Abstract
Objectives
Proteins p27 and c-myc are both key players in the cell cycle. While p27, a tumor suppressor, inhibits progression from G1 to S phase, c-Myc, a proto-oncogene, plays a key role in cell cycle regulation and apoptosis. The objective of our study was to determine the association between expression of c-Myc and the loss of p27 by immunohistochemistry (IHC) in the four major subtypes of breast cancer (BC) (Luminal A, Luminal B, HER2, and Triple Negative) and with other clinicopathological factors in a population of 202 African-American (AA) women.
Materials and Methods
Tissue microarrays (TMAs) were constructed from FFPE tumor blocks from primary ductal breast carcinomas in 202 AA women. Five micrometer sections were stained with a mouse monoclonal antibody against p27 and a rabbit monoclonal antibody against c-Myc. The sections were evaluated for intensity of nuclear reactivity (1–3) and percentage of reactive cells; an H-score was derived from the product of these measurements.
Results
Loss of p27 expression and c-Myc overexpression showed statistical significance with with ER negative (p<0.0001), PR negative (p<0.0001), triple negative (TN) (p<0.0001), grade 3 (p=0.038), and overall survival (p=0.047). There was no statistical significant association between c-myc expression/p27 loss and luminal A/B and Her2 overexpressing subtypes.
Conclusion
In our study, a statistically significant association between c-Myc expression and p27 loss and the triple negative breast cancers (TNBC) was found in AA women. A recent study found that constitutive c-Myc expression is associated with inactivation of the axin 1 tumor suppressor gene. p27 inhibits cyclin dependent kinase2/cyclin A/E complex formation. Axin 1 and CDK inhibitors may represent possible therapeutic targets for TNBC.
Keywords: p27, c-Myc, cyclin and cyclin dependent kinase, Axin I tumor suppressor gene, triple negative breast cancer, African American
Introduction
Breast cancer is the most common cause of cancer morbidity and the second most common cause of cancer mortality in women worldwide. Histologically, breast neoplasia is divided into two major types, ductal and lobular. Molecular classification of ductal breast cancer by gene expression profiling has identified five major subgroups (Luminal A, Luminal B, Her-2, Normal breast like and basal phenotype) that differ in clinical behavior [1,2,3]. Luminal A and B, are estrogen and/or progesterone receptors(ER/PR) hormone receptor positive. They are generally low grade cancers with good prognosis, increased overall survival and can be treated with hormone receptor inhibitors [1,2]. Her2 overexpressing tumors are aggressive, carry poor prognosis, but have available targeted therapy. The treatment of these tumors with trastuzumab (HER2 inhibitor) has significantly improved prognosis. The triple negative breast cancers (TNBC), tumors lacking expression of ER, PR and HER2 receptors, are generally high grade ductal cancers with established aggressive clinical course, high proliferative index, decreased overall survival and increased incidence of distant metastasis[1,2]. They might be resistant to conventional chemotherapy. Currently, no targeted therapy is available for these aggressive tumors. The basaI-like TNBC subtype expresses CK5. However, all the TNBC are not basal type and vice versa.
Recent studies have shown that cell cycle dysregulation plays an important role in the pathogenesis of TNBC [17,18]. Still, the significance of c-Myc expression, p27 loss and cell cycle dysregulation in breast carcinogenesis is poorly understood. The high proliferative activity of TNBC supports the upregulation of cell cycle driver genes and the downregulation of cell cycle inhibitors as potential pathogenetic mechanisms.
In particular, c-Myc is a proto-oncogene, located on chromosome 8, that regulates the expression of many target genes involved in cell growth, cell cycle regulation, and apoptosis [14,32]. Constitutive expression of c-Myc can result in uncontrolled cell proliferation. C-Myc activation promotes formation of cyclin A/E and cyclin dependent kinase 2 complex (CDK2 and cyclinA/E), which are critical for progression from the G1 to the S phase of the cell cycle. It also downregulates p21; this inhibits progression from the G2 to the M phase [17]. A recent study has found that c-Myc stabilization by selective phosphorylation results in c-Myc with enhanced oncogenic activity due to inactivation of the axin 1 tumor suppressor gene, an important regulator of survival, growth, and stress pathways [40,41]. Protein p27 (cyclin-dependent kinase inhibitor 1B) is a tumor suppressor protein, encoded by the CDKN1B gene. It inhibits formation of CDK2/cyclin A/E complex and prevents progression of the cell cycle from the G1 phase to the S phase [9].
Notably, Breast cancers in African American (AA) women present at a younger age, have a higher grade and stage at diagnosis, and are associated with a higher mortality [38,39]. We hypothesize that the inverse expression of c-myc with p27 could be associated with more adverse and aggressive clinical phenotypes. However, no study has specifically investigated the combined expression of c-Myc and p27 in breast cancer in AA women, who manifest an increased incidence of TNBCs. For this study, we compared the immunohistochemical expression of c-Myc and p27 in the four major subtypes of breast cancer (BC) (Luminal A, Luminal B, HER2, and Triple Negative), and determined that association with other clinicopathological features including grade, stage, disease-free, and overall survival in a population of 202 AA women.
Materials and Methods
Tissue Samples
This study was reviewed and formally exempted by the Howard University Institutional Review Board (IRB-10-MED-24). We analyzed invasive breast ductal carcinomas (IDCs) from 202 AA women diagnosed and treated at the Howard University Hospital between 2000 and 2010. Demographic and clinical information was obtained through the Howard University Cancer Center Tumor Registry.
Tissue Microarrays
A series of tissue microarrays (TMAs) was constructed containing the consecutive primary IDCs (Pantomics, Inc., Richmond, CA). The TMAs consisted of 10 × 16 arrays of 1.0-mm tissue cores from well preserved, morphologically representative tumor cells in archived, formalin-fixed, paraffin-embedded (FFPE) surgical blocks. A precision tissue arrayer (Beecher Instruments, Silver Spring, MD) with two separate core needles for punching the donor and recipient blocks was used. The device also had a micrometer-precise coordinate system for tissue assembly on a multitissue block. Two separate tissue cores of IDC represented each surgical case in the TMA. Each tissue core was assigned a unique TMA location number, which was subsequently linked to an Institutional Review Board-approved database containing demographic and clinical data. Using a microtome, 5-μm sections were cut from the TMA blocks and mounted onto Superfrost Plus microscope slides.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on TMA sections of FFPE tumor tissue. The polymer-HRP system was utilized for immunostaining. Following deparaffinization and rehydration of the tissue sections, heat-induced epitope retrieval at pH 9.0 was performed. Five micrometer sections were stained with mouse monoclonal antibodies against p27Kip1 (SX53G8, Cell Marque, Rocklin, CA). Additional five micrometer sections were stained with a rabbit monoclonal antibody against c-Myc (EP121, Cell Marque, Rocklin, CA). Primary antibody detection was carried out using a polymer-based detection system with staining development achieved by incubation with 3,3′-diaminobenzadine (DAB) and DAB Enhancer (Envision Plus, DAKO, Carpinteria, CA). IHC staining was performed at Quest Diagnostics (Chantilly, VA). Immunohistochemical stains were scored by two independent observers (TN and FK) blinded to the clinical outcome. The sections were evaluated for intensity of nuclear reactivity (1–3) and percentage of reactive cells. The results were entered into a secure research database. An H-score was derived from the product of these measurements. Cases were categorized as having decreased (score ≤50) or increased (score >50) nuclear expression.
Breast subtypes were defined using immunohistochemical expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 based on criteria established in the literature. Luminal A was characterized by strong expression of ER and PR (H-score ≥200), Ki-67 proliferation <14%, and HER2 negativity. Luminal B was characterized by Ki-67 proliferation ≥14% and often weaker expression of ER/PR (H-score <200). Luminal B cases were HER2-negative or HER2 positive. The HER2 subtype was hormone receptor-negative with only HER2 positivity. The triple-negative subtype lacked expression of ER, PR, and HER2.
Statistical Analysis
To determine the association between c-MYC, p27 and ER, PR, HER2, subtype, grade, stage, age, overall survival, and recurrence, bivariate analysis was performed using the chi square test or Fisher’s exact test, as appropriate. To examine the correlation between variables, overall survival, and disease-free survival, Kaplan-Meier survival analyses were carried out. Estimates were considered statistically significant for two-tailed values of P< 0.05. All analyses were carried out using the SPSS 22.0 statistical program (SPSS Inc., Chicago, IL).
Results
Characteristics of the Study Population
Clinical and pathological characteristics of the study population are summarized in Table 1. There were 202 patients diagnosed with infiltrating ductal carcinomas, with adequate FFPE tumor tissue for analysis, at our institution from 2000 to 2010. The majority of the tumor blocks (67.8%) came from women over the age of 50 (mean=57.65, SD=13.03), most of whom (76.2%) had no cancer recurrence. In this population, 43%, 47.7%, and 13.9% of tumors were ER−, PR−, and HER2-positive, respectively. The most prevalent breast cancer subtype was luminal A (ER+ or PR+, HER2−; 43.5%), followed by triple-negative (ER−, PR−, HER2−; 33.7%). Greater than 70% of all tumors were stage I and II; however, they tended to be of higher grade, with Grade 3 tumors comprising 67.4% of the tumors in the study population.
Table 1.
Clinical and pathologic characteristics of study population
| Characteristic | Number | % |
|---|---|---|
| n=202 | ||
| Age | ||
| <50 | 62 | 32.1 |
| >50 | 131 | 67.8 |
| Estrogen Receptor | ||
| Positive | 110 | 57 |
| Negative | 83 | 43 |
| Progesterone Receptor | ||
| Positive | 92 | 47.7 |
| Negative | 101 | 52.3 |
| HER2 Status | ||
| Positive | 27 | 14 |
| Negative | 166 | 86 |
| Subtype* | ||
| Luminal A | 84 | 43.5 |
| Luminal B | 27 | 14 |
| Her2 | 17 | 8.8 |
| Triple Negative | 65 | 33.7 |
| Pathologic Stage Group | ||
| 1 | 60 | 31.1 |
| 2 | 80 | 41.5 |
| 3 | 40 | 20.7 |
| 4 | 12 | 6.2 |
| Unknown | 1 | 0.5 |
| Grade | ||
| I | 9 | 4.6 |
| II | 54 | 28 |
| III | 130 | 67.4 |
| Recurrence | ||
| None | 147 | 76.2 |
| Loco-regional | 8 | 4.1 |
| Distant | 21 | 10.9 |
| Never Disease free | 8 | 4.1 |
| Unknown | 9 | 4.7 |
+N=202
ER, estrogen receptor; PR, progesterone receptor.
Luminal A: ER+ or PR+, HER2−; luminal B: ER+ or PR+, HER2+; triple-negative: ER−, PR−, HER2−; Her2+: ER−, PR−, HER2+
Immunohistochemistry and Analysis
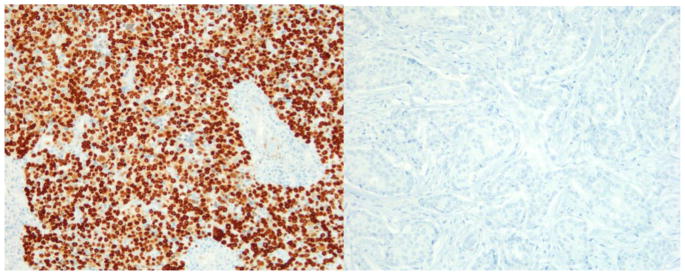
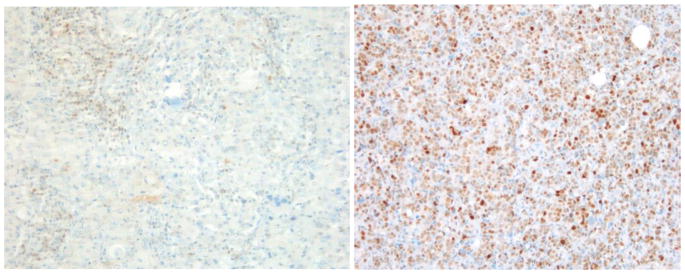
Because some cores were lost in deeper TMA sections, only 197 cases were included in the analysis. The association between clinicopathological features with c-Myc and p27 expression (no expression/co-expression, c-Myc positive/p27 negative and c-Myc negative/p27 positive) can be found in table 2. Loss of p27 expression and c-Myc overexpression showed statistical significance with ER negative (p<0.0001), PR negative (p<0.0001), triple negative (TN) (p<0.0001) and grade 3 (p=0.038) breast cancers in AA women. There was no statistical significant association between c-myc expression/p27 loss and luminal A/B and Her2 overexpressing subtypes. Immunohistochemical detection of c-Myc in triple negative subtype with basal phenotype and luminal A subtype is shown in figures 1a and 1b, respectively. The triple negative subtype shows strong and diffuse expression of c-Myc and the luminal A subtype shows no expression of c-Myc. p27 expression in triple negative subtype with basal phenotype and luminal A subtype is shown in figures 2a and 2b, respectively. The triple negative subtype shows no expression of p27and the luminal A subtype shows strong expression of p27. No association was seen between c-Myc expression/p27 loss and age, tumor size, node status, or stage.
Table 2.
Association between ER, PR, molecular subtype, grade, cMyc, and p27 co-expression
| No expression or co expression | % | p27+ cMyc − | % | p27− cMyc + | % | p-Value | |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
| Estrogen Receptor Expression | |||||||
| Negative | 28 | 50.91% | 12 | 21.82% | 15 | 27.27% | |
| Positive | 33 | 55.93% | 25 | 42.37% | 1 | 1.69% | <0.0001 |
| Progesterone Receptor Expression | |||||||
| Negative | 35 | 55.56% | 13 | 20.63% | 15 | 23.81% | |
| Positive | 26 | 50.98% | 24 | 47.06% | 1 | 1.96% | <0.0001 |
| Breast Cancer Molecular Subtype | |||||||
| Luminal A | 24 | 54.55% | 20 | 45.45% | 0 | 0.00% | |
| Luminal B | 10 | 62.50% | 5 | 31.25% | 1 | 6.25% | |
| HER 2+ | 12 | 75.00% | 2 | 12.50% | 2 | 12.50% | |
| Triple Negative Breast Cancer | 15 | 39.47% | 10 | 26.32% | 13 | 34.21% | <0.0001 |
| Grade | |||||||
| I | 2 | 50.00% | 2 | 16.67% | 0 | 0.00% | |
| II | 21 | 60.00% | 14 | 40.00% | 0 | 0.00% | |
| III | 38 | 50.67% | 21 | 28.00% | 16 | 21.33% | 0.038 |
Figure 1.
a) Triple negative subtype with basal-like phenotype showing diffuse and strong nuclear expression of c-Myc (X200); b) Luminal A subtype showing no expression of c-Myc (X200)
Figure 2.
a) Triple negative subtype with basal-like phenotype showing loss of expression of p27 (X200); b) Luminal A subtype showing strong expression of p27 (X200)
Discussion
Our study evaluated c-Myc and p27 expression in breast cancers in a minority population of AA women. Our results showed a significant association of c-Myc overexpression and loss of p27 in TNBCs (P<0.0001) in AA females with high sensitivity, specificity, PPV, and NPV. These findings further support the results of previous studies, which have linked either c-Myc overexpression or p27 loss to breast cancers with negative-hormone-receptor and basal-like phenotypes. No study has specifically investigated the combined expression of c-Myc and p27 in breast cancer in AA women. In our study, a statistically significant association was found between p27 loss and c-Myc overexpression in TNBCs with decreased overall survival. Earlier studies also showed worse prognosis and decreased disease-free and overall survival in c-Myc expressing breast cancers. Our study showed the association between c-Myc overexpression and p27 loss in TNBC in AA women and establishes the combined role of these key cell cycle modulators in these tumors.
Biological differences are found between breast cancers in AA and Caucasian women. In United States, the incidence of breast cancer is rising in AA women since 2008 and the overall incidence is equal in AA and Caucasian women since 2012 [37]. The breast cancer mortality is high in AA women with lower five year survival rate (77%) as compared to Caucasian women (90%) [38]. TNBC is more frequent in premenopausal AA women (39%) compared to either postmenopausal AA Women (14%) or in non-African Americans of any age (16%) [39]. More rapid progression and decreased survival in AA women may be attributed to the higher incidence of TNBCs in AA women. Approximately 70% of TNBCs are basal-like, characteristically displaying a stem cell-like phenotype, high proliferative activity, and increased incidence of lymph node and distant metastasis. In our study, 67% of TNBCs expressed CK5, a basal marker.
C-Myc, a strong proto oncogene, encodes a nuclear transcription factor that regulates expression of many genes involved in cell cycle progression, cell growth, and apoptosis through binding on Enhancer Box sequences and recruiting histone acetyltransferases [14,32]. c-Myc drives the cell cycle by promoting progression from G1 to S phase and G2 to M phase. G1 to S phase progression is facilitated by activating CDK2 and cyclin A/E complex formation, which leads to phosphorylation of the retinoblastoma protein (Rb); this causes inactivation of the Rb protein bypassing a critical checkpoint of the cell cycle [17,18,19]. G2 to M phase progression is facilitated by downregulating the p21 gene, that inhibits progression in this phase of cell cycle. A recent study found that constitutive c-Myc expression is associated with inactivation of the axin 1 tumor suppressor gene [40,41]. c-Myc is a multifunctional protein that promotes cell growth and also regulates apoptosis via downregulation of the BCL2 protein. Our study demonstrates constitutive expression of c-Myc in TNBCs when compared to luminal A and B tumors in AA women; this finding suggests that cell cycle dysregulation may play a significant role in the pathogenesis of these cancers. TNBCs are highly aggressive tumors with increased incidence of distant metastasis. They are resistant to currently available targeted therapy since they lack hormone and HER2 receptors. Axin 1 tumor suppressor gene or other target genes regulated by c-Myc may represent potential therapeutic targets for TNBCs in AA women [7].
P27 is a tumor suppressor gene that inhibits progression from G1 to S phase of cell cycle. It inhibits the formation of CDK2 and cyclin A/E complexes and keeps Rb protein in a hypophosphorylated state [9,18,21]. Loss of p27 suggests loss of this critical checkpoint of the cell cycle. Our study demonstrates loss of p27 in TNBCs when compared to luminal A and B tumors in AA women. Cyclin dependent kinase inhibitors may represent potential therapeutic targets for TNBCs in AA women.
In summary, our study shows significant association between c-Myc overexpression and p27 loss in TNBCs, a subtype, which correlates with progression and decreased survival, in AA women. TNBCs often have an aggressive clinical course, high proliferative activity, increased incidence of distant metastasis, and are resistant to targeted therapy due to lack of hormone and HER2 receptors. Breast cancers have heterogeneous carcinogenesis at the molecular level. Identification and understanding of molecular pathogenesis that underlie TNBC is critical to develop therapeutic targets. Cell cycle dysregulation with increased proliferative activity is central to the pathogenesis of TNBC. c-Myc overexpression and p27 loss cause uncontrolled activation of the cell cycle through several mechanisms. Axin 1 tumor suppressor gene stabilizers and cyclin dependent kinase inhibitors may represent potential molecular targets for therapy in triple-negative and hormone-negative breast cancers, occurring with increased incidence in minority populations.
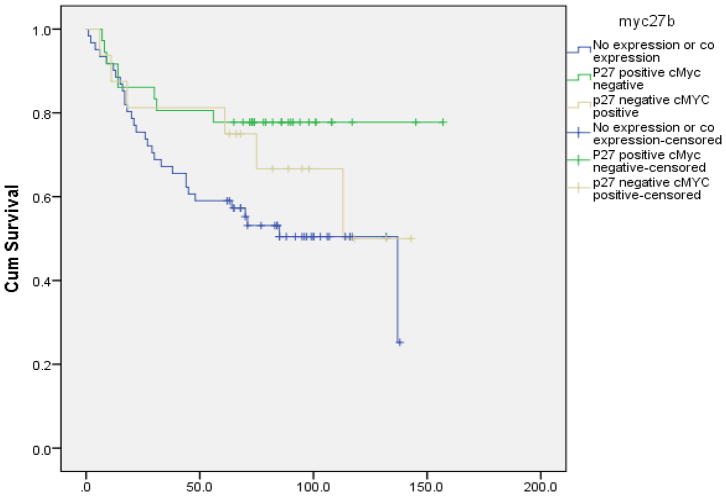
Figure 3.
Overall survival analysis of p27 and cMyc expression
Table 3.
Mean survival for p27 and cMYC expression
| Meana | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | P-Value | ||
| Lower Bound | Upper Bound | ||||
|
|
|
|
|
|
|
| No expression or co expression | 84.82 | 7.23 | 70.65 | 98.99 | |
| P27 positive cMyc negative | 126.81 | 9.50 | 108.19 | 145.42 | |
| p27 negative cMYC positive | 102.58 | 13.32 | 76.48 | 128.69 | 0.047b |
|
| |||||
| Overall | 103.754 | 6.345 | 91.318 | 116.19 | |
Estimation is limited to the largest survival time if it is censored;
Log Rank Chi-square=6.136 (2df)
Acknowledgments
Financial Support: This project has been funded in whole or in part with Federal funds (UL1RR031975) from the National Center for Research Resources (NCRR), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise,” (G12 RR003048) from the RCMI Program at Howard University, Division of Research Infrastructure, National Center for Research Resources, NIH and (U54 CA091431) the Howard University Cancer Center/Johns Hopkins Cancer Center Partnership, National Cancer Institute, NIH.
We would like to acknowledge Pantomics, Inc. for constructing the TMAs and Quest Diagnostics for performing the immunohistochemical staining of the TMAs.
The project is approved by IRB Howard University/Cancer Center.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charafe-Jauffret E, Ginestier C, Monville F, et al. How to best classify breast cancer: conventional and novel classifications (review) Int. J Oncol. 2005;27(5):1307–1313. [PubMed] [Google Scholar]
- 4.Corzo C, Corominas JM, Tusquets I, et al. The MYC oncogene in breast cancer progression: from benign epithelium to invasive carcinoma. Cancer Genet Cytogenet. 2006;165(2):151–156. doi: 10.1016/j.cancergencyto.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Deming SL, Nass SJ, Dickson RB, Trock BJ. C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. Br J Cancer. 2000;83:1688–1695. doi: 10.1054/bjoc.2000.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 7.Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16(4):318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27Kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama KI, Hatakeyama S, Nakayama K. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem Biophys Res Commun. 2001;282:853–860. doi: 10.1006/bbrc.2001.4627. [DOI] [PubMed] [Google Scholar]
- 10.Yang HY, Zhou BP, Hung MC, Lee MH. Oncogenic signals of Her-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J Biol Chem. 2000;275:24735–24739. doi: 10.1074/jbc.C000147200. [DOI] [PubMed] [Google Scholar]
- 11.Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, Vinci F, Chiapetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 12.Cowling VH, Cole MD. Mechanism of transcriptional activation by the Myc oncoproteins. Semin Cancer Biol. 2006;16:242–252. doi: 10.1016/j.semcancer.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Blancato J, Singh B, Liu A, Liao DJ, Dickson RB. Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer. 2004;90(8):1612–1619. doi: 10.1038/sj.bjc.6601703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robanus-Maandag EC, Bosch CA, Kristel PM, et al. Association of C-MYC amplification with progression from the in situ to the invasive stage in C-MYC-amplified breast carcinomas. J Pathol. 2003;201(1):75–82. doi: 10.1002/path.1385. [DOI] [PubMed] [Google Scholar]
- 16.Scorilas A, Trangas T, Yotis J, Pateras C, Talieri M. Determination of c-myc amplification and overexpression in breast cancer patients: evaluation of its prognostic value against c-erbB-2, cathepsin-D and clinicopathological characteristics using univariate and multivariate analysis. Br J Cancer. 1999;81(8):1385–1391. doi: 10.1038/sj.bjc.6693404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiuchi D, Kusdra L, Huskey NE, Chandriani S, Lenburg ME, Gonzalez-Angulo AM, Creasman KJ, Bazarov AV, Smyth JW, Davis SE, Yaswen P, Mills GB, Esserman LJ, Goga A. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. doi: 10.1084/jem.20111512. Published March 19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Tsihlias J, Kapusta L, Slingerland J. The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med. 1999;50:401–423. doi: 10.1146/annurev.med.50.1.401. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Rosai J, Nguyen HT, Capodieci P, Cordon-Cardo C, Koff A. p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc Natl Acad Sci USA. 1999;96:6382–6387. doi: 10.1073/pnas.96.11.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Jin G, Hu Z, Zhai X, Chen W, Wang S, Wang X, Qin J, Gao J, Liu J, Wei Q, Shen H. Variant genotypes of CDKN1A and CDKN1B are associated with an increased risk of breast cancer in Chinese women. Int J Cancer. 2006;119:2173–2178. doi: 10.1002/ijc.22094. [DOI] [PubMed] [Google Scholar]
- 23.Adhikary S, Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 24.Pelengaris S, Khan M. The many faces of c-MYC. Arch Biochem Biophys. 2003;416(2):129–136. doi: 10.1016/s0003-9861(03)00294-7. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22(56):9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso C, Glendon G, Anson-Cartwright L, Shi EJ, Andrulis I, Knight J. Ethnicity, but not cancer family history, is related to response to a population-based mailed questionnaire. Ann Epidemiol. 2004;14:36–43. doi: 10.1016/s1047-2797(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 27.Connolly J, Kempson R, LiVolsi V, Page D, Patchefsky A, Silverberg S. Recommendations for the reporting of breast carcinoma. Association of Directors of Anatomic and Surgical Pathology. 2004 [Google Scholar]
- 28.Lagios MD, Margolin FR, Westdahl PR, Rose MR. Mammographically detected duct carcinoma in situ. Frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer. 1989;63:618–624. doi: 10.1002/1097-0142(19890215)63:4<618::aid-cncr2820630403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.NCCN clinical practice guidelines in oncology. Breast Cancer. 2010:2. [Google Scholar]
- 30.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology. 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Chen Y, Olopade O. MYC and Breast Cancer. Genes Cancer. 2010 Jun;1(6):629–640. doi: 10.1177/1947601910378691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 34.Escot C, Theillet C, Lidereau R, et al. Genetic alteration of the c-myc protooncogene (MYC) in human primary breast carcinomas. Proc Natl Acad Sci USA. 1986;83(13):4834–4838. doi: 10.1073/pnas.83.13.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayberry RM, Stoddard-Wright C. Breast cancer risk factors among black women and white women: similarities and differences. Am J Epidemiol. 1992;136:1445–1456. doi: 10.1093/oxfordjournals.aje.a116465. [DOI] [PubMed] [Google Scholar]
- 36.Mayberry RM. Age-specific patterns of association between breast cancer and risk factors in black women, ages 20 to 39 and 40 to 54. Ann Epidemiol. 1994;4:205–213. doi: 10.1016/1047-2797(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 37.DeSantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. doi: 10.3322/caac.21320. http://onlinelibrary.wiley.com/doi/10.3322/caac.21320/epdf. [DOI] [PubMed]
- 38.American Cancer Society. Cancer Facts and Figures for African Americans 2009–2010. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 39.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Farrell AS, Daniel CJ, Arnold H, Scanlan C, Laraway BJ, Janghorban M, Lum L, Chen D, Troxell M, Sears R. Mechanistic insight into Myc stabilization in breast cancer involving aberrant Axin1 expression. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2790–5. doi: 10.1073/pnas.1100764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold HK, et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28(5):500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein–protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 44.Grushko TA, Dignam JJ, Das S, et al. MYC is amplified in BRCA1-associated breast cancers. Clin Cancer Res. 2004;10(2):499–507. doi: 10.1158/1078-0432.ccr-0976-03. [DOI] [PubMed] [Google Scholar]
- 45.Boyd KE, Farnham PJ. Identification of target genes of oncogenic transcription factors. Proc Soc Exp Biol Med. 1999;222(1):9–28. doi: 10.1111/j.1525-1373.1999.09992.x. [DOI] [PubMed] [Google Scholar]
- 46.Philipp-Staheli J, Kim KH, Payne SR, Gurley KE, Liggitt D, Longton G, Kemp CJ. Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell. 2002;1:355–368. doi: 10.1016/s1535-6108(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 47.Pruitt K, Der CJ. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 2001;171:1–10. doi: 10.1016/s0304-3835(01)00528-6. [DOI] [PubMed] [Google Scholar]
- 48.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 49.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. Akt/PKB mediates cell cycle progression by phosphorylation of p27kip1 on threonine157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 50.Singh SP, Lipman J, Goldman H. Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 51.Sotillo R, Dubus P, Martin J, de la Cueva E, Ortega S, Malumbres M, Barbacid M. Wide spectrum of tumors in knock-in mice carrying a cdk4 protein insensitive to Ink4 inhibitors. EMBO J. 2001;20:6637–6647. doi: 10.1093/emboj/20.23.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St Croix B, Florenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS. Impact of the cyclin-dependent kinase inhibitor p27Kip1 on resistance of tumor cells to anticancer agents. Nat Med. 1996;2:1204–1210. doi: 10.1038/nm1196-1204. [DOI] [PubMed] [Google Scholar]
- 53.Supriatno HK, Hoque MO, Bando T, Yoshida H, Sato M. Overexpression of p27(Kip1) induces growth arrest and apoptosis in an oral cancer cell line. Oral Oncol. 2002;38:730–736. doi: 10.1016/s1368-8375(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 54.Alles MC, Gardiner-Garden M, Nott DJ, Wang Y, Foekens JA, Sutherland RL, Musgrove EA, Ormandy CJ. Meta-analysis and gene set enrichment relative to er status reveal elevated activity of MYC and E2F in the “basal” breast cancer subgroup. PLoS ONE. 2009;4:e4710. doi: 10.1371/journal.pone.0004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vennstrom B, Sheiness D, Zabielski J, Bishop JM. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aulmann S, Adler N, Rom J, Helmchen B, Schirmacher P, Sinn HP. c-myc amplifications in primary breast carcinomas and their local recurrences. J Clin Pathol. 2006;59(4):424–428. doi: 10.1136/jcp.2005.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bieche I, Laurendeau I, Tozlu S, et al. Quantitation of MYC gene expression in sporadic breast tumors with a real-time reverse transcription-PCR assay. Cancer Res. 1999;59(12):2759–2765. [PubMed] [Google Scholar]
- 58.Chrzan P, Skokowski J, Karmolinski A, Pawelczyk T. Amplification of c-myc gene and overexpression of c-Myc protein in breast cancer and adjacent non-neoplastic tissue. Clin Biochem. 2001;34(7):557–562. doi: 10.1016/s0009-9120(01)00260-0. [DOI] [PubMed] [Google Scholar]
- 59.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol. 2005;58:225–236. doi: 10.1136/jcp.2003.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 61.Blain SW, et al. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3(2):111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 62.Achenbach TV, Slater EP, Brummerhop H, Bach T, Muller R. Inhibition of cyclin-dependent kinase activity and induction of apoptosis by preussin in human tumor cells. Antimicrob Agents Chemother. 2000;44:2794–2801. doi: 10.1128/aac.44.10.2794-2801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone MV, Chiappetta G, Vento MT, Spiezia S, et al. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Invest. 1999;104:856–874. doi: 10.1172/JCI6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carneiro C, Jiao MS, Hu M, Sharrer D, Vidal A, Park M, Pandolfi PP, Cordon-Cardo C, Koff A. p27 deficiency desensitizes Rb−/− cells to signals that trigger apoptosis during pituitary tumor development. Oncogene. 2002;22:361–369. doi: 10.1038/sj.onc.1206163. [DOI] [PubMed] [Google Scholar]
- 65.Ciaprrone M, Yamamoto H, Yao Y, Sgambato A, Cattorett E, Tomita N, Monden T, Rotterdam H, Weinstein IB. Localization and expression of p27Kip1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58:114–122. [PubMed] [Google Scholar]
- 66.Cipriano SC, Chen L, Burns KH, Koff A, Matzuk MM. Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol Endocrinol. 2001;15:985–986. doi: 10.1210/mend.15.6.0650. [DOI] [PubMed] [Google Scholar]
- 67.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massagué J, Scher HI. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- 68.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27 Kip1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 69.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai L, Broudy V, Perlmutter RM, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 70.Hershko D, Bornstein G, Ben-Izhak O, Carrano A, Pagano M, Krausz MM, Hershko A. Inverse relation between levels of p27Kip1 and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer. 2001;91:1745–1751. doi: 10.1002/1097-0142(20010501)91:9<1745::aid-cncr1193>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 71.Hurteau JA, Allison BM, Brutkiewicz SA, Boebl MG, Heilman DK, Bigsby RM, Harrington MA. Expression and subcellular localizationof the cyclin-dependent kinase inhibitor p27(KLp1) in epithelial ovariancancer. Gynecol Oncol. 2001;83:292–298. doi: 10.1006/gyno.2001.6376. [DOI] [PubMed] [Google Scholar]
- 72.Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H, Sun H. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr Biol. 2001;11:263–267. doi: 10.1016/s0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 73.Marins CP, Berns A. Loss of p27Kip1 but not p21Cip1 decreases survival and synergizes with MYC in murine lymphomagenesis. EMBO J. 2002;21:3739–3748. doi: 10.1093/emboj/cdf364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naidu R, Wahab NA, Yadav M, Kutty MK. Protein expression and molecular analysis of c-myc gene in primary breast carcinomas using immunohistochemistry and differential polymerase chain reaction. Int J Mol Med. 2002;9(2):189–196. [PubMed] [Google Scholar]
- 75.Lutterbach B, Hann SR. Overexpression of c-Myc and cell immortalization alters c-Myc phosphorylation. Oncogene. 1997;14(8):967–975. doi: 10.1038/sj.onc.1200920. [DOI] [PubMed] [Google Scholar]
- 76.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 77.Alkarain A, Slingerland J. Deregulation of p27 by oncogenic signaling and its prognostic significance in breast cancer. Breast Cancer Res. 2004;6:13–21. doi: 10.1186/bcr722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schondorf T, Eisele L, Gohring UJ, Valter MM, Warm M, Mallmann P, Becker M, Fechteler R, Weisshaar MP, Hoopmann M. The V109G polymorphism of the p27 gene CDKN1B indicates a worseoutcome in node-negative breast cancer patients. Tumour Biol. 2004;25:306–312. doi: 10.1159/000081396. [DOI] [PubMed] [Google Scholar]
- 79.Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–3986. doi: 10.1158/0008-5472.CAN-03-3900. [DOI] [PubMed] [Google Scholar]
- 80.Al-Kuraya K, Schraml P, Sheikh S, et al. Predominance of high-grade pathway in breast cancer development of Middle East women. Mod Pathol. 2005;18(7):891–897. doi: 10.1038/modpathol.3800408. [DOI] [PubMed] [Google Scholar]
- 81.Rodrik V, Gomes E, Hui L, Rockwell P, Foster DA. Myc stabilization in response to estrogen and phospholipase D in MCF-7 breast cancer cells. FEBS Lett. 2006;580(24):5647–5652. doi: 10.1016/j.febslet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]