Pancreatic cancer is one of the most notorious cancers, affecting almost 55,000 people in the United States alone every year. The 5-year survival rate for this disease is a dismal 7%.1 Aggressive biology leading to immense therapeutic resistance is one of the main reasons for such poor survival statistics.2 The approved standard of care, gemcitabine, adds only 6 weeks of survival benefit to patients diagnosed with the disease. A combination of gemcitabine/Abraxane (Celgene, NJ), approved for metastatic disease, adds marginally to the survival advantage. Thus, development of novel therapeutic targets that will improve survival is the major area of focus in pancreatic cancer research.
The existence of gastrin and its receptor cholecystokinin B (CCKBR) has been acknowledged for almost 100 years.3, 4 However, their role in cancer, specifically in pancreatic cancer, has not been well studied. CCKB receptors have been shown to be overexpressed in pancreatic cancer by earlier studies from Smith et al.5, 6 These studies have shown that the gastrointestinal trophic peptide, gastrin, stimulates the growth of pancreatic adenocarcinoma via interaction with the overexpressed CCK receptor in the malignant cells.
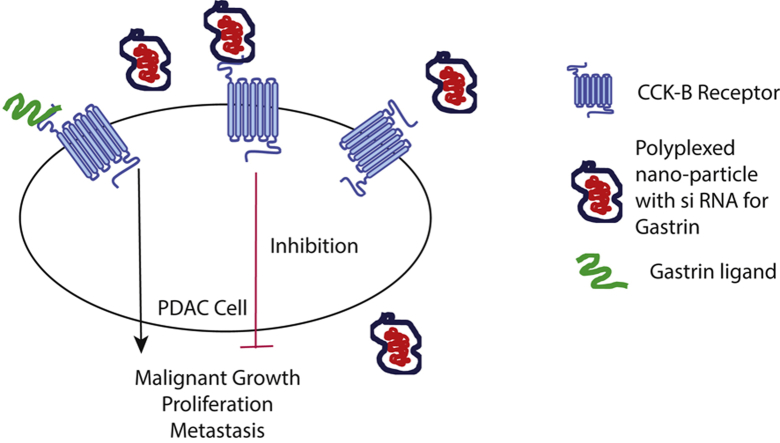
CCKB receptors have very low expression in normal pancreas tissue. As the tumor develops through the early precancerous pancreatic intraepithelial neoplasia lesions (PanIN), their expression increases. In a fully developed tumor, there is significant overexpression of the CCKB receptor. Studies have shown that CCKB receptors are present on the pancreatic stellate cells (PSC) as well and they can be targeted to revert fibrosis, which is a classic signature of the pancreatic adenocarcinoma. Gastrin peptide is one of the major ligands of CCKB receptor and its autocrine signaling via CCKB receptor regulates malignant growth, proliferation, and the metastatic ability of the tumor cells. This makes it an attractive target for the development of novel therapy. The study by Burks et al7 published in this issue highlights the development of a polyplex nanoparticle that is biodegradable and nontoxic and serves as a vehicle to carry CCKB small interfering RNA (siRNA) without off-target effects.
Theranostic nanomedicines are evolving as an encouraging therapeutic model. They take advantage of the high capacity of the different nanoplatforms to deliver inhibitory agents (drugs, siRNA) to tumor cells. Fluorescently labeled or magnetically conjugated nanoplatforms can be developed further as imaging tools to detect and diagnose different cancers.8 The interest in the field of targeted nanomedicines in pancreatic cancer reached its peak with the approval of nab-paclitaxel (Abraxane) in which 130 nm albumin bound nanoparticle formulation of the age-old drug paclitaxel. This conjugation was able to increase the payload of paclitaxel within the tumor cell, thereby improving survival in patients receiving gemcitabine/Abraxane combination therapy.9 Similarly, Onivyde (Merrimac Pharmaceuiticals Inc, Cambridge, MA), a nanoliposomal irinotecan formulation, currently is being evaluated in pancreatic cancer.10, 11 In this context, development of a targeted nanoconjugate, as studied by Burks et al, that can improve delivery of targeted siRNA to tumor cells is extremely timely.
Because of their role in promoting tumor proliferation and invasion, natural ligands of CCKB receptors such as gastrin and CCK have been attached to nanocarriers, radionuclides, and imaging agents to facilitate their uptake by tumor cells. A study from 2017 by Clawson et al12 described conjugating the CCKBR to DNA aptamers to improve their uptake in orthotopic pancreatic adenocarcinoma. In the study by Burk et al, a polyplex nanoparticle designed to target the CCKB receptor is effectively able to deliver the gastrin siRNA to the tumor cell, resulting in decreased primary tumor size as well as metastasis. The study further showed that this polyplex nanoparticle platform also may be used for knocking down other major stimulatory proteins and may be used in combination with other siRNAs to improve efficacy.
Theranostic imaging involves delivery of a therapeutic payload to cancer-specific targets that can be imaged in a noninvasive manner. Apart from the therapeutic approach that this study highlights, the polyplexed nanoparticle targeting the CCKBR also can be developed as a noninvasive imaging platform for pancreatic cancer by fluorescent conjugation of this receptor. Because one of the challenges in the field of pancreatic cancer is the inability to detect the tumor sufficiently early, this potentially can be developed as an early detection imaging tool.
In conclusion, theranostic nanoparticles as described in Burk et al need to be exploited further to develop not only better targeted therapy, but also better detection techniques that can be conjugated with multiple noninvasive imaging platforms to detect pancreatic cancer early. This has the potential for providing a wider window for treatment of patients diagnosed with this devastating disease with the available and novel therapies.
Footnotes
Conflicts of interest The authors disclose the following: The University of Minnesota holds a patent for Minnelide (WO/2010/129918/Triptolide Prodrugs), which has been licensed to Minneamrita Therapeutics, LLC, and Ashok K. Saluja is the co-founder and the Chief Scientific Officer and Sulagna Banerjee is a compensated consultant; this relationship is managed by the University of Miami.
Funding Supported by National Institutes of Health grants R01-CA170946 and CA124723 (A.K.S.) and R01-CA184274 (S.B.).
Supplementary Material
Supplemental Graphical Summary.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers (Basel) 2017;9. [DOI] [PMC free article] [PubMed]
- 3.Ballinger A., Ahmed M., Kumar P., Clark M. Gastrin effects on growth and exocrine secretion in the neonatal rat pancreas. Pancreas. 1997;14:295–300. doi: 10.1097/00006676-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Tang C., Biemond I., Lamers C.B. Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study. Gastroenterology. 1996;111:1621–1626. doi: 10.1016/s0016-5085(96)70025-2. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.P., Fonkoua L.K., Moody T.W. The role of gastrin and CCK receptors in pancreatic cancer and other malignancies. Int J Biol Sci. 2016;12:283–291. doi: 10.7150/ijbs.14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith J.P., Solomon T.E. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91–G101. doi: 10.1152/ajpgi.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burks J., Nadella S., Mahmud A., Mankongpaisarnrung C., Wang J., Hahm J.I., Tucker R.D., Shivapurkar N., Stern S.T., Smith J.P. Cholecystokinin receptor-targeted polyplex nanoparticle inhibits growth and metastasis of pancreatic cancer. Cell Mol Gastroenterol Hepatol. 2018;6:17–32. doi: 10.1016/j.jcmgh.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau J., Lin K.S., Benard F. Past, present, and future: development of theranostic agents targeting carbonic anhydrase IX. Theranostics. 2017;7:4322–4339. doi: 10.7150/thno.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoy S.M. Albumin-bound paclitaxel: a review of its use for the first-line combination treatment of metastatic pancreatic cancer. Drugs. 2014;74:1757–1768. doi: 10.1007/s40265-014-0291-8. [DOI] [PubMed] [Google Scholar]
- 10.Lamb Y.N., Scott L.J. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs. 2017;77:785–792. doi: 10.1007/s40265-017-0741-1. [DOI] [PubMed] [Google Scholar]
- 11.Passero F.C., Jr., Grapsa D., Syrigos K.N., Saif M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev Anticancer Ther. 2016;16:697–703. doi: 10.1080/14737140.2016.1192471. [DOI] [PubMed] [Google Scholar]
- 12.Clawson G.A., Abraham T., Pan W., Tang X., Linton S.S., McGovern C.O., Loc W.S., Smith J.P., Butler P.J., Kester M., Adair J.H., Matters G.L. A Cholecystokinin B receptor-specific DNA aptamer for targeting pancreatic ductal adenocarcinoma. Nucleic Acid Ther. 2017;27:23–35. doi: 10.1089/nat.2016.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]