Abstract
Background & Aims
Early life adversity is considered a risk factor for the development of gastrointestinal diseases, including inflammatory bowel disease. We hypothesized that early life colonic inflammation causes susceptibility to aggravated overexpression of interleukin (IL)1β.
Methods
We developed a 2-hit rat model in which neonatal inflammation (NI) and adult inflammation (AI) were induced by trinitrobenzene sulfonic acid.
Results
Aggravated immune responses were observed in NI + AI rats, including a sustained up-regulation of IL1β and other cytokines. In parallel with exacerbated loss of inhibitor of kappa B alpha expression, NI + AI rats showed hyperacetylation of histone H4K12 and increased V-Rel Avian Reticuloendotheliosis Viral Oncogene Homolog A binding on the IL1B promoter, accompanied by high levels of norepinephrine/epinephrine. Propranolol, a β-blocker, markedly ameliorated the inflammatory response and IL1β overexpression by mitigating against epigenetic modifications. Adrenalectomy abrogated NI-induced disease susceptibility whereas yohimbine sensitized the epithelium for exacerbated immune response. The macrophages of NI rats produced more IL1β than controls after exposure to lipopolysaccharide (LPS), suggesting hypersensitization; incubation with LPS plus Foradil (Sigma, St. Louis, MO), a β2-agonist, induced a greater IL1β expression than LPS alone. Epinephrine and Foradil also exacerbated LPS-induced IL1β activation in human THP-1–derived macrophages, by increasing acetylated H4K12, and these increases were abrogated by propranolol.
Conclusions
NI sensitizes the colon epithelium for exacerbated IL1β activation by increasing stress hormones that induce histone hyperacetylation, allowing greater access of nuclear factor-κB to the IL1B promoter and rendering the host susceptible to aggravated immune responses. Our findings suggest that β blockers have a therapeutic potential for inflammatory bowel disease susceptibility and establish a novel paradigm whereby NI induces epigenetic susceptibility to inflammatory bowel disease.
Keywords: Early Life Adversity, Inflammatory Bowel Disease, Epinephrine, Histone Acetylation, NF-κB
Abbreviations used in this paper: AI, adult inflammation; ChIP, chromatin immunoprecipitation; Ctl, control; H4K12ac, acetylated HRK12; HDAC, histone deacetylase; IBD, inflammatory bowel disease; IκB, inhibitor of kappa B alpha; IL, interleukin; LPS, lipopolysaccharide; MPO, myeloperoxidase; mRNA, messenger RNA; NF-κB, nuclear factor-κB; NI, neonatal inflammation; PCR, polymerase chain reaction; PMA, phorbol 12-myristate 13-acetate; RelA, V-Rel Avian Reticuloendotheliosis Viral Oncogene Homolog A; RNAP II, RNA polymerase II; TNBS, 2,4,6-trinitrobenzene sulfonic acid; Tnf, tumor necrosis factor
Graphical abstract
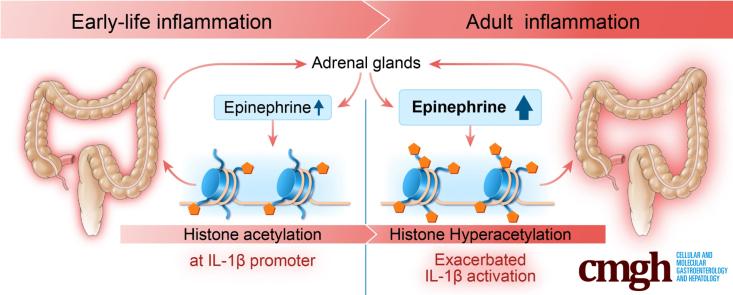
Summary.
This investigation showed that neonatal inflammation increases susceptibility to inflammatory bowel disease by epigenetically sensitizing the interleukin 1β promoter for exacerbated overexpression when exposed to another episode of inflammation later in life. Propranolol might mitigate against the inflammatory bowel disease susceptibility by reversing epigenetic modifications.
Inflammatory bowel disease (IBD) is a chronic, progressive, relapsing, and immunologically mediated disorder that often targets the young and remains a lifelong affliction. Epidemiologic studies have suggested that the incidence of IBD is increasing worldwide.1, 2, 3 Current models of human IBD posit that the inflammatory pathogenesis arises from, and is perpetuated by, interactions between host genetic and immune factors, gastrointestinal microbes, and environmental triggers.4 Accumulating clinical evidence has shown that early life infection is a risk factor for the development of pediatric and adult IBD,5, 6, 7, 8, 9, 10, 11, 12 and gastrointestinal infection in adolescents and adults is a trigger for its onset or exacerbation.13, 14, 15, 16, 17 However, it is unclear how these events cause an aggravated and prolonged immune response, which is the hallmark of IBD.
Early postnatal life is a uniquely vulnerable period, characterized by epigenetic plasticity, in which neonates are susceptible to environmental influences that induce durable-epigenetic changes that persist in the adult.18, 19 It now is well recognized that adverse early life events have an important role in perinatal programming and maturation of the immune system that make the host susceptible to complex diseases,20, 21, 22, 23, 24 including IBD.8, 10, 18 However, the molecular mechanisms by which adverse early life experiences predispose to IBD remain unknown.
A variety of cytokines, including interleukin (IL)1, have been implicated in the pathogenesis of IBD.25 The IL1 family of cytokines comprises 11 proteins (IL1F1–IL1F11) encoded by 11 distinct genes in human beings and mice. IL1-type cytokines are major mediators of innate immune reactions, and blockade of IL1 by the IL1 receptor antagonist has shown an essential role of IL1 in a number of human autoinflammatory diseases.26 IL1β, a proinflammatory cytokine with a wide range of systemic and local effects, has received considerable attention as a potential mediator of inflammatory cell infiltration and mucosal barrier disruption that accompanies gut inflammation.27 It can modulate the function of both immune and nonimmune cells. IL1β also appears to promote inflammation by stimulating the production of other cytokines (eg, IL6) and chemokines (eg, C-X-C motif chemokine ligand 1, C-X-C motif chemokine ligand 8, IL8).28, 29, 30 Stimulation with IL1β promotes the activation and effector functions of dendritic cells, macrophages, and neutrophils.31 It also induces neutrophilia and promotes neutrophil migration.32 IL1β promotes T-cell activation and survival,33 and acts in concert with other proinflammatory cytokines to promote the differentiation of CD4+ Th17 cells.34, 35, 36, 37 Because of the potent inflammatory activity of IL1β, tight mechanisms are in place to regulate its secretion. However, our understanding of IL1β activation in the pathogenesis of IBD is limited, and it is unclear whether early life adversity, such as neonatal colonic inflammation, aggravates IL1β overexpression to exacerbate immune responses when subjected to a second inflammatory insult later in life.
The present investigation sought to test the hypothesis that neonatal colonic inflammation epigenetically aggravates IL1β activation in rat colon epithelium when the host is exposed to a second episode of inflammation as an adult. Our findings provide compelling evidence that neonatal colonic inflammation triggers aberrant increases in norepinephrine and epinephrine to enhance histone acetylation at the IL1B gene promoter. Notably, the altered chromatin status persists, facilitating nuclear factor-κB (NF-κB) recruitment and IL1β overexpression when subjected to an additional insult in adult life.
Materials and Methods
Reagents
Propranolol hydrochloride was purchased from Tocris Bioscience (Bristol, UK). Epinephrine, norepinephrine, lipopolysaccharide (LPS, from Escherichia coli O111:B4, cat. L4391), formoterol fumarate dihydrate (Foradil), phorbol 12-myristate 13-acetate (PMA), sodium butyrate, yohimbine, and 2,4,6-trinitrobenzene sulfonic acid (TNBS) were from Sigma (St. Louis, MO).
Cell Culture
THP-1 cells were purchased from ATCC (Manassas, VA) and maintained in RPMI 1640 medium with 2 mmol/L L-glutamine, 10% fetal bovine serum, and 0.05 mmol/L 2-mercaptoethanol. To induce differentiation, THP-1 cells seeded at 2 × 105 cells/mL were incubated with 100 nmol/L PMA for 3 days.
Animals and Procedures
Male Sprague Dawley rat littermates were used in the preclinical studies. Five-day-old and 6-week-old Sprague Dawley rats were purchased from Harlan Laboratories (Houston, TX). The work was approved by the Institutional Animal Care and Use Committee at The University of Texas Medical Branch at Galveston.
Rat littermates were divided randomly into 4 groups: (1) vehicle treatment in both neonatal and adult-life stages (controls, Ctl); (2) sham treatment as neonates followed by an inflammatory insult as adults (adult inflammation [AI]) (Ctl + AI); (3) neonatal inflammatory insult (neonatal inflammation [NI]) and then sham treatment as adults; and (4) NI plus AI in combination (NI + AI) (Figure 1A). Experimenters were blinded to treatment assignment. To induce neonatal inflammation, TNBS (130 mg/kg, 2.86 mg for a 22-g pup, dissolved in 200 μL saline containing 10% ethanol) was injected intraluminally 2 cm into the colon of male pups on postnatal day 10.38 The animals were kept in a head-down position while the anus was held closed for 1 minute to prevent leakage. Rats in the sham treatment groups received 200 μL of saline. Six to 8 weeks later, animals were subjected to a secondary TNBS insult (65 mg/kg, 13 mg for a 200-g rat), as AI. Under light anesthesia, 250 μL of TNBS in phosphate-buffered saline containing 40% ethanol was injected intrarectally via a catheter, advanced to 8 cm into the colon. Control rats were given 250 μL of saline. One to 8 weeks after the second TNBS treatment, animals were decapitated under anesthesia, blood samples were collected for plasma preparation. For histologic examination, a full-thickness colon specimen located 3 cm above the anal canal was obtained, fixed in 4% paraformaldehyde in phosphate-buffered saline, embedded in paraffin, sectioned, and stained with H&E. The mucosa/submucosa were dissected from the full-length colon, snap-frozen in liquid nitrogen, pulverized, and stored at -80°C for molecular studies.
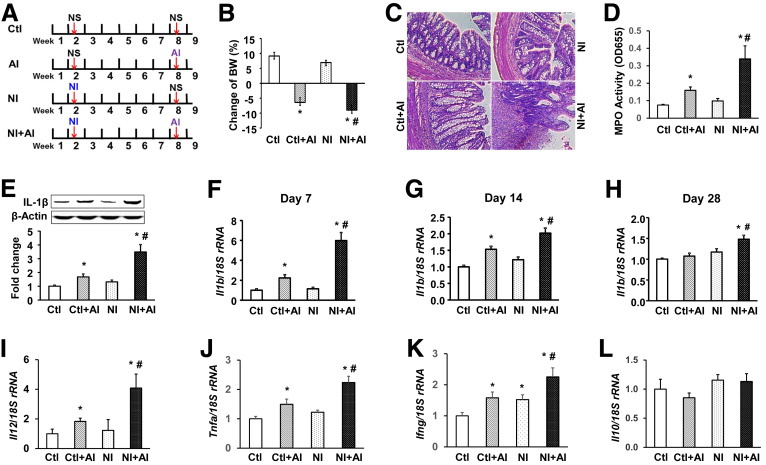
Figure 1.
Neonatal colonic inflammation induces aggravated immune responses when subjected to a secondary insult. (A) Schematic presentation of the animal protocol. NS, normal saline. (B) Body weight changes of 4 groups of rats after the second TNBS treatment. (C) H&E staining of the colon, from muscularis to lumen. Scale bar: 100 μm. (D) MPO activity in colon mucosa/submucosa. (E) IL1β protein expression in the mucosa/submucosa dissected from the entire colon. β-Actin served as the loading control. Top: representative images of Western blots. Bottom: relative optical band density ratio between IL1β and β-actin. Real-time reverse-transcription PCR was performed to detect IL1β mRNA levels in the colon mucosa/submucosa at (F) 1 week, (G) 2 weeks, or (H) 4 weeks after AI, as well as the mRNA accumulation of (I) Il12, (J) Tnfa, (K) interferon γ (Ifng), and (L) Il10 at 7 days after AI. Values are presented as means ± SEM (n = 12). Two-way analysis of variance. *P < .05 vs Ctl. #P < .05 vs Ctl + AI. rRNA, ribosomal RNA.
We also induced AI with TNBS (130 mg/kg) in 6- to 8-week-old rats followed by a second AI (65 mg/kg TNBS) 6–8 weeks later (AI + AI), to determine whether a delayed first-time AI also aggravates the immune response, comparable with NI treatment.
For intervention experiments with propranolol, all 4 groups of rats were given propranolol hydrochloride (2 mg/kg dissolved in saline) by daily intraperitoneal injection for 7 days, starting right before the AI. Control groups received normal saline. Animals were killed 3 hours after the last injection.
To ablate epinephrine/norepinephrine, adrenalectomy was performed in 6-week-old control and NI rats. Briefly, rats were anesthetized with 2%–3% isoflurane. The left flank of the rat (landmark the adrenals: caudal end of the ribs on left lateral side of the animal) was shaved with electric clippers and the fur was removed. A 1.5- to 2-cm dorsal incision was made over the left adrenal. The adrenals, left and right, were externalized from the abdominal cavity and a ligature was placed below each gland. The adrenals were excised using forceps, and the peritoneal cavity was closed with Vicryl sutures (Ethicon, Somerville, NJ), and the skin with Prolene sutures (Ethicon, San Lorenzo, Puerto Rico). The rats were given normal saline in place of drinking water and they were given 2 weeks to recover before the next procedure. Sham surgery also was performed and served as control. All 4 groups of animals then were treated with TNBS (65 mg/kg) and killed 7 days later.
To increase endogenous levels of epinephrine/norepinephrine in animals, we treated 10-day-old rat pups with LPS-free yohimbine (2.5 mg/kg dissolved in saline) by intraperitoneal injection twice weekly for 6 weeks. The control group received saline. Four hours after the last injection, blood was collected under light anesthesia from the saphenous vein for the measurement of epinephrine levels, and the rats then were treated with TNBS (65 mg/kg) via intrarectal injection. All animals were euthanized 7 days later for tissue collection.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed as described previously.38, 39 Antibodies for immunoprecipitation were from the following sources: V-Rel Avian Reticuloendotheliosis Viral Oncogene Homolog A (RelA) (cat. 06-418; Millipore, Temecula, CA), histone H4 acetyl lysine 12 (H4K12ac, cat. 39165), histone deacetylase 3 (HDAC3; cat. 40968), and RNA polymerase II (cat. 39097; Active Motif, Carlsbad, CA). Precipitated DNA, SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and primers specific to the human IL1B or rat IL1β gene promoter (Table 1) were used for real-time quantitative polymerase chain reaction (PCR). Fold differences in precipitated DNA were normalized against input.
Table 1.
Primers Specific to the Human IL1B and Rat II1b Promoters Used in ChIP–Quantitative PCR Assays
| Species | Primer name | Primer sequence |
|---|---|---|
| Human | ||
| IL1B-NF-κB-F | 5’-TGGCCCTTCATTGTACCCAT-3’ | |
| IL1B-NF-κB-R | 5’-TCGTTGTGCAGTTGATGTCC-3’ | |
| IL1B-core-F | 5’-CTCAGTTTATTAGTCCCCTCCCC-3’ | |
| IL1B-core-R | 5’-CTCCCTCGCTGTTTTTATGGC-3’ | |
| Rat | ||
| II1b-NF-κB-F | 5’-GCTCCCTCAGCTTAAGTCCA-3’ | |
| II1b-NF-κB-R | 5’-CATTATTTCCCCCTGGACAA-3’ | |
| II1b-core-F | 5’-ATTCCCACCAAGCTTCTTCC-3’ | |
| II1b-core-R | 5’-TGGAGAGGATCCCAGATGAG-3’ |
F, forward; R, reverse.
Real-Time Reverse-Transcription PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). Complementary DNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). IL1B and IL1β messenger RNA (mRNA) levels were quantitated using TaqMan-based quantitative PCR, as reported.38 18S ribosomal RNA served as an internal control.
Immunoblotting
Western blot was performed as described previously.38, 39 Primary antibodies were as follows: anti-IL1β rabbit polyclonal (cat. sc-7884), anti-inhibitor of kappa B alpha (IκBα) rabbit polyclonal (cat. sc-371, 1:200) (Santa Cruz, Dallas, TX), and anti–β-actin mouse monoclonal antibody (cat. A5441, 1:5000; Sigma). All blots were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Band density was determined using LI-COR Image Studio Software.
Isolation of Macrophages by Fluorescence-Activated Cell Sorting
Cell sorting was performed using a BD FACSAria IIU instrument (Becton Dickinson, Franklin Lakes, NJ). Mucosa/submucosa tissue from the entire colon of each rat was dissociated in 10 mL Hanks’ balanced salt solution containing 0.1 mg/mL Liberase (Sigma) and 0.1 mg/mL DNase I, and passed through 70-micron filters. The associated macrophages were isolated by sorting CD163+ and propidium iodide–negative cells (viable cells) from a 40% Percoll fractionation of dissociated mucosal cells obtained from 4 groups of rats, euthanized 7 days after AI. This time point provided the optimal isolation of mature tissue macrophages, based on pilot studies (not shown). Expression of CD163, a member of the scavenger receptor cysteine-rich family class B, was restricted to cells of the monocyte/macrophage lineage, and it is expressed in most subpopulations of mature tissue macrophages.
Myeloperoxidase Assay
Frozen colon mucosa/submucosa was pulverized in liquid nitrogen, homogenized in 20 mmol/L phosphate buffer (pH 7.4), and centrifuged at 4°C for 10 minutes. Pellets were sonicated in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethyl ammonium bromide and centrifuged at 4°C for 5 minutes. The supernatant (100 μL) was incubated with 16 mmol/L tetramethyl benzidine in 50% ethanol, 0.3 mmol/L H2O2, and 8 mmol/L sodium phosphate buffer (pH 5.4) for 3 minutes. Myeloperoxidase (MPO) activity was measured by reading the absorbance at 655 nm in a microplate reader.40
Norepinephrine and Epinephrine Assays
Plasma norepinephrine and epinephrine levels were measured using the Noradrenaline Research Enzyme Immunoassay kit and the Epinephrine Research Enzyme-Linked Immunosorbent Assay kit (Rocky Mountain Diagnostics, Colorado Springs, CO), respectively, according to the manufacturer’s instructions.
Statistics
All data were expressed as means ± SEM. We used 1-way analysis of variance followed by the Tukey post hoc analysis for comparison of more than 2 means, and the Student t test to compare between 2 means, and considered P < .05 to be statistically significant.
Results
Neonatal Inflammation in the Colon Induces Aggravated Immune Responses When Subjected to a Second Inflammatory Insult as Adults
We reported that neonatal colonic inflammation activates α1C1b gene transcription by up-regulating histone acetylation on the α1C1b promoter, causing motility dysfunction in the colon.38 The latter work suggested that neonatal inflammation could induce sustained epigenetic changes, which might make the host susceptible to aggravated immune responses when exposed to a secondary challenge in adult life. To test this hypothesis, we performed an experiment in vivo with 4 groups of rats (Figure 1A). Notably, TNBS-induced inflammation in adult life caused significant loss of body weight in both the Ctl + AI and NI + AI groups, when compared with control rats (n = 12) (Figure 1B). There was no significant difference between NI and Ctl rats. Importantly, NI + AI rats showed a significantly greater decrease in body weight compared with Ctl + AI rats (P < .05). H&E staining of the colons showed severe local diffuse destruction, higher histologic scores, and neutrophil infiltration in NI + AI vs Ctl + AI rats (Figure 1C). A significantly greater increase in MPO activity was found in the colon mucosa/submucosa of NI + AI rats vs Ctl + AI rats (Figure 1D), NI rats, and Ctl rats (P < .01). Immunoblotting showed a marked up-regulation of mature IL1β protein in colon mucosa/submucosa of Ctl + AI and NI + AI rats, compared with Ctl rats (Figure 1E), and this was significantly greater for NI + AI vs Ctl + AI rats. There was no significant difference between NI and Ctl rats.
To investigate transcriptional changes, IL1β mRNA levels were examined by real-time reverse-transcription PCR in the colon mucosa/submucosa of rats at 7, 14, and 28 days after AI (Figure 1F–H). One week after AI, IL1β mRNA levels were increased by 2.5- and 6-fold in Ctl + AI and NI + AI rats, respectively, compared with Ctl rats (Figure 1F). The increase in NI + AI rats was significantly greater than that in Ctl + AI rats (P < .05). At 2 weeks, IL1β mRNA levels were increased 1.5- and 2-fold in Ctl + AI and NI + AI rats, respectively, which was statistically significant compared with Ctl rats (Figure 1G). IL1β mRNA levels were significantly greater in NI + AI rats vs Ctl + AI rats. At 4 weeks (Figure 1H), IL1β mRNA accumulation remained significantly increased for NI + AI rats compared with all other treatment groups (P < .05). There were no significant differences between Ctl + AI, NI, and Ctl rats by day 28. The mRNA levels of Il12 (Figure 1I), tumor necrosis factor α (Tnfa) (Figure 1J), and interferon γ (Figure 1K), but not Il10 (Figure 1L), also were increased significantly in NI + AI rats vs all other treatment groups. These findings strongly support our hypothesis that neonatal inflammation makes the host vulnerable to aggravated immune responses when subjected to a secondary insult in adult life.
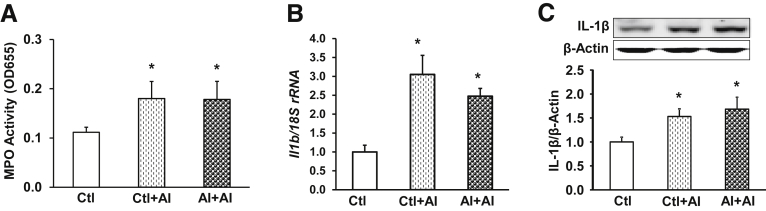
We next sought to investigate whether NI was a prerequisite for the increased susceptibility later in life, or could be substituted with a delayed first hit as an adult. Thus, colonic inflammation was first induced with TNBS in 6- to 8-week-old adult rats (AI rats, saline served as control),41 followed by another dose of TNBS 6 weeks later (AI + AI rats), and tissue was collected 7 days later. There was no difference in MPO activities of colon mucosa/submucosa for AI + AI and Ctl + AI rats (n = 8) (Figure 2A), both being significantly greater than Ctl (P < .05). The second AI also increased IL1β mRNA (Figure 2B) and protein (Figure 2C) levels in colon mucosa/submucosa of Ctl + AI and AI + AI rats, respectively, but no significant differences were found between Ctl + AI and AI + AI rats. These findings suggested that a first time AI in adulthood cannot substitute for NI in exacerbating immune responses when the host is exposed to an inflammatory challenge later in life.
Figure 2.
First-time AI does not induce aggravated IL1β overexpression when the host is exposed to another episode of AI later in life. Adult rats were subjected to an intrarectal injection of TNBS and received a second treatment 6 weeks later. Sham controls received normal saline. Tissue was collected 7 days after the second treatment. (A) MPO activity, (B) IL1β mRNA levels, and (C) IL1β protein expression were quantified in the colon mucosa/submucosa. mRNA was quantified by quantitative reverse-transcription PCR and protein levels were determined by Western blot and densitometry. Data are given as means ± SEM (n = 8). Analysis of variance. *P < .05 vs Ctl. rRNA, ribosomal RNA.
Exacerbated IL1β Overexpression in the Colon Mucosa/Submucosa of NI + AI Rats Is Mediated by NF-κB
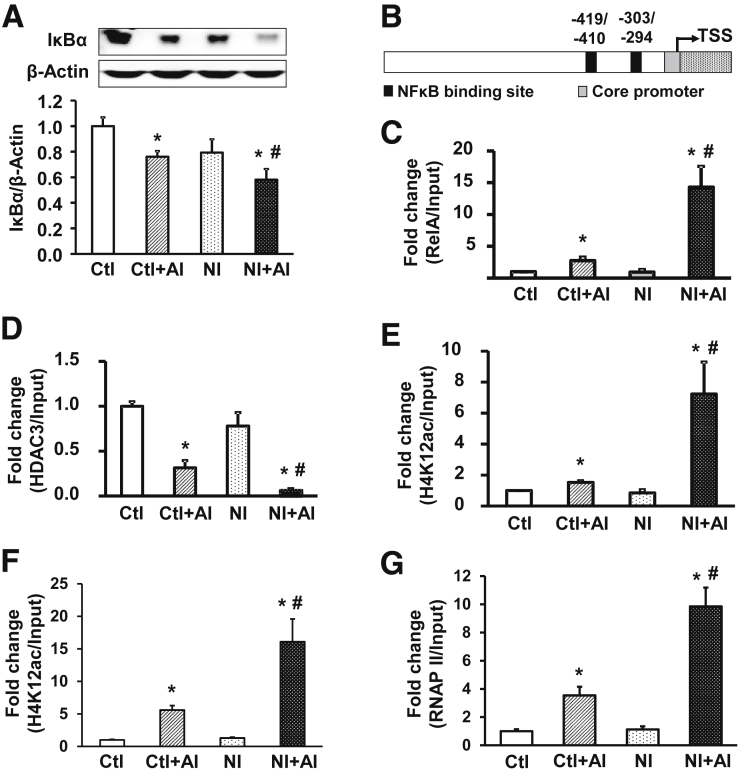
It is well known that NF-κB activation plays an important role in the production of proinflammatory cytokines, such as IL1β. To investigate whether NF-κB was activated in NI + AI rats, we first assessed the levels of IκBα. The latter protein inhibits NF-κB by masking nuclear localization signals on NF-κB proteins, keeping them sequestered in an inactive state in the cytoplasm,42 and thereby blocking the ability of NF-κB transcription factors to tether to DNA.43 We found that IκBα protein levels were significantly attenuated in colon mucosa/submucosa of both Ctl + AI and NI + AI rats (Figure 3A). More importantly, down-regulation of IκBα protein expression was significantly greater in NI + AI vs Ctl + AI rats (P < .05), suggesting an exacerbated activation of NF-κB in NI + AI rats. To show a cause-and-effect relationship between NF-κB activation and IL1β up-regulation, we identified 2 NF-κB binding motifs (-419/-410 and -303/-294) in the rat IL1β promoter (Figure 3B). ChIP–quantitative PCR assays with RelA (p65) antibody showed low, constitutive RelA interactions on IL1β for the Ctl and NI groups (Figure 3C). These interactions were enhanced significantly in Ctl + AI and NI + AI groups, compared with Ctl. Notably, RelA interactions with IL1β were increased significantly in NI + AI rats compared with Ctl + AI. There was no significant difference in RelA binding between NI and Ctl groups (Figure 3C).
Figure 3.
Neonatal inflammation epigenetically aggravates IL1β activation through NF-κB. (A) IκBα protein expression in the colon mucosa/submucosa of 4 groups of rats. Top: representative images of Western blots. Bottom: relative optical band density ratio between IκBα and β-actin (n = 6). *P < .05 vs Ctl. #P < .05 vs Ctl + AI. (B) Schematic of the rat IL1β promoter with 2 NF-κB binding motifs. Nucleotide numbering is relative to the transcription start site (TSS). (C) ChIP–quantitative PCR analysis of RelA (p65) association with the NF-κB binding sites of the rat IL1β promoter in the colon mucosa/submucosa. (D) HDAC3 interaction with the NF-κB binding motifs at the rat IL1β promoter. (E) Acetylation status of H4K12ac around the NF-κB binding region of the rat IL1β promoter in colon mucosa/submucosa. (F) Acetylated histone H4K12 at the IL1β core promoter region. (G) RNAP II association with the IL1β core promoter in the colon mucosa/submucosa of 4 groups of rats. Chromatin was immunoprecipitated with specific antibodies, as indicated. Precipitated chromatin was quantified by real-time PCR using primers specific to the NF-κB binding region, or the core promoter of the rat IL1β gene, and normalized to inputs. Means ± SEM. N = 3 independent experiments. Two-way analysis of variance. *P < .05 vs Ctl. #P < .05 vs Ctl + AI.
Chromatin can exist in open or closed states, depending on the circumstances,44 and chromatin remodeling is required for the recruitment of NF-κB and subsequent activation of target genes.45 We investigated the role of HDAC3, a pivotal HDAC in the colonic epithelium,46 and H4K12ac status near the NF-κB binding region of the IL1β promoter. In colonic mucosa/submucosa samples, HDAC3 binding was significantly repressed in NI + AI vs Ctl + AI, and control rats (Figure 3D). A significant repression also was observed in Ctl + AI vs NI and control rats. There was no significant difference between Ctl and NI groups. When histone acetylation was examined for the NF-κB binding region of IL1β, significantly greater H4K12ac levels were detected in NI + AI vs Ctl + AI, NI, and Ctl groups (Figure 3E). Levels of H4K12ac in NI + AI and Ctl + AI levels were significantly higher than in Ctl and NI groups, whereas Ctl and NI groups did not differ markedly from each other. We concluded that a reciprocal relationship exists, involving increased H4K12ac and decreased HDAC3 interactions in the vicinity of the NF-κB binding region of IL1β in NI + AI vs Ctl + AI rats.
We also examined H4K12ac and RNA polymerase II (RNAP II) recruitment near the IL1β core promoter (-141/+42) (Figure 3B). As with the NF-κB binding region (Figure 3E), H4K12ac levels were increased significantly in the core promoter of IL1β for NI + AI rats vs Ctl + AI rats, NI rats, and control rats (Figure 3F). This was interpreted as evidence of increased chromatin relaxation, and greater accessibility of the preinitiation complex, on the IL1β promoter. Core promoter H4K12ac levels were significantly greater in Ctl + AI vs NI and Ctl rats, but there was no significant difference between NI and Ctl groups. Concomitantly, RNAP II association with the IL1β core promoter was significantly augmented in NI + AI vs Ctl + AI, NI, and Ctl groups (Figure 3G).
Stress Hormones Play an Important Role in the Aggravated IL1β Activation in NI + AI Rats
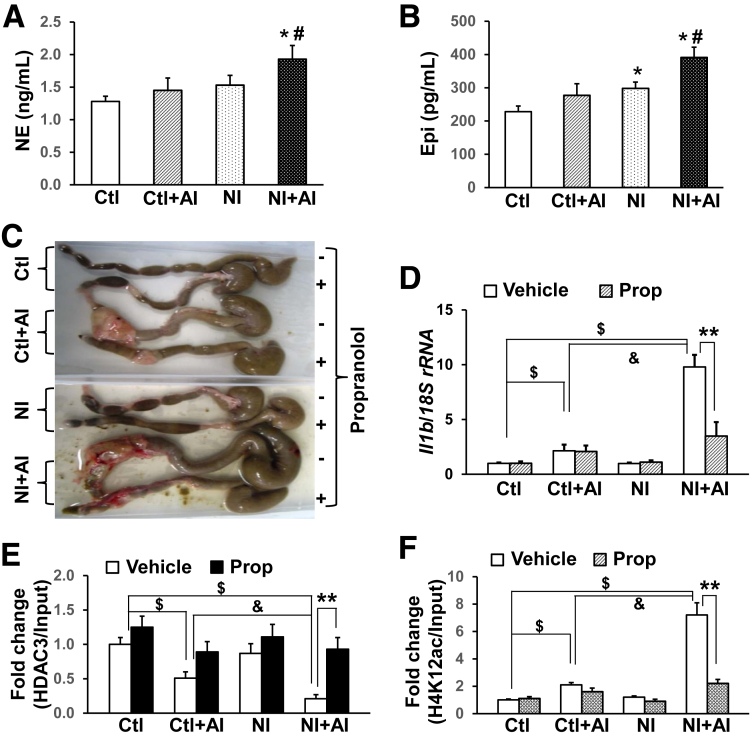
Alterations in the brain-gut axis are considered a pillar of the modern view of irritable bowel syndrome47 and IBD pathogenesis.48 Stress responses represent a defense against real or perceived threats, which stimulate the adrenal medulla to increase plasma levels of norepinephrine and epinephrine. When blood norepinephrine and epinephrine levels were examined in the present investigation, we found that norepinephrine (Figure 4A) and epinephrine (Figure 4B) levels were increased significantly in NI + AI rats compared with Ctl + AI, NI, and Ctl groups, respectively. There were also increases of norepinephrine (n = 30; P = .068) and epinephrine (P = .047) in NI vs Ctl rats (Figure 4A and B). Thus, stress hormones were implicated only in the aggravated immune responses of rats after NI + AI treatment.
Figure 4.
NI induces exacerbated IL1β activation through stress hormones. (A and B) Plasma norepinephrine (NE) and epinephrine (Epi) content in 4 groups of rats measured using enzyme immunoassay and enzyme-linked immunosorbent assay kits, respectively (n = 30). *P < .05 vs Ctl. #P < .05 vs the other 3 groups. (C) Representative images of colons from 4 groups of rats treated with vehicle or propranolol, a nonselective β-blocker. Propranolol (2 mg/kg) was dissolved in saline and given to the animals by daily intraperitoneal injection. Another 4 groups of animals received saline and served as controls. (D) IL1β mRNA levels in the colon mucosa/submucosa quantified by quantitative reverse-transcription PCR; 18S ribosomal RNA (rRNA) served as an internal control. n = 8. (E) ChIP–quantitative PCR analysis of HDAC3 binding to the NF-κB binding sites at the rat IL1β promoter. (F) ChIP–quantitative PCR data of H4K12ac levels surrounding the NF-κB binding region of the IL1β promoter. Data are shown as means ± SEM. N = 3 independent experiments. Two-way analysis of variance. $P < .05, &P < .05, **P < .05.
Based on the findings for norepinephrine and epinephrine, we postulated that stress hormones mediate the aggravated immune responses caused by NI + AI exposure. To test this hypothesis, we treated all 4 groups (Ctl, Ctl + AI, NI, and NI + AI) with propranolol hydrochloride (2 mg/kg/day intraperitoneally), a nonselective β blocker, for 7 days, starting immediately before AI. Propranolol markedly ameliorated TNBS-induced swelling, cecal enlargement, and tissue damage in NI + AI rats (Figure 4C). Significant improvement also was observed in Ctl + AI rats after the treatment. The exacerbated up-regulation of IL1β mRNA in the colon mucosa/submucosa of NI + AI rats was significantly repressed by propranolol (Figure 4D). Thus, aggravated immune responses in NI + AI rats appear to be mediated, at least in part, by heightened levels of stress hormones. Notably, IL1β mRNA levels in Ctl + AI remained unchanged by propranolol.
To investigate whether blockade of adrenoceptors by propranolol mitigates against NI-induced epigenetic modifications on the IL1β promoter, HDAC3 and H4K12ac levels were assessed, as described earlier. We found that the NI-reduced HDAC3 interactions near the NF-κB binding region of IL1β essentially were reversed by propranolol treatment in NI + AI rats (Figure 4E). Less dramatic increases in HDAC3 binding also were noted in Ctl, Ctl + AI, and NI groups treated with propranolol. Accordingly, propranolol reversed the histone hyperacetylation on IL1β in NI + AI rats (Figure 4F). No significant changes were detected for H4K12ac in Ctl + AI, NI, or Ctl rats given propranolol, compared with the corresponding vehicle controls.
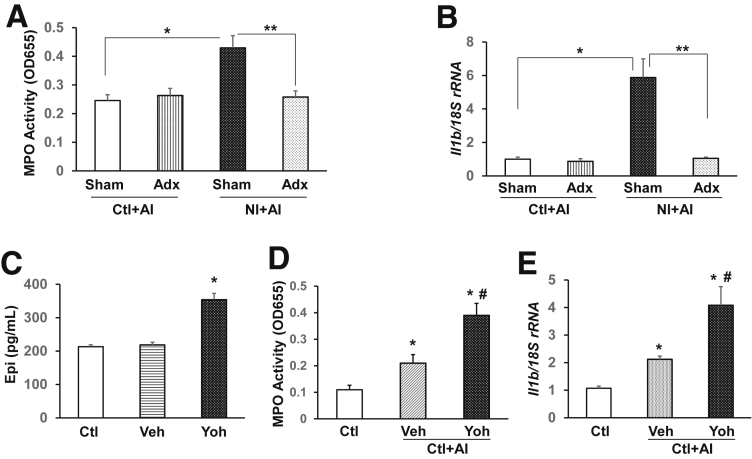
To verify that stress hormones mediate the disease sensitization, we depleted norepinephrine and epinephrine by adrenalectomy in Ctl and NI rats, and applied AI when the animals were 8 weeks old. Ablation of norepinephrine and epinephrine almost completely abrogated the significant increases of MPO activity (Figure 5A) and IL1β activation (Figure 5B) by NI and had little effect on control rats, suggesting that high constitutive levels of norepinephrine and epinephrine are critical to the NI-induced disease susceptibility. To further show that an increase of norepinephrine and epinephrine increases inflammatory responses after AI, we administered yohimbine, an α2 antagonist proven to increase norepinephrine and epinephrine,49 for 6 weeks starting from postnatal day 10. Serum levels of epinephrine were increased significantly by yohimbine treatment in naive rats compared with vehicle-treated rats (P < .05) (Figure 5C). Importantly, yohimbine-treated animals showed significantly higher levels of MPO activity (Figure 5D) and IL1β mRNA (Figure 5E) in the colon mucosa/submucosa after the inflammatory insult with TNBS, clearly showing sensitization in the colon.
Figure 5.
Stress hormones play a key role in aggravating inflammatory responses in the colon. Adrenalectomy abolished aggravated increases of (A) MPO activity and (B) IL1β mRNA expression by NI in the colon mucosa/submucosa. Sham surgery was performed in control groups (n = 6). *P < .05, **P < .05. Long-term administration of yohimbine (Yoh, LPS-free) by twice-weekly intraperitoneal injection not only (C) increased the serum levels of epinephrine (Epi), but also (D) exacerbated the up-regulation of MPO activity and IL1β mRNA (E) after AI (n = 6). One-way analysis of variance. *P < .05 vs Ctl. #P < .05 vs vehicle (Veh). Data are presented as the means ± SEM. Adx, adrenalectomy.
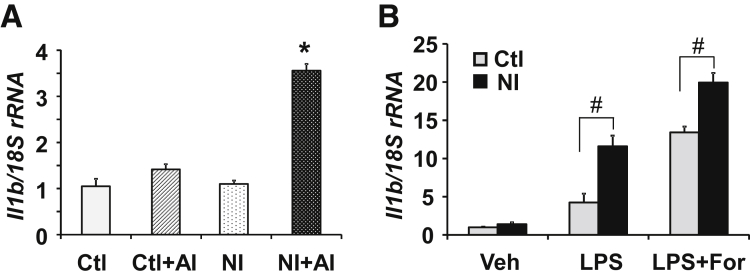
Neonatal Inflammation Sensitizes Macrophages in the Colon
Macrophages play a critical role in the initiation, maintenance, and resolution of inflammation, and they function in both innate immunity and adaptive immunity of vertebrate animals.50 We hypothesized that neonatal inflammation sensitizes macrophages in the colonic mucosa, and makes them more sensitive to inflammatory stimuli. To test this hypothesis, we isolated macrophages from the colon mucosa/submucosa by fluorescence-activated cell sorting, and analyzed IL1β mRNA expression by quantitative reverse-transcription PCR. IL1β mRNA levels were significantly greater in NI + AI rats vs Ctl + AI rats, NI rats, and Ctl rats (Figure 6A). No significant differences were noted for IL1β mRNA in the other treatment groups. To corroborate that NI induced macrophage sensitization in the colon, we incubated the macrophages of Ctl or NI rats with either 100 ng/mL LPS or a combination of 100 ng/mL LPS and 1 μmol/L Foradil, a selective β2-adrenergic agonist, and examined IL1β mRNA expression. Real-time PCR analyses showed that LPS induced a significantly greater increase of IL1β mRNA in the macrophages isolated from NI rats vs Ctl rats (Figure 6B), strongly supporting our hypothesis that NI sensitizes macrophages in the colon. Compared with LPS alone, a combination of LPS and Foradil resulted in significantly greater increases of IL1β in both NI and Ctl rat macrophages, providing additional evidence that aberrant levels of stress hormones exacerbate immune responses. The IL1β mRNA levels were significantly greater in NI vs Ctl rat macrophages after the treatment of LPS/Foradil (Figure 6B).
Figure 6.
Neonatal colonic inflammation sensitizes the macrophages in the rat colon. (A) IL1β mRNA expression levels in the macrophages isolated from the colon mucosa/submucosa of 4 groups of animals by fluorescence-activated cell sorting. IL1β mRNA was quantified by quantitative reverse-transcription PCR (n = 6). *P < .05 vs the other 3 groups. (B) IL1β mRNA in macrophages treated with LPS or the combination of LPS and Foradil, a β2-adrenergic agonist. Macrophages were isolated from the colon mucosa/submucosa of Ctl and NI rats by fluorescence-activated cell sorting and incubated for 24 hours in complete RPMI 1640 medium containing 100 ng/mL LPS, or 100 ng/mL LPS plus 1 μmol/L Foradil. Means ± SEM. N = 3 independent experiments. Two-way analysis of variance. #P < .05.
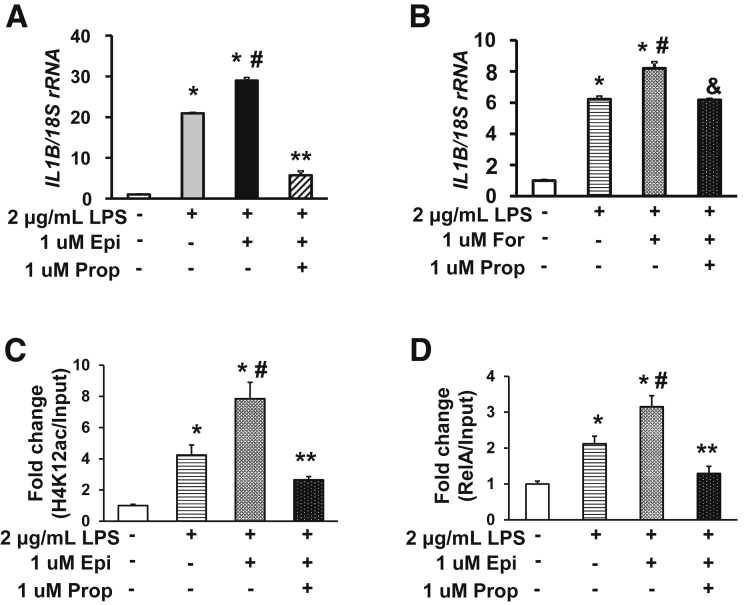
LPS-Induced IL1β Activation Is Aggravated by Epinephrine in Human THP-1–Derived Macrophages
To further validate that stress hormones aggravate IL1β activation in inflammatory conditions, we used human THP-1 cells, which differentiate into macrophages after treatment with PMA. Incubation with LPS dramatically increased IL1B mRNA expression in THP-1–derived macrophages (Figure 7A), as expected. In the presence of LPS, epinephrine (1 μmol/L) further increased IL1B expression. Surprisingly, norepinephrine (1 μmol/L) had little effect (data not shown). Propranolol treatment not only completely abrogated the IL1B overexpression exacerbated by epinephrine, but also significantly mitigated against LPS-induced IL1B activation. Similar to epinephrine, Foradil also further increased IL1B mRNA levels in the presence of LPS, and the increase was reversed by propranolol (Figure 7B).
Figure 7.
Propranolol ameliorates the aggravated up-regulation of IL1β by epinephrine or Foradil in human THP-1 cells. THP-1 cell–derived macrophages were treated with 0.1 μg/mL LPS, 1 μmol/L Foradil (For), or their combination for 24 hours. IL1B mRNA levels were evaluated by quantitative reverse-transcription PCR and normalized to 18S ribosomal RNA (rRNA). ChIP–quantitative PCR were performed to quantify RelA binding and H4K12ac levels. (A) Propranolol (Prop) markedly repressed the IL1B mRNA up-regulation by LPS and Epi (n = 3). *P < .05 vs control, #P < .05 vs LPS, **P < .05 vs LPS + Epi. (B) Exacerbated IL1B up-regulation by Foradil was abrogated by propranolol (n = 3). *P < .05 vs control, #P < .05 vs LPS, &P < .05 vs LPS + For. (C) H4K12ac levels at the core promoter of the human IL1B promoter. (D) ChIP–quantitative PCR evaluation of RelA association with the NF-κB binding sites on the human IL1B promoter. Means ± SEM. N = 3 independent experiments. Two-way analysis of variance. *P < .05 vs Ctl, #P < .05 vs LPS, **P < .05 vs LPS + Epi.
We next incubated THP-1 macrophages with LPS, epinephrine, or their combination in the presence or absence of propranolol for 24 hours, and performed ChIP–quantitative PCR assays on the human IL1B promoter. At the NF-κB binding region, H4K12ac was increased significantly in cells treated with LPS (Figure 7C), compared with control cells. Addition of epinephrine plus LPS further increased H4K12ac levels surrounding the NF-κB binding motifs; this increase was mitigated significantly by propranolol. Consistently, we found that the association of RelA with NF-κB binding motifs (-413/-404 and -297/-288) on the human IL1B promoter was increased significantly in LPS-treated cells vs control cells (Figure 7D). The NF-κB binding was increased further in cells treated with LPS + epinephrine, compared with LPS alone, and the exacerbated increase was ameliorated by propranolol. These results suggest that epinephrine aggravates LPS-induced IL1B overexpression by enhancing histone acetylation, as in the preclinical rat model.
Discussion
During the perinatal period, at a time of epigenetic plasticity, environmental conditions can modulate gene expression via epigenetic reprogramming.51 It is believed that adverse early life events can interfere with the perinatal programming and maturation of the immune system, predisposing the host to complex diseases20, 21, 22, 23, 24 such as IBD.8, 10, 18 A major deficiency in the field involves our understanding of how epigenetic regulation might predispose the host to IBD, and whether this occurs preferentially during the neonatal period. More specifically, it is unclear whether and how neonatal colonic inflammation epigenetically interferes with the activation of IL1β, which is highly overexpressed in patients with active IBD, and animals with experimental colitis. Identification of new IBD mechanisms, particularly molecules that mediate the exacerbation of IL1β expression, will facilitate the development of novel therapies and interventional strategies against IBD and/or IBD susceptibility associated with neonatal injury. In this report, a 2-hit chemical injury model was used to investigate epigenetic mechanisms linked to aggravated IL1β overexpression in colonic epithelium of the rat. We found that neonatal inflammation, followed by an additional inflammatory insult later in adult life, caused aberrant increases in colonic MPO activity, and IL1β overexpression was linked to epigenetic reprogramming of the IL1β gene transcription, via increased histone acetylation (H4K12ac) and RelA binding coupled to loss of HDAC3.
Recent findings point to a critical window of early postnatal development during which gene expression may be persistently re-programed.52 Adversity in the early stages of development can have a profound impact on psychological and physical health.53 Indeed, human and animal studies have provided converging evidence that adverse early life experiences, such as prenatal exposure to stress,54, 55, 56 nutritional deprivation,57 postnatal neglect and abuse,58, 59, 60 and neonatal inflammation,38, 61, 62 can have a significant long-term impact with implications for the emergence of various complex diseases, including IBD. The underlying mechanisms remain almost as much a mystery today as they were a century ago.23 It is understandable that early life adversity might cause a myriad of transient alterations. However, some durable epigenetic changes have been identified, which may underlie enduring physiological, neurologic, or pathologic outcomes. DNA methylation and histone modifications have been examined after certain early life experiences, with changes noted in several key genes involved in the regulation of the hypothalamic–pituitary–adrenal axis.63, 64, 65 A key question that remains unanswered is what mediates those epigenetic changes.
We found that circulating levels of both norepinephrine and epinephrine were increased in NI rats, which is consistent with previous observations,62, 66 and there was a greater increase in NI + AI rats. Norepinephrine is a catecholamine with multiple physiological functions, including acting as a hormone and a neurotransmitter. It is best known for its role in the fight-or-flight stress response, along with epinephrine, but there is accumulating evidence for the modulation of inflammatory outcomes. In 2007, Rommelfanger and Weinshenker67 reported that norepinephrine suppresses the expression of proinflammatory molecules, such as TNF-α and IL1β, and increased the expression of anti-inflammatory molecules, such as IκB, by signaling through α1-, α2-, and β-adrenergic receptors on astrocytes and glia. This regulates the expression of inflammatory genes and nitric oxide, which are thought to contribute to neurodegenerative diseases. Another study by Takayanagi et al68 in 2012 found that norepinephrine regulates intestinal mucosal immune responses. Norepinephrine suppresses the production of interferon-γ and TNF-α in murine intestinal intraepithelial lymphocytes via the β1 adrenoceptor. A major noradrenergic center of the brain is the locus coeruleus, which signals to sympathetic preganglionic cholinergic neurons in the spinal cord.69 These sympathetic nerve endings release norepinephrine, which has been found to have anti-inflammatory effects by interacting with the adrenoceptors expressed on lymphocytes and macrophages. In this way, norepinephrine has activity at both ends of the pathway: initiating signaling from the locus coeruleus and interacting with immune adrenoceptors to exert an immunomodulatory effect. In the case of IBD, it is well known that various stressors can trigger relapse, or exacerbate the inflammatory condition.70 Our findings suggest that persistently increased levels of norepinephrine and epinephrine in NI + AI rats sensitize the IL1β promoter in intestinal macrophages, resulting in aggravated IL1β activation; significantly greater increases of IL1β also were observed in NI rat macrophages incubated with LPS or LPS plus Foradil, a β2-adrenergic agonist. This notion is strongly supported by our findings showing that propranolol, a nonselective β blocker, markedly abrogated the aberrant overexpression of IL1β in NI + AI rats, and ameliorated against the heightened inflammatory response in the colon. Moreover, ablation of norepinephrine/epinephrine by adrenalectomy abrogated NI-induced susceptibility, and increase of norepinephrine/epinephrine by yohimbine sensitized the colon epithelium for exacerbated immune responses. In addition, incubation of human THP-1–derived macrophages with epinephrine or Foradil, but not norepinephrine, aggravated LPS-induced IL1β up-regulation; the aggravation was completely blocked by propranolol. These results provide additional evidence that epinephrine functions as a mediator of IL1β activation, and β-blockers could be beneficial to IBD patients.
IL1β is produced by a wide range of cells and is a major player in immune and inflammatory processes.31 However, molecular mechanisms by which epinephrine mediates or aggravates IL1β activation remain unclear. Epigenetic sensitization of the IL1β promoter by neonatal inflammation is virtually unknown. Our hypothesis was that epinephrine modulates histone modifications to remodel the chromatin state surrounding the IL1β promoter. ChIP–quantitative PCR data showed that HDAC3 association with the corresponding NF-κB binding region was repressed significantly in NI + AI rats vs Ctl + AI rats, although H4K12ac was increased markedly. Consistently, H4K12Ac and RNAP II binding at the core promoter of IL1β also were increased significantly, leading to heightened IL1β overexpression. Importantly, these epigenetic alterations were reversed by propranolol. Furthermore, LPS-induced histone acetylation on the human IL1B promoter in THP-1–derived macrophages was aggravated significantly by epinephrine, and this was alleviated by propranolol. Our findings show that epinephrine sensitizes the rat IL1β and human IL1B gene promoters via histone hyperacetylation.
Two NF-κB binding motifs exist in both human IL1B (-413/-404 and -297/-288) and rat IL1β promoters (-419/-410 and -303/-294), implicating NF-κB as a key regulator of IL1β expression in different species. We found significant down-regulation of IκBα in the colonic epithelia of NI + AI rats, and the underlying mechanisms are currently under investigation. It is noteworthy, however, that NF-κB alone cannot tether to DNA without chromatin relaxation, which is regulated by multiple factors generating and maintaining a cell-specific chromatin landscape. Although NF-κB is able to influence the chromatin state through a variety of mechanisms, chromatin remodeling remains a key determinant for DNA access and NF-κB binding activity.71 Epigenetic modifications, especially acetylation of the lysine residues on the N-terminal tails of histone proteins, are essential to such chromatin dynamics. The lysine residue at H4K12 can be acetylated but not methylated, and is part of a backbone of histone modifications associated with active promoters.72 Thus, histone hyperacetylation around the NF-κB binding motifs on the IL1B/IL1β promoter becomes critical for NF-κB–mediated IL1β activation. Indeed, RelA association with the IL1β promoter was significantly greater in the colon mucosa/submucosa of NI + AI rats, compared with the other 3 groups, which strongly correlates with the histone acetylation levels. We also observed aggravated RelA binding to the human IL1B promoter in THP-1–derived macrophages treated with both LPS and epinephrine. These findings provide strong evidence that adrenal signaling epigenetically modulates IL1β expression. Although changes in HDAC3 and H4K12ac were seen on the IL1β promoter in response to propranolol, we cannot rule out mechanisms such as up-regulation of miRNAs that target IL1β for down-regulation, such as miR-146a, which plays a critical role in gut homeostasis.73 Pyrosequencing showed only subtle changes in DNA methylation on the IL1β promoter after NI (data not shown); future studies might focus on long-range genomic interactions impacted by altered chromatin states in IBD.74
Importantly, rats given the 2-hit chemical insult protocol only as adults did not recapitulate the findings for NI + AI, strongly implicating early epigenetic reprograming in the neonate as a critical regulator of the exacerbated IL1β overexpression in the colon. Adult rats exposed to NI alone were normal in appearance, and their levels of MPO activity, IL1β expression, and H4K12 acetylation at the IL1β promoter were not significantly different from controls. Thus, individuals subjected to early life adversity might not be permanently predisposed to increased susceptibility for IBD. An increase of H4K12ac in NI + AI rats, based on ChIP–quantitative PCR assays, suggests the need to examine other epigenetic modifications that might prime the IL1B promoter for greater NF-κB access, and heightened IL1β protein expression. Comprehensive ChIP sequencing experiments are planned for the future to interrogate the epigenetic landscapes for IL1β, and other key inflammatory regulators that might be important mediators of NI responses and IBD susceptibility.
In summary, neonatal colonic inflammation, but not acute adult inflammation, aggravates the expression of stress hormones, particularly epinephrine, to induce histone hyperacetylation and allow greater access of NF-κB to the IL1β promoter, resulting in IL1β overexpression in the colonic epithelium. By doing so, NI renders the rat susceptible to an aggravated immune response, a hallmark of IBD, when they are exposed to an additional inflammation later in life. Propranolol ameliorated histone hyperacetylation and the exacerbated IL1β overexpression induced by NI. Thus, in some patients, IBD susceptibility might be circumvented via a precision medicine approach, using β-blockers and possibly HDAC or histone acetyltransferase modulators75, 76, 77, 78 to target critical points in disease pathogenesis.
Acknowledgments
The authors would like to acknowledge Karen Prince (Department of Pathology, Texas Children’s Hospital) for her artwork on the graphical abstract.
Footnotes
Author contributions Xiaoying S. Zhong, John H. Winston, Yingzi Cong, Tor C. Savidge, Roderick H. Dashwood, Don W. Powell, and Qingjie Li performed the study concept and design; Xiaoying S. Zhong, John H. Winston, Xiuju Luo, Kevin T. Kline, Syed Z. Nayeem, and Qingjie Li performed the experiments; Xiaoying S. Zhong, John H. Winston, Xiuju Luo, Kevin T. Kline, Syed Z. Nayeem, Yingzi Cong, Tor C. Savidge, Roderick H. Dashwood, Don W. Powell, and Qingjie Li analyzed and interpreted the data; Xiaoying S. Zhong, Roderick H. Dashwood, and Qingjie Li drafted the manuscript; Xiaoying S. Zhong, John H. Winston, Xiuju Luo, Yingzi Cong, Tor C. Savidge, Roderick H. Dashwood, Don W. Powell, and Qingjie Li critically revised the manuscript for important intellectual content; and Yingzi Cong, Tor C. Savidge, Roderick H. Dashwood, Don W. Powell, and Qingjie Li obtained funding.
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant R21 AI126097 and American Heart Association grant 17GRNT33460395 (Q.L.); National Institutes of Health/National Cancer Institute grants CA122959 and HHSN26100004, the John S. Dunn Foundation, and a Chancellors Research Initiative (R.H.D.); National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases R01 grants DK098370, DK105585, and DK112436 (Y.C.); National Institutes of Health/National Institute of Allergy and Infectious Diseases grants U011AI24290-01 and R01AI10091401 (T.C.S.); and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants R21DK096323-01 and P30 DK56338, which support the Texas Medical Center Digestive Diseases Center.
References
- 1.Loftus E.V., Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Farrokhyar F., Swarbrick E.T., Irvine E.J. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:2–15. doi: 10.1080/00365520150218002. [DOI] [PubMed] [Google Scholar]
- 3.Russel M.G., Stockbrugger R.W. Epidemiology of inflammatory bowel disease: an update. Scand J Gastroenterol. 1996;31:417–427. doi: 10.3109/00365529609006759. [DOI] [PubMed] [Google Scholar]
- 4.Sartor R.B. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 5.Wurzelmann J.I., Lyles C.M., Sandler R.S. Childhood infections and the risk of inflammatory bowel disease. Dig Dis Sci. 1994;39:555–560. doi: 10.1007/BF02088342. [DOI] [PubMed] [Google Scholar]
- 6.Delco F., Sonnenberg A. Exposure to risk factors for ulcerative colitis occurs during an early period of life. Am J Gastroenterol. 1999;94:679–684. doi: 10.1111/j.1572-0241.1999.00936.x. [DOI] [PubMed] [Google Scholar]
- 7.Hutfless S., Li D.K., Heyman M.B., Bayless T.M., Abramson O., Herrinton L.J. Prenatal and perinatal characteristics associated with pediatric-onset inflammatory bowel disease. Dig Dis Sci. 2012;57:2149–2156. doi: 10.1007/s10620-012-2128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonntag B., Stolze B., Heinecke A., Luegering A., Heidemann J., Lebiedz P., Rijcken E., Kiesel L., Domschke W., Kucharzik T., Maaser C. Preterm birth but not mode of delivery is associated with an increased risk of developing inflammatory bowel disease later in life. Inflamm Bowel Dis. 2007;13:1385–1390. doi: 10.1002/ibd.20206. [DOI] [PubMed] [Google Scholar]
- 9.Roberts S.E., Wotton C.J., Williams J.G., Griffith M., Goldacre M.J. Perinatal and early life risk factors for inflammatory bowel disease. World J Gastroenterol. 2011;17:743–749. doi: 10.3748/wjg.v17.i6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baron S., Turck D., Leplat C., Merle V., Gower-Rousseau C., Marti R., Yzet T., Lerebours E., Dupas J.L., Debeugny S., Salomez J.L., Cortot A., Colombel J.F. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut. 2005;54:357–363. doi: 10.1136/gut.2004.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aspberg S., Dahlquist G., Kahan T., Källén B. Fetal and perinatal risk factors for inflammatory bowel disease. Acta Paediatr. 2006;95:1001–1004. doi: 10.1080/08035250600573151. [DOI] [PubMed] [Google Scholar]
- 12.Gilat T., Hacohen D., Lilos P., Langman M.J. Childhood factors in ulcerative colitis and Crohn's disease. An international cooperative study. Scand J Gastroenterol. 1987;22:1009–1024. doi: 10.3109/00365528708991950. [DOI] [PubMed] [Google Scholar]
- 13.Mann E.A., Saeed S.A. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28:24–29. doi: 10.1097/MOG.0b013e32834c453e. [DOI] [PubMed] [Google Scholar]
- 14.Lidar M., Langevitz P., Shoenfeld Y. The role of infection in inflammatory bowel disease: initiation, exacerbation and protection. Isr Med Assoc J. 2009;11:558–563. [PubMed] [Google Scholar]
- 15.Kalischuk L.D., Buret A.G. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am J Physiol Gastrointest Liver Physiol. 2010;298:G1–G9. doi: 10.1152/ajpgi.00193.2009. [DOI] [PubMed] [Google Scholar]
- 16.Gradel K.O., Nielsen H.L., Schønheyder H.C., Ejlertsen T., Kristensen B., Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Hansen R., Thomson J.M., El-Omar E.M., Hold G.L. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol. 2010;45:266–276. doi: 10.1007/s00535-009-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renz H., Brandtzaeg P., Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 19.Schuurmans C., Kurrasch D.M. Neurodevelopmental consequences of maternal distress: what do we really know? Clin Genet. 2013;83:108–117. doi: 10.1111/cge.12049. [DOI] [PubMed] [Google Scholar]
- 20.Flavahan W.A., Gaskell E., Bernstein B.E. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:6348. doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinaudo P., Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012;74:107–130. doi: 10.1146/annurev-physiol-020911-153245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatchwell E., Greally J.M. The potential role of epigenomic dysregulation in complex human disease. Trends Genet. 2007;23:588–595. doi: 10.1016/j.tig.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Bale T.L., Baram T.Z., Brown A.S., Goldstein J.M., Insel T.R., McCarthy M.M., Nemeroff C.B., Reyes T.M., Simerly R.B., Susser E.S., Nestler E.J. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulic M.K., Hodder M., Forsberg A., McCarthy S., Richman T., D'Vaz N., van den Biggelaar A.H., Thornton C.A., Prescott S.L. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. 2011;127:470–478. doi: 10.1016/j.jaci.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida H., Russell J., Senchenkova E.Y., Almeida Paula L.D., Granger D.N. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am J Pathol. 2010;177:2774–2781. doi: 10.2353/ajpath.2010.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber A., Wasiliew P., Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 27.Coccia M., Harrison O.J., Schiering C., Asquith M.J., Becher B., Powrie F., Maloy K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witowski J., Tayama H., Ksiazek K., Wanic-Kossowska M., Bender T.O., Jörres A. Human peritoneal fibroblasts are a potent source of neutrophil-targeting cytokines: a key role of IL-1beta stimulation. Lab Invest. 2009;89:414–424. doi: 10.1038/labinvest.2009.1. [DOI] [PubMed] [Google Scholar]
- 29.Kwon K.H., Murakami A., Hayashi R., Ohigashi H. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337:647–654. doi: 10.1016/j.bbrc.2005.09.107. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C.A. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 32.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Sasson S.Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C.A., Paul W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. Immunity. 2009;31:331–341. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., Mills K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. J Exp Med. 2006;203:1685–1691. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 37.Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q., Winston J.H., Sarna S.K. Developmental origins of colon smooth muscle dysfunction in IBS-like rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G503–G512. doi: 10.1152/ajpgi.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q., Sarna S.K. Nuclear myosin II regulates the assembly of preinitiation complex for ICAM-1 gene transcription. Gastroenterology. 2009;137:1051–1060. doi: 10.1053/j.gastro.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi K., Chen J., Mitra S., Sarna S.K. Impaired integrity of DNA after recovery from inflammation causes persistent dysfunction of colonic smooth muscle. Gastroenterology. 2011;141:1293–1301. doi: 10.1053/j.gastro.2011.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q., Sarna S.K. Nitric oxide modifies chromatin to suppress ICAM-1 expression during colonic inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G103–G110. doi: 10.1152/ajpgi.00381.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs M.D., Harrison S.C. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 43.Verma I.M., Stevenson J.K., Schwarz E.M., Van Antwerp D., Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 44.Bevington S.L., Cauchy P., Cockerill P.N. Chromatin priming elements establish immunological memory in T cells without activating transcription: T cell memory is maintained by DNA elements which stably prime inducible genes without activating steady state transcription. Bioessays. 2017;39:2. doi: 10.1002/bies.201600184. [DOI] [PubMed] [Google Scholar]
- 45.Natoli G. Control of NF-kappaB-dependent transcriptional responses by chromatin organization. Cold Spring Harb Perspect Biol. 2009;1:a000224. doi: 10.1101/cshperspect.a000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer E.A., Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brzozowski B., Mazur-Bialy A., Pajdo R., Kwiecien S., Bilski J., Zwolinska-Wcislo M., Mach T., Brzozowski T. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14:892–900. doi: 10.2174/1570159X14666160404124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murburg M.M., Villacres E.C., Ko G.N., Veith R.C. Effects of yohimbine on human sympathetic nervous system function. J Clin Endocrinol Metab. 1991;73:861–865. doi: 10.1210/jcem-73-4-861. [DOI] [PubMed] [Google Scholar]
- 50.Navegantes K.C., de Souza Gomes R., Pereira P.A.T., Czaikoski P.G., Azevedo C.H.M., Monteiro M.C. Immune modulation of some autoimmune diseases: the critical role of macrophages and neutrophils in the innate and adaptive immunity. J Transl Med. 2017;15:36. doi: 10.1186/s12967-017-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucassen P.J., Naninck E.F., van Goudoever J.B., Fitzsimons C., Joels M., Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–631. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Karsten C.A., Baram T.Z. How does a neuron “know” to modulate its epigenetic machinery in response to early-life environment/experience? Front Psychiatry. 2013;4:89. doi: 10.3389/fpsyt.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kundakovic M., Lim S., Gudsnuk K., Champagne F.A. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry. 2013;4:78. doi: 10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winston J.H., Li Q., Sarna S.K. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterol Motil. 2014;26:715–730. doi: 10.1111/nmo.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Kinsella M.T., Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. 2009;52:425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lumey L.H., Stein A.D., Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horwitz A.V., Widom C.S., McLaughlin J., White H.R. The impact of childhood abuse and neglect on adult mental health: a prospective study. J Health Soc Behav. 2001;42:184–201. [PubMed] [Google Scholar]
- 59.De Bellis M.D. The psychobiology of neglect. Child Maltreat. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- 60.Anda R.F., Felitti V.J., Bremner J.D., Walker J.D., Whitfield C., Perry B.D., Dube S.R., Giles W.H. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacRae M., Macrina T., Khoury A., Migliore M.M., Kentner A.C. Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience. 2015;298:455–466. doi: 10.1016/j.neuroscience.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 62.Winston J.H., Sarna S.K. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology. 2013;144:570–579. doi: 10.1053/j.gastro.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weaver I.C., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 64.Korosi A., Shanabrough M., McClelland S., Liu Z.W., Borok E., Gao X.B., Horvath T.L., Baram T.Z. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murgatroyd C., Patchev A.V., Wu Y., Micale V., Bockmühl Y., Fischer D., Holsboer F., Wotjak C.T., Almeida O.F., Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 66.Aguirre J.E., Winston J.H., Sarna S.K. Neonatal immune challenge followed by adult immune challenge induces epigenetic-susceptibility to aggravated visceral hypersensitivity. Neurogastroenterol Motil. 2017;29:9. doi: 10.1111/nmo.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rommelfanger K.S., Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 68.Takayanagi Y., Osawa S., Ikuma M., Takagaki K., Zhang J., Hamaya Y., Yamada T., Sugimoto M., Furuta T., Miyajima H., Sugimoto K. Norepinephrine suppresses IFN-γ and TNF-α production by murine intestinal intraepithelial lymphocytes via the β₁ adrenoceptor. J Neuroimmunol. 2012;245:66–74. doi: 10.1016/j.jneuroim.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Westlund K.N., Bowker R.M., Ziegler M.G., Coulter J.D. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- 70.Mawdsley J.E., Rampton D.S. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatt D., Ghosh S. Regulation of the NF-κB-mediated transcription of inflammatory genes. Front Immunol. 2014;5:71. doi: 10.3389/fimmu.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z., Zang C., Rosenfeld J.A., Schones D.E., Barski A., Cuddapah S., Cui K., Roh T.Y., Peng W., Zhang M.Q., Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Runtsch M.C., Round J.L., O'Connell R.M. MicroRNAs and the regulation of intestinal homeostasis. Front Genet. 2014;5:347. doi: 10.3389/fgene.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dekker J., Mirny L. The 3D genome as moderator of chromosomal communication. Cell. 2016;164:1110–1121. doi: 10.1016/j.cell.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajendran P., Williams D.E., Ho E., Dashwood R.H. Metabolism as a key to histone deacetylase inhibition. Crit Rev Biochem Mol Biol. 2011;46:181–199. doi: 10.3109/10409238.2011.557713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajendran P., Delage B., Dashwood W.M., Yu T.W., Wuth B., Williams D.E., Ho E., Dashwood R.H. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011;10:68. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajendran P., Ho E., Williams D.E., Dashwood R.H. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin Epigenetics. 2011;3:4. doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajendran P., Dashwood W.M., Li L., Kang Y., Kim E., Johnson G., Fischer K.A., Löhr C.V., Williams D.E., Ho E., Yamamoto M., Lieberman D.A., Dashwood R.H. Nrf2 status affects tumor growth, HDAC3 gene promoter associations, and the response to sulforaphane in the colon. Clin Epigenetics. 2015;7:102. doi: 10.1186/s13148-015-0132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]