Abstract
The chief concerns for antiretroviral therapy (ART) programs considering removal of CD4+ cell count thresholds for treatment are the increased incidence of ART-related adverse events. A nationwide observational cohort study was conducted among patients who initiated ART in 2012. We divided the eligible patients into three groups: an early ART group with a baseline CD4+ cell count of 500 cells/μL or greater, a standard ART group with a baseline CD4+ cell count between 350 and 499 cells/μL, and a late ART group with a baseline CD4+ cell count between 200 and 349 cells/μL. These patients were followed up to December 31, 2014 and observed for three outcomes: virological failure, treatment nonretention, or time to death. Patients who met the eligibility criteria numbered at 26,752. Out of all study participants, 20,827 participants were in late ART group, 4336 were in standard ART group, and 1589 were in early ART group. Patients in late ART group were more likely to become virally suppressed 12 and 24 months after treatment initiation than patients in early ART group [adjusted odds ratio (aOR) 0.81; 95% CI, 0.69–0.95 and aOR, 0.78; 95% CI, 0.65–0.94]. Treatment nonretention was also less likely to occur among patients in late ART group than early ART group 12 months after treatment initiation (aOR, 0.85; 95% CI, 0.75–0.96). Compared with early ART group, neither standard ART group nor late ART group had a statistically significant difference in the time-to-death analysis. Late ART initiates were more likely to be virally suppressed and retained on treatment than early ART initiates. The importance of treatment retention and adherence should be emphasized for high CD4+ patients newly initiated to ART therapy through education and counseling programs.
Keywords: CD4+, early treatment, HIV/AIDS
Introduction
The 90-90-90 campaign outlined in the AIDS 2014 conference detailed new targets for testing and treatment.1 Early treatment and medication adherence are contributing factors for realizing the goal.2,3 As to early treatment, for several years, medical professionals and HIV policymakers have maintained a consensus that ART should be initiated in all patients with a CD4+ cell count of 350 cells/μL or less.4–6 In 2013, WHO published HIV treatment guidelines with a new recommendation for a CD4+ cell count treatment threshold of 500 cells/μL,7 following similar recommendations made in national guidelines of developed countries. In September, 2015, WHO released revised HIV treatment guidelines recommending removing CD4+ cell count thresholds for HIV treatment entirely,8 citing recently published interim results of the Strategic Timing of Antiretroviral Therapy study (START).
The chief concerns for ART programs considering removal of CD4+ cell count thresholds for treatment are increased incidence of ART-related adverse events,9–13 poor adherence stemming from greater numbers of patients who are less motivated to start and adhere to ART because they feel well,14 and fostering conditions conducive to transmission of ART-resistant strains of HIV. The START trial has provided a well-grounded scientific rationale for removal of CD4+ thresholds for ART initiation.15 However, observational studies are still needed to understand fully the range of benefits and harms of ART taken over a lifetime, for treatment retention maybe different among patients in clinical trial and real world. It’s sure that people tend not to take medications to prevent conditions because they do not receive immediate benefits in the sense of feeling better.16
Chinese treatment guidelines were revised in 2014 to increase CD4+ cell count thresholds for ART initiation to 500 cells/μL.17 China is considering adopting core features of the new WHO treatment guidelines, including removal of the CD4+ threshold for treatment. We analyzed outcomes of Chinese PLWH initiated on ART at a range of different initial CD4+ counts over the last several years to help inform policy changes under consideration. China’s national ART database provides a valuable resource for longitudinal evaluation of more than 200,000 PLWH initiated on ART at different CD4+ thresholds, including subpopulations for whom Chinese national guidelines recommended initiation of ART without respect to CD4+ count (e.g., HIV-positive members of serodiscordant couples). The objective of this study is to compare treatment nonretention, virological failure, and mortality between three categories of HIV-positive patients on ART: (1) those who initiated ART with a baseline CD4+ cell count more than 500 cells/μL (“early ART group”); (2) those who started ART with a baseline CD4+ cell count between 350 and 499 cells/μL (“standard ART group”); and (3) those who initiated ART with a baseline CD4+ cell count between 200 and 349 cells/μL (“late ART group”).
Methods
Ethical approval
The study protocol was reviewed and approved by the Institutional Review Board of National Centre for AIDS/STD Control and Prevention (NCAIDS), China CDC (#X150619380) and the Associate Director for Science, IS CDC.
Data source
For our nationwide observational cohort study, we used the National Free Antiretroviral Treatment Program (NFATP) database managed by the NCAIDS. The NFATP database has been described in previous publications.18–20 Standardized case report forms (CRFs) are used by China CDC public health workers to gather information from local ART clinic staff on patients meeting criteria for inclusion in the NFATP database. Different CRFs are used to track five treatment statuses of a patient’s treatment: an initial patient assessment, treatment follow-up, treatment regimen change, treatment/follow-up termination, and transfer of care. The local CDC public health worker is responsible for assessing the patient’s treatment status and completing one or more of these forms. The county CDC then transmits the completed forms to the NFATP database. The NFATP database includes all HIV-diagnosed Chinese citizens who meet contemporaneous national treatment criteria and were referred to NFATP since 2002. Key outcome events, such as death, are ascertained by local CDC staff through direct contact with ART clinic staff and entered into the NFATP database.
Study subjects
Using the NFATP database, we selected for analysis patients who first initiated ART treatment between January 1, 2012 and December 31, 2012. Patients were excluded if they were not ART naive, younger than 15 years of age at the time of ART initiation, began treatment with a nonstandard ART regimen (standard ART regimen: zidovudine/stavudine [d4T] + lamivudine + nevirapine/efavirenz or tenofovir + lamivudine + nevirapine/efavirenz), or had missing followup data. A patient has complete follow-up data if information from additional visits to an ART clinic is recorded in the NFATP database, in addition to the baseline information recorded from his/her first visit.
Description of the study outcomes
Outcomes for our analysis were separately assessed at 12 and 24 months after ART initiation. We compared among the three ART groups (1) differences in the rate of treatment nonretention, (2) differences in the rate of experiencing virological failure, and (3) differences in time to death for an AIDS-related death. The analytic observation period for each study subject begins on the day of ART initiation and extends 12 or 24 months following treatment initiation, or upon reaching an end-point for each of our study outcomes as described below. Therefore, this study consists of two assessments. One is a serial cross-sectional assessment, and the other is a time-to-event assessment.
Assessment of differences in treatment retention
A patient’s treatment retention status was based on regrouping five follow-up statuses in the NFATP database: currently on treatment, stopped treatment, lost to follow-up, deceased, or transferred to another treatment facility.21 Upon initiating ART treatment, the patient is required to retrieve medications every 3 months. Patients who consistently retrieve medications every 3 months are “currently on-treatment.” Should the patient fail to retrieve medications at the next 3-month interval, the patient is still considered to be “currently on-treatment.” In this situation, an additional 90-day grace period is given for the patient to show up before she/he is considered to be “lost-to-follow-up.” Patients who have died in the interim before their next scheduled 3-month appointment may be classified as “died,” depending on the timing of ascertainment of death information. The “stopped-treatment” status is assigned to patients who have informed their physician that they wish to discontinue ART treatment.
For the purposes of the assessing differences in the rate of treatment retention between the treatment groups, patients who died, stopped treatment, or were lost to follow-up within the analytic observation period were grouped into a “treatment non-retention” category. Patients reported to be “currently on-treatment” or “transferred-to another-treatment-facility” are considered “retained,” meaning they are on a consistent ART regimen. Comparisons of the three treatment group’s rate of treatment retention were assessed separately at 12 and 24 months following treatment initiation. If a patient was assigned a “non-retained” status at any time between treatment initiation and 12 months after treatment initiation, she/he was included for analysis at the 12-month end-point. If a patient was assigned a “non-retained” status at any time between 12 months following ART initiation and 24 months following ART end-point, she/he was included for analysis at the 24-month end-point.
Assessment of differences in virological failure
We defined virological failure as having an HIV viral load of 400 copies/mL or greater on a blood sample drawn 6–24 months following initiation of ART.22 We used sensitivity analyses to determine if the population rate of virological failure is affected by the time intervals surrounding the study end-points, within which viral load test results were assessed (i.e., 3, 5, 6, 7, and 9 months). Since no differences in virological failure rate were observed among these various intervals, 6 months was chosen to be the interval surrounding the study end-points. Only patients who received a viral load test during this time interval are included for our analysis comparing virological failure rate among different treatment groups. If a patient received a viral load test within 6 months before or after 12 or 24 months following treatment initiation, she/he will be included for analysis for those respective study end-points. If a patient received several viral load tests during this time period, the test done on a date closest to the study end-points will be used for analysis.
Assessment of differences in time to death
Based on physician discretion and judgment, deaths of people living with HIV (PLHIV) were grouped into one of five mutually exclusive categories in the NFATP database: AIDS-related death, suicide, deaths from injuries or accidents, death from other known causes, or death from unknown causes.21 We excluded from our time-to-death analysis patients who died from suicide, injuries or accidents, and other known causes. Patients who died from AIDS-related events or unknown events were considered to have died from an AIDS-related event. The time to AIDS-related death was defined as the duration between treatment initiation and the date of death from AIDS-related event as defined above. Since deaths happened infrequently during the observational period of our study, time to death was only assessed at 24 months following treatment initiation.
Statistical analyses
Summary statistics were used to characterize the study population and describe the demographic profile of the study patients. We used logistic regression to evaluate odds ratios and 95% confidence intervals between our treatment group variable and the virological failure and treatment retention outcomes at 12 and 24 months. Because the exact time of death is recorded in the NFATP database, we used the Cox proportional hazards regression to evaluate hazard ratios and 95% confidence intervals between our treatment group variable and the time-to-death outcome at the end of 24 months following treatment initiation. The proportional hazards assumption was tested by considering an interaction term between death and log-transformed follow-up time. For our multivariate analyses, we included covariates that were biologically relevant (i.e., age, sex). Statistical significance was assigned to p-values less than 0.05. All analyses were performed using SAS version 9.3.
Results
Study patients
The number of HIV-positive individuals with an initial CD4+ cell count exceeding 200 cells/μL who started combination antiretroviral therapy (ART) numbered at 28,778 between January 1, 2012 and December 31 (Fig. 1). Those who were not ART naive (n = 376), who were younger than 15 years of age (n = 10), or whose initial antiretroviral regimen was not zidovudine/stavudine + lamivudine + nevirapine/ efavirenz or tenofovir + lamivudine + nevirapine/efavirenz (n = 1631) were excluded from our analysis. Twenty-seven percent of patients who had started with nonstandard regimens (n = 452) had started without triple therapy regimen or with unknown regimen. So we did a sensitive analysis with the rest 1179 patients. We got similar results with our study. Nine patients were excluded for missing follow-up data, leaving 26,752 patients for our analysis.
FIG. 1.
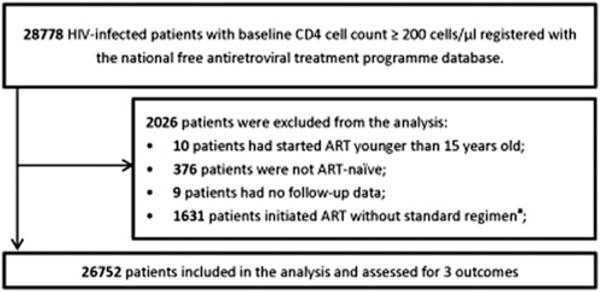
Selection of HIV-infected patients who initiated ART between January 1, 2012 and December 31, 2012. aStandard regimen: zidovudine/stavudine + lamivudine + nevirapine/efavirenz or tenofovir + lamivudine + nevirapine/ efavirenz. ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Of all the study patients meeting the eligibility criteria, 77.85% belonged in the late ART initiation group, 16.21% in the standard ART initiation group, and 5.94% in the early ART initiation group. Most of the study patients were between 15 and 44 years of age (72.41%) at the time of ART initiation. Most study patients had reported heterosexual sex as their only HIV transmission risk factor (60.34%). Most patients received zidovudine/stavudine + lamivudine + nevirapine/efavirenz as their initial regimen (80.04%). Table 1 describes the demographic, behavioral, and HIV treatment characteristics of the study patients.
Table 1.
Baseline Demographic and Behavioral Characteristics of Adult HIV Patients (N= 26,752) Who Initiated ART in 2012
| Variables | Baseline CD4+ count in cells/μL, n (%) | |||
|---|---|---|---|---|
| 200–349 (n = 20,827) | 350–499 (n = 4336) | ≥500 (n = 1589) | ||
| Sex | ||||
| Male | 18,256 | 14,553 (69.88) | 2673 (61.65) | 1030 (64.82) |
| Female | 8496 | 6274 (30.12) | 1663 (38.35) | 559 (35.18) |
| Age at start of ART (years) | ||||
| Mean (std.) | 39.21 (12.91) | 37.89 (11.78) | 36.19 (11.06) | |
| 15–44 | 19,371 | 14,795 (71.04) | 3300 (76.11) | 1276 (80.30) |
| 45–59 | 5184 | 4276 (20.05) | 768 (17.71) | 240 (15.10) |
| ≥60 | 2197 | 1856 (8.91) | 268 (6.18) | 73 (4.59) |
| Marital statusa | ||||
| Married or living with partners | 16,173 | 12,039 (58.04) | 2944 (68.02) | 1190 (75.03) |
| Single, divorced or windowed | 10,483 | 8703 (41.96) | 1384 (31.98) | 996 (24.97) |
| HIV transmission mode | ||||
| MSM | 4945 | 4383 (21.04) | 463 (10.68) | 99 (6.23) |
| Heterosexual | 16,142 | 12,444 (59.75) | 2750 (63.42) | 948 (59.66) |
| IDU | 3559 | 2444 (11.73) | 737 (17.00) | 378 (23.79) |
| Othersb | 2106 | 1556 (7.47) | 386 (8.90) | 164 (10.32) |
| Type of treatment center | ||||
| County or higher level hospital | 24,220 | 18,945 (90.96) | 3842 (88.61) | 1433 (90.18) |
| Township hospital | 2532 | 1882 (9.04) | 494 (11.39) | 156 (9.82) |
| Initial ART regimen | ||||
| AZT/d4T+3TC+EFV/NVP | 21,414 | 17,397 (83.53) | 3119 (71.93) | 898 (56.51) |
| TDF +3TC+EFV/NVP | 5338 | 3430 (16.47) | 1217 (28.07) | 691 (43.49) |
All demographic and behavioral characteristics included were shown to be statistically significant with respect to differences between the three treatment groups.
Some of the individual counts do not sum to the totals due to missing data.
“Others” indicate that the marital status of participants is unknown.
3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; IDU, injection drug use; MSM, men who have sex with men; NVP, nevirapine; TDF, tenofovir.
Comparisons of treatment nonretention
At the end of 12 and 24 months following ART initiation, 26,742 and 24,274 participants, respectively, had complete follow-up data in the NFATP database. When we did not control for demographic or behavioral factors, the odds of treatment nonretention in patients from the late ART group was lower than patients in the early ART group at 12 [unadjusted odds ratio (uOR) = 0.77; 95% CI = 0.68–0.87] and 24 months (uOR = 0.82; 95% CI = 0.70–0.96). When we adjusted for sex, age, and marital status the odds of treatment nonretention was also lower in the late ART group than the early ART group at 12 [adjusted odds ratio (aOR) = 0.75; 95% CI = 0.66–0.85] and 24 months (aOR = 0.80; 95% CI = 0.68–0.94). However, when we adjusted for sex, age, marital status, treatment center type, initial ART regimen, and transmission mode, odds of treatment nonretention was lower in the late ART group than the early ART group at 12 months only (aOR=0.85; 95% CI = 0.75–0.96) (Fig. 2, Table 2).
FIG. 2.
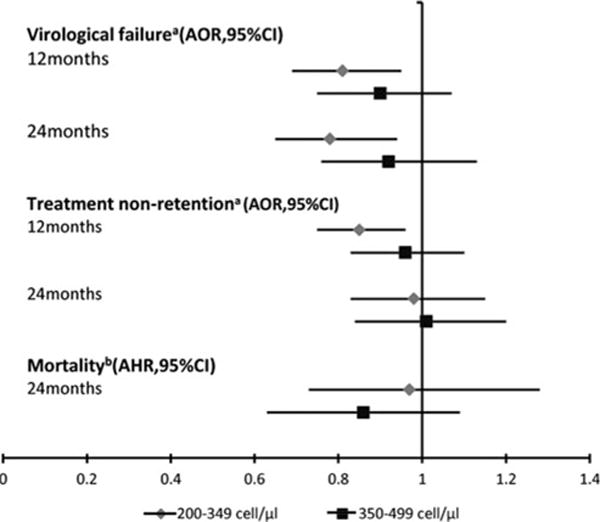
Multivariable comparisons between early initiation of ART and later initiation of ART with respect to the three assessed outcomes. aControlled for age, sex, marital status, HIV transmission mode, type of treatment center, and initial ART regimen; bControlled for age, sex, and HIV transmission mode. AHR, adjusted hazard ratios; AOR, adjusted odd ratios; CI, confidence interval.
Table 2.
Comparisons Between Early Antiretroviral Therapy (ART) Initiation and Standard/ Late ART Initiation with Respect to Treatment Nonretention Among Adult HIV Patients Who Started ART in 2012
| Baseline CD4+ count in cells/μL, n (%)
|
|||
|---|---|---|---|
| 200–349 | 350–499 | ≥500 | |
| Twelve months after initiating ART (n = 26,742) | |||
| Number of treatment nonretentions | 3896 (18%) | 927 (21%) | 366 (23%) |
| Number retained on treatment at study end-point | 20,821 | 4333 | 1588 |
| Unadjusted OR (95% CI), p-value | 0.77 (0.68–0.87), <0.0001 | 0.91 (0.79–1.04), 0.17 | 1 |
| OR adjusted by age and sex (95% CI), p-value | 0.76 (0.67–0.86), <0.0001 | 0.91 (0.79–1.04), 0.17 | 1 |
| OR adjusted by age, sex and transmission mode (95% CI), p-value | 0.89 (0.78–1.01), 0.063 | 0.98 (0.85–1.12), 0.74 | 1 |
| OR adjusted by age, sex, and marital status (95% CI), p-value | 0.75 (0.66–0.85), <0.0001 | 0.91 (0.79–1.04), 0.16 | 1 |
| OR adjusted by all factorsa but not transmission mode (95% CI), p-value | 0.77 (0.68–0.87), <0.0001 | 0.92 (0.80–1.05), 0.22 | 1 |
| OR adjusted by all factorsa and transmission mode (95% CI), p-value | 0.85 (0.75–0.96), 0.011 | 0.96 (0.83–1.10), 0.53 | 1 |
| Twenty-four months after initiating ART (n = 24,274) | |||
| Number of treatment nonretentions | 2283 (12%) | 524 (13%) | 203 (14%) |
| Number retained on treatment at study end-point | 18,921 | 3938 | 1415 |
| Unadjusted OR (95% CI), p-value | 0.82 (0.70–0.96), 0.012 | 0.82 (0.77–1.10), 0.33 | 1 |
| OR adjusted by age and sex (95% CI), p-value | 0.81 (0.69–1.09), 0.008 | 0.92 (0.77–1.09), 0.33 | 1 |
| OR adjusted by age, sex, and transmission mode (95% CI), p-value | 0.98 (0.84–1.15), 0.80 | 0.99 (0.83–1.19), 0.95 | 1 |
| OR adjusted by age, sex and marital status (95% CI), p-value | 0.80 (0.68–0.94), 0.0053 | 0.92 (0.77–1.10), 0.35 | 1 |
| OR adjusted by all factorsa but not transmission mode (95% CI), p-value | 0.87 (0.74–1.02), 0.08 | 0.96 (0.81–1.15), 0.66 | 1 |
| OR adjusted by all factorsa and transmission mode (95% CI), p-value | 0.98 (0.83–1.15), 0.81 | 1.01 (0.84–1.20), 0.95 | 1 |
All factors here include sex, age, marital status, type of treatment center, initial ART regimen.
ART, antiretroviral therapy; CI, confidence interval; OR, odds ratio.
Comparisons of virological failure
At the end of 12 and 24 months following ART initiation, 21,159 and 17,768 participants, respectively, had complete viral load test results in the NFATP database. Patients in the late ART group were less likely to experience virological failure than were patients in the early ART group at 12 and 24 months (uOR, 0.72; 95% CI, 0.62–0.84 and uOR, 0.68; 95% CI, 0.57–0.80). When we adjusted for age and sex, the likelihood of virological failure was also lower in the late ART group than the early ART group at 12 and 24 months (aOR = 0.72; 95% CI = 0.61–0.84 and aOR = 0.68; 95% CI = 0.57–0.81). Significant odds ratios were also observed when we adjusted for age, sex, and transmission mode at 12 and 24 months; age, sex, marital status, treatment center type, and initial ART regimen at 12 and 24 months; and age, sex, marital status, treatment center type, initial ART regimen, and transmission mode at 12 and 24 months (Fig. 2, Table 3).
Table 3.
Comparisons Between Early Antiretroviral Therapy (Art) Initiation And Standard/Late Art Initiation With Respect To Virological Failure Among Adult Hiv Patients Who Started Art In 2012
| Baseline CD4+ count in cells/μL, n (%) | |||
|---|---|---|---|
| 200–349 | 350–499 | >500 | |
| Twelve months after initiating ART (n = 21,159) | |||
| Number of failures | 2185 (13%) | 525 (15%) | 212 (17%) |
| Number of people on treatment | 16,524 | 3421 | 1214 |
| Unadjusted OR (95% CI), p-value | 0.72 (0.62–0.84), <0.0001 | 0.86 (0.72–1.02), 0.08 | 1 |
| OR adjusted by age and sex (95% CI), p-value | 0.72 (0.61–0.84), <0.0001 | 0.86 (0.72–1.02), 0.08 | 1 |
| OR adjusted by age, sex, and transmission mode (95% CI), p-value | 0.81 (0.69–0.94), 0.007 | 0.91 (0.76–1.08), 0.28 | 1 |
| OR adjusted by all factorsa but not transmission mode (95% CI), p-value | 0.75 (0.64–0.88), 0.0003 | 0.87 (0.73–1.03), 0.11 | 1 |
| OR adjusted by all factorsa and transmission mode (95% CI), p-value | 0.81 (0.69–0.95), 0.008 | 0.90 (0.75–1.07), 0.22 | 1 |
| Twenty-four months after initiating ART (n = 17,768) | |||
| Number of failures | 1644 (11%) | 433 (14%) | 168 (16%) |
| Number of people on treatment | 13,826 | 2933 | 1009 |
| Unadjusted OR (95% CI), p-value | 0.68 (0.57–0.80), <0.0001 | 0.87 (0.71–1.05), 0.15 | 1 |
| OR adjusted by age and sex (95% CI), p-value | 0.68 (0.57–0.81), <0.0001 | 0.87 (0.72–1.06), 0.16 | 1 |
| OR adjusted by age, sex, and transmission mode (95% CI), p-value | 0.77 (0.65–0.92), 0.0038 | 0.93 (0.76–1.13), 0.48 | 1 |
| OR adjusted by all factorsa but not transmission mode (95% CI), p-value | 0.73 (0.61–0.87), 0.0004 | 0.89 (0.73–1.08), 0.22 | 1 |
| OR adjusted by all factorsa and transmission mode (95% CI), p-value | 0.78 (0.65–0.94), 0.0075 | 0.92 (0.76–1.13), 0.43 | 1 |
All factors here include sex, age, marital status, type of treatment center, initial ART regimen.
ART, antiretroviral therapy; CI, confidence interval; OR, odds ratio.
Comparisons of time-to-death
At the end of 24 months following ART initiation, 953 patients were deceased, 836 (87.72%) of whom died of an AIDS-related event. The percentage of patients from our defined treatment categories who died from an AIDS-related event was 3.40% in the early ART group, 2.86% in the standard ART group, and 3.16% in the late ART group. Neither the standard ART group nor the late ART group had statistically significant differences in time to AIDS-related death compared with the early ART group (Fig. 2, Table 4).
Table 4.
Comparisons Between Early Antiretroviral Therapy (Art) Initiation And Standard/Late Art Initiation With Respect To Mortality Among Adult Hiv Patients Who Started Art In 2012
| Baseline CD4+ count in cells/μL, n (%) | |||
|---|---|---|---|
| 200–349 | 350–499 | ≥500 | |
| Twenty-four months after initiating ART (N = 24,274) | |||
| Number of noninjury death | 658 (3%) | 124 (2%) | 54 (3%) |
| Number of people on treatment | 20,827 | 4336 | 1589 |
| Unadjusted HR (95% CI), p-value | 0.90 (0.68–1.19), 0.46 | 0.82 (0.59–1.13), 0.22 | 1 |
| HR adjusted by all factorsa but not transmission mode (95% CI), p-value | 0.77 (0.58–1.01), 0.11 | 0.77 (0.56–1.06), 0.062 | 1 |
| HR adjusted by all factorsa and transmission mode (95% CI), p-value | 0.97 (0.73–1.28), 0.82 | 0.86 (0.63–1.19), 0.36 | 1 |
All factors here include sex and age. Factors such as type of treatment center and initial ART regimen were not included in the analysis, because they were shown to be insignificant from our previous studies. Marital status was not included because it was shown to be insignificant in the univariate analysis.
ART, antiretroviral therapy; HR, hazard ratios.
Discussion
This is the first study to our knowledge in China to compare HIV treatment outcomes between Chinese PLHIV who started ART at high CD4+ cell counts and those who started later in the course of their disease. The results of our study demonstrate that adult HIV-infected patients who started ART early in the course of their disease were more likely to be nonadherent with their treatment. The reported experiences in other parts of the world corroborate these findings,23–25 although a recent study showed a disconnect between viral load suppression and retention in care.26 Many studies claimed that virological failure and nonretention are inextricably linked, where ART adherence is the strongest predictor for virological suppression.27,28 Although we do not have data in China to enable exploration of potential reasons for the difference between the adherence of the early treatment group and the later treatment groups, previous studies show that drug side effects and toxicities29 may lead to incomplete adherence and virological failure among PLHIV on ART. Assuming there is some degree of universality to the drivers of PLHIVs’ adherence-related behavior, the challenge of adherence for PLHIV who initiate treatment at high CD4+ cell counts is an important question in China, where all CD4 thresholds to ART initiation will be removed for ART enrollment.
When we controlled for sex, age, marital status, type of treatment center, initial ART regimen, and transmission mode in our multivariate logistic regression, the early ART group had a lower treatment retention rate 12 months after ART initiation than the late ART group. However, this difference was no longer significant at 24 months. The presence of side effects to ART leading to discontinuation during the first year may explain this transient difference in retention after treatment initiation. China’s limited number of options in its ART formulary may explain why those in the early ART group with side effects were not retained on an alternate regimen.
Patients with higher baseline CD4+ cell counts have likely spent most of their time following infection without experiencing opportunistic infections and other complications associated with later HIV disease, and may therefore be less cognizant of the capacity of HIV infection to lead to life-threatening illness.
Our results did not show a significant relationship between early treatment and death after 24 months of observation on mortality. This may indicate that 2 years is not a sufficient follow-up period to assess the effect of early treatment on mortality. In addition, previous observational studies do not conclusively show a relationship between early ART treatment and mortality.30–33 Data from the North America AIDS Cohort Collaboration on Research and Design (NAAC-CORD) demonstrated a benefit of early initiation of ART therapy in reducing AIDS-related death,30 while the Antiretroviral Therapy Cohort Collaborative (ARTCC) and the Concerted Action on Seroconversion to AIDS and Death (CASCADE) did not.31,32
Our analysis is subject to several limitations. First, although we adjusted for factors shown in the literature to be biologically and socially relevant, residual confounding may still occur. Second, we did not consider the potential impact of common HIV coinfections, such as hepatitis B and C viruses, that are particularly prevalent in China as monoinfections.34,35 Patients in China who have a coinfection of HIV, hepatitis B virus, and hepatitis C virus have been shown to be more likely to have virological failure and be lost to follow-up.34 Third, although all physicians are required to provide counseling on the importance of ART adherence to PLHIV starting ART, the extent to which this requirement implemented is unknown. Lack of adherence counseling may have contributed to poor adherence among members of our observational cohort. Finally, unlike other studies investigating the difference between deferred and early therapy initiation, we did not include endpoints for other indicators besides death, virological failure, and treatment nonretention. These indicators include serious non-AIDS-related opportunistic infections, tuberculosis, malignant lymphoma, non-AIDS defining cancer,15 and rates of sexual transmission.3 This makes our results less comparable to the results of these studies. Although studies that included these additional end-points offer a relative complete representation of possible health outcomes relevant to PLHIV, our three study end-points also offer a holistic picture on the natural progression of HIV disease and treatment.
Our study showed that the early ART group was more likely to experience virological and treatment failure than the late ART groups, which suggests that implementation-related concerns (adherence support, side effects management, smooth transfer of HIV care services to mobile patients) may cloud the prospects of realizing the benefits of early treatment shown in the controlled and resource-rich environment of clinical trials. ART adherence may be particularly influenced in China by the stigma associated with HIV infection,36,37 and the economic burden of treatment, which remains substantial despite the provision of ART medications at no charge.38 Recent studies highlighted several major barriers of engagement in HIV care in China.39,40 Two of these barriers are psychological burden of committing to HIV care and perceived discrimination from healthcare workers.40 Thus, China had a high percentage of participants who did not disclose at all.39 The importance of treatment adherence should be emphasized for patients newly initiated to ART therapy through education and counseling programs.41 ART patients may benefit from patient support groups or other strategies aimed at promoting skills for coping with societal HIV-related stigma. Early treatment has the potential to accelerate declines in AIDS-related mortality and decrease HIV transmission,3,15 but these benefits can only be realized if a program also adopts a patient-centered approach that includes adherence promotion and stigma-reduction efforts.
Acknowledgments
We thank the staff of the local counties’ Centers for Disease Control, who spent numerous hours and effort working with us in obtaining, verifying, and cleaning the data used in this study. This project has also been supported (in part) by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The National Center for AIDS/STD Control and Prevention, China Center for Disease Control and Prevention, and Beijing Ditan Hospital.
Footnotes
CDC Authorship Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS. Ambitious treatment targets: Writing the final chapter on the AIDS epidemic. 2014 Jul; Available at: www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/JC2670_UNAIDS_Treatment_Targets_en.pdf (Last accessed July 2, 2017).
- 2.Corless IB, Hoyt AJ, Tyerviola L, et al. 90-90-90-Plus: Maintaining adherence to antiretroviral therapies. AIDS Patient Care STDS. 2017;31:227–236. doi: 10.1089/apc.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Cock KM, El-Sadr WM. When to start ART in Africa—An urgent research priority. N Engl J Med. 2013;368:886–889. doi: 10.1056/NEJMp1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 6.Writing G, Williams I, Churchill D, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013. All changed text is cast in yellow highlight) HIV Med. 2014;15(Suppl 1):1–85. doi: 10.1111/hiv.12119. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1 (Last accessed July 15, 2017). [PubMed]
- 8.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Sep; Available at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1 (Last accessed July 15, 2017). [PubMed]
- 9.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 10.Sanne I, Mommeja-Marin H, Hinkle J, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 11.Jim Y, Juliane SF, Fux CA, et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS. 2012;26:567–575. doi: 10.1097/QAD.0b013e32834f337c. [DOI] [PubMed] [Google Scholar]
- 12.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 13.Lene R, Amanda M, Ole K, et al. Exposure to antiretrovirals (ARVs) and risk of renal impairment among HIV-positive persons with normal baseline renal function: The D:A:D study. J Infect Dis. 2013;207:1359–1369. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundgren JD, Babiker AG, Gordin FM, Borges AH, Neaton JD. When to start antiretroviral therapy: The need for an evidence base during early HIV infection. BMC Med. 2013;11:1322–1324. doi: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group ISS. Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz MH. Pre-exposure prophylaxis for HIV: Can it be implemented in the real world? Am J Prevent Med. 2013;44(1 Suppl 2):S161. doi: 10.1016/j.amepre.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Commission NHaFP. Criteria Revision for Free ART Initiation by the National Health and Family Planning Commission in 2014. 2014 [Google Scholar]
- 18.Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: Challenges and responses. AIDS. 2007;21(Suppl 8):S143–S148. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 19.Ye M, Fujie Z, Yan Z, et al. Cohort profile: The Chinese national free antiretroviral treatment cohort. Int J Epidemiol. 2010;39:973–979. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han M, Chen Q, Hao Y, et al. Design and implementation of a China comprehensive AIDS response programme (China CARES), 2003–08. Int J Epidemiol. 2010;39(Suppl 2):ii47–ii55. doi: 10.1093/ije/dyq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F. China Free ART Manual. Beijing: Chinese Center for Disease Control and Prevention; 2012. [Google Scholar]
- 22.Ma Y, Zhao D. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50:264–271. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesh KK, Srikrishnan AK, Mayer KH, et al. Predictors of nonadherence to highly active antiretroviral therapy among HIV-infected South Indians in clinical care: Implications for developing adherence interventions in resource-limited settings. AIDS Patient Care STDS. 2010;24:795–803. doi: 10.1089/apc.2010.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adakun SA, Siedner MJ, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62:317–321. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muya AN, Geldsetzer P, Hertzmark E, et al. Predictors of nonadherence to antiretroviral therapy among HIV-infected adults in Dar es Salaam, Tanzania. J Int Assoc Provid AIDS Care. 2014;14:163–171. doi: 10.1177/2325957414539193. [DOI] [PubMed] [Google Scholar]
- 26.Feller DJ, Agins BD. The dissociation between viral load suppression and retention in care. AIDS Patient Care STDS. 2016;30:103–105. doi: 10.1089/apc.2015.0209. [DOI] [PubMed] [Google Scholar]
- 27.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Li X, Lin Z, et al. Side effects, adherence selfefficacy, and adherence to antiretroviral treatment: A mediation analysis in a Chinese sample. AIDS Care. 2016;28:919–926. doi: 10.1080/09540121.2015.1124984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients:A collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study [J] Ann Intern Med. 2011;154:509. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujie Z, Hao Z, Yasong W, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–12: A retrospective observational cohort study. Lancet Infect Dis. 2014;14:1065–1072. doi: 10.1016/S1473-3099(14)70946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jie L, Daiming F. Hepatitis B in China. Lancet. 2007;369:1582–1583. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 36.Merati DT, Supriyadi, Yuliana F. The disjunction between policy and practice: HIV discrimination in health care and employment in Indonesia. AIDS Care. 2005;17(Suppl 2):175–179. doi: 10.1080/09540120500119932. [DOI] [PubMed] [Google Scholar]
- 37.Okta S, Merati TP, Paxton S, et al. AIDS-related discrimination in Asia. AIDS Care. 2005;17:413–424. doi: 10.1080/09540120412331299807. [DOI] [PubMed] [Google Scholar]
- 38.Moon S, Van LL, Durier N, et al. Out-of-pocket costs of AIDS care in China: Are free antiretroviral drugs enough? AIDS Care. 2008;20:984–994. doi: 10.1080/09540120701768446. [DOI] [PubMed] [Google Scholar]
- 39.Mona L, Margaret J, Sharon W, et al. The association between HIV disclosure status and perceived barriers to care faced by women living with HIV in Latin America, China, Central/Eastern Europe, and Western Europe/Canada. AIDS Patient Care STDS. 2016;30:435–444. doi: 10.1089/apc.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu L, Osborn CY, Qian HZ, et al. Barriers and facilitators of linkage to and engagement in HIV care among HIV-positive men who have sex with men in China: A qualitative study. AIDS Patient Care STDS. 2016;30:70–77. doi: 10.1089/apc.2015.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horberg MA, Hurley LB, Klein DB, et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDS. 2015;29:582–590. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]


