Abstract
Thearubigins (TRs) are the major components of black tea, which are formed during the fermentation reactions. Although anti-inflammatory and anti-cancer activities of TRs have been reported, the prepared TRs according to the literature methods still contain many floating peaks. It is puzzling whether the observed activities are from TRs or these floating peaks. Thus, it is urgent to develop a method to prepare pure TRs and redefine them. In the present study, we developed a new method, the combination of caffeine precipitation and Sephadex LH-20 column chromatography, to prepare pure TRs. The floating peaks on the hump of the crude TRs were removed, and pure TRs were prepared. The chemical profile of the floating peaks was established using LC/MS, and the major compounds in this fraction were identified as apigenin glycosides, quercetin glycosides, kaempferol glycosides, theaflavins, theasinensin, and galloylglucoses based on the analysis of their tandem mass spectra and in comparison with literature data. This study will pave the way to further study the chemistry and biological activities of TRs and the health effects of black tea consumption.
Keywords: black tea, thearubigins, caffeine precipitation, Sephadex LH-20 column, flavonoids
1. Introduction
Black tea is one of the most popular beverages in the world, produced from young green shoots of the tea plant (Camellia sinensis) by fermentation [1]. Consumption of black tea has been associated with many health benefits including the prevention of cardiovascular disease [2], cancer [3, 4], and obesity [5]. These effects are attributed to the polyphenol compounds in black tea [6, 7], which include catechins, phenolic acids, theaflavins(TFs) and thearubigins (TRs) [ 8–11].
Aside from TRs, other polyphenols in black tea have been well characterized structurally. TRs were thought to be the major components of black tea which are believed to account for up to 60% of the solids in a black tea infusion [12, 13]. However, the content of TRs was overestimated because of a lack in an effective method to prepare pure TRs. On reverse-phase high performance liquid chromatography (HPLC), the crude TRs appeared as a large Gaussian-shape hump that is overlaid by a number of well-resolved sharp peaks [1, 14]. In the 1960s, Roberts reported the crude TRs as three fractions, SI, SII, and SIa, which showed a broad streak on two-dimensional paper chromatography [15, 16]. In the 1990s, Bailey reinterpreted the issue with division of the crude TRs into groups I, II, and III with the development of HPLC techniques [17, 18]. At that time, Powell began to investigate the TRs via the caffeine precipitation method [19]. During the past sixty years, many scientists have been working on the isolation, purification, and characterization of TRs [1, 18, 20–23]. Most of the reports on the separation of TRs were based on and modified from the Roberts’ method and the caffeine precipitation method. However, Roberts’ method involves many steps and cannot produce pure TRs, and the TRs from the caffeine precipitation method still showed a hump with many floating peaks [20]. Ulf W Stodt prepared the more clean TRs via Amberlite XAD-7 resin and high-speed counter-current chromatography but with low yields [20].
Numerous studies have reported the biological activities of TRs. Murad and colleagues reported that TRs protected against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice [24], and improved the sildenafil-induced delayed gut motility in mice [25]. Halder et al. found that TRs have significant antimutagenic and anticlastogenic effects in Salmonella assay in vitro and in vivo in bone marrow cells of mice [26]. TRs have also been reported to protect against the neuromuscular blocking action of botulinum neurotoxin types A, B, and E by binding with the toxins [27, 28]. However, the TRs used in these studies were prepared based onthe Roberts’ method and the caffeine precipitation method, which are crude TRs and contain many floating peaks. It is puzzling whether the observed activities are from TRs or these floatingpeaks. In the present study, we developed a new method by combination of caffeine precipitation and the Sephadex LH-20 column chromatography to remove the floating peaks and prepare pure TRs. We also analyzed the chemical profile of these floating peaks and elucidated their structures based on the analysis of their tandem mass spectra and in comparison with literature data.
2. Materials and methods
2.1. Chemicals and reagents
Black tea was purchased from Yunnan Province of China in 2016 (Baoshan Changninghong Tea Industry Group Co., LTD, Yunnan, China). Caffeine and Sephadex LH-20 were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). ACS-grade ethanol, acetone, HPLC grade methanol, LC/MS-grade solvents and other reagents were obtained from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Preparation of black tea extract
Black tea was soaked with 70% methanol at room temperature for 4 days and repeated for four times. The extracts were then concentrated by a rotary evaporator under vacuum at 35°C to remove the methanol, and then freeze-dried to obtain the black tea extract (yield: 33% ).
2.3. Preparation of crude TRs
In our study, considering the effects of 1) concentrations of caffeine to precipitate tea polyphenols, 2) the order of decaffeination by chloroform and the partition against ethyl acetate, and 3) the pre-extraction of ethyl acetate before caffeine precipitation on the purity and amount of the TRs, six methods (methods 1, 2, 3, 1′, 2′, and 3′) were used to prepare the crude TRs. Their schematic depictions of the procedure are shown in Figure 1. The only difference between methods 1, 2, and 3 and methods 1′, 2′, and 3′, respectively, is the concentration of caffeine. We used 20 mM caffeine in methods 1, 2, and 3, and 40 mM caffeine in methods 1′, 2′, and 3′. For method 1, TRs were prepared according to the method of caffeine precipitation with modification [1, 23]. Briefly, black tea extract (1 g) was added to boiling water (60 mL) with 20 mM of caffeine, stirred to ensure dissolution (Figure 1). The solutionwas allowed to stand at 4 °C for 2 h, and then centrifuged at 8819 rpm for 10 min to form the precipitate. The precipitate was suspended in boiling water again and extracted by ethyl acetate (100 mL/time, 6 times), which produced the aqueous fraction and the ethyl acetate fraction. The aqueous phase was partitioned against chloroform (100 mL/time, 4 times) to remove caffeine, then the decaffeinated aqueous phase was dried by a rotary evaporator under vacuum at 35 °C, to get the crude TRs fraction. Similarly, in method 2, the caffeine precipitate was suspended in boiling water and extracted by chloroform first, and then ethyl acetate instead of ethyl aceate first and then chloroform in method 1. Then, the aqueous phase was dried by a rotary evaporator to get the crude TRs fraction. While, in method 3, black tea extract was extracted by ethyl acetate, then the aqueous phase was precipitated by caffeine. Thecrude TRs were obtained by decaffeination of the precipitate.
Figure 1.
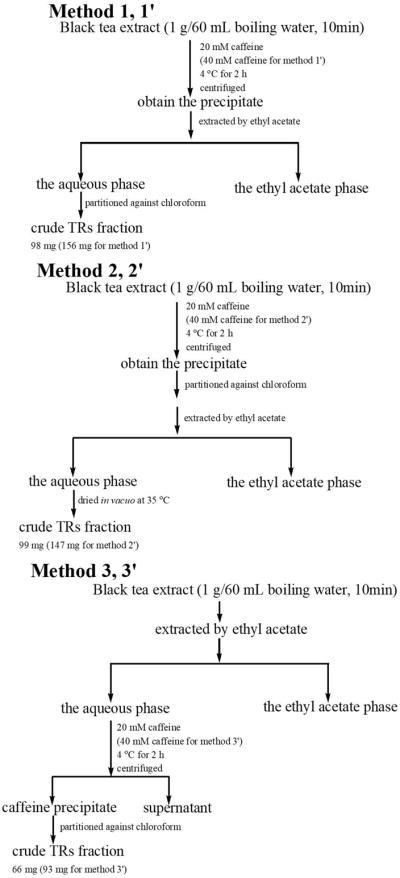
Schematic depictions of the procedure of methods 1, 1′, 2, 2′, 3, and 3′ for preparation of the crude thearubigins (TRs)fraction from black tea extract.
2.4. Separation of the floating peaks from the crude TRs and preparation of pure TRs fractions
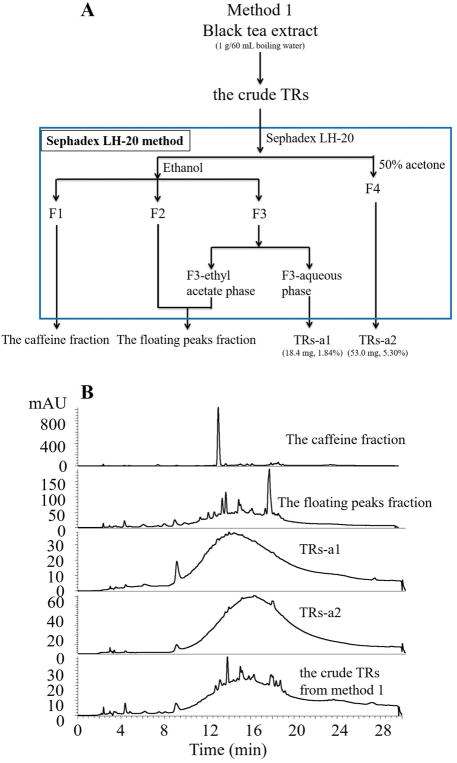
The crude TRs fraction prepared based on method 1 was further chromatographed over Sephadex LH-20 column (38 × 3.2 cm) with ethanol and 50% aqueous acetone as eluents. HPLC-DAD was used to monitor the separation. All fractions containing caffeine were combined and named as the caffeine fraction (F1). Fractions with just the floating peaks were combined as fraction F2. Fractions with a hump with some floating peaks were combined as fraction F3. F3 was partitioned against ethyl acetate to further remove the floating peaks to get the ethyl acetate phase and the aqueous phase. The ethyl acetate phase was combined with F2 to obtain the floating peaks fraction. The aqueous phase was named as the TRs-a1 fraction. Fractions eluted by 50% acetone showed a clean hump and were combined as the TRs-a2 fraction. The detailed procedure was shown in Figure 2A, and the HPLC chromatograms were shown in Figure 2B.
Figure 2.
(A) Flowchart of Sephadex LH-20 method to prepare pure thearubigins (TRs), TRs-a1 and TRs-a2, from the crude TRs fraction obtained from method 1, (B) The HPLC-DAD chromatograms of the caffeine fraction, the floating peaks fraction, TRs-a1 fraction, and TRs-a2 fraction from the crude TRs fraction of method 1, and the crude TRs fraction prepared from method 1.
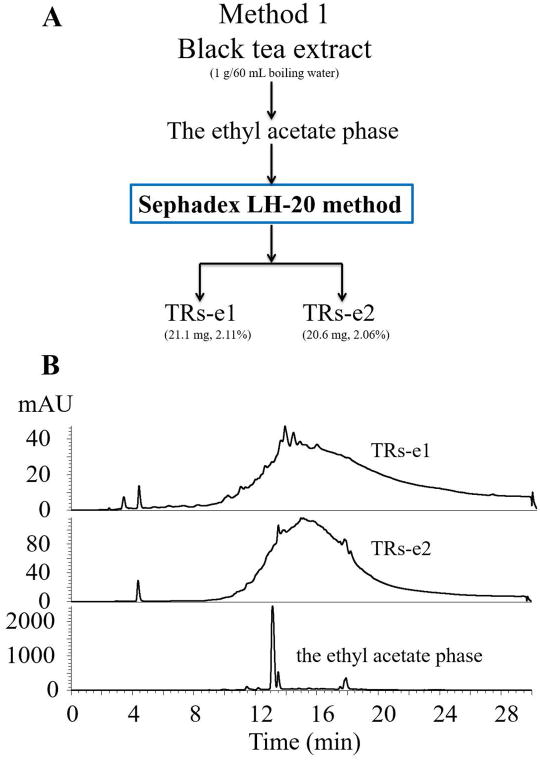
The ethyl acetate fraction obtained from method 1 was also loaded to Sephadex LH-20 column (38 × 3.2 cm) with ethanol and 50% aqueous acetone as eluents following the same process as described above (Figure 2A) to remove caffeine and all the peaks, to get the pure TRs fractions: TRs-e1, and TRs-e2 (Figure 3).
Figure 3.
(A) Flowchart of the modified method to prepare the two thearubigins (TRs) fractions, TRs-e1 and TRs-e2, from the ethyl acetate phase of method 1, (B) The HPLC-DAD chromatograms of the TRs-e1, TRs-e2, and the ethyl acetate phase from method 1.
2.5. New method to prepare TRs
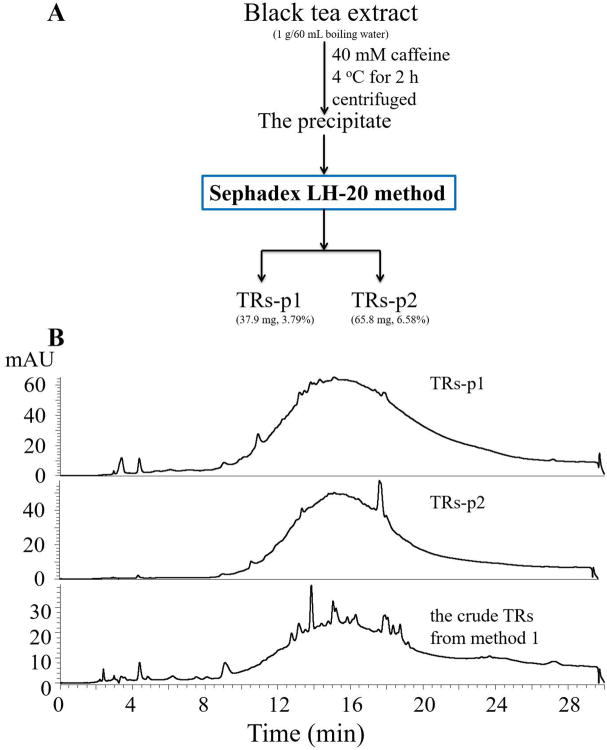
Black tea extract (1 g) was added to boiling water (60 mL) with 40 mM of caffeine, stirred to ensure dissolution (Figure 4A). The solution was allowed to stand at 4 °C for 2 h, and then centrifuged at 8819 rpm for 10 min to form the precipitate. After centrifugation, the precipitant containing TRs was directly suspended in 90% aqueous ethanol, and subjected to Sephadex LH-20 column (38 × 3.2 cm) eluted by ethanol and 50% aqueous acetone according to the method described above (Figure 2A), to remove caffeine and all the known peaks and get the pure TRs fractions: TRs-p1 and TRs-p2 (Figure 4).
Figure 4.
(A) Flowchart of the new method for preparation of pure thearubigins (TRs), TRs-p1 and TRs-p2, from black tea extract, (B) The HPLC-DAD chromatograms of the TRs-p1 and TRs-p2 fraction, and the crude TRs fraction prepared from method 1.
2.6. HPLC-DAD analysis
HPLC analysis was carried out on a Thermo scientific HPLC equipped with Dionex Ultimate 3000 RS Pump, RS Autosampler, and diode array detector (Thermo Fisher Scientific Inc., Waltham, MA, USA). Chromatographic separation was performed using a Luna 5 μm Phenyl-Hexyl C18 column (250 mm × 4.6 mm i.d., 5 μm) (Phenomenex). Mobile phases were composed of 0.1% formic acid in water with 5% methanol (mobile phase A) and 0.1% formic acid in methanol with 5% water (mobile phase B). The flow rate was 1 mL/min, and the linear gradient elution had the following profile: 20% B from 0 to 5 min; 20–100% B from 5 to 20 min; 100% B from 20 to 26.5 min; 100–20% B from 26.5 to 27 min; and then 20% B from 27 to 30 min. The injection volume was 20 μL, and the detection wavelength was set at 278 nm.
2.7. HPLC-MS/MS analysis
LC/MS analysis was performed with a Thermo-Finnigan Spectra System equipped with Dionex Ultimate 3000 degasser, RS Pump, RS Autosample, diode array detector, RS column compartment, an Accela refrigerated autosampler, and an LTQ Velos Pro ion trap mass detector incorporated with heated electrospray ionization interfaces (Thermo Electrom, San Jose, CA, USA). The same column, solvents A and B, and gradient system as shown under section 2.6. HPLC-DAD Analysis were used for the LC/MS analysis. The flow rate was 1 mL/min with 30% imported into the MS detector by a three-way valve. The injection volume was 10 μL. The electrospray ionization interface was operated at negative-ion mode using a nebulizer at approximately 3.6 kV. Nitrogen gas was used as the sheath gas at a flow rate of 34 arb and the aux gas at a flow rate of 10 arb. Optimized parameters, including temperature (300 °C), voltage of the capillary (45 V), and voltage of the tube lens offset (120 V). It was tuned using authentic theaflavin. Selected ion monitoring (SIM) mode was used to search flavonoids. For MS-MSn (n = 2–4) analysis, collision induced dissociation (CID) was conducted using an isolation width of 1.5 Da and the normalized collision energy of 35 values. The mass range was measured from 50 to 2000 m/z. Data acquisition and analysis were performed using Xcalibur 2.0 (Thermo Electron, San Jose, CA, USA).
3. Results and Discussion
3.1. Preparation of crude TRs
To investigate the impacts of 1) concentrations of caffeine to precipitate tea polyphenols, 2) the order of decaffeination by chloroform and the partition against ethyl acetate, and 3) the pre-extraction of ethyl acetate before caffeine precipitation on the purity and amount of the TRs, we tried six different methods to prepare the crude TRs (Figure 1). In the reported caffeine precipitation method [20], 20 mM of caffeine was used. We proposed that higher concentrations of caffeine could precipitate more polyphenols. Therefore, two parallel sets of experiments were taken with 20 mM of caffeine for methods 1, 2, and 3 and 40 mM caffeine for methods 1′, 2′, and 3′. To determine the impact of the order of decaffeination by chloroform and the partition against ethyl acetate to the purity and amount of the crude TRs, the precipitant was extracted by ethyl acetate first, and then the resulting solution was decaffeinated by chloroform in methods 1 and 1′, and in methods 2 and 2′ the precipitant was decaffeinated first, and then the resulting solution was extracted by ethyl acetate (Figure 1). While in methods 3 and 3′, ethyl acetate was used to extract the ethyl acetate soluble TFs-rich fraction out first, and then the remaining ethyl acetate insoluble fraction was precipitated by caffeine to produce crude TRs (Figure 1). Based on the amount of crude TRs generated from each method (1: 98 mg; 1′: 156 mg; 2: 99 mg; 2′: 147 mg; 3: 66 mg; and 3′: 93 mg), we concluded that 1) the 40 mM of caffeine could produce more crude TRs than 20 mM of caffeine; 2) the order of decaffeination by chloroform and the partition against ethyl acetate had no impact on the amount of crude TRs; and 3) pre-extraction using ethyl acetate before caffeine precipitation significantly decreased the amount of crude TRs.
3.2. Separation of the floating peaks from the crude TRs and preparation of pure TRs fractions
The crude TRs prepared based on literature methods has many floating peaks on the TRs hump. We developed the Sephadex LH-20 method to separate caffeine and the floating peaks from the TRs hump. As shown in Figure 2A, the crude TRs obtained from method 1 was loaded to the Sephadex LH-20 column eluted by ethanol to wash out caffeine first as fraction F1, most of the floating peaks as fraction F2, and the TRs hump with some floating peaks on it as fraction F3, and followed by 50% aqueous acetone to generate fraction F4. F3 was further extracted by ethyl acetate to remove the floating peaks from the TRs hump to generate the clean TRs hump named TRs-a1 (1.84%) (Figure 2B). The ethyl acetate phase containing the floating peaks was combined with fraction F2 to obtain the fraction of the floating peaks (Figure 2B). The fraction F4 eluted by 50% acetone contained a clean hump and was named the TRs-a2 fraction (5.30%) ( Figure 2B ).
Using this Sephadex LH-20 method, the ethyl acetate phase obtained from method 1 was also loaded to Sephadex LH-20 column to remove caffeine and all the peaks to generate two pure TRs fractions named TRs-e1 (2.11%) and TRs-e2 (2.06%) (Figure 3). These results indicated that some TRs could be extracted away by ethyl acetate, which explained why the pre-extraction of ethyl acetate before caffeine precipitation (methods 3 and 3′) generated the lowest amount of crude TRs amongst all the methods. TRs-e1 and TRs-e2 could be the TRs fraction that was included in Roberts’ SI fraction, which contains large proportions of catechin monomers and TFs [20].
3.3. New method to prepare TRs
Since the crude TRs fraction could be purified by Sephadex LH-20 column to obtain pure TRs, and some TRs could be extracted away by ethyl acetate, we proposed a simple method to prepare pure TRs by directly loading the caffeine precipitate to Sephadex LH-20 column. The precipitate produced by 40 mM of caffeine was loaded to the Sephadex LH-20 column eluted by ethanol and 50% aqueous acetone according to the Sephadex LH-20 method in Figure 2, to obtain pure TRs-p1 (3.79%) and TRs-p2 (6.58%) which showed a hump in each of their HPLC chromatograms (Figure 4). The total yield of TRs from black tea extract is 10.37%. Compared to the published methods, we used less organic solvents by using ethanol and a small amount of acetone to elute the column, and avoided using toxic chloroform for decaffeination.
3.4. Identification of the floatingpeaks on the crude TRs using LC -MS/MS analysis
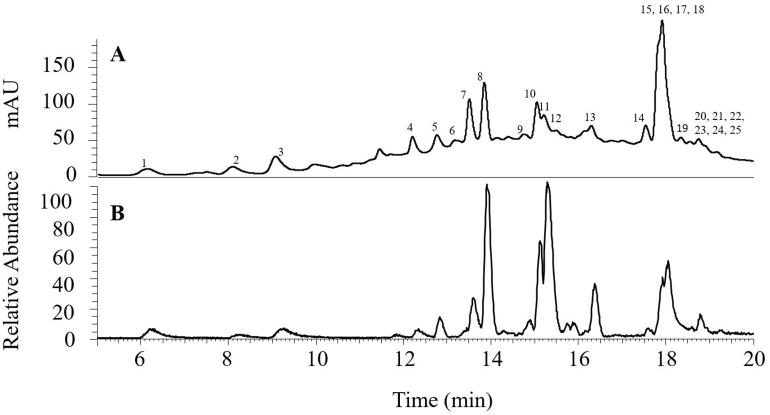
In order to show the crude TRs that was prepared based on the literature method contains many known peaks, we studied the chemical profile of the floating peaks fraction obtained from method 1 by LC/MS with data-dependent acquisition. With data -dependent MS/MS analysis by collecting the MS 2 and MS3 spectra of the most intense ions in the sample, we were able to tentatively identify 25 compounds in this fraction. Then, we used selected-ion-monitoring (SIM) model to conduct the MSn (n=2–4) of these 25 compounds to further confirm their structures. LC chromatograms recorded at 278 nm and extracted from the Total Ion Chromatogram (TIC) are shown in Figure 5. The retention times (tR), deprotonated molecules ([M-H]−), and the major fragment ions are listed in Table 1. Peak identification was based on analysis of their tandem mass spectra and compared to published data in literature [29–37]. Twenty-five compounds were detected and identified as apigenin glycosides, quercetin glycosides, kaempferol glycosides, catechins, theaflavins, theasinensin, and galloylglucoses (Table 1).
Figure 5.
(A) The HPLC-DAD chromatogram of the floating peaks fraction from method 1 and (B) the LC chromatogram of the extracted mass spectrum under Total Ion Chromatogram (TIC).
Table 1.
Mass spectrometric data of possible compounds identified from the resolved peaks*
| No | tR | [M-H] − | MS2 | MS3 | MS4 | compounds |
|---|---|---|---|---|---|---|
| 1 | 6.15 | 761 | 609, 591 | 591, 565, 471 | theasinensin-gallate | |
| 2 | 8.09 | 483 | 331, 313, 271, 211, 169 | 331/169 313/169 | digalloylglucose | |
| 3 | 9.07 | 633 | 615, 481, 463, 421, 331, 301 | 257, 229, 185 | galloyl-HHDP-glucose | |
| 4 | 12.2 | 635 | 483, 465, 423, 313 | 423, 331, 313, 271, 169 | trigalloylglucose | |
| 5 | 12.76 | 593 | 503, 473, 413, 293 | 445, 383, 353, 311 | apigenin-6, 8-C-dihexoside | |
| 6 | 13.44 | 563 | 545, 503, 473, 443, 383, 353 | 425, 383, 353 | apigenin-6-C-glucosyl-8-C- arabinoside | |
| 7 | 13.51 | 441 | 289, 169 | 245, 205 | epicatechin-gallate | |
| 8 | 13.85 | 563 | 545, 503, 473, 443, 383, 353 | 425, 383, 353 | apigenin-6-C-arabinosyl-8-C- glucoside | |
| 9 | 14.75 | 771 | 609, 343, 301 | 343, 301 | quercetin-162-162-146 | |
| 10 | 15.05 | 533 | 515, 473, 443, 383, 353, 311 | 473/413 443/383 | apigenin-6, 8-C-dipentoside | |
| 11 | 15.21 | 609 | 301, 343 | rutin | ||
| 12 | 15.89 | 755 | 593, 285 | kaempferol-3-O-glucosylrutinoside | ||
| 13 | 16.29 | 593 | 447, 285 | 357, 327, 285 | kaempferol-162-146 | |
| 14 | 17.54 | 699 | 681, 427, 409, 383, 289 | 409, 383, 289 | theaflavate B | |
| 15 | 17.91 | 563 | 545, 425, 407, 379 | theaflavin | ||
| 16 | 17.91 | 715 | 563, 545, 527, 407 | theaflavin-monogallate | ||
| 17 | 17.91 | 867 | 715, 697, 527 | theaflavin-digallate | ||
| 18 | 18.13 | 901 | 755 | 609, 591, 301 | 343, 301 | quercetin-146-146-146-162 |
| 19 | 18.34 | 1033 | 887 | 741, 301 | 591, 301 | quercetin-146-146-146-162-132 |
| 20 | 18.6 | 885 | 739, 431 | 593, 453, 285 | 285 | kaempferol-146-146-146-162 |
| 21 | 18.79 | 901 | 755 | 609, 591, 301 | 343, 301 | quercetin-146-146-146-162 |
| 22 | 18.81 | 1017 | 871 | 725, 585, 285 | 575, 285 | kaempferol-146-146-146-162-132 |
| 23 | 18.9 | 1033 | 887 | 741, 301 | 591, 301 | quercetin-146-146-146-162-132 |
| 24 | 19.22 | 885 | 739, 431 | 593, 453, 285 | 285 | kaempferol-146-146-146-162 |
| 25 | 19.25 | 1017 | 871 | 725, 285 | 285 | kaempferol-146-146-146-162-132 |
162: glycosyl or galactosyl, 146: rhamnosyl or p-coumaroyl, 132: arabinosyl
Four C-glycosylated apigenins (peaks 5, 6, 8, and 10, Figure 5 and Table 1) were detected in the floating peaks fraction. Peak 8 had a molecular ion at m/z 563 [M-H]− with fragment ions at m/z 473 (loss of 90 amu) and 443 (loss of 120 amu) indicated that there is a C-hexosyl group [29–31]. The tandem mass spectrum of m/z 443 (MS3: 443/563 [M- H]−) had fragment ion at m/z 383 (loss of 60 amu) which indicated that there is a C-pentosyl group [29–31]. All of these suggested that peak 8 was apigenin with one C- hexoside group and one C-pentosyl group. In addition, we could detect a small peak with the same molecular weight as peak 8 at m/z 563 (peak 6, tR 13.44 min) and similar MS2 data with those of peak 8, indicating peak 6 also contains one C-hexosyl group and one C-pentosyl group. Apigenin-6-C-glucosyl-8-C-arabinoside and apigenin-6-C-arabinosyl-8-C-glucoside have been previously reported in tea, and the retention time of apigenin-6-C-glucosyl-8-C-arabinoside was shorter than that of apigenin-6-C-arabinosyl-8-C-glucoside [32]. Thus, peaks 6 and 8 were tentatively identified as apigenin-6-C-glucosyl-8-C-arabinoside and apigenin-6-C-arabinosyl-8-C-glucoside, respectively. In a similar manner, peak 5 (tR 12.76 min) had a molecular ion at m/z 593 [M-H]− with fragment ions at m/z 473 (loss of 120 amu) and 503 (loss of 90 amu), indicating it was apigenin-6, 8-C-dihexoside. However, we could not determine whether the hexosyl group is glucosyl or galactosyl. Peak 10 (tR 15.05 min) had a molecular ion at m/z53 3 [M-H] − with fragment ions at m/z 473 (loss of 60 amu) and 443 (loss of 90 amu). The tandem mass spectra of the fragment ions m/z 473 and 443 (MS3: 473/533 and 443/533) had the fragment ions at m/z 413 and 383, which lost 60 amu, respectively. All of these features suggested that peak 10 was apigenin-6, 8-C-dipentoside.
Six quercetin O-glucosides (peaks 9, 11, 18, 19, 21, and 23) were detected in the floating peaks fraction. They were identified as quercetin O-glycosides as listed in Table 1, due to the observation of the aglycone ion at m/z 301 (molecular ion of quercetin under negative-ion mode), and the fragment ions which lost 146 amu, 162 amu or 132 amu. Here 146 amu could indicate a rhamnosyl or p-coumaroyl group, 162 amu could suggest a glucosyl or galactosyl group, and 132 amu could represent an arabinosyl group. Without authentic standards, we could not determine the exact structures of these six compounds.
Six kaempferol O-glycosides (peaks 12, 13, 20, 22, 24, and 25) were detected in the floating peaks fraction. Peak 12 (tR 15.89 min) had a molecular ion at m/z 755 [M-H] − with fragment ions at m/z 593 (loss of 162 amu) and 285 (loss of 162, 162 and 146 amu). Kaempferol-3-O-glucosylrutinoside has been reported in tea previously [32]. Thus, peak 12 could be tentatively identified as kaempferol-3-O-glucosylrutinoside. Peak 13 (tR 16.29 min) had a molecular ion at m/z593 [M-H] − with fragment ions at m/z447 (loss of 146 amu) and 285 (loss of 146 and 162 amu), and the tandem mass spectrum of the fragment ion m/z 447 (MS3: 447/593 [M-H] −) had a fragment ion at m/z 285, the loss of 162 amu from m/z 447. These fragments suggested that this peak was kaempferol glycoside, in which the loss of 162 amu could due to a glucosyl or galactosyl group, and 146 amu could due to a rhamnosyl or p-coumaroyl group. In a similar way, peaks 20, 22, 24, and 25 all had the fragment ion at m/z 285, and were provisionally identified as kaempferol glycosides as listed in Table 1.
TFs were also detected from this fraction. Peak 15 (tR 17.91 min) had a molecular ion at m/z 563 [M-H] −, with fragment ions at m/z 545, 425, 407, and 379 which were identified to those of the authentic theaflavin standard obtained in our lab. Similarly, peaks 16 (tR 17.91 min, m/z 715 [M-H] −) and 17 (tR 17.91 min, m/z 867 [M-H] −) were identified as theaflavin-monogallate and theaflavin-digallate based on the comparison with the authentic standards, respectively[ 33].
Three galloylglucoses (peaks 2–4) were detected in the floating peaks fractions. Peak 2 (tR 8.09 min) had a molecular ion at m/z 483 [M-H] − with a fragment ion at m/z 331 (loss of 152 amu), and the fragment ions at m/z 331 and 313 had MS3 fragment ion at m/z 169, indicating that there were two galloyl groups, and this compound could be tentatively identified as digalloyl glucose [34]. Peak 3 (tR 9.07 min) had a molecular ion at m/z 633 [M-H] − with fragment ions at m/z 481 (loss of 152 amu), 463 (loss of 170 amu), and 301 (loss of 332 amu). The fragment of 301 was indicative of an hexahydroxydiphenoyl (HHDP) group [34]. Therefore, this compound could be tentatively identified as galloyl-HHDP-glucose. Peak 4 (tR 12.2 min) had a molecular ion at m/z 635 [M-H] − with fragment ion at m/z 483 (loss of 152 amu), the tandem mass spectrum of m/z 483 (MS3: 483/635 [M-H] −) had fragment ions at m/z 331 and 313, which were similar with the tandem mass spectrum of peak 2, so this compound could be tentatively identified as trigalloylglucose [34].
We also detected a theasinensin, a catechin, and theaflavate B in the floating peaks fraction. Peak 1 (tR 6.15 min) had a molecular ion at m/z 761 [M-H] − with fragment ion at m/z 609 (loss of 152 amu), and the fragment ion at m/z 609 had the characteristic MS3 fragment ion of theasinensins at m/z 471, which lost 138 amu corresponding to a retro Diels-Alder fragmentation [35]. This indicated that the peak 1 could be a theasinensin-gallate. Peak 7 (tR 13.51 min) had a molecular ion at m/z 441 [M-H] − with a fragment ion at m/z 289 (loss of 152 amu) indicated that this compound could be epicatechin-gallate. Peak 14 (tR 17.54 min) had a molecular ion at m/z 699 [M-H] − with a fragment ion at m/z 427 (loss of 272 amu) and 271 (loss of 427 amu), and it was 152 amu less than that of theaflavate A. These indicate that this compound could be theaflavate B [22, 36, 37].
Theaflavins have been reported to have strong anti-inflammatory and anti-cancer activities via the NF-κB and MAPKs pathways [38]. Rutin and other flavonoids have also been shown to have the anti-inflammatory and anti-cancer activities [39, 40]. Altogether, the polyphenols present in the crude TRs could overestimate or underestimate our understanding of the biological activities of TRs.
4. Conclusion
In the present study, we developed a novel method, the combination of caffeine precipitation and Sephadex LH-20 column chromatography, to remove the floating peaks in the crude TRs used in literature and obtain pure TRs. Compared to literature methods, our method is simple, requires less organic solvents, and can be used for large scale preparation of TRs. We have used this method to prepare a large scale of TRs (100 g) to further study how TRs are metabolized by gut microbiota and how microbiome affects the effects of TRs on gastrointestinal health. We also identified the structures of the floating peaks in the crude TRs using LC/MS. Many of these compounds possess certain biological activities, which complicate our understanding of the beneficial health effects of TRs. This study will pave the way to further study the chemistry and biological activities of TRs and the health effects of black tea consumption. Additionally, our results showed that only 10% TRs could be prepared from black tea extract indicating that the amount of TRs was overestimated in the literature.
Highlights.
Different factors on the preparation of crude thearubigins were investigated.
A newmethod was developed to prepare pure thearubigins.
The chemical profile of the floating peaks was established.
Acknowledgments
The authors wish to thank Mr. Hunter Snooks who assisted in the proofreading of the manuscript. This work was supported by NIH R01 grant AT008623 to S. Sang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuhnert N. Unraveling the structure of the black tea thearubigins. Arch Biochem Biophys. 2010;501:37–51. doi: 10.1016/j.abb.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Hartley LC, Flowers N, Clarke A, Stranges S, Hooper L, Rees K. PP10 Green and Black Tea for the Primary Prevention of Cardiovascular Disease (CVD): A Cochrane Systematic Review. J Epidemiol Community Health. 2013;67(Suppl 1):A52–A53. doi: 10.1002/14651858.CD009934.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode AM, Dong Z. Molecular and cellular targets. Mol Carcinog. 2006;45:422. doi: 10.1002/mc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140:446. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo JM, Munekata PES. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac J Trop Biomed. 2016;6:709–719. [Google Scholar]
- 7.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698s–1702s. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- 10.Satoh E, Ishii T, Shimizu Y, Sawamura SI, Nishimura M. Black tea extract, thearubigin fraction, counteract the effects of botulinum neurotoxins in mice. Br J Pharmacol. 2001;132:797–798. doi: 10.1038/sj.bjp.0703883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh E, Ishii T, Shimizu Y, Sawamura SI, Nishimura M. The mechanism underlying the protective effect of the thearubigin fraction of black tea (Camellia sinensis) extract against the neuromuscular blocking action of botulinum neurotoxins. Basic Clin Pharmacol Toxicol. 2002;90:199–202. doi: 10.1034/j.1600-0773.2002.900405.x. [DOI] [PubMed] [Google Scholar]
- 12.Haslam E. Thoughts on thearubigins. Phytochemistry. 2003;64:61–73. doi: 10.1016/s0031-9422(03)00355-8. [DOI] [PubMed] [Google Scholar]
- 13.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Drynan JW, Clifford MN, Obuchowicz J, Kuhnert N. The chemistry of low molecular weight black tea polyphenols. Nat Prod Rep. 2010;27:417–462. doi: 10.1039/b912523j. [DOI] [PubMed] [Google Scholar]
- 15.Roberts EAH, Smith RF. The phenolic substances of manufactured tea. IX. -the spectrophotometric evaluation of tea liquors. J Sci Food Agric. 1963;14:689–700. [Google Scholar]
- 16.Roberts EAH, Cartwright RA, Oldschool MM. The phenolic substances of manufactured tea. I. -Fractionation and paper chromatography of water-soluble substances. J Sci Food Agric. 1957;8:72–80. [Google Scholar]
- 17.Bailey R, Nursten H, McDowell I. Comparative study of the reversed-phase high-performance liquid chromatography of black tea liquors with special reference to the thearubigins. J Chromatogr A. 1991;542:115–128. [Google Scholar]
- 18.Bailey R, Nursten H, McDowell I. Isolation and high-performance liquid chromatographic analysis of thearubigin fractions from black tea. J Chromatogr A. 1994;662:101–112. [Google Scholar]
- 19.Powell C, Clifford MN, Opie SC, Ford MA, Robertson A, Gibson CL. Tea cream formation: The contribution of black tea phenolic pigments determined by HPLC. J Sci Food Agric. 1993;63:77–86. [Google Scholar]
- 20.Stodt UW, Stark J, Engelhardt UH. Comparison of three strategies for the isolation of black tea thearubigins with a focus on countercurrent chromatography. J Food Compos Anal. 2015;43:160–168. [Google Scholar]
- 21.Kuhnert N, Dairpoosh F, Yassin G, Golon A, Jaiswal R. What is under the hump? Mass spectrometry based analysis of complex mixtures in processed food–lessons from the characterisation of black tea thearubigins, coffee melanoidines and caramel. Food Funct. 2013;4:1130–1147. doi: 10.1039/c3fo30385c. [DOI] [PubMed] [Google Scholar]
- 22.Menet MC, Sang S, Yang CS, Ho CT, Rosen RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem. 2004;52:2455–2461. doi: 10.1021/jf035427e. [DOI] [PubMed] [Google Scholar]
- 23.Kuhnert N, Drynan JW, Obuchowicz J, Clifford MN, Witt M. Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Commun Mass Spectrom. 2010;24:3387–3404. doi: 10.1002/rcm.4778. [DOI] [PubMed] [Google Scholar]
- 24.Murad HA, Habib H, Kamel Y, Alsayed S, Shakweer M, Elshal M. Thearubigins protect against acetaminophen-induced hepatic and renal injury in mice: biochemical, histopathological, immunohistochemical, and flow cytometry study. Drug Chem Toxicol. 2016;39:190–198. doi: 10.3109/01480545.2015.1070170. [DOI] [PubMed] [Google Scholar]
- 25.Murad HA, Abdallah HM. Black Tea Extract and its Thearubigins Relieve the Sildenafil-Induced Delayed Gut Motility in Mice: A Possible Role of Nitric Oxide. Phytother Res. 2014;28:1687–1691. doi: 10.1002/ptr.5183. [DOI] [PubMed] [Google Scholar]
- 26.Halder B, Pramanick S, Mukhopadhyay S, Giri AK. Inhibition of benzo[a]pyrene induced mutagenicity and genotoxicity by black tea polyphenols theaflavins and thearubigins in multiple test systems. Food Chem Toxicol. 2005;43:591–597. doi: 10.1016/j.fct.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Satoh E, Ishii T, Shimizu Y, Sawamura S, Nishimura M. Black tea extract, thearubigin fraction, counteract the effects of botulinum neurotoxins in mice. Br J Pharmacol. 2001;132:797–8. doi: 10.1038/sj.bjp.0703883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh E, Ishii T, Shimizu Y, Sawamura S, Nishimura M. The mechanism underlying the protective effect of the thearubigin fraction of black tea (Camellia sinensis) extract against the neuromuscular blocking action of botulinum neurotoxins. Pharmacol Toxicol. 2002;90:199–202. doi: 10.1034/j.1600-0773.2002.900405.x. [DOI] [PubMed] [Google Scholar]
- 29.Omar MH, Mullen W, Crozier A. Identification of proanthocyanidin dimers and trimers, flavone C-glycosides, and antioxidants in Ficus deltoidea, a Malaysian herbal tea. J Agric Food Chem. 2011;59:1363–1369. doi: 10.1021/jf1032729. [DOI] [PubMed] [Google Scholar]
- 30.Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 31.Lin LZ, Harnly JM. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J Agric Food Chem. 2007;55:1084. doi: 10.1021/jf062431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin LZ, Chen P, Harnly JM. New phenolic components and chromatographic profiles of green and fermented teas. J Agric Food Chem. 2008;56:8130–8140. doi: 10.1021/jf800986s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Parks TA, Chen X, Gillitt ND, Jobin C, Sang S. Structural identification of mouse fecal metabolites of theaflavin 3, 3′-digallate using liquid chromatography tandem mass spectrometry. J Chromatogr A. 2011;1218:7297–7306. doi: 10.1016/j.chroma.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Kortesniemi M, Liu P, Karonen M, Salminen JP. Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem. 2012;60:8672–8683. doi: 10.1021/jf302925v. [DOI] [PubMed] [Google Scholar]
- 35.Yassin GH, Grun C, Koek JH, Assaf KI, Kuhnert N. Investigation of isomeric flavanol structures in black tea thearubigins using ultraperformance liquid chromatography coupled to hybrid quadrupole/ion mobility/time of flight mass spectrometry. J Mass Spectrom. 2014;49:1086–1095. doi: 10.1002/jms.3406. [DOI] [PubMed] [Google Scholar]
- 36.Verloop AJ, Vincken JP, Gruppen H. A tandem mass spectrometry method based on selected ions detects low-abundance phenolics in black tea-theatridimensins as products of the oxidative cascade. Rapid Commun Mass Spectrom. 2016;30:1797–1805. doi: 10.1002/rcm.7658. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JR, Davis AL, Cai Y, Davies AP, Wilkins JP, Pennington M. Theaflavate B, isotheaflavin-3′-O-gallate and neotheaflavin-3-O-gallate: three polyphenolic pigments from black tea. Phytochemistry. 1998;49:2511–2519. [Google Scholar]
- 38.Wang Y, Ho CT. Flavors in Noncarbonated Beverages. ACS Publications; 2010. Functional contribution of polyphenols in black tea; pp. 45–59. [Google Scholar]
- 39.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 40.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]