Abstract
Wnt signal cascade is an evolutionarily conserved, developmental pathway that regulates embryogenesis, injury repair, and pathogenesis of human diseases. It is well established that Wnt ligands transmit their signal via canonical, β-catenin-dependent and noncanonical, β-catenin-independent mechanisms. Mounting evidence has revealed that Wnt signaling plays a key role in controlling early nephrogenesis and is implicated in the development of various kidney disorders. Dysregulations of Wnt expression cause a variety of developmental abnormalities and human diseases, such as congenital anomalies of the kidney and urinary tract, cystic kidney, and renal carcinoma. Multiple Wnt ligands, their receptors, and transcriptional targets are upregulated during nephron formation, which is crucial for mediating the reciprocal interaction between primordial tissues of ureteric bud and metanephric mesenchyme. Renal cysts are also associated with disrupted Wnt signaling. In addition, Wnt components are important players in renal tumorigenesis. Activation of Wnt/β-catenin is instrumental for tubular repair and regeneration after acute kidney injury. However, sustained activation of this signal cascade is linked to chronic kidney diseases and renal fibrosis in patients and experimental animal models. Mechanistically, Wnt signaling controls a diverse array of biologic processes, such as cell cycle progression, cell polarity and migration, cilia biology, and activation of renin–angiotensin system. In this chapter, we have reviewed recent findings that implicate Wnt signaling in kidney development and diseases. Targeting this signaling may hold promise for future treatment of kidney disorders in patients.
1. INTRODUCTION
Wnt signaling is one of the most highly conserved pathways throughout evolution, and it plays important roles in a diverse array of biologic processes, such as embryonic development, metabolism, tumorigenesis, and stem cell renewal.1–3 The first Wnt-1 gene, also known as Wingless in Drosophila and Int-1 in mammalians, was discovered based on the activation by integration of virus DNA in mouse breast tumors in 1982.4 Sequence analyses revealed that Wnt-1 was actually the homolog of the Wingless (wg) gene in Drosophila that controls segment polarity in the formation of the body axis during embryonic development. Since then, totally 19 Wnt ligands in mammalian genomes are identified, which possess unique structural features and distinct expression patterns. The signal is initiated through the engagement of Wnt ligands to the extracellular domain of frizzled (Fzd) receptor and coreceptors, the low-density lipoprotein receptor-related protein 5 and 6 (LRP5 and LRP6).5 Depending on the involvement of key intracellular molecule β-catenin, Wnt signaling is typically classified as either β-catenin-dependent, canonical or β-catenin-independent, noncanonical pathways. The latter includes Wnt/planar cell polarity (PCP) and Wnt/Ca2+ signaling routes.5–7
Wnt ligands and their receptors are expressed in developing kidney during nephron formation.8,9 Although this signal is relatively silent in adult, it becomes reactivated rapidly after a wide variety of injuries.10–12 In the past decades, tremendous efforts have been made to delineate the expression, function, and targets of Wnt/β-catenin signaling in the developing kidneys, as well as in damaged kidneys after injury.6,13 In this chapter, we provide a comprehensive review on the expression, regulation, and function of Wnt/β-catenin signaling in the context of kidney development and disorders.
2. WNT SIGNALING: COMPONENTS AND ROUTES
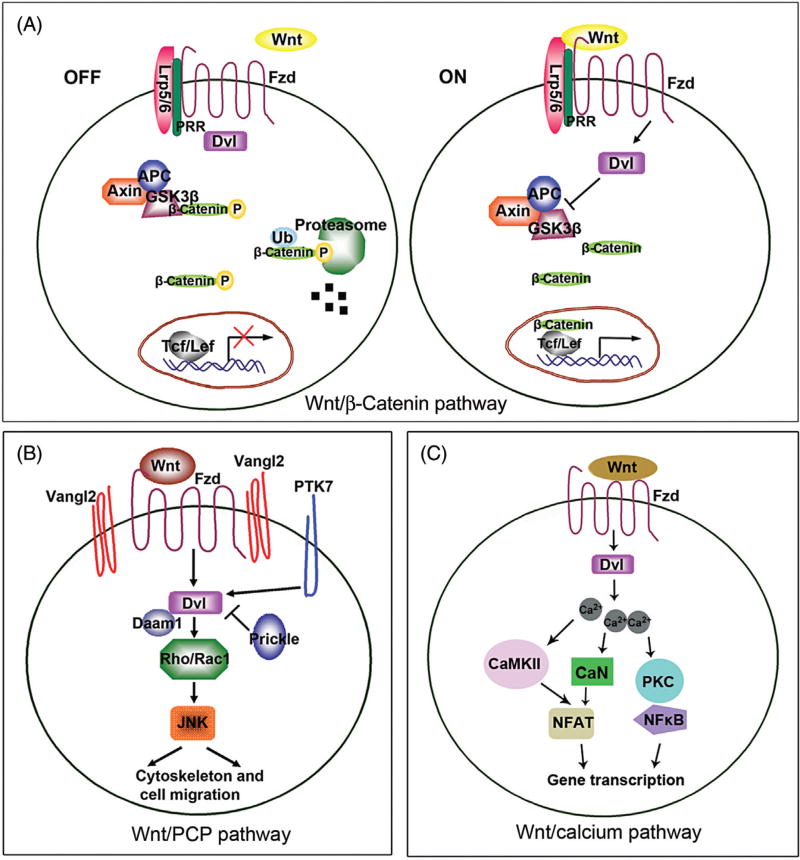
The canonical pathway of Wnt signaling is mediated by intracellular protein β-catenin (Fig. 1). In the absence of Wnt ligands-receptors engagement, cellular β-catenin levels are limited due to the phosphorylation-triggered proteasome degradation (Fig. 1A). Upon activation, Wnt ligands bind to the Fzd and LRP5/6 receptors, thereby activating the cytoplasmic protein dishevelled (Dvl). Dvl then inhibits the β-catenin destruction complex, consisting of the scaffold proteins Axin, adenomatous polyposis coli (APC), serine/threonine kinases of glycogen synthase kinase-3β (GSK-3β), and casein kinase 1 (CK1). Unphosphorylated β-catenin accumulates in the cytoplasm and enters the nucleus, where it interacts with T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) family of transcription factors to regulate Wnt target genes (Fig. 1A).14
Fig. 1.
Different Wnt signaling pathways. (A) Canonical Wnt/β-catenin signal pathway. When the signal is in “ON” state, Wnt ligands bind to Frizzled (Fzd) receptors and LRP5/6 coreceptors, which causes Dishevelled (Dvl) to inhibit the β-catenin destruction complex consisting of adenomatous polyposis coli (APC), Axin, and GSK-3β. Pro(renin) receptor (PRR) is also an obligatory component of the Wnt receptor complex and required for canonical Wnt/β-catenin signaling. Stabilized β-catenin is able to enter the nucleus and acts with T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) transcription factor for specific gene expression. When the signal is “OFF,” Dvl cannot act on the destruction complex and β-catenin is then phosphorylated by GSK-3β, leading phosphorylated β-catenin for degradation by ubiquitin (Ub)-proteasome system. (B) Noncanonical Wnt/PCP signal pathway. In Wnt/PCP pathway, Wnt ligands can bind Fzd, Vangl2, and PTK7, then acts with Rho/Rac1 small GTPases and JNK kinases through Dvl. Subsequently, cytoskeleton organization and cell migration are regulated in response to the signal. Daam1 acts with Dvl for downstream effectors, while Prickle inhibits this function. (C) Noncanonical Wnt/Ca2+ pathway. Wnt ligand binding activates Fzd/Dvl and induces a rise in intracellular Ca2+ levels. Elevated intracellular Ca2+ activates calcium calmodulin-dependent protein kinase II (CaMKII), calcinurin (CaN), and protein kinase C (PKC), which activate nuclear transcription factors (NFAT and NF-κB) and promote the expression of downstream target genes.
Recent studies indicate that pro(renin) receptor (PRR) may function as an amplifier of the canonical Wnt/β-catenin signaling (Fig. 1A).15 PRR is a transmembrane protein with multiple distinct functions.16 We reported that PRR is an essential component of the Wnt receptor complex and is obligatory for its signal transduction. Meanwhile, Wnt/β-catenin controls PRR gene and induces its expression in kidney tubular epithelial cells. Therefore, PRR induction and Wnt/β-catenin activation constitute a self-perpetuating cycle, which leads to the amplification of Wnt/β-catenin signaling.15,17
Wnts are also able to transmit their signals via β-catenin-independent, noncanonical pathways (Fig. 1B–C).3,6 The Wnt/PCP pathway is evolutionarily conserved and plays a major role in embryonic tissue patterning, cell polarization, migration, and morphogenesis (Fig. 1B).18 The Wnt/PCP ligands, such as Wnt5, Wnt7, and Wnt11, bind to the 7-transmembrane Fzd receptor and then recruit the cytoplasmic scaffold protein Dvl to the plasma membrane. Additional downstream components of the Wnt/PCP pathway include Van Gogh homolog 2 (Vangl2), Celsr1, Prickle, protein tyrosine kinase 7 (Ptk7), and Scrib, which work together to establish planar polarity. The Wnt/PCP effectors then regulate cytoskeleton dynamics directing asymmetric distribution of cellular components and migration. Many targets, such as Daam1, Rho, Rac, Rho kinase, C-Jun N-terminal kinase (JNK) are involved in these final steps (Fig. 1B).19
Another noncanonical Wnt signaling is the Wnt/Ca2+ pathway, which leads to the release of intracellular Ca2+, possibly via G-proteins (Fig. 1C).3,6 This pathway involves activation of phospholipase C (PLC) and protein kinase C (PKC). Elevated Ca2+ can activate the phosphatase calcineurin, which leads to dephosphorylation of the nuclear factor of activated T-cells (NFAT) and its accumulation in the nucleus (Fig. 1C). The Ca2+-mediated pathway has critical roles in dorsal/ventral patterning, gastrulation, and cardiac development. While the involvement of Wnt/β-catenin and Wnt/PCP signaling in regulating kidney development and disease is well studied, little is known about the role of Wnt/Ca2+ pathway in these processes.
3. WNT SIGNALING AND KIDNEY DEVELOPMENT
3.1 Major Events in Nephrogenesis
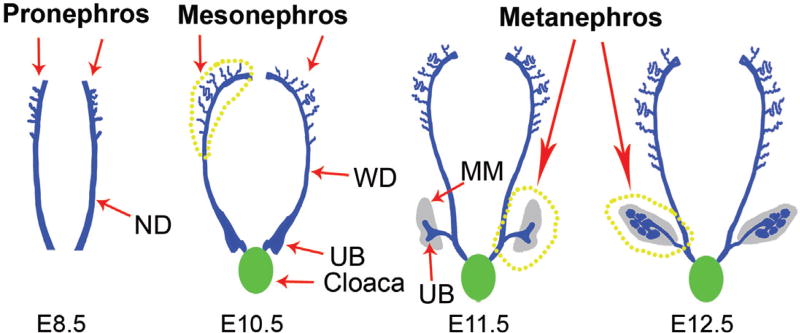
Significant advances have been made in deciphering molecular events in kidney development, making it possible to recognize the temporal and spatial pattern of embryonic kidney and its progenitors (Fig. 2).20 The mammalian kidney originates from intermediate mesoderm (IM) after gastrulation, and then lies along the anteroposterior axis between paraxial mesoderm and IM.21 There are three stages of kidney development in a temporal and craniocaudal sequence, and each is marked by the successive development of a more advanced kidney: pronephros, mesonephros, and metanephros (Fig. 2). The pronephros, also named primitive kidneys, develops at embryonic day 8.5 (E8.5) in a mouse embryo and at approximately the sixth somite in humans, and consists of 6–10 pairs of tubules.22 This organ is considered as transient structures in mammalians and completely disappears, but it is essential for the next coming cells and tissues, including the mesonephric kidney. The mesonephros, also named middle kidney, develops by the formation of mesonephric tubules from the IM and is physiologically functional during early embryonic life with the structure of mesonephric tubules and ducts [Wolffian ducts (WD)] at E10 in mouse embryo. The mesonephric duct reaches the cloaca with the proximal end and convoluted tubules blending into the duct. An important aggregate adjacent to the duct is called the metanephric mesenchyme (MM). The mesonephros gradually degenerates, but it also contributes to other organs, such as reproductive tracts of the testes, epididymal ducts, and vas deferens in all vertebrates. The metanephros, also named permanent kidney, begins in week 5 of gestation in humans and at E10.5 in mice. Metanephros is also derived from the IM, and finally becomes the functional adult kidney in mammalians (Fig. 2).23
Fig. 2.
Diagram depicts the major events in mammalian nephrogenesis. There are three stages of kidney development in a temporal sequence: pronephros, mesonephros, and metanephros. The pronephros develops at embryonic day 8.5 (E8.5) in mice. The mesonephros develops by the formation of mesonephric tubules from the intermediate mesoderm. The metanephros, begins at E10.5 in mice, finally becomes the permanent and functional kidney in mammalians. MM, Metanephric mesenchyme; ND, nephric duct; UB, ureteric bud; WD, Wolffian ducts.
The process of metanephric nephrogenesis is initiated by the invasion and reciprocal interaction between the ureteric bud (UB) and MM.24 The UB arises from an outgrowth of the Wolffian duct and invades the MM, and induces the surrounding mesenchyme to undergo a mesenchymal to epithelial transition (MET), further forming the renal vesicles (RVs). The RVs subsequently differentiate through comma- and S-shaped body stages, and give rise to glomerular podocytes, parietal epithelium cells, proximal, and distal tubule cells25; whereas the UB undergoes branching morphogenesis to form collect duct. As such, the metanephros finally gives rise to ureteric tree and mature kidney.26
3.2 Wnt Ligands and Nephron Formation
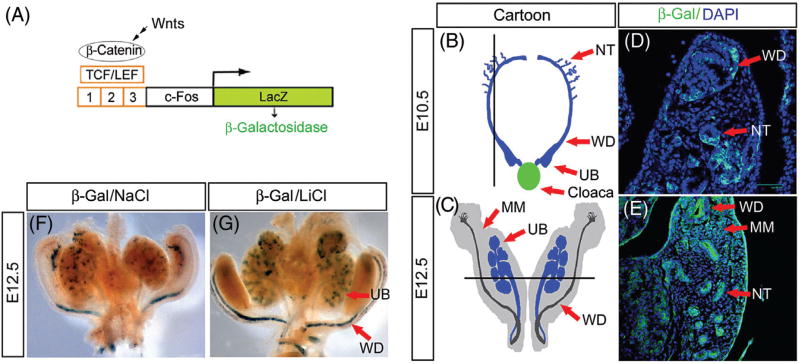
Wnt signaling plays critical roles in several processes during kidney development, such as UB induction and nephrongenesis (Table 1).27 Using β-catenin responsive TCF/β-gal reporter mice (Fig. 3A),28 canonical Wnt signaling activation was detected in the epithelia of branching UB and MM.29 This signal is quickly decreased in mature nephrons and disappeared in postnatal kidney. The Wnt inhibitor, Dickkopf-1 (Dkk1), could disrupt UB growing in cultured fetal kidney explants, which confirms the intense canonical Wnt signaling in branching nephrogenesis.29 Active β-catenin signal is detectable within the mesenchymal progenitor pool of RVs in mice, which is both necessary and sufficient to regulate RVs and induces the expected molecular responses.30 Both microarray and genetic analysis revealed that there is Wnt-dependent pathway regulated by protein kinase A (PKA) via Wnt induction during progression of the MM to tubular differentiation.31 Wnt signaling also patterns the proximal-distal nephron axis through enriching in the distal and decreasing in the proximal region of the forming nephron in chick models.32 It is concluded that high Wnt levels induce tubular components, whereas areas with low level of Wnt ligands give rise to glomerular elements.32
Table 1.
Wnt Components Involved in Kidney Development.
| Wnt Components |
Developmental Roles | KO Renal Defects | References |
|---|---|---|---|
| Wnt1 | Inducing tubulogenesis | ? | 34 |
| Wnt2b | Inducing ureter branching | ? | 37 |
| Wnt4 | Initiating renal development | Müller duct regression and renal dysgenesis | 38–44 |
| Wnt5a | Regulating metanephric mesenchyme | CAKUT | 45–48 |
| Wnt6 | Inducing ureter bud branching | ? | 49 |
| Wnt7b | Maintaining cortico-medullary axis | Fails to form medullary zone and concentrate urine | 50 |
| Wnt9b | Regulating progenitors in metanephric mesenchyme | Disrupts tubular cell division and increases diameter | 51–55 |
| Wnt11 | Autocrine factor in nephric duct and ureteric epithelium | Disrupts UB branching and causes renal hypoplasia | 56–58 |
| Lrp6 | Acting through Ret signal pathway for renal defects | Hypoplasia cystic kidney | 59 |
| Frizzle4 | Coordinating with Frizzle8 | Frizzle4/8 double KO exhibits reduced ureteric bud growth | 61 |
| Frizzle8 | Coordinating with Frizzle4 to maintain normal ureteric epithelial function | Frizzle4/8 double KO exhibits reduced ureteric bud growth | 60 |
| GSK-3β | Inducing early nephrogenesis | ? | 62,63 |
| β-Catenin | Critical for nephron progenitor maintenance and nephrogenesis | Conditional deletion at different lineages causes various renal defects, including aplasia, hypoplasia and cysts | 64–68 |
| Vangl2 | Indispensable for normal morphogenesis of both UB and MM-derived structures | Impairs branching morphogenesis and causes cystic kidney | 73 |
| Fat4 | Regulating cell division and tubular elongation via acting with vangl2 | Cystic kidney | 75 |
| Daam1 | Promoting pronephric tubulogenesis | Disrupts tubule elaboration and branching in pronephic proximal segments | 76 |
CAKUT, Congenital anomalies of the kidney and urinary tract; MM, metanephric mesenchyme; UB, ureteric bud; ?, undefined.
Fig. 3.
Wnt/β-catenin signaling is activated during kidney development. (A) Diagram depicts the construction of the Wnt/β-catenin responsive reporter TOPgal mice. (B–E) Wnt/β-catenin is activated in different time points during kidney development. The black lines in Panels B and C indicate dissection planes for corresponding embryonic kidneys of Panels D and E, respectively. At E10.5 (D) and E12.5 (E), Wnt/β-catenin signaling reporter TOPgal is activated in the nephric tube (NT), Wolffian ducts (WD), and metanephric mesenchyme (MM). (F–G) GSK-3β inhibition by lithium chloride leads to β-catenin-mediated gene expression in TOPgal reporter mice. Maternal injection of the Wnt agonist LiCl significantly enhanced the activity of the reporter β-galactosidase (G), compared with the NaCl-treated mouse embryonic kidneys (F). LEF, Lymphoid enhancer-binding factor; TCF, T cell factor; UB, ureteric bud.
Deficiencies in Wnt ligands have been linked to serious renal developmental defects.33 The first significant observation indicates that Wnt1 could replace UB for the induction of tubulogenesis.34 Thus far, a total of 7 Wnt ligands have been found during kidney ontogeny, including Wnt2b, Wnt4, Wnt5a, Wnt6, Wnt7b, Wnt9b, and Wnt11 (Table 1).35,36 Wnt2b and Wnt4 are expressed in the kidney mesenchymal cells.37,38 Studies suggest that Wnt4 plays central roles in the initial stages of renal development,39 and it does so by both canonical and noncanonical pathways.40,41 Furthermore, Wnt4 is able to coordinate with bone morphogenetic protein 4 (BMP4) to regulate the smooth muscle cells in medullary stroma for renal vascular development and maturation.42 Patients with aWnt4 mutation are associated with Müllerian-duct regression and renal defects.43,44
Wnt5a is also critical for early kidney development, as suggested by recent studies.45 Wnt5a is shown to act through Ror2 for the induction of metanephric mesenchyme and renal morphogenesis via a noncanonical pathway.46,47 Mutation of Wnt5a could lead to urogenital defects of the congenital anomalies of the kidney and urinary tract (CAKUT), which disrupts multiple tissue differentiation and development.48 Wnt6, Wnt7b, Wnt9b, and Wnt11 are all expressed in the UB during the early stages of development. Aside from its role in UB branching morphogenesis, Wnt6 could rescue the Wnt4 mutant embryos and upregulate Wnt4 transcription for ureter epithelialization.49 Wnt7b acts in cortico-medullary axis, and embryonic kidneys fail to form medullary zone and are unable to concentrate urine normally in Wnt7b−/− null mice.50
Wnt9b is one of the major ligands in the organization of mammalian urogenital system.51 The Wnt9b-expressing cells functionally act for the inductive response in metanephric mesenchyme, which is critical for the development of mesonephric/metanephric tubules and extension of the Müllerian duct.51 In order to keep the balance between maintenance of renal mesenchymal progenitor and mesenchymal to epithelial transition (MET) differentiation, Wnt9b controls subsets of progenitor cell’s state for differentiation or undifferentiation.52 Further evidences demonstrate that active Wnt9b/β-catenin signaling in Six2 positive progenitors is required for their renewal/proliferation.52 The transgenic mice with overexpression of Wnt9b in Six2 lineage display a disrupted cell fate decisions and severe renal defects, such as CAKUT.53 After deletion of β-catenin specifically in the kidney stroma, the fibroblasts in kidney capsule, cortex, and medulla, the expression of Wnt9b was markedly reduced in adjacent ureteric epithelial cells, suggesting the role of Wnt9b as effector downstream of β-catenin during induction of nephron progenitors.54 It is interesting to note that Wnt9b participates in the planar cell polarity of epithelium and contributes to the size of tubular diameters. The mice with attenuation of Wnt9b exhibit randomly oriented cell divisions and significantly increase tubular diameter, which act through the noncanonical Rho/JNK pathway during kidney morphogenesis.55
Wnt11 was first identified to be expressed in the nephric duct prior to the outgrowth of UB in control of its branching.56 This expression is independent of Wnt4 and MM specific factors, suggesting that it acts as an autocrine factor inside the ureteric epithelium.56 The Wnt11 gene in human embryos displays its restricted expression in the tips of the UB. There is an important feedback loop among Wnt11, Ret tyrosine kinase receptor, and glial cell-derived neurotrophic factor (GDNF).57 Deficiency of the Wnt11 gene disrupted the UB branching morphogenesis and led to renal hypoplasia in embryonic mice, which was rescued by maintaining GDNF expression.57 Furthermore, Wnt11 expression is reduced in the absence of Ret/GDNF signaling.57 These results illustrate the synergistic interaction of Wnt11/GDNF/Ret and their positive autoregulatory mechanism during normal ureteric branching morphogenesis. The role of Wnt11 in later stage of mammalian kidney organogenesis is established and supported by the anomalies in kidney tubular system and secondary glomerular cysts in Wnt11−/− null mice, which is consistent with its early involvement in UB branching.58 These effects presumably act through several downstream genes implicated in kidney development, such as Wnt9b, Six2, Foxd1, Hox10, and Dvl2.58
3.3 Wnt Signaling Effectors and Kidney Development
Our previous studies exhibit that the key Wnt coreceptor LRP6 plays critical roles during early kidney development.59 The LRP6 knockout mice exhibit severe urogenital defects with hypoplasia cystic kidney (Table 1). Further studies reveal that the UB inductive factor of Ret acts downstream of LRP6-mediated Wnt signaling, which is most likely responsible for renal defects in mutant mice.59 After inhibition of Xenopus frizzled-8 (Xfz8), one of the Fzd receptor of Wnts, defects in pronephric tubule branching are evident, suggesting a role for Xfz8 in maintaining normal epithelium structure of pronephric duct and tubules.60 The phenotypes of targeted mutations in Fzd4 and Fzd8 result in disrupted UB growth and reduction of kidney size, but there might be homeostatic network regulations in the mutants of Fzd4−/− or Fzd4−/−; Fzd8−/− cells compared with wild-type controls, which can account for discrepancies in renal phenotypes.61 Through inhibition of GSK-3β, the Wnt agonist lithium is able to induce early stages of epithelial differentiation in isolated nephrogenic mesenchyme, indicating the possible molecular mechanisms in the early events of nephrogenesis.62 These results are further confirmed by independent group using GSK-3β inhibitor lithium or 6-bromoindirubin-3′-oxime (BIO), which result in abundant epithelial differentiation and full segregation of nephrons.63 Furthermore, stabilized β-catenin and upregulated LEF1 and TCF1 are capable of inducing nephron differentiation in isolated kidney mesenchymes as well.63
As β-catenin is the principal intracellular mediator in canonical Wnt signaling, more attentions have been paid to elucidate its roles during kidney development. Active form of β-catenin is detectable as early as E6.5 in mouse embryo, and contributes to anterior–posterior axis, primitive streak, and mesoderm formation.64 In vitro and in vivo studies indicate that Six2-dependent self-renewal and β-catenin-directed differentiation institutes a regulatory complex, which keeps the balance between nephron progenitor maintenance and nephrogenesis.65 Conditional deletion of β-catenin in mouse WD epithelium with Hoxb7-Cre causes defect in branching morphogenesis and results in renal aplasia or hypoplasia.66 Multiple putative targets of β-catenin/TCF transcripts, such as cyclin D1 and Emx2, are detected along renal epithelial differentiation in organ culture of rat embryonic kidney.67 In order to delineate the role of β-catenin at the late S-shaped body stage, mice with conditional deletion of β-catenin was created in the developing kidney by using Pax8-Cre. The phenotypes of these mice include abnormal kidneys with reduced renal function, hypoplastic renal parenchyma with a thin cortex, missing of superficial layer of renal tubules, and absence of parietal epithelial cells in Bowman’s capsule.68 These results suggest an indispensable role of Wnt/β-catenin signaling in the late stages of nephrogenesis, although one cannot completely exclude the possibility that some effects of β-catenin ablation may result from its role in regulating adherent junctions.
3.4 Wnt/PCP Pathway and Nephron Maturation
Increasing evidence suggests a critical role for Wnt/PCP signaling in kidney development and function. The PCP is defined for the cells in tissue perpendicular to the apical–basal axis, which was first described in Drosophila.69 Deficiency of core PCP genes, such as Vangl2, Celsr1, Fzd3/6, and Dvl1/2, have been shown to disrupt asymmetric localization, convergent extension, hair cell organization, neural tube closure, and to cause cystic kidney in mammals.70 Some PCP components are expressed in the renal developing epithelia, such as UB, RVs, and S-shaped body, suggesting their functions in cell division orientation, movements, adhesion, and contribution to morphogenesis of the mature nephron.71 Several systematic analyses have implicated the function of PCP in UB branching and elongation to determine tubular diameter, length, and shape.72,73
Transgenic mouse models with mutations in Wnt ligands of the PCP pathway (Wnt5a, Wnt7b, Wnt9b, and Wnt11) exhibit UB branching defects. The noncanonical Wnt5a/Ror2 signaling in IM-derived nephric duct controls UB expansion, and Wnt5a knockout mice exhibit duplicated ureters and kidneys because of abnormal GDNF signaling and spatiotemporally aberrant interaction between MM and WD.46,47 The absence of Wnt7b ligand displayed normal development of cortical epithelium but was incapable of forming medullary zone and concentrated urine.50 This phenotype is explained by the disorganization of cell division planes in the collecting duct epithelium of the emerging medullary zone. Likewise, Wnt9b is suggested to be essential in establishing the length and diameter of the kidney tubules, while deletion ofWnt9b during kidney morphogenesis disrupts PCP in the epithelium and leads to polycystic kidney disease (PKD).55 Besides its role in convergent extension in fish, Wnt11 regulates Ret/GDNF loop for UB branching and metanephric development.57
The key PCP moleculeVangl2 is expressed in UB, collecting duct, MM, and nephron, whose mutation impairs branching morphogenesis and causes cystic kidney.74 After podocyte-specific ablation of Vangl2 gene, the mutants show significantly smaller glomeruli with maturation defects.75 These results indicate the important role of Vangl2 in podocytes morphology and function.75 Another Drosophila PCP protein, fat, is essential in vertebrate PCP pathway. Deletion of fat4 caused cystic kidney owing to disrupted cell division and tubule elongation upon interaction with Vangl2.76 The PCP component of Daam1 is expressed at developing pronephric anlagen and interacts with the novel weak-similarity Rho-GEF (WGEF). Knockdown of Daam1 led to downregulation of late pronephric epithelial markers, possible through Daam1/Rho-GEF axis to inhibit pronephric tubulogenesis.77 Therefore, Wnt/PCP is fundamental for kidney development and function and requires to be the focus of more research.
3.5 Wnt Signaling and Cystogenesis
Wnt/β-catenin signaling is involved in renal polycystic lesions, which was first illustrated by overexpression of β-catenin in epithelial cells of the kidney. The transgenic mice exhibited severe polycystic kidney with abnormal renal function, similar to human autosomal dominant polycystic kidney disease (ADPKD).78 After conditional deletion of APC in renal epithelium, there is multiple cysts formation with increasing levels of β-catenin protein.79 Aquaporin-1 (AQP1), the molecular marker of renal proximal tubules and thin descending limb of Henle, could interact with β-catenin in models of ADPKD. The AQP1 knockout mice increased cysts development with upregulation of Wnt/β-catenin signal.80 These results show that the activation of Wnt/β-catenin signaling is required for renal cystogenesis. Consistently, conditional stabilization of β-catenin with Hoxb7-Cre from mouse tubular epithelium led to cystic kidney and hydronephrosis.66 On the other hand, the cystic kidney and abnormalities are also induced by loss of the Jouberin (Jbn) protein, owing to a downregulation of endogenous Wnt activity.81 Jbn was found to interact with and facilitate β-catenin nuclear accumulation, resulting in positive modulation of its downstream transcription.81 Our previous studies also demonstrate that global deletion of LRP6 causes renal hypoplasia and cysts formation, further confirming the role of LRP6-mediated Wnt/β-catenin signaling in the process.59 However, there are controversies in the literature regarding the role of canonical Wnt/β-catenin signaling in cystogenesis. Mice with Inversin gene mutation had developmental abnormalities and renal cysts formation, although canonical Wnt/β-catenin signaling was not altered as defined in the Wnt reporter BATlacZ mice.82 Additional studies also indicate normal TCF/β-catenin activity in two different models of PKD.83 Therefore, the unique and precise regulation of Wnt/β-catenin signaling is required during kidney development and disruption of which leads to a cystic abnormality in a content-dependent manner.
Polycystic kidneys are also caused by the genetic mutations, which are related to primary cilia and Wnt.84 The impaired cilia led to a disturbed fluid flow and calcium influx that initiate cyst formation. Because the tubular epithelial cells undergo oriented cell division during tubular elongation, the Wnt/PCP signal might be critical for the cyst formation.85 There is evidence that maturation of nephrons is associated with mitotic orientation and intrinsic PCP signal, and abnormality of this pathway causes polycystic kidney in animal models.86 It is interesting to point out that gene mutation of cilia-associated protein Inversin led to nephronophthisis type II, which is an autosomal recessive cystic kidney disease with extensive renal cysts formation.87 It becomes apparent that Inversin acts between canonical and noncanonical Wnt signaling for normal tubular differentiation.87 Based on these observations, it is conceivable that Wnt/β-catenin signaling is sufficient to induce cysts formation, but compromised Wnt/PCP pathway is also involved.88 Therefore, both canonical and noncanonical Wnt signaling are important in kidney development, and disruption of either pathway can cause cystic kidney disease.89 As many proteins, including Vangl, Fz, and Dvl, have been found to localize in the ciliary basal body,90 changes in their expression and structure would eventually cause defective primary cilia in cystic kidney disease.
4. WNT SIGNALING AND KIDNEY DISEASES
4.1 Wnt Signaling in Acute Kidney Injury
Progresses have been made in recent years to demonstrate a key role of Wnt/β-catenin signaling in kidney injury and fibrosis, which is summarized in several recent reviews (Table 2).6,13 In both acute kidney injury (AKI) and chronic kidney disease (CKD) animal models, Wnt/β-catenin is activated, which could function as either renal protective or detrimental mechanisms.91,92 The AKI is described as a rapid decrease in kidney function, which involves hemodynamic alterations, inflammation, and endothelial and epithelial cell injury.93 The outcome of AKI is either adaptive leading to the restoration of epithelial integrity or maladaptive progressing to CKD.94 At different time points after AKI, the Wnt4-mediated β-catenin is activated, which contributes to recovery from renal injury. The activated β-catenin is able to promote cell cycle progression through its transcriptional targets, such as cyclin D1 in LLC-PK1 cells.95 Using the BATgal and Axin2-lacz Wnt signaling reporter mice, it has been shown that activation of Wnt7b signaling is renoprotective after ischemia reperfusion injury (IRI), and macrophages are the sources of Wnt7b during ischemic injury and repair process.96 In vitro, β-catenin signaling reduces Bax-mediated apoptosis and improves cell survival after induction of metabolic stress in proximal tubular epithelial cells. Constitutively activated β-catenin also regulated Bax activation and translocation to mitochondria upon acute stress injury.97 Consistent with these in vitro findings, mice with tubule-specific β-catenin knockout are more susceptible to AKI following either ischemic or toxic insults.92 These conditional knockout mice exhibit higher mortality rate and increased serum creatinine, and worsen the morphological injury. There is more tubular cell apoptosis in the β-catenin null kidneys exhibiting high levels of p53 and Bax.92 Another study using the Wnt agonist, a synthetic pyrimidine, also showed renal protective effects of Wnt signaling after IRI in rats through attenuating inflammation and oxidative stress, suggesting the potential pharmacological application of manipulating Wnt activity on preventing kidney injury.98
Table 2.
Wnt Components and Kidney Diseases.
| Diseases | Wnt Components | Roles | References |
|---|---|---|---|
| AKI | Wnt4 | Promotes tubular cell cycle progression | 94 |
| Wnt7b | Protective effects after IRI | 95 | |
| Wnt agonist | Improves renal function after IRI | 97 | |
| Tubular β-catenin | Protects against ischemic and toxic AKI | 96–98 | |
| CKD | Wnt ligands | Increased renal expressions after UUO | 10,102,103 |
| Wnt1 | Tubular induction of Wnt1 causes renal fibrosis | 103 | |
| Dkk1 | Inhibition of renal fibrosis after UUO | 10 | |
| sFRP4 | Inhibition of renal fibrosis | 12 | |
| Wntless | Tubular KO reduces renal fibrosis after UUO | 102 | |
| Klotho | Sequesters Wnts and inhibits kidney fibrosis | 113,114 | |
| Fibroblast β-Catenin | Activation of β-catenin in interstitial pericytes/fibroblasts causes renal fibrosis | 101 | |
| Tubular β-catenin | Tubular deletion of β-catenin fails to affect renal fibrosis after UUO | 135 | |
| PRR | Amplifies Wnt/β-catenin signaling and aggravates renal fibrosis | 15 | |
| Podocytopathy | Wnt1 | Wnt1 overexpression induces podocyte injury and albuminuria | 11 |
| β-Catenin | Podocyte-specific deletion of β-catenin reduces proteinuria | 11,13,115,118 | |
| β-Catenin | Podocyte-specific stabilization of β-catenin causes podocytopathy | 116 | |
| Cancer | Wnt1 | Positively correlates with tumor progression in RCC | 127 |
| Wnt7a | Tumor suppressor properties in RCC | 129 | |
| Wnt10a | Independent risk factors for RCC carcinogenesis | 128 | |
| Fzd5, Fzd8 | Biomarkers in RCC | 130 | |
| Fzd7 | High expression in RCC | 131 | |
| β-Catenin | Abnormal accumulation in RCC carcinogenesis, mutation involved in WTs | 127 |
AKI, acute kidney injury; CKD, chronic kidney disease; IRI, ischemia reperfusion injury; PRR, pro(renin) receptor; RCC, renal cell carcinoma; UUO, unilateral ureteral obstruction.
Severe or repeated AKI will lead to incomplete renal recovery and often progresses to CKD. We have shown that mice subjected to 20 min IRI exhibit transient Wnt/β-catenin activation, moderate AKI with complete recovery of renal function; whereas 30 min IRI causes sustained activation of this signaling and severe AKI, and eventually progresses to CKD characterized by renal fibrosis.99 A sustained activation of Wnt/β-catenin accelerates AKI to CKD progression characterized by interstitial myofibroblast activation and excessive extracellular matrix deposition, while blockade of Wnt/β-catenin prevents AKI to CKD progression.99 Therefore, the magnitude and duration of Wnt/β-catenin activation plays a decisive role in determining the outcomes of AKI.6,100
4.2 Wnt Signaling in Chronic Kidney Disease
Wnt/β-catenin signaling is also activated in fibrotic kidney initiated by unilateral ureteral obstruction (UUO), as well as in many other models of CKD (Table 2).10,12,101,102 The regulation and actions of Wnt/β-catenin signaling in the pathogenesis of fibrotic CKD has been reviewed previously.91,101 Secreted frizzled-related protein 4 (sFRP4), an endogenous extracellular Wnt antagonist, inhibits β-catenin activation and reduces renal fibrosis after UUO presumably via preventing Wnts binding to their receptors.12 A systematic analysis for the Wnt ligands in mouse model of UUO revealed that 16 out of 19 different Wnts are induced in the kidney in different levels at some points during the injury.10 Notably, the cellular source of Wnt ligands in the fibrotic kidneys is largely from the tubular epithelium.103,104 The activation of Wnt signaling leads to dramatic accumulation of β-catenin and upregulation of its target genes, such as c-Myc, Twist, TCF1, and fibronectin in renal epithelial cells.10 Both in vitro and in vivo studies illustrate that Wnt/β-catenin controls several key fibrosis-related downstream genes, such as Snail1, fibronectin, plasminogen activator inhibitor-1 (PAI-1), matrix metalloproteinase 7 (MMP-7), and multiple components of the renin–angiotensin system (RAS), including angiotensinogen, renin, angiotensin converting enzyme (ACE), and angiotensin receptor type 1 (AT1).11,105–108 Targeted inhibition of Wnt/β-catenin signaling by ICG-001, a small molecule peptidomimetic that selectively inhibits β-catenin signaling in a CBP-dependent fashion,109,110 suppresses matrix expression and ameliorates renal interstitial fibrosis.111 Therefore, inhibition of Wnt/β-catenin signaling may be an effective strategy to ameliorate kidney injury and fibrotic lesions in various models of CKD.91
The role of Wnt/β-catenin in renal fibrogenesis is supported by using the soluble form of Klotho as a secreted Wnt antagonist in renal fibrotic models. Klotho is antiaging protein that is highly expressed in renal tubular epithelium of normal kidney, which is suppressed in the elderly and in patients with CKD.112,113 Klotho is present as full-length, transmembrane protein or secreted, soluble form. Both membranous and soluble forms of Klotho can bind with multiple Wnt ligands and represses their target genes transcription.114 Overexpression of Klotho clearly hampers the activation of Wnt signal in mice with UUO, accompanied by reducing extracellular matrix deposition, bypassing the G2/M arrest and diminishing fibrotic cytokine production.114,115 TGF-β1, a master regulator of tissue fibrosis, could suppress Klotho expression and concomitantly activates β-catenin to promote myofibroblast activation and renal fibrosis.114 Klotho also attenuates Wnt1-triggered activation of RAS in a dose-dependent manner in renal epithelial cells.116 It is concluded that Klotho protects against renal fibrosis through sequestering and antagonizing Wnt/β-catenin signaling.
4.3 Wnt Signaling in Podocytopathy and Proteinuria
Activation of Wnt/β-catenin signaling also play a crucial role in mediating podocyte dysfunction, proteinuria, and glomerulosclerosis, leading to end stage renal disease (ESRD).13 Glomerular β-catenin is activated in adriamycin-induced kidney injury characterized by podocyte damage and albuminuria. In contrast, podocyte-specific β-catenin knockout mice are protected against albuminuria after adriamycin treatment.11,117 These results confirm a critical role for Wnt/β-catenin signaling in the development of podocytopathy and proteinuria. Further studies using the same conditional knockout mice show that β-catenin is instrumental in disrupting podocyte slit diaphragm (SD), leading to an impaired glomerular filtration barrier.117,118 Studies also demonstrated that Wnt/β-catenin plays a role in mediating the transforming growth factor-β1 (TGF-β1)-induced podocyte injury and proteinuria in vitro and in vivo.119 Overexpression of Wnt antagonist Dkk-1 gene alleviates TGF-β1-triggered podocyte damage and albuminuria. The action of β-catenin on podocytes includes its role in triggering ubiquitin-mediated degradation of Wilms’ tumor 1 (WT1), a key podocyte-specific transcription factor that is critical to maintain podocyte integrity and function.120 In that regard, β-catenin specifically targets WT1 for protein degradation, finally leading to podocyte dedifferentiation and mesenchymal transformation.120
Given the importance of β-catenin activation in mediating podocyte injury, it is conceivable that strategies modulating its activity may be effective in ameliorating podocytopathy and proteinuria. Indeed, vitamin D analogs, such as paricalcitol, preserves podocyte integrity and function, inhibits proinflammatory cytokines, suppresses expression of the fibrogenic genes, and also ameliorates established proteinuria in mice after adriamycin injection.121 Studies also indicate that angiotensin II (Ang II), the principal effector of RAS activation, acts at upstream of Wnt/β-catenin signaling, impairs podocyte integrity and causes albuminuria in mice.122 The Wnt antagonist Dkk1 attenuates Ang II-induced podocyte injury in vitro and in vivo.122 In addition, high glucose is shown to impair podocyte integrity by suppressing podocin and nephrin and inducing (pro)reninreceptor (PRR), Wnt3a, β-catenin, and Snail1. These results suggest a potential role of PRR-Wnt/β-catenin-Snail1 pathway in high glucose-triggered podocyte injury.123 More interestingly, all RAS genes, including angiotensinogen, renin, PRR, ACE, and AT1 are found to be novel downstream targets of Wnt/β-catenin, as overexpressions of either β-catenin or different Wnt ligands induces, but β-catenin inhibitor ICG-001 inhibits, their expression.15,108,124 Thus, there might be a vicious cycle between Wnt/β-catenin and RAS in the process of podocyte injury, and disruption of this cycle formation will preserve podocyte function and prevent proteinuria.13
4.4 Wnt Signaling and Human Kidney Disease
Aside from its roles in cultured cells and animal models, Wnt/β-catenin is also activated in clinical human samples of CKD.103 Active β-catenin is detectable in the podocytes of human kidney biopsy of CKD, such as diabetic nephropathy (DN) and focal segmental glomerulosclerosis (FSGS).11 Increased nuclear β-catenin accumulation is discovered in the peripheral blood leukocytes from immunoglobulin A nephropathy (IgAN) patients, suggesting that a hyperactivation of Wnt signal might contribute to the pathogenesis of IgAN.125 Upregulation of Wnt/β-catenin components is documented in the glomeruli and podocytes of diabetic kidney disease (DKD) patients, who exhibit glomerular basement membrane (GBM) abnormalities and albuminuria.118 There is increased activation of Wnt signaling in human collapsing glomerulopathies, indicating a potential role of Wnt pathway in the regulation of mature podocytes cell cycle progression and proliferation in renal disease state.126 In lupus nephritis patients, active Wnt/β-catenin signal is accompanied by increasing levels of plasmatic Dkk-1,127 probably reflecting that Dkk1 is a target of the β-catenin signaling.128 These alterations suggest a close relationship between hyperactive Wnt signaling and the pathogenesis of lupus nephritis.127 Collectively, the activation of Wnt signaling in AKI and CKD after injury triggers adaptive or maladaptive responses depending on the injury severity and duration. This activation could be indispensable for kidney repair and regeneration, or promotes disease progression and fibrosis in humans as well.6,100
4.5 Wnt Signaling and Kidney Cancer
As there are similarities between embryonic growth and abnormal cell proliferation in cancer, the Wnt signaling molecules are considered as candidates for the development of kidney cancers. The renal cell carcinoma (RCC) is the major form of kidney tumor with high frequency. Studies showed that Wnt1 is upregulated and associated with increased tumor size, more advanced stage and invasiveness in clear cell RCC (ccRCC).129 After screening normal kidney and RCC cell lines and tissues, investigators found that Wnt10a is significantly induced and acts as an independent risk factor for RCC carcinogenesis and progression.130 However,Wnt7a gene is inactivated in ccRCC and exhibits tumor suppressor properties, and there is positive correlation between tumor stage and Wnt7a hypermethylation.131 These observations demonstrate unique differences for canonical Wnts (Wnt1, Wnt10a) and noncanonical Wnts (Wnt7a) during tumorigenesis. The mRNA levels of Wnt receptors Fzd5 and Fzd8 are increased in RCC accompanied by active β-catenin signal and high levels of Wnt target cyclin D1, so Fzd5 and Fzd8 are considered as biomarkers in RCC.132 Another receptor, Fzd7, also shows higher levels of expression compared with surrounding normal tissues in RCC, which might be activated by a Wnt3a-mediated pathway.133 Altered expression of β-catenin is also detected in RCC, and abnormal accumulation of β-catenin, at least partially, contributes to renal carcinogenesis.129 Overall, Wnt signaling is constitutively activated in RCC and functional loss of Wnt antagonists may be one of the reasons for that process, and could be candidate for targeted therapies.134 Additional evidences showed that Wnt signal not only participates in RCC, but also in the pathogenesis of embryonic kidney-derived tumor, Wilms’ tumor (WT) or nephroplastoma, one of the most common pediatric malignancies.135 Because Wnt4 serves as critical renal developmental factor, the mutations of its downstream β-catenin gene might cause WTs. A protein named WTX, which is mutant and encoded by Wilms’ tumor gene in the X chromosome, could form a complex with β-catenin to promote its ubiquitination and degradation.136 Thus, both RCC and WTs require β-catenin signal participation for the process of tumorigenesis.
5. CONCLUSIONS
Normal kidney development requires complex and precise cell-cell communications, in which Wnt signaling is one of the main mediators. Extensive studies in last decades have revealed that Wnt signaling is indispensable not only in normal nephrogenesis but also in kidney repair and regeneration after AKI, as well as in the evolution of various CKD characterized by tissue fibrosis and renal insufficiency. Both canonical and noncanonical Wnt signaling are instrumental for UB induction, nephron formation, and maturation. Dysregulation of Wnt signaling is implicated in a wide variety of kidney disorders ranging from fibrosis, cystic formation, proteinuria to tumorigenesis. There is a tremendous progress in the field during last several years through the discovery of novel targets of Wnt/β-catenin signaling and delineating new mechanisms of its action. As there are many Wnt antagonists, such as small molecule inhibitor ICG-001 and soluble Klotho are currently available, it is hopeful that manipulation of this signaling pathway by diverse strategies will eventually translate into effective therapies for patients with various kidney disorders.
Acknowledgments
This work was supported by the National Science Foundation of China Grants 81521003 and 81770715, and the National Institutes of Health (NIH) Grants DK064005 and DK106049.
References
- 1.Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31:2685–2696. doi: 10.1038/emboj.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Zhou D, Tan RJ, Fu H, Liu Y. Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest. 2016;96:156–167. doi: 10.1038/labinvest.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 10.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Liu Y. Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–545. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y. (Pro)renin receptor Is an amplifier of Wnt/beta-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol. 2017;28:2391–2408. doi: 10.1681/ASN.2016070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010;21:18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 17.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 18.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokol SY. Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development. Semin Cell Dev Biol. 2015;42:78–85. doi: 10.1016/j.semcdb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AC, Muthukrishnan SD, Guay JA, Adams DC, Schafer DA, Fetting JL, Oxburgh L. Role for compartmentalization in nephron progenitor differentiation. Proc Natl Acad Sci USA. 2013;110:4640–4645. doi: 10.1073/pnas.1213971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietila I, Vainio SJ. Kidney development: an overview. Nephron Exp Nephrol. 2014;126:40. doi: 10.1159/000360659. [DOI] [PubMed] [Google Scholar]
- 23.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker KA, Bertram JF. Kidney development: core curriculum 2011. Am J Kidney Dis. 2011;57:948–958. doi: 10.1053/j.ajkd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev. 2004;14:550–557. doi: 10.1016/j.gde.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech Dev. 1997;62:105–120. doi: 10.1016/s0925-4773(97)00667-9. [DOI] [PubMed] [Google Scholar]
- 27.Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317:83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 28.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 29.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007;293:F494–F500. doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- 30.Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 31.Gallegos TF, Kouznetsova V, Kudlicka K, Sweeney DE, Bush KT, Willert K, Farquhar MG, Nigam SK. A protein kinase A and Wnt-dependent network regulating an intermediate stage in epithelial tubulogenesis during kidney development. Dev Biol. 2012;364:11–21. doi: 10.1016/j.ydbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider J, Arraf AA, Grinstein M, Yelin R, Schultheiss TM. Wnt signaling orients the proximal-distal axis of chick kidney nephrons. Development. 2015;142:2686–2695. doi: 10.1242/dev.123968. [DOI] [PubMed] [Google Scholar]
- 33.Halt K, Vainio S. Coordination of kidney organogenesis by Wnt signaling. Pediatr Nephrol. 2014;29:737–744. doi: 10.1007/s00467-013-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzlinger D, Qiao J, Cohen D, Ramakrishna N, Brown AM. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev Biol. 1994;166:815–818. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- 35.Merkel CE, Karner CM, Carroll TJ. Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatr Nephrol. 2007;22:1825–1838. doi: 10.1007/s00467-007-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4:55–59. doi: 10.4161/org.4.2.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y, Liu A, Zhang S, Ruusunen T, Kreidberg JA, Peltoketo H, Drummond I, Vainio S. Induction of ureter branching as a response to Wnt-2b signaling during early kidney organogenesis. Dev Dyn. 2001;222:26–39. doi: 10.1002/dvdy.1164. [DOI] [PubMed] [Google Scholar]
- 38.Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 39.Shan J, Jokela T, Skovorodkin I, Vainio S. Mapping of the fate of cell lineages generated from cells that express the Wnt4 gene by time-lapse during kidney development. Differentiation. 2010;79:57–64. doi: 10.1016/j.diff.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Saulnier DM, Ghanbari H, Brandli AW. Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol. 2002;248:13–28. doi: 10.1006/dbio.2002.0712. [DOI] [PubMed] [Google Scholar]
- 41.Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, Yamaguchi TP, Rodriguez LG, Perantoni AO. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. 2011;352:58–69. doi: 10.1016/j.ydbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itaranta P, Chi L, Seppanen T, Niku M, Tuukkanen J, Peltoketo H, Vainio S. Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev Biol. 2006;293:473–483. doi: 10.1016/j.ydbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Mullerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Xiao A, Choi SY, Kan Q, Zhou W, Chacon-Heszele MF, Ryu YK, McKenna S, Zuo X, Kuruvilla R, Lipschutz JH. Wnt5a is necessary for normal kidney development in zebrafish and mice. Nephron Exp Nephrol. 2014;128:80–88. doi: 10.1159/000368411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun K, Ajima R, Sharma N, Costantini F, Mackem S, Lewandoski M, Yamaguchi TP, Perantoni AO. Non-canonical Wnt5a/Ror2 signaling regulates kidney morphogenesis by controlling intermediate mesoderm extension. Hum Mol Genet. 2014;23:6807–6814. doi: 10.1093/hmg/ddu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishita M, Qiao S, Miyamoto M, Okinaka Y, Yamada M, Hashimoto R, Iijima K, Otani H, Hartmann C, Nishinakamura R, Minami Y. Role of Wnt5a-Ror2 signaling in morphogenesis of the metanephric mesenchyme during ureteric budding. Mol Cell Biol. 2014;34:3096–3105. doi: 10.1128/MCB.00491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pietila I, Prunskaite-Hyyrylainen R, Kaisto S, Tika E, van Eerde AM, Salo AM, Garma L, Miinalainen I, Feitz WF, Bongers EM, Juffer A, Knoers NV, Renkema KY, Myllyharju J, Vainio SJ. Wnt5a deficiency leads to anomalies in ureteric tree development, tubular epithelial cell organization and basement membrane integrity pointing to a role in kidney collecting duct patterning. PLoS One. 2016;11:e0147171. doi: 10.1371/journal.pone.0147171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itaranta P, Lin Y, Perasaari J, Roel G, Destree O, Vainio S. Wnt-6 is expressed in the ureter bud and induces kidney tubule development in vitro. Genesis. 2002;32:259–268. doi: 10.1002/gene.10079. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. AWnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 52.Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiefer SM, Robbins L, Rauchman M. Conditional expression of Wnt9b in Six2-positive cells disrupts stomach and kidney function. PLoS One. 2012;7:e43098. doi: 10.1371/journal.pone.0043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boivin FJ, Sarin S, Lim J, Javidan A, Svajger B, Khalili H, Bridgewater D. Stromally expressed beta-catenin modulates Wnt9b signaling in the ureteric epithelium. PLoS One. 2015;10:e0120347. doi: 10.1371/journal.pone.0120347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- 57.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- 58.Nagy II, Xu Q, Naillat F, Ali N, Miinalainen I, Samoylenko A, Vainio SJ. Impairment of Wnt11 function leads to kidney tubular abnormalities and secondary glomerular cystogenesis. BMC Dev Biol. 2016;16:30. doi: 10.1186/s12861-016-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Stokes A, Duan Z, Hui J, Xu Y, Chen Y, Chen HW, Lam K, Zhou CJ. LDL receptor-related protein 6 modulates Ret proto-oncogene signaling in renal development and cystic dysplasia. J Am Soc Nephrol. 2016;27:417–427. doi: 10.1681/ASN.2014100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Satow R, Chan TC, Asashima M. The role of Xenopus frizzled-8 in pronephric development. Biochem Biophys Res Commun. 2004;321:487–494. doi: 10.1016/j.bbrc.2004.06.166. [DOI] [PubMed] [Google Scholar]
- 61.Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development. 2011;138:1161–1172. doi: 10.1242/dev.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davies JA, Garrod DR. Induction of early stages of kidney tubule differentiation by lithium ions. Dev Biol. 1995;167:50–60. doi: 10.1006/dbio.1995.1006. [DOI] [PubMed] [Google Scholar]
- 63.Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of beta-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J Am Soc Nephrol. 2007;18:1130–1139. doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- 64.Mohamed OA, Clarke HJ, Dufort D. Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 65.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, Yang J, Paragas N, Wallace VA, Dufort D, Pavlidis P, Jagla B, Kitajewski J, Barasch J. Beta-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development. 2007;134:3177–3190. doi: 10.1242/dev.006544. [DOI] [PubMed] [Google Scholar]
- 68.Grouls S, Iglesias DM, Wentzensen N, Moeller MJ, Bouchard M, Kemler R, Goodyer P, Niggli F, Grone HJ, Kriz W, Koesters R. Lineage specification of parietal epithelial cells requires beta-catenin/Wnt signaling. J Am Soc Nephrol. 2012;23:63–72. doi: 10.1681/ASN.2010121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 70.McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol. 2009;20:2104–2111. doi: 10.1681/ASN.2008111173. [DOI] [PubMed] [Google Scholar]
- 71.Carroll TJ, Das A. Planar cell polarity in kidney development and disease. Organogenesis. 2011;7:180–190. doi: 10.4161/org.7.3.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schnell U, Carroll TJ. Planar cell polarity of the kidney. Exp Cell Res. 2016;343:258–266. doi: 10.1016/j.yexcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papakrivopoulou E, Dean CH, Copp AJ, Long DA. Planar cell polarity and the kidney. Nephrol Dial Transplant. 2014;29:1320–1326. doi: 10.1093/ndt/gft484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, Dean CH. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocque BL, Babayeva S, Li J, Leung V, Nezvitsky L, Cybulsky AV, Gros P, Torban E. Deficiency of the planar cell polarity protein Vangl2 in podocytes affects glomerular morphogenesis and increases susceptibility to injury. J Am Soc Nephrol. 2015;26:576–586. doi: 10.1681/ASN.2014040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 77.Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol. 2011;22:1654–1664. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 79.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–3945. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Li F, Sun Y, Lei L, Zhou H, Lei T, Xia Y, Verkman AS, Yang B. Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. FASEB J. 2015;29:1551–1563. doi: 10.1096/fj.14-260828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int. 2011;79:957–965. doi: 10.1038/ki.2010.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller MM, Iglesias DM, Zhang Z, Corsini R, Chu L, Murawski I, Gupta I, Somlo S, Germino GG, Goodyer PR. T-cell factor/beta-catenin activity is suppressed in two different models of autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:146–153. doi: 10.1038/ki.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 86.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 87.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wuebken A, Schmidt-Ott KM. WNT/beta-catenin signaling in polycystic kidney disease. Kidney Int. 2011;80:135–138. doi: 10.1038/ki.2011.87. [DOI] [PubMed] [Google Scholar]
- 89.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349–360. doi: 10.1016/j.molmed.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goggolidou P. Wnt and planar cell polarity signaling in cystic renal disease. Organogenesis. 2014;10:86–95. doi: 10.4161/org.26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/beta-catenin signaling and kidney fibrosis. Kidney Int Suppl. 2011;2014(4):84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–208. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 94.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 96.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20:1919–1928. doi: 10.1681/ASN.2009030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuncewitch M, Yang WL, Corbo L, Khader A, Nicastro J, Coppa GF, Wang P. WNT agonist decreases tissue damage and improves renal function after ischemia-reperfusion. Shock. 2015;43:268–275. doi: 10.1097/SHK.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y. Sustained activation of Wnt/beta-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 101.Maarouf OH, Ikeda Y, Humphreys BD. Wnt signaling in kidney tubulointerstitium during disease. Histol Histopathol. 2015;30:163–171. doi: 10.14670/HH-30.163. [DOI] [PubMed] [Google Scholar]
- 102.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou D, Fu H, Zhang L, Zhang K, Min Y, Xiao L, Lin L, Bastacky SI, Liu Y. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol. 2017;28:2322–2336. doi: 10.1681/ASN.2016080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD. Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol. 2016;27:781–790. doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J Am Soc Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, Tian J, Fu H, Hou FF, Liu Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol. 2017;28:598–611. doi: 10.1681/ASN.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Y, Masiello D, McMillian M, Nguyen C, Wu Y, Melendez E, Smbatyan G, Kida A, He Y, Teo JL, Kahn M. CBP/catenin antagonist safely eliminates drug-resistant leukemia-initiating cells. Oncogene. 2016;35:3705–3717. doi: 10.1038/onc.2015.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis. 2017;3:15–23. doi: 10.1159/000452880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303:F1641–F1651. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol. 2015;185:3211–3223. doi: 10.1016/j.ajpath.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heikkila E, Juhila J, Lassila M, Messing M, Perala N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H. Beta-catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 118.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/beta-catenin pathway in podocytes integrates cell adhesion, differentiation and survival. J Biol Chem. 2011;286:26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L, Li Y, He W, Zhou D, Tan RJ, Nie J, Hou FF, Liu Y. Mutual antagonism of Wilms’ tumor 1 and beta-catenin dictates podocyte health and disease. J Am Soc Nephrol. 2015;26:677–691. doi: 10.1681/ASN.2013101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang L, Xu L, Song Y, Li J, Mao J, Zhao AZ, He W, Yang J, Dai C. Calmodul-independent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J Biol Chem. 2013;288:23368–23379. doi: 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-beta-catenin-snail signaling pathway. PLoS One. 2014;9:e89233. doi: 10.1371/journal.pone.0089233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou L, Liu Y. Wnt/beta-catenin signaling and renin-angiotensin system in chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25:100–106. doi: 10.1097/MNH.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cox SN, Sallustio F, Serino G, Pontrelli P, Verrienti R, Pesce F, Torres DD, Ancona N, Stifanelli P, Zaza G, Schena FP. Altered modulation of WNT-beta-catenin and PI3K/Akt pathways in IgA nephropathy. Kidney Int. 2010;78:396–407. doi: 10.1038/ki.2010.138. [DOI] [PubMed] [Google Scholar]
- 126.Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockman SA, Cheung P, Lee E, Kuhnert F, Olson JL, Kuo CJ, Gharavi AG, D’Agati VD, Artandi SE. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2011;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang XD, Huang XF, Yan QR, Bao CD. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS One. 2014;9:e84852. doi: 10.1371/journal.pone.0084852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 129.Kruck S, Eyrich C, Scharpf M, Sievert KD, Fend F, Stenzl A, Bedke J. Impact of an altered Wnt1/beta-catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int J Mol Sci. 2013;14:10944–10957. doi: 10.3390/ijms140610944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hsu RJ, Ho JY, Cha TL, Yu DS, Wu CL, Huang WP, Chu P, Chen YH, Chen JT, Yu CP. WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/beta-catenin pathway. PLoS One. 2012;7:e47649. doi: 10.1371/journal.pone.0047649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kondratov AG, Kvasha SM, Stoliar LA, Romanenko AM, Zgonnyk YM, Gordiyuk VV, Kashuba EV, Rynditch AV, Zabarovsky ER, Kashuba VI. Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One. 2012;7:e47012. doi: 10.1371/journal.pone.0047012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janssens N, Andries L, Janicot M, Perera T, Bakker A. Alteration of frizzled expression in renal cell carcinoma. Tumour Biol. 2004;25:161–171. doi: 10.1159/000081098. [DOI] [PubMed] [Google Scholar]
- 133.Xu R, Zeng S, Xie W, Sun C, Chen YL, Chen MJ, Zhang L. The expression and function of Frizzled-7 in human renal cell carcinoma. Clin Transl Oncol. 2016;18:269–276. doi: 10.1007/s12094-015-1362-3. [DOI] [PubMed] [Google Scholar]
- 134.Saini S, Majid S, Dahiya R. The complex roles of Wnt antagonists in RCC. Nat Rev Urol. 2011;8:690–699. doi: 10.1038/nrurol.2011.146. [DOI] [PubMed] [Google Scholar]
- 135.Maiti S, Alam R, Amos CI, Huff V. Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res. 2000;60:6288–6292. [PubMed] [Google Scholar]
- 136.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]