that the radiation-associated mortality of 7-9 Gy GIARS-mice are reduced after the Pheno2b-Mφ elimination by CCL1 antisense ODN.
Figure 3.
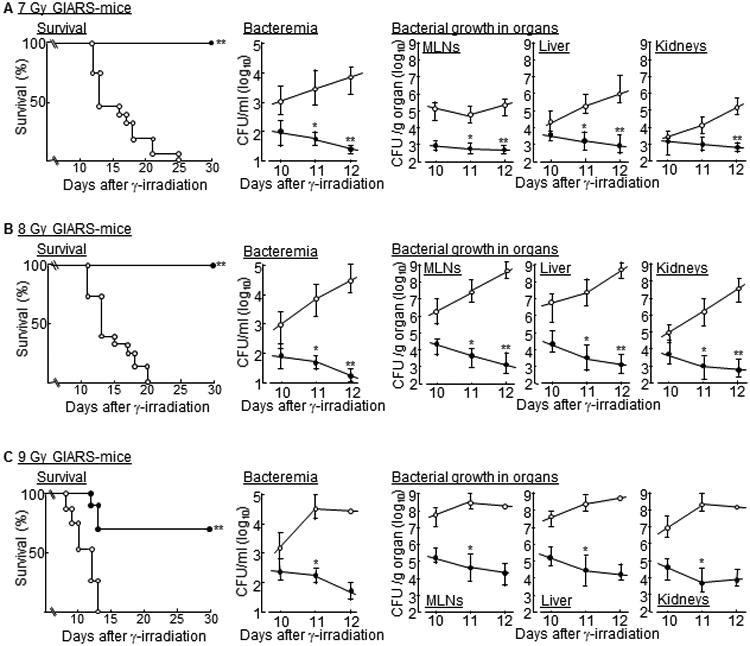
Survival rates and bacterial growth in the organs of 7-9 Gy GIARS-mice treated with CCL1 antisense ODN. GIARS-mice exposed to 7 Gy (A), 8 Gy (B), and 9 Gy (C) of γ-rays were treated s.c. with 25 ng/mouse of CCL1 antisense ODN (●) or scrambled ODN (○) twice daily until end of experiments starting 3 days after the irradiation. The survival of these mice was monitored twice a day for 30 days (A-C, left panels). Data are representative of two independent experiments. Ten to 15 mice per group were used in each independent experiment. *p <0.001 (log-rank test). Bacterial growth in the blood (A-C, middle panels) and various organs (A-C, right panels) of these mice were determined by the colony counting method. Data are representative of two independent experiments. Four to five mice per group were used in each independent experiment. Data are displayed by the mean ± SE. *p <0.01, **p <0.001 (Student's t test).
Recovery of GI damage and antibacterial resistance of 10 Gy GIARS-mice
In contrast, 10 Gy GIARS-mice died even when they were decontaminated with an antibiotic mixture (Fig. 4A) or treated with CCL1 antisense ODN (Fig. 4B). These results indicate that the mortality of 10 Gy GIARS-mice is not directly associated with gut bacteria-associated sepsis influenced by the Pheno2b-Mφ polarization. As shown in Fig 4D, severe damages to the crypt/villus units were demonstrated in the ileum of 10 Gy GIARS-mice. Therefore, we tried to heal the GI damage of 10 Gy GIARS-mice in the first step, then the infection-associated mortality of these mice were reduced by the Pheno2b-Mφ elimination using CCL1 antisense ODN. In the results, 7 days post-irradiation, GI damage in the ileum of 10 Gy GIARS-mice was markedly improved after the transplantation of ES-ICs (2 × 106 cells/mouse, i.v., 2 and 4 days post-irradiation) (Fig. 4D). ES-ICs were created from murine embryonic stem cells after the activation of Wnt/β-catenin and inhibition of Notch signaling pathways (Fig. 4C), as previously described (11). ES-ICs were shown to be a mixture of enterocytes (Cd×2+, 68.9%), goblet cells (Muc2+, 4.6%), enteroendocrine cells (Chga+, 16.7%), and Paneth cells (Lyz1+, 5.8%) (Supplemental Fig. 3). Also, the number of crypts and the length of villi were recovered in the ileum of 10 Gy GIARS-mice transplanted with ES-ICs (Fig. 4D and E). These results indicate that crypt/villus units are significantly renewed in 10 Gy GIARS-mice transplanted with ES-ICs. Therefore, in subsequent studies, the mortality rate of 10 Gy GIARS-mice was examined after the transplantation of ES-ICs and treatment with CCL1 antisense ODN in combination. As a result, all of the 10 Gy GIARS-mice given both CCL1 antisense ODN and ES-ICs survived, while mice given either of them died within 20 days of the γ-irradiation.
Figure 4.
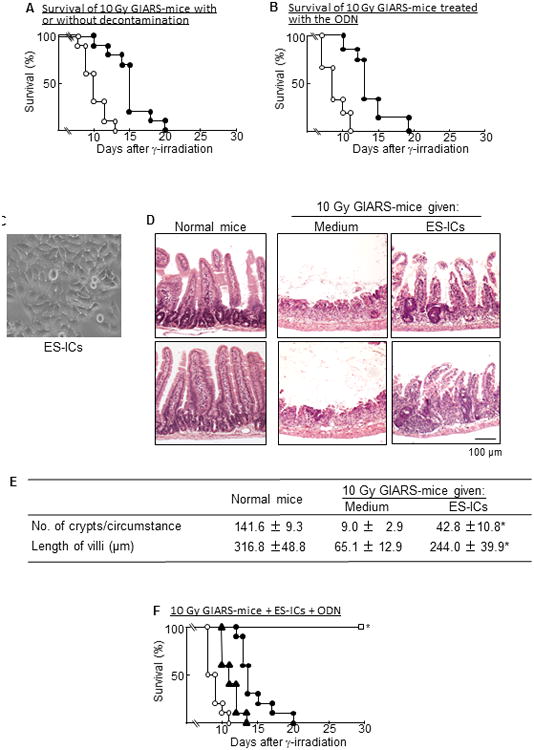
Gastrointestinal damages and antibacterial resistance of 10 Gy GIARS-mice. The survival of 10 Gy GIARS-mice (○) and dGIARS-mice (●) was monitored twice a day for 30 days (A). Data are representative of two independent experiments. Ten mice per group were used in each independent experiment. Ten Gy GIARS-mice were treated s.c. with 25 μg/mouse of CCL1 antisense ODN (●) or scrambled ODN (○) twice daily until end of experiments starting 3 days after the irradiation, and the survival of these mice was monitored twice daily for 30 days (B). Data are representative of two independent experiments. Fifteen mice per group were used in each independent experiment. ES-ILCs (C), differentiated from murine embryonic stem cells, were transplanted (2 × 106 cells/mouse, i.v.) to 10 Gy GIARS-mice 2 and 4 days post-irradiation. Light microscopic images (100× magnification) of the H&E ileum section were obtained from these mice 7 days post-irradiation (D). Data shown in C and D are representative of three independent experiments. Three mice per group were used in each independent experiment. Light microscopic images obtained in D were digitized, and the number of crypts per circumstance and the length of villi were measured 7 days post-irradiation by analySIS docu software (E). Data are shown as means ± SE. *p <0.001 versus GIARS-mice treated with media (Student's t test). (F) Ten Gy GIARS-mice were given ES-ICs alone (2 × 106 cells/mouse, i.v.) 2 and 4 days post-irradiation ▲, 25 μg/mouse of CCL1 antisense ODN alone (●), or both (□). Untreated 10 Gy GIARS-mice served as a control (●). The survival of these mice was monitored twice a day for 30 days. Data are representative of two independent experiments. Ten mice per group were used in each independent experiment. *p <0.001 versus control (log-rank test).
Discussion
Gut bacteria-associated sepsis is a serious concern in persons who suffer from gastrointestinal acute radiation syndrome (GIARS) caused by lethal doses (6-15 Gy) of γ-irradiation (1-4). In our previous studies, Pheno2b-Mφ were predominantly isolated from the MLNs of mice exposed to a non-lethal dose (5 Gy) of γ-rays, and these mice were shown to be highly susceptible to E. faecalis oral infection, a model used for sepsis stemming from bacterial translocation (5). Pheno2b-Mφ located in the bacterial translocation sites of mice exposed to 5 Gy of γ-rays were characterized as cells responsible for the increased susceptibility to E. faecalis translocation (5). However, these mice did not die without any infections with external pathogens (5). On the other hand, without any infections with external pathogens, all GIARS-mice exposed to 7-10 Gy of γ-rays died within 2-3 weeks of the γ-irradiation. Therefore, in this study, we tried to reduce the radiation-associated mortality of 7-10 Gy GIARS-mice. Infectious complications shown by bacterial growth in the blood and organs were demonstrated in 7-9 Gy GIARS-mice. After decontamination with an antibiotic mixture, however, these mice (dGIARS-mice) were shown to be resistant against radiation-associated mortalities. In our studies, bacterial load dramatically reduced to 102∼103 CFU/gram ileum of normal mice 2 days after 7 day-decontamination by an antibiotic mixture. Ten days after the end of decontamination, bacterial number in the intestine of these mice recovered up to 1010∼1011 CFU/gram ileum (same as compared to the levels shown before the antibiotic treatment). The composition of gut microbiota seemed to be altered by the antibiotic decontamination. In fact, the majority (70%) of bacteria in the ileum of mice just after the decontamination was shown to be Gram-negative, as compared with that of Gram-positive bacteria (65%) demonstrated in the ileum of normal mice. Gram-negative bacteria (68%) were counted as a majority of microbiota in the ileum of mice 10 days after the decontamination.
Then, mice 10 days after the antibiotic decontamination were exposed to 7 Gy whole body γ-irradiation. In the results, all of these mice did not die for a month, as compared with the same mice without decontamination died within 20 days of the γ-irradiation. These results suggest that the composition of gut microbiota influenced by an antibiotic mixture is not very important factors on the decontamination-associated survival of 7 Gy GIARS-mice. Also, all of the 7 Gy dGIARS-mice died when they were subjected to a model of sepsis caused by bacterial translocation. These results indicate that the mortality of 7 Gy GIARS-mice is associated with bacterial translocation. In addition, we measured the endotoxin levels in the sera of normal mice and 7 Gy GIARS-mice using a LAL Chromogenic Endotoxin Quantitation Kit. The concentration of endotoxin in the sera of normal mice was 0.65 to 1.1 EU/ml, while 1.4, 5.0 or 12 EU/ml of endotoxin was detected in the sera of 7 Gy GIARS-mice 4, 7 or 10 days post-irradiation. These results indicate that some infections occur in mice irradiated with γ-rays. Intestinal permeability is well-known to be increased in mice exposed to whole body γ-irradiation. We have previously measured the intestinal permeability of GIARS-mice by the FITC-dextran method. It was increased 5 times in 10 Gy GIARS-mice 7 days post-irradiation, as compared to that of normal mice, while a slight increase of intestinal permeability (1.1 to 1.4-times) was demonstrated in mice 7 Gy GIARS-mice 7 days post-irradiation. From these, the increased intestinal permeability seems to be involved in the bacterial translocation and sepsis developed in mice exposed to higher doses of γ-rays. The Pheno2b-Mφ polarization was occurred in the MLNs of 7-10 Gy GIARS-mice markedly earlier than that in the MLNs of 5 Gy WBI-mice, and it was controlled in these mice by the administration of CCL1 antisense ODN. Pheno2b-Mφ require CCL1 for their prolongation (9), and the ODN suppresses the CCL1 gene expression in Pheno2b-Mφ (5, 6, 9, 17). Therefore, the radiation-associated mortality of 7-9 Gy GIARS-mice was dramatically reduced after treatment with CCL1 antisense ODN.
The mortality of 10 Gy GIARS-mice, however, was not effectively reduced by the ODN treatment. It has been shown that the lethal doses of γ-irradiation cause the depression of intestinal stem cells (24-26), which exist at the intestinal crypt base. The loss of self-renewing crypt/villus units causes the diminished length of villi in the intestine. As such, the number of crypts and the length of villi were severely reduced in the ileum of 10 Gy GIARS-mice 7 days post-irradiation, as compared to those of the ileum in normal mice. Such gastrointestinal damage (GI damage) in the ileum of 10 Gy GIARS-mice was not seen in mice exposed to a non-lethal dose (5 Gy) of γ-rays, thereby gut bacteria-associated sepsis was not commonly developed in these mice (5). When 10 Gy GIARS-mice were transplanted with ES-ICs, both the number of crypts and the length of villi recovered. ES-ICs were a mixture of enterocytes, goblet cells, enteroendocrine cells, and Paneth cells, which were differentiated from murine embryonic stem cells (day 3.5 blastocysts from BALB/cJ mice) under the cultivation with a Wnt/β-catenin activator and a Notch signaling pathway inhibitor for 10 days, as reported previously (11). Ten Gy GIARS-mice did not survive even when they were transplanted with ES-ICs alone or treated with CCL1 antisense ODN alone; however, all of the 10 Gy GIARS-mice given both ES-IC transplantation and CCL1 antisense ODN treatment survived for a month post-irradiation. These results indicate that the mortality rates of 7-10 Gy GIARS-mice are reduced by treatment with CCL1 antisense ODN for 7-9 Gy and in combination with ES-IC transplantation for 10 Gy. In the ES-ILC preparation utilized in this study, Lgr5+ intestinal stem cells were not included. To determine the paracrine effects of the ES-IC preparation on the recovery of crypt numbers and the length of villi in the ileum of 10 Gy GIARS-mice, further studies will be needed by the tracking of each cell component in ES-ICs after transplantation.
In our previous studies (5, 6, 10), Pheno1-Mφ distributed in the bacterial translocation sites have been characterized as a major host defense effector cell against infectious complications stemming from gut-associated bacteria. Mφ have been described to be radioresistant (8), highly plastic and undergo reprogramming with the emergence of a spectrum of distinct functional phenotypes in response to diverse signals derived from pathogens and injured/infected tissues (27-31). In our current study, following the stimulation with a bacterial antigen, dNormal mouse Mφ switched to Pheno1-Mφ in cultures, while 7 Gy dGIARS-mouse MLN-Mφ did not. Thus, Pheno2b-Mφ possess very low plasticity. The properties of Pheno2b-Mφ were maintained for 7 days or more in cultures not supplemented with any cytokines or growth factors (9). Pheno2b-Mφ disappeared when CCL1 was depleted from their cultures (9). CCL1 is an essential chemokine for prolonging the life of Pheno2b-Mφ (9). Because Pheno2b-Mφ are CCL1 producer cells (32, 33), they do not change their properties as long as CCL1 is present.
Pheno2b-Mφ were originally demonstrated under co-stimulation of quiescent Mφ via Tolllike receptor (TLR) 4 and Fc receptors together (34). Subsequently, other stimuli including apoptotic neutrophils and PGE2 have been described as stimulators for the Pheno2b-Mφ polarization (35). In the case of fungal infections, several components of the fungal cell wall have been shown as stimulators for the Pheno2b-Mφ polarization via dectin-1 activation (36). Pheno2b-Mφ inhibit the switching of Mφ to Pheno1-Mφ by suppressing the expression of KLF6, a critical molecular switch regulating the Mφ polarization (37). With these stimulations, Mφ influenced by the irradiation were shown to be switched to Pheno2b-Mφ. Mφ influenced by the irradiation expressed miR-222 excessively, and the Pheno2b-Mφ polarization was promoted under the less expression of GAS5 RNA. miR-222 suppressed the GAS5 expression through the activation of the NMD pathway. Thus, the reduced expression of GAS5 RNA was shown to be responsible for the predominance of Pneno2b-Mφ in GIARS-mice. The reduction of GAS5 RNA expression is maintained in Mφ throughout the lifetime (approximately 3 weeks), and mice with Pheno2b-Mφ are susceptible to sepsis caused by gut-associated bacterial translocation. Therefore, we think that the increased susceptibility of the γ-irradiated mice is maintained till the appearance of new Mφ with GAS5 RNA. We have recently demonstrated that the level of HMGB1 increases (5 to 8-fold) in the sera of mice exposed to 7-9 Gy of γ-rays. This protein has been shown to induce miR-222 expression (38) which can bind directly to GAS5 and reduce its RNA level (20). miR-222 expression is minimal in normal mouse Mφ, while the expression of miR-222 increases (4 to 7-fold) in the same Mφ cultured with 100 ng/ml of HMGB1 for 24 h. miR-222 expression was greatly inhibited by glycyrrhizin, an HMGB1 antagonist, in Mφ cultures supplemented with HMGB1. Glycyrrhizin has been proven to bind directly to HMBG1 (Kd approximately 150 μM, as shown by NMR and fluorescence studies) and interfere with the binding of HMGB1 to DNA in living cells (39, 40). Also, the interaction of HMGB1 with its receptors (RAGE and TLR4) is shown to be blocked by glycyrrhizin (41). Therefore, we will hypothesize that HMGB1 plays a role on the induction of miR-222 expression in GIARS-mice. This hypothesis will be examined in the following studies. In our studies, BALB/c mice are more susceptible to radiation-associated injury, as compared with that of C57BL/6 mice. BALB/c mice exposed to 9 Gy of γ-rays died within 2 weeks of the irradiation. However, 20% of C57BL/6 mice did not die after the same γ-irradiation. The BALB/c strain of mice has a double-strand DNA relative repair defect (polymorphism in DNA-dependent protein kinase catalytic subunit) (42), and this defect seems to be involved in the enhanced radiation susceptibility. In this study, miR-222 gene expression in Mφ was stimulated after exposure to a lethal dose of γ-rays and GAS5 NMD was activated by miR-222. This resulted in the reduced GAS5 RNA in Mφ. Because the reduction of GAS5 RNA causes the Pheno2b-Mφ polarization influenced by LPS and immune complex in combination (16), the degree of radiation-induced miR-222 expression may be different between BALB/c mice and C57BL/6 mice. In our future studies, these questions will be examined.
Mφ isolated from a bacterial translocation site organ (MLNs) were utilized in this study. As shown in Supplemental Fig. 4, peritoneal Mφ from 7 Gy GIARS-mice also produced enough amounts of CCL1 and IL-10 (biomarkers of Pheno2b-Mφ) in their cultures. However, Mφ from the spleen of these mice produced a minimum amount of these biomarkers. This indicates that Mφ in the spleen of 7 Gy GIARS-mice are not completely polarized to the M2b phenotype. Mφ in the bacterial translocation site organs seems to be the first host defense effector cells against invaded pathogens. Splenic Mφ may play a role as a host defense effector cell after spreading the invaded bacteria throughout the whole body.
Radiation-associated mast cell hyperplasia and activation have been demonstrated in the rectal tissues of patients treated with radiotherapy (43) and mice exposed 27 Gy of γ-rays on the colorectal region (43). Histamine released from activated mast cells increases vascular permeability. Mast cells and vasoactive intestinal peptide (a peptide hormone) have been described to be involved in the increased translocation of commensal and pathogenic live bacteria in colonic epithelial tissues from patients with irritable bowel syndrome (44). Therefore, we think that mast cells and some gut hormones play a role on the increased bacterial translocation in irradiated victims, in addition to Mφ with the M2b phenotype. To clarify this, further studies will be required.
We have demonstrated that the mortality of 7-9 Gy GIARS-mice was associated with infectious complications stemming from gut microbiota. Pheno2b-Mφ were predominantly isolated from the bacterial translocation sites (MLNs) of these mice. The mortality rates associated with bacterial translocation was markedly reduced in these mice after the elimination of Pheno2b-Mφ. Although 10 Gy GIARS-mice died even after decontamination by the antibiotic mixture or CCL1 antisense ODN treatment, severe GI damage was considered to play a role on their mortalities. GI damage was markedly improved after transplantation with ES-ICs, and all of the 10 Gy GIARS-mice given both CCL1 antisense ODN and ES-ICs in combination survived for month after the irradiation. Infectious complications were not severely developed in 10 Gy GIARS-mice subjected to both ODN treatment and ES-IC transplantation. In conclusion, the mortality rates of 7-10 Gy GIARS-mice are reducible by the CCL1 antisense ODN treatment, with higher Gy levels needing an ES-IC transplantation in addition.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant U01 AI107355 (to F.S.).
Abbreviations in this article
- dGIARS-mice
decontaminated GIARS-mice
- ES-ICs
intestinal lineage cells differentiated from murine embryonic stem cells
- GAS5
growth arrest-specific transcript 5
- GFP
green fluorescent protein
- GIARS-mice
mice with gastrointestinal acute radiation syndrome
- LP
lamina propria
- LPS/IC
a mixture of LPS and immune complex
- miR
microRNA
- MLNs
mesenteric lymph nodes
- ODN
oligodeoxynucleotide
- Pheno2b-Mφ
macrophages with the M2b phenotype
- WBI
whole body γ-irradiation
References
- 1.Leibowitz BJ, Wei L, Zhang L, Ping X, Epperly M, Greenberger J, Cheng T, Yu J. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. doi: 10.1038/ncomms4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naftalin R. Alterations in colonic barrier function caused by a low sodium diet or ionizing radiation. J Environ Pathol Toxicol Oncol. 2001;23:79–97. doi: 10.1615/jenvpathtoxoncol.v23.i2.10. [DOI] [PubMed] [Google Scholar]
- 3.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 4.Brook I, Elliott TB, Ledney GL, Shoemaker MO, Knudson GB. Management of postirradiation infection: lessons learned from animal models. Mil Med. 2004;169:194–197. doi: 10.7205/milmed.169.3.194. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Nakamura K, Cornforth M, Suzuki F F. Role of M2b macrophages on the acceleration of bacterial translocation and subsequent sepsis in mice exposed to whole body 137Cs γ-irradiation. J Immunol. 2012;189:296–303. doi: 10.4049/jimmunol.1200350. [DOI] [PubMed] [Google Scholar]
- 6.Ohama H, Asai A, Ito I, Suzuki S, Kobayashi M, Higuchi K, Suzuki F. M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. Am J Pathol. 2015;185:420–431. doi: 10.1016/j.ajpath.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 8.Heylmann D, Rödel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846:121–129. doi: 10.1016/j.bbcan.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Asai A, Nakamura K, Kobayashi M, Herndon DN, Suzuki F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J Leukoc Biol. 2012;92:859–867. doi: 10.1189/jlb.0212107. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchimoto Y, Asai A, Tsuda Y, Ito I, Nishiguchi T, Garcia MC, Suzuki S, Kobayashi M, Higuchi K, Suzuki F. M2b monocytes provoke bacterial pneumonia and gut bacteria-associated sepsis in alcoholics. J Immunol. 2015;195:5169–577. doi: 10.4049/jimmunol.1501369. [DOI] [PubMed] [Google Scholar]
- 11.Ogaki S, Shiraki N, Kume K, Kume S. Wnt and Notch signals guide embryonic stem cell differentiation into the intestinal lineages. Stem Cells. 2013;31:1086–1096. doi: 10.1002/stem.1344. [DOI] [PubMed] [Google Scholar]
- 12.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 14.Nieto C, Espinosa M. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid. 2003;49:281–285. doi: 10.1016/s0147-619x(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu K, Kogiso M, Kobayashi M, Herndon DN, Suzuki F. Effect of CCL2 antisense oligodeoxynucleotides on bacterial translocation and subsequent sepsis in severely burned mice orally infected with Enterococcus faecalis. Eur J Immunol. 2012;42:158–164. doi: 10.1002/eji.201141572. [DOI] [PubMed] [Google Scholar]
- 16.Ito I, Asai A, Suzuki S, Kobayashi M, Suzuki F. M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Commun. 2017;493:170–175. doi: 10.1016/j.bbrc.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Nishiguchi T, Ito I, Lee JO, Suzuki S, Suzuki F, Kobayashi M. Macrophage polarization and MRSA infection in burned mice. Immunol Cell Biol. 2017;95:198–206. doi: 10.1038/icb.2016.84. [DOI] [PubMed] [Google Scholar]
- 18.Marta GN, Garicochea B, Carvalho AL, Real JM, Kowalski LP. MicroRNAs, cancer and ionizing radiation: where are we? Rev Assoc Med Bras. 2015;61:275–281. doi: 10.1590/1806-9282.61.03.275. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Guo F, Wang P, Hong S, Zhang C. miR-221/222 confers radioresistance in glioblastoma cells through activating Akt independent of PTEN status. Curr Mol Med. 2014;14:185–195. doi: 10.2174/1566524013666131203103147. [DOI] [PubMed] [Google Scholar]
- 20.Yu F, Zheng J, Mao Y, Dong P, Lu Z, Li G, Guo C, Liu Z, Fan X. Long non-coding RNA growth arrest-specific transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism of competing endogenous RNA. J Biol Chem. 2015;290:28286–28298. doi: 10.1074/jbc.M115.683813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Q, Wang N, Qi J, Gu Z, Shen H. Long non-coding RNA-GAS5 acts as a tumor suppressor in bladder transitional cell carcinoma via regulation of chemokine (C-C motif) ligand 1 expression. Mol Med Rep. 2016;13:27–34. doi: 10.3892/mmr.2015.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: to translate or to degrade. EMBO J. 2008;27:471–481. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belew AT, Meskauskas A, Musalgaonkar S, Advani VM, Sulima SO, Kasprzak WK, Shapiro BA, Dinman JD. Ribosomal frameshifting in the CCR5 mRNA is regulated by miRNAs and the NMD pathway. Nature. 2014;512:265–269. doi: 10.1038/nature13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton EB, Jr, Varney TR. Mesenchymal stem cell therapy for acute radiation syndrome: innovative medical approaches in military medicine. Mil Med Res. 2015;2:2. doi: 10.1186/s40779-014-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 1998;78:993–1003. doi: 10.1038/bjc.1998.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berbée M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M M. γ-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU. 1 pathway Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015;53:676–688. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- 29.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–13. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, Van Damme J, Sozzani S, Martini A, Sinigaglia F, Vecchi A, Mantovani A. Differential regulation of chemokine production by Fcγ receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J Leukoc Biol. 2006;80:342–349. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 33.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2016;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 35.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF, Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One. 2013;8:e60086. doi: 10.1371/journal.pone.0060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Date DR Das, Narla G, Simon DI, Jain MK, Mahabeleshwar GH. Kruppel-like transcription factor 6 regulates inflammatory macrophage polarization. J Biol Chem. 2014;289:10318–10329. doi: 10.1074/jbc.M113.526749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, Zicari A, Antonaci A. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012;28:2285–2289. doi: 10.3892/or.2012.2058. [DOI] [PubMed] [Google Scholar]
- 39.Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Sitia G, Iannacone M, Müller S, Bianchi ME, Guidotti LG LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol. 2006;81:100–107. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 41.Zhao F, Fang Y, Deng S, Li X, Zhou Y, Gong Y, Zhu H, Wang W. Glycyrrhizin protects rats from sepsis by blocking HMGB1 signaling. Biomed Res Int. 2017;2017:9719647. doi: 10.1155/2017/9719647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okayasu R, Suetomi K, Yu Y, Silver A, Bedford JS, Cox R, Ullrich RL. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- 43.Blirando K, Milliat F, Martelly I, Sabourin JC, Benderitter M, François A. Mast cells are an essential component of human radiation proctitis and contribute to experimental colorectal damage in mice. Am J Pathol. 2011;178:640–651. doi: 10.1016/j.ajpath.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bednarska O, Walter SA, Casado-Bedmar M, Ström M, Salvo-Romero E, Vicario M, Mayer EA, Keita AV. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153:948–960. doi: 10.1053/j.gastro.2017.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


