Abstract
Background & Aims
Pancreatic ductal adenocarcinoma (PDAC) remains the most aggressive malignancy with the lowest 5-year survival rate of all cancers in part owing to the lack of tumor-specific therapy and the rapid metastatic nature of this cancer. The gastrointestinal peptide gastrin is a trophic peptide that stimulates growth of PDAC in an autocrine fashion by interaction with the cholecystokinin receptor that is overexpressed in this malignancy.
Methods
We developed a therapeutic novel polyplex nanoparticle (NP) that selectively targets the cholecystokinin receptor on PDAC. The NP was characterized in vitro and stability testing was performed in human blood. The effects of the target-specific NP loaded with gastrin small interfering RNA (siRNA) was compared with an untargeted NP and with an NP loaded with a scrambled siRNA in vitro and in 2 orthotopic models of PDAC. A polymerase chain reaction metastasis array examined differentially expressed genes from control tumors compared with tumors of mice treated with the targeted polyplex NP.
Results
The polyplex NP forms a micelle that safely delivers specific gastrin siRNA to the tumor without off-target toxicity. Consistent with these findings, cellular uptake was confirmed only with the targeted fluorescently labeled NP by confocal microscopy in vitro and by IVIS fluorescent based imaging in mice bearing orthotopic pancreatic cancers but not found with untargeted NPs. Tumor uptake and release of the gastrin siRNA NP was verified by decreased cellular gastrin gene expression by quantitative reverse-transcription polymerase chain reaction and peptide expression by immunohistochemistry. Growth of PDAC was inhibited in a dose-related fashion in cell culture and in vivo. The targeted NP therapy completely blocked tumor metastasis and altered tumor-specific genes.
Conclusions
Our polyplex nanoparticle platform establishes both a strong foundation for the development of receptor-targeted therapeutics and a unique approach for the delivery of siRNA in vivo, thus warranting further exploration of this approach in other types of cancers.
Keywords: CCK Receptor, Gene Therapy, Gastrin, Nanotechnology, Orthotopic
Abbreviations used in this paper: CCK, cholecystokinin; Ex/Em, maximal excitation and emission wavelengths; Ga-10, gastrin 10 peptide; mRNA, messenger RNA; MW, molecular weight; N/P, ratio of “amines” of poly (L-lysine) unit and “phosphates” of siRNA complexed in the polyplex; NMR, nuclear magnetic resonance; NP, nanoparticle; PanIN, pancreatic intraepithelial neoplasia; PBS, phosphate-buffered saline; PDAC, pancreatic ductal adenocarcinoma; PEG, polyethylene glycol; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; siRNA, small interfering RNA
Graphical abstract
Summary.
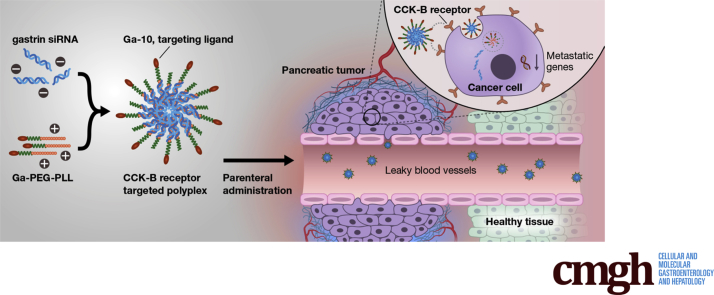
Here, we report the development of a polyplex nanoparticle that selectively targets the cholecystokinin receptor on human pancreatic cancer and delivers small interfering RNAs specific to gastrin to block cancer cell growth in vitro and in vivo. One remarkable finding in our investigation was that this therapeutic approach completely prevented metastasis—the most common cause of death in this condition.
Pancreatic ductal adenocarcinoma (PDAC) has a dismal prognosis,1, 2 and the current chemotherapeutic regimens provide a stagnant 5-year survival rate of only approximately 7%.3 With the recent increase in the incidence of pancreatic cancer it is anticipated that this malignancy will surpass colon and breast cancer in the next decade to become the second leading cause of cancer-related deaths in the United States.4 In this new era of precision medicine and genomic profiling, targeted therapies directed at cancer-specific receptors have improved the outcome of many recalcitrant cancers.5, 6 Reasons for the poor outcome in pancreatic cancer includes its propensity to metastasize rapidly1 and the lack of available targeted therapies.6
We previously showed that pancreatic cancer overexpresses the cholecystokinin-B (CCK-B) receptor.7 Although CCK-B receptors are present at a very low density in normal pancreas tissue,8 their expression increases in early precancerous pancreatic intraepithelial neoplasia (PanIN) lesions of the pancreas9 and becomes markedly overexpressed in cancer.7, 8 Down-regulation of the CCK-B receptor in pancreatic cancer cells has been shown to reduce cancer cell proliferation, decrease DNA synthesis, induce cell-cycle arrest, increase apoptosis, and decrease cell migration.10
CCK receptors also are expressed on pancreatic stellate cells,11, 12 the cell responsible for the dense fibrosis in the pancreatic tumor microenvironment,13, 14 and blockade of the CCK-B receptor in (Pdx1 [pancreatic and duodenal homeobox 1] promoter; Cre recombinase; Lox-Stop-Lox; G12D mutation results in an amino acid substitution at position 12 in KRAS [Kirsten rat sarcoma virus], from a glycine (G) to an aspartic acid [D]) Pdx1-Cre/LSL-KrasG12D transgenic mice halts progression of the precancerous PanIN lesions and also reverses the fibrosis.9 Gastrin is the major ligand for the CCK-B receptor,15 and gastrin stimulates the growth of pancreatic cancer in an autocrine fashion.16 Although gastrin is expressed in the fetal pancreas,17, 18, 19 its expression is turned off at week 14 of gestation and gastrin is not found in the normal adult human pancreas.20 However, the gastrin peptide becomes re-expressed in precancerous pancreatic PanIN lesions,21 and is expressed markedly in pancreatic cancer.20 Down-regulation of gastrin messenger RNA (mRNA) by RNA interference techniques inhibits growth and metastasis of human pancreatic cancer.22, 23 These properties make gastrin mRNA and its receptor, the CCK-B receptor, ideal targets for cancer therapeutics. RNA interference is an effective tool for studying gene expression in vitro; however, applying this technique in the clinic has been challenging. Various vehicles have been attempted to transport small interfering RNA (siRNA) to tissues in vivo, although safe and effective delivery methods remain problematic. Furthermore, drugs or molecules that target selective cancer cell membrane–associated receptors significantly improve efficacy and limit off-site toxicity.
Because the CCK-B receptor is overexpressed on PDAC, researchers have been trying to develop imaging strategies for CCK-B–receptor–positive cancers using 111In-labeled-CCK24 and 68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-minigastrin.25 Currently, radiopeptide imaging with 111In-minigastrin labeling to detect CCK-B receptors is established and in clinical use for medullary cancer (another cancer that expresses CCK-B receptors).26 By using a similar maleimide coupling technique to target the CCK-B receptor as previously described,27 we developed a polyplex nanoparticle (NP) that selectively targets the CCK-B receptor and serves as a target-specific vehicle to deliver gene therapy to inhibit growth and metastasis of PDAC. Biodegradable nontoxic nanoparticles that serve as vehicles to carry siRNA without off-target toxicity, such as the polyplex NP described in this work, have the potential to revolutionize cancer therapeutics.
Materials and Methods
Synthesis of the CCK-B–Receptor–Targeted Polyplex
The targeted polyplex was synthesized from gastrin-10 peptide conjugated poly (ethylene glycol)-block-poly (L-lysine) (Ga–polyethylene glycol [PEG]5k-PLL27) (5k indicates PEG molecular weight [MW] and 27 is the PLL degree of polymerization; termed Ga-PEG-PLL). Ga-PEG-PLL was synthesized from thiol functionalized PEG5K-PLL27 (sulfhydryl [SH]-PEG-PLL). Briefly, SH-PEG-PLL was synthesized from trityl-S-poly(ethylene glycol)-block-poly (L-lysine) (Tr-S-PEG-PLL) (average MW, 9700 g/mol; PEG MW, 5000 g/mol; Tr-S-PEG-PLL: custom synthesized; Alamanda Polymers, Huntsville, AL) by reducing with trifluoroacetic acid and triethylsilane (98:2 vol/vol). Maleimide functionalized gastrin 10 peptide (3-maleimido-propionyl-Glu-Glu-Glu-Ala-Tyr-Gly-Trip-Met-Asp-Phe-NH2; MW, 1426.48 g/mole) (Ga-10) was conjugated to the resulting SH-PEG-PLL via Michael addition reaction at pH 7 in deoxygenated HEPES buffer (100 mmol/L) under an inert atmosphere. Next, the reaction mixture was dialyzed (Spectrapor [Rancho Dominguez, CA] RC membrane; MW cut off, 8–10 kilodaltons) overnight against deionized water to remove any salts or other reaction reagents, and lyophilized for 48 hours to obtain white dry Ga-PEG-PLL. The peptide-conjugated polymer was purified further to remove any unreacted Ga-10 peptide using fast-phase liquid chromatography. Briefly, the fast-phase liquid chromatography system consisted of a UV detector set at λ = 220 nm, a size exclusion column (HiPrep 16/60 Sephacryl S-500 HR; GE Healthcare Life Sciences, Pittsburgh, PA), and a mobile phase consisting of sodium phosphate buffer (pH 7.0, 0.3 mol/L NaCl), at a flow rate of 1 mL/min. The isolated fraction corresponding to intact Ga-PEG-PLL was lyophilized and characterized. The synthesized Ga-PEG-PLL polymer was characterized by nuclear magnetic resonance (1H NMR) (400 MHz) and gel permeation chromatography (Shimadzu, Columbia, MD) equipped with both UV (λ = 220 nm) and fluorescent detectors for detection of tryptophan (Ex/Em (Excitation (Ex) and emission (Em) wavelengths, λ = 280/350 nm) as a fluorescent marker, and a Shodex (New York, NY) Protein KW 403-4F (mobile phase: sodium phosphate buffer, pH 7.0 + 0.3 mol/L NaCl, flow rate: 0.33 mL/min) (Shodex, Japan).
Finally, the polyplex was prepared by mixing 1 mg/mL of polymer (Ga-PEG5K-PLL27) for targeted and PEG5K-PLL30 (27 and 30 indicate the number of lysine units in PLL) for untargeted polyplex with gastrin siRNA (GUGCUGAGGAUGAGAACUA) (Invitrogen, Thermo Scientific, Waltham, MA) (19 mol phosphate/mol of siRNA) at the N/P ratio of 5 in 20 mmol/L HEPES buffered saline (pH 7.4), followed by a 30-minute incubation at room temperature to allow polyplex formation. The N/P molar ratios were calculated using moles of PEG-PLL primary amines to moles siRNA phosphates.
Characterization of the CCK-B–Receptor–Targeted Polyplex
The prepared targeted polyplex (Ga-PEG5K-PLL27) and untargeted polyplex (PEG5K-PLL30) were characterized for their hydrodynamic size distribution by dynamic light scattering using a S Nano Zetasizer (Malvern, Westborough, MA) with a low-volume quartz cuvette (b = 10 mm, 25°C, 633-nm laser, 173° scattering angle). The physiological stability of the gastrin siRNA polyplex was evaluated in 90% fresh human serum. Polyplex were prepared from untargeted PEG-PLL polymers of varied PEG vs PLL lengths (PEG MW, 5K–20K; PLL, 30–100 lysine units) at the N/P ratio of 5 in HEPES buffered saline (pH 7.4). Briefly, the gastrin siRNA polyplex (1.5 μL) was mixed with 13.5 μL of fresh human serum (100%) at a final siRNA concentration of 6.7 μmol/L and incubated at 37°C with 5% CO2 for various time points for up to 7 hours. At specific time points, the incubated samples were removed, serum nuclease activity was terminated, and samples were immediately frozen in liquid nitrogen and stored at -80°C. The integrity of the siRNA displaced from nanoparticles was analyzed by gel electrophoresis in 20% polyacrylamide gel performed at 140 volts for 1.5 hours, the siRNA bands were visualized under a UV transilluminator at 497 nm, and the image was captured with an equipped camera.
Gastrin siRNA Polyplex Effects on Human Pancreatic Cancer Cell Growth In Vitro
Human pancreatic cancer cells were cultured in appropriate media (RPMI for AsPC-1 and BxPC-3 cells, and DMEM for PANC-1 cells). For cell growth studies, 300,000 cells were plated onto each well of a 6-well tissue culture plate. Cells then were treated for 48 hours with phosphate-buffered saline (PBS) vehicle control, polyplex with scrambled siRNA, or polyplex with gastrin siRNA, at siRNA concentrations of 120, 240, or 480 nmol/L. Viable cell counts then were performed by the trypan blue exclusion technique.
To show that the siRNA polyplex indeed was taken up into the cytoplasm of the cancer cells, cancer cells were treated for 48 hours with either PBS (control) or Cy3-labeled siRNA (Dharmacon, Lafayette, CO) polyplex (240 or 480 nmol/L). Nuclei were stained with 4′,6-diamidino-2-phenylindole. Images were taken with a 40× objective on a Zeiss (San Diego, CA) (LSM-510) confocal Microscope.
RNA was extracted from pancreatic cancer cells (Qiagen Gaithersburg, MD) after treatment with PBS, scrambled RNA polyplex, or gastrin siRNA polyplex for 48 hours. Pancreatic cancer cells were treated with polyplex carrying gastrin siRNA at 120, 240, or 480 nmol/L, scrambled siRNA control polyplex, or PBS vehicle for 48 hours, and quantitative gastrin mRNA analysis was performed. RNA was extracted from treated cells and subjected to real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) using SYBR Green (Life Technologies) and the following gastrin oligonucleotide primers: forward: 5’-GCCTCTCATCATCGAAGGCA-3’ and reverse: 5’-GCCGAAGTCCATCCATCCAT-3’, with glyceraldehyde-2-phosphate dehydrogenase as the internal control.
In addition to showing that the polyplex loaded with gastrin siRNA decreased gastrin mRNA, we also confirmed that the peptide was decreased by performing immunohistochemistry on pancreatic cancer cells treated with siRNA-loaded polyplex compared with controls. A total of 150,000 cancer cells were plated onto round coverslips. The following day, cells were treated with 120, 240, or 480 nmol/L of gastrin siRNA polyplex and scrambled siRNA polyplex for 48 hours. The cells were washed, fixed, and incubated with a polyclonal gastrin antibody (1:1000; Peninsula Labs, Carlsbad, CA) overnight at 4°C, followed by incubation with a secondary goat anti-rabbit rhodamine-labeled antibody (1:200; Thermo Scientific) for 1 hour at room temperature in the dark. Coverslips were mounted with EverBrite hardset media with 4′,6-diamidino-2-phenylindole (Biotium, Hayward, CA), and imaged by fluorescent microscopy using an Olympus (Center Valley, PA) IX-71 inverted epifluorescence microscope.
Effects of CCK-B–Receptor–Targeted Gastrin siRNA Polyplex Therapy on Growth and Metastasis of Pancreatic Cancer In Vivo
The next series of experiments were conducted using 2 human pancreatic cancer cell lines grown orthotopically in the pancreas of athymic nude mice. All animal studies were performed in an ethical fashion under a protocol approved by the Georgetown University Institutional Animal Care and Use Committee. In the orthotopic tumor experiments, each mouse received an inoculum of tumor cells (900,000 for BxPC-3 cells and 1 × 106 for PANC-1 cancer cells) orthotopically into the pancreas with a 28–30G needle in a volume of 0.1 mL. Before the orthotopic injection of tumor cells, all mice were fully anesthetized with a mixture of ketamine-HCl (100 mg/kg) and zylazine (5 mg/kg) intraperitoneally or inhaled isoflurane 3% for induction and 0.25%–2% for maintenance. Incisions were treated with 1% lidocaine to minimize pain after surgery and staple suturing. Mice in each group were allowed to recover from surgery for 1 week. Because the tumors were orthotopic, we used cancer cells that had been transfected with luciferase for measurements and visualization. Seven days after tumor cell inoculation, all the mice were imaged and then placed randomly into 1 of 5 different treatment groups such that the mean tumor size by luciferase imaging was equal in all the groups at baseline. Animals then were treated 3 times per week (0.1 mL volume, intraperitoneally) with PBS vehicle control or 240 nmol/L (BxPC-3) or 480 nmol/L siRNA (PANC-1) of targeted gastrin siRNA polyplex (targeted siRNA), targeted scrambled siRNA control polyplex (targeted scrambled), untargeted gastrin siRNA polyplex (untargeted siRNA), or untargeted scrambled siRNA control polyplex (untargeted scrambled). One set of mice that was not used for the growth experimentation was allowed to let the orthotopic tumors grow for several weeks, then these mice were injected intraperitoneally with either CCK-B–receptor–targeted or –untargeted polyplex loaded with 240 nmol/L of fluorescent Cy3-labeled gastrin siRNA (Dharmacon) and imaged immediately and again after 5 hours with the IVIS Lumina III In Vivo Optical Imaging System (Perkin Elmer, Bridgeville, PA) to determine if the targeted NPs increased delivery to the tumors.
Ten minutes before imaging, luciferin (Nanolight Technology, Pinetop, AZ) was administered to mice (using a 27 g needle intraperitoneally) at a concentration of 135 mg/kg in a volume of 100 μL. Tumor size was determined using software on an IVIS imaging system in the animal facility of the Georgetown University College of Medicine. Tumor volumes were measured by IVIS imaging weekly, and after 4 weeks of therapy for BxPC-3 tumors and 7 weeks for PANC-1 tumors, mice were euthanized, and tumors and metastases were dissected.
Tissue Analysis and Immunohistochemistry
Excised tumors were weighed and divided for study by flash-freezing tissues, and placing part in RNAlater (ThermoFisher, Waltham, MA), and placing part in formalin. The number, size, and location of metastases also were recorded. Metastases were confirmed histologically by H&E staining in the core histopathology laboratory of Georgetown Lombardi Cancer center. For immunohistochemistry, tumors were sectioned from paraffin-embedded blocks (10 μm) and fixed on slides. Antigen retrieval was performed in citrate buffer (pH = 6) and specimens were blocked with 3% peroxidase in Tris-buffered saline with Tween, then blocked with 10% normal goat serum in Tris-buffered saline with Tween for 10 minutes at room temperature. Tissue sections then were reacted with a polyclonal anti-goat gastrin antibody (T-4347; Peninsula Labs) at a titer of 1:200 at 4°C overnight and then reacted with a rabbit anti-goat horseradish peroxidase secondary antibody for 30 minutes at room temperature for BxPC-3 tumors. Analysis of gastrin immunoreactivity was performed by a technician blinded to the treatment using software by ImageJ (National Institutes of Health, Bethesda, MD). Immunoreactivity for Ki67 was performed with an anti-Ki67 antibody (NB600-1252; Novus Biological, Oceanside, CA) at a titer 1:100 at 4°C overnight, and then reacted with the rabbit anti-goat horseradish peroxidase secondary antibody for 30 minutes at room temperature. Manual Ki67 cell counts were performed in a blinded fashion with 5–8 low-power fields analyzed and averaged per group.
For gastrin mRNA analysis, poly-A RNA was extracted from PANC-1 tumors and subjected to qRT-PCR on an Applied Biosystems (Foster City, CA) Step-one plus Real-Time PCR System using fast cycling. The reaction was performed with SYBR Green (Life Technologies) and gastrin primers (as described earlier).
Metastasis Array Analysis
Metastases were counted and removed for histologic confirmation by H&E staining. RNA was extracted (RNeasy; Qiagen) from BxPC-3 primary control tumors and tumors from mice treated with targeted gastrin siRNA. RNAs were subjected to a PCR metastases array (PAHS-028Z; Qiagen) analysis following the protocol provided by the manufacturer. This array included 84 genes that encode several classes of protein factors including cell adhesion, extracellular matrix components, cell cycle, cell growth and proliferation, apoptosis, transcription factors and regulators, and other genes related to tumor metastasis.
Statistical Analysis
Results were expressed as means ± SE and parametric analysis was performed when the results assumed a normal bell-shaped distribution. In this situation, analysis of variance and Student t tests were used to evaluate statistical significance with a P < .05 considered statistically significant. When the data were skewed, a nonparametric analysis was performed using either the Kruskal–Wallis or Mann–Whitney tests. RT-PCR results were expressed as a pairwise Student t tests on the normalized mean change in cycle threshold (the difference between the cycle count of the gene of interest minus the count of an endogenous control) values for each group according to the method of Livak and Schmittgen.28
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Synthesis of the CCK-B–Receptor–Targeted Polyplex Ga-PEG-PLL
Development of the targeted polyplex platform from a thiol functionalized polyethylene glycol-block-poly (L-lysine) (SH-PEG-PLL) is shown in Figure 1A. Grafting of PEG to the surface of the NPs extends their circulation lifetime and prevents interactions with the biological in vivo environment (ie, uptake into hematopoietic cells).29 NPs, functionalized with PEG moieties, accumulate within solid tumors via the enhanced permeation retention effect.30, 31 Although enhanced permeation retention serves as an effective passive targeting strategy, improved uptake and less off-target toxicity occurs when the NP can actively and selectively target receptor proteins on cancerous cells to deliver genes and agents.
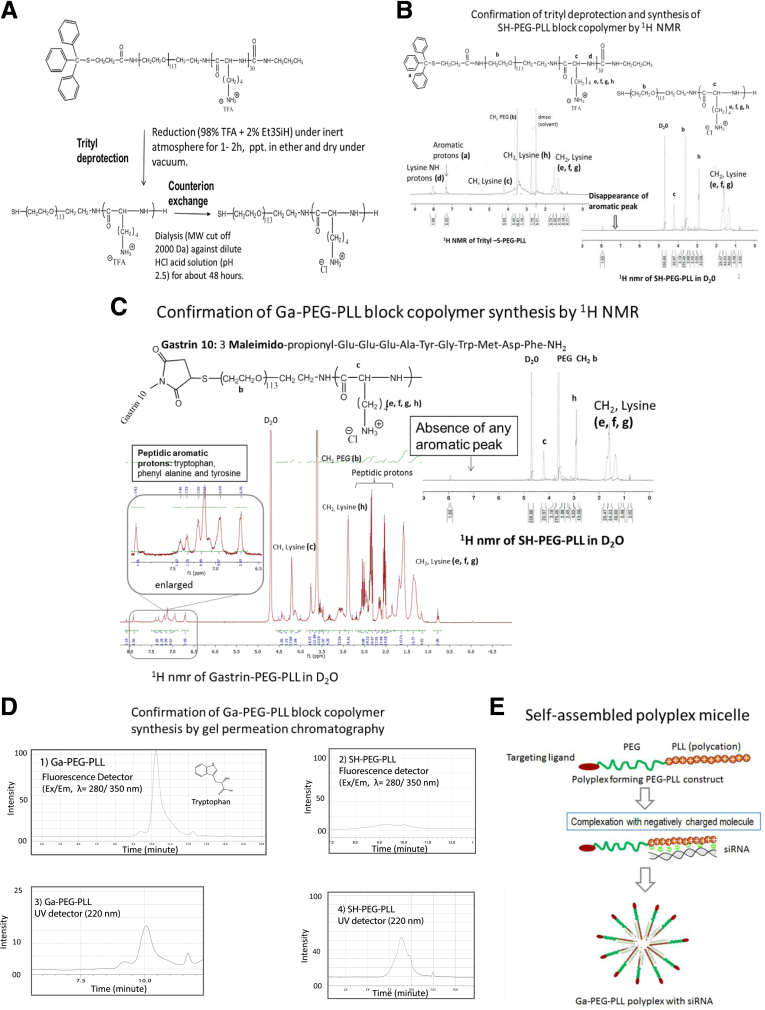
Figure 1.
Synthesis of the gastrin-targeted polyplex. (A) Synthesis of thiol-functionalized polyplex forming block copolymer SH-PEG-PLL. SH-PEG-PLL block copolymer was synthesized from Tr-S-PEG-PLL (Tr-S-PEG-PLL) by reducing with trifluoroacetic acid and triethylsilane (98:2 vol/vol). (B) 1H NMR of trityl protected polymer and thiol-functionalized polymer SH-PEG-PLL (absence of aromatic peaks at δ, 7.35 ppm). (C) Synthesis of CCK-B–receptor–targeted PEG-PLL block copolymer (Ga-PEG-PLL). A CCK-receptor–specific peptide, Ga-10, was conjugated to the SH-PEG-PLL block copolymer via a thiol-maleimide–coupling reaction. 1H NMR of purified Ga-PEG-PLL indicates the presence of an aromatic proton (δ, 7.5–6.8 ppm) from the Ga-10 peptide. (D) Both Ga-PEG-PLL and SH-PEG-PLL were assessed by gel permeation chromatography to confirm the Ga-10 conjugation to the polymer backbone using a Shodex protein KW 403-4F column using both fluorescence (Ex/Em: λ= 280/350 nm) and UV detector (220 nm). Because of the presence of fluorescent tryptophan (Ex/Em: λ= 280/350 nm) in the Ga-10 peptide, the target-specific polymer, Ga-PEG-PLL elution was strongly detected via fluorescent detector (D1), whereas the untargeted precursor, SH-PEG-PLL elution was barely detected (D2) at an identical polymer concentration. On the other hand, both targeted and untargeted polymers showed an identical elution profile and intensity via UV detector at 220 nm (D3 and D4). (E) Finally, the targeted polyplex was prepared by mixing the Ga-PEG-PLL with selective gastrin siRNA to form the polyplex micelle.
Characterization of the untargeted thiol functionalized polymer by 1H-NMR confirmed the NP molecular weight of approximately 9400 daltons. Trityl deprotection (Figure 1B) and subsequent conjugation of Ga-10 polymer were confirmed by 1H-NMR, which showed complete removal of the trityl group and more than 70% conjugation (Figure 1C), rendering the NPs target-specific to the CCK-B receptor.27, 31 The conjugation of Ga-10 to the PEG-PLL polymer backbone was confirmed further by gel permeation chromatography using Ga-10–associated tryptophan fluorescence (ex/em, 280/350) from the Ga-PEG-PLL polymer (Figure 1D1). Untargeted polymer also was evaluated under identical conditions and no fluorescent peak was detected, indicating the absence of any peptide-associated fluorescent moiety in the polymer Figure 1D2). Both targeted and untargeted polymers showed identical elution profiles and intensity via UV detection at 220 nm (Figures 1D3 and D4).
The positively charged lysine polycation tail (PLL) of the Ga-PEG-PLL polymer allows for electrostatic complexation with negatively charged siRNA, and when mixed together with the siRNA in an aqueous solution, a self-assembled polyplex micelle forms owing to the amphiphilic nature of the block copolymer complexed siRNA (Figure 1E). This pegylated core-shell structured morphology shields the host from the polycationic charge that could induce off-target toxicity (which occurs commonly with cationic liposome formulations), and protects the siRNA from nuclease-mediated degradation.
Characterization of the CCK-B–Receptor–Targeted Polyplex
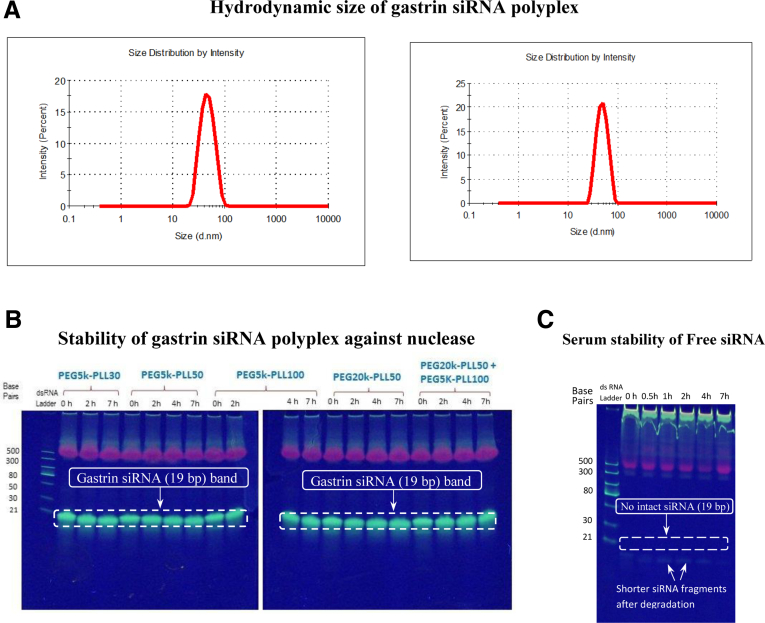
Both the targeted and untargeted synthesized polyplex NPs were characterized for their hydrodynamic size by dynamic light scattering, with resulting size distributions of 44.3 ± 0.3 and 48.2 ± 0.3 nm for the targeted and untargeted polyplex, respectively (Figure 2A), indicating that the addition of the Ga-10 moiety for targeting did not significantly increase the size of the NP. Nanoparticles are characteristically very small (<100 nm) and can penetrate the fenestrated vasculature and dense fibrotic microenvironment characteristic of pancreatic cancer.32 To be clinically useful, siRNA delivery platforms also must be stable in human blood. Therefore, the physiological stability of the polyplex NP was compared with free siRNA in fresh human serum. The PEG-PLL polyplex encapsulating gastrin siRNA was protected efficiently from serum degradation for at least 7 hours, the last incubation time point (Figure 2B). In contrast, free siRNA was completely degraded within a few minutes of incubation and no nucleic acid band was detected at even the earliest incubation time point of 0.5 hours (Figure 2C). The siRNA size also was confirmed by gel electrophoresis and consistent with 19 bp (Figure 2C).
Figure 2.
Characterization of the targeted polyplex. (A) Hydrodynamic size of both targeted (Ga-PEG-PLL) and untargeted (PEG-PLL) polyplex measured by dynamic light scattering technique. The targeted polyplex was synthesized from Ga-PEG5K-PLL27 and the untargeted version from PEG5K-PLL30 block copolymer by complexing with gastrin siRNA at the N/P ratio of 5. Attachment of the targeting ligand, Ga-10, on the targeted polyplex surface did not change the polyplex size significantly. (B) A series of untargeted PEG-PLL polyplex formulations from varied PEG (5k–20k) and PLL block lengths (30–100 lysine units) encapsulating gastrin siRNA (19 bp) at the N/P ratio of 5 showed remarkable enhancement in serum stability compared with free siRNA. No siRNA degradation was observed for at least 7 hours in 90% fresh human serum. PEG5K-PLL30 was chosen as an untargeted polyplex for all in vitro and in vivo investigations. (C) Unencapsulated (free) siRNA degrades very fast in human serum (serum half-life, <10 min).
Effects of Polyplex on Pancreatic Cancer Growth in Cell Culture
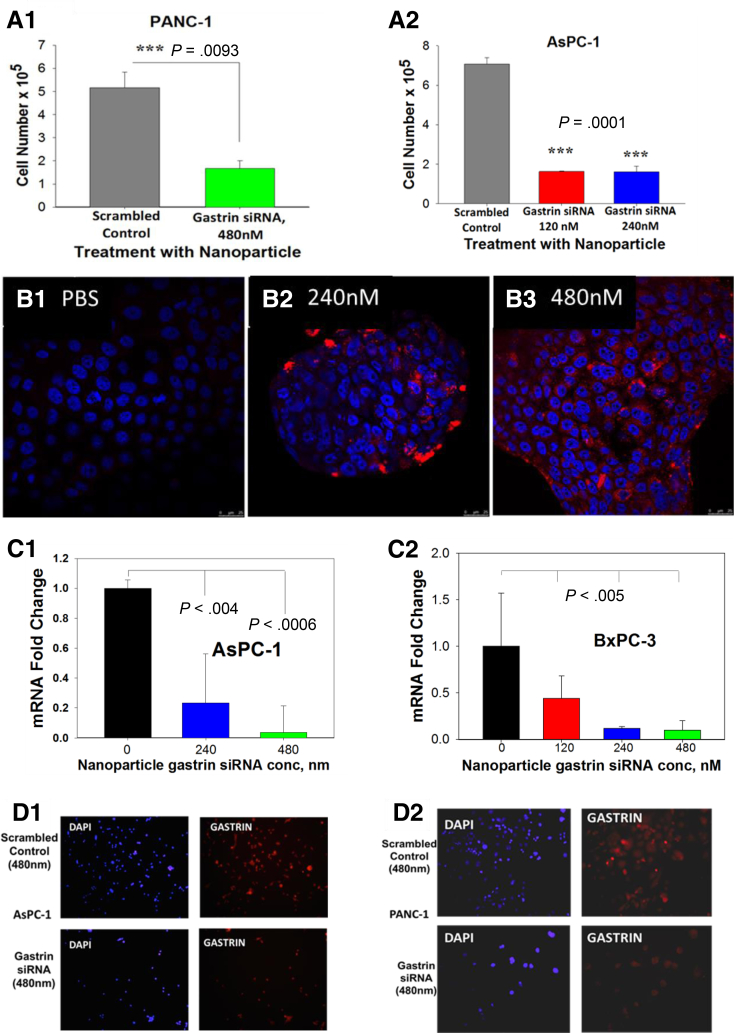
Three different human PDAC cell lines were studied in vitro. All 3 cancer cells overexpress gastrin mRNA and peptide in varying amounts: AsPC-1 > BxPC-3 > PANC-1.33 CCK-B receptors also are overexpressed on all of these cells (as determined by radioligand-receptor binding assays), ranging from PANC-1 with a binding capacity with a Bmax of 283 fmol/mg protein to BxPC-3 with a Bmax of 125 fmol/mg protein.7, 20 The effects of gastrin siRNA-loaded polyplex NPs on the growth of pancreatic cancer cells were evaluated in vitro and compared with that of cells treated with a scrambled siRNA control. Polyplex NP complexed with the gastrin siRNA significantly reduced cellular proliferation compared with cancer cells treated with the nonselective scrambled siRNA-loaded polyplex in a dose-related fashion with the 480 nmol/L dose having the greatest inhibition (Figure 3A). To confirm that NPs are taken up into the cancer cells and release the siRNA, we fluorescently labeled the gastrin siRNA with the fluorophore Cy3 and imaged the cellular compartments by confocal microscopy; imaging confirmed localization of the fluorescently tagged gastrin siRNA in the cytoplasm (Figure 3B), with the greatest fluorescence observed at the higher (480 nmol/L) concentration of siRNA (Figure 3B3). By using the 2 human PDAC cancer cell lines (AsPC-1 and BxPC-3) that express the greatest amount of gastrin,22 we showed that treatment of these pancreatic cancer cells in culture with polyplex NP loaded with gastrin siRNA significantly decreased gastrin mRNA by qRT-PCR in a dose-responsive decrease in (480 nmol/L > 240 nmol/L > 120 nmol/L > PBS) compared with cells treated with NPs loaded with scrambled control siRNA (Figure 3C1 and C2). To confirm delivery and release of the siRNA and its ability to prevent peptide translation, we analyzed gastrin peptide knockdown by immunofluorescence. The NP carrying gastrin siRNA significantly decreased in gastrin peptide compared with AsPC-1 or PANC-1 cancer cells treated with a scrambled siRNA control polyplex (Figure 3D). These data show that polyplex micelle nanoparticles are taken up into the cancer cells in vitro, and deliver the antigastrin siRNA payload, which then down-regulates expression of the gastrin peptide and subsequently cancer cell growth. Because the human cancer cells produce varying amounts of gastrin mRNA and peptide, our NP platform shows an efficient means for decreasing gastrin production and growth from a variety of pancreatic cancer cells.
Figure 3.
Effects of gastrin siRNA polyplex on growth of pancreatic cancer in vitro. (A) Effects of siRNA-loaded NPs on growth of pancreatic cancer in vitro. Growth of pancreatic cancer cells PANC-1 (A1) and AsPC-1 (A2) was decreased significantly with the addition of polyplex loaded with the gastrin siRNA compared with scrambled control RNA. (B) Localization of polyplex in cancer cells after uptake. Confocal microscopy (Zeiss) images of BxPC-3 human pancreatic cancer cells in culture treated with PBS (control, B1) or polyplex loaded with fluorescently labeled Cy3 siRNA shows uptake and localization of the siRNA in the cancer cell cytoplasm when treated with 240 nmol/L (B2) or 480 nmol/L (B3) of siRNA. (C) Measurement of target mRNA after treatment with siRNA-loaded polyplex. Quantitative RT-PCR showed a dose-dependent decrease in gastrin mRNA in AsPC-1 (C1) and BxPC-3 (C2) cells treated with polyplex carrying gastrin siRNA at 120, 240, or 480 nmol/L, but not in vehicle controls. (D) Analysis of gastrin peptide expression by immunofluorescence in AsPC-1 (D1) and PANC-1 (D2) cells after treatment with siRNA polyplex confirms that the polyplex loaded with gastrin siRNA also decreased peptide expression.
Effects of CCK-B–Receptor–Targeted Polyplex on Growth and Metastases In Vivo
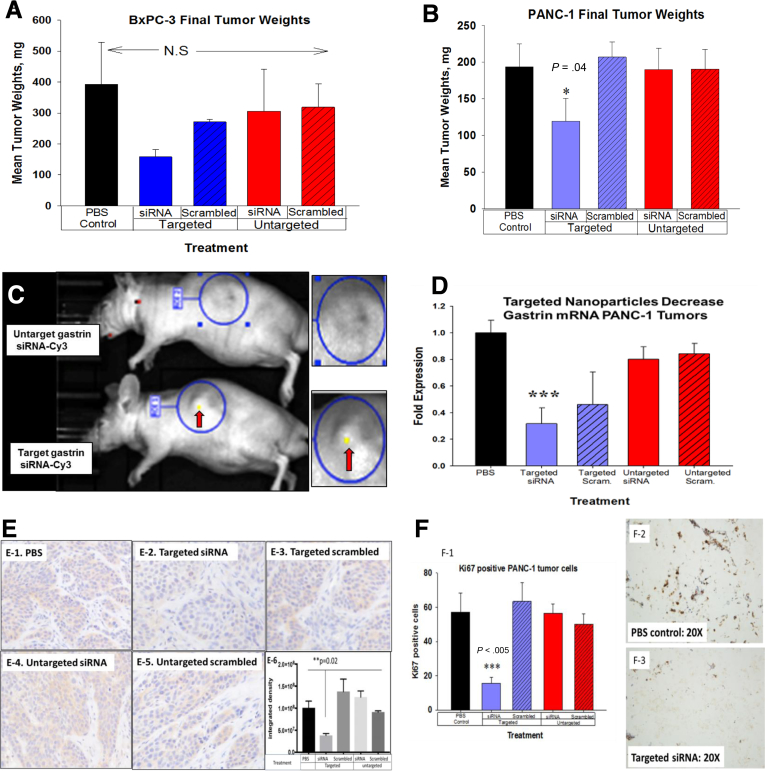
Because selective receptor targeting enhances delivery and uptake to tumors, we evaluated the effects of targeted gastrin siRNA polyplex NPs on pancreatic cancer growth in comparison with untargeted NPs in 2 unique murine orthotopic tumor models. There was no evidence of toxicity related to the NP therapy by histologic examination of uninvolved livers and all the mice gained weight equally in all groups (data not shown). In our animal models, we compared CCK-receptor–targeted specific polyplex NPs carrying gastrin siRNA gene therapy with that of 4 other control treatments: PBS, targeted NPs with scrambled siRNA, untargeted NPs with gastrin siRNA, and untargeted NPs with scrambled siRNA. The importance of these controls was to show that the selective receptor targeting of the NPs markedly improves tumor uptake and knockdown of the gene of interest (gastrin). In addition, we compared targeted specific NPs carrying the gastrin siRNA with targeted NPs carrying a scrambled siRNA control with the same nucleotide composition to show that both receptor targeting and selective mRNA down-regulation of a proliferative peptide are required to inhibit growth and metastasis of pancreatic cancer. The treatment of mice bearing human BxPC-3 tumors with 240 nmol/L of target-specific NPs showed a decrease in final tumor weights (Figure 4A) compared with untargeted and control (PBS-treated) mice, but these decreases did not reach statistical significance. Although the primary tumor mass was not statistically less, none of the BxPC-3 tumor-bearing mice treated with targeted gastrin loaded siRNA NPs had evidence of metastases. Therefore, to improve efficacy for primary tumor growth inhibition, we increased the NP dose to 480 nmol/L of gastrin siRNA in a second set of in vivo experiments with PANC-1 tumor-bearing mice. At this higher dose, PANC-1 primary tumor weights were indeed significantly smaller in the mice treated with the targeted siRNA polyplex NP compared with all of the other control treatment groups (Figure 4B). Similar to the BxPC-3, none of the mice bearing PANC-1 pancreatic cancer treated with the target-specific gastrin siRNA polyplex NP had evidence of metastatic disease. These in vivo experiments support the in vitro studies and show the dose-responsive effectiveness of the CCK-receptor–targeted nanoparticle polyplex to inhibit cancer growth. These in vivo studies are important because they show the ability of the targeted polyplex NP to inhibit metastases in 2 different models. The studies also show the efficient uptake of the NPs with CCK-receptor targeting and knockdown of the gene of interest: gastrin.
Figure 4.
Selective uptake of targeted gastrin siRNA polyplex decreases orthotopic pancreatic tumor growth in vivo. (A) Final BxPC-3 tumor weights tended to be smaller in mice treated with 240 nmol/L siRNA, but this was not statistically significant. (B) Mice bearing human PANC-1 tumors and treated with 480 nmol/L targeted siRNA had significantly smaller tumors compared with controls. (C) Targeted siRNA are taken up into BxPC-3 orthotopic pancreatic tumors in mice. Polyplex that were either targeted to the CCK-B receptor or untargeted were loaded with fluorescently labeled Cy3 gastrin siRNA and injected intraperitoneally and imaged by fluorescent microscopy. Only the mice receiving the targeted siRNA showed fluorescent uptake within the tumors after 5 hours (arrows showing fluorescence). There was no uptake of fluorescent particles detected in the mice treated with untargeted polyplex circles show area of tumor and magnified view. (D) qRT-PCR for gastrin mRNA. Treatment with targeted siRNA had significantly less gastrin mRNA in PANC-1 tumors. (E1-5) Immunohistochemistry for gastrin peptide is shown from a representative BxPC-3 tumor from each treatment group. (E6) Densitometry analysis of immunostaining for gastrin reactivity showed significantly less gastrin (P = .02) in the tumors of mice treated with targeted siRNA. (F) Ki67 staining proliferation index was markedly reduced in PANC-1 tumors of mice treated with targeted siRNA (F1). PBS control Ki67 immunoreactivity (F2). Tumors treated with targeted NP with gastrin siRNA have reduced Ki67 immunoreactivity (F3). ∗P < .05, ∗∗∗P < .005.
Although the primary tumor size of the BxPC-3 tumors were not statistically different from each other, the polyplex NPs still decreased gastrin expression and prevented metastases. Reasons for the significant decrease in the primary tumor weights of the PANC-1 orthotopic mice treated with the targeted antigastrin siRNA polyplex compared with the mice bearing BxPC-3 tumors may be related to the dosing. The polyplex dose was increased to 480 nmol/L 3 times per week in the PANC-1 mice compared with 240 nmol/L in the BxPC-3 mice. Perhaps a more frequent dosing schedule or a higher dose also significantly would have decreased the primary BxPC-3 tumor size. The amount of gastrin produced by BxPC-3 cells is higher than the amount produced by PANC-1 cells,22 and the PANC-1 cancer cells have greater CCK-B–receptor density as determined by radioligand binding studies.7 We previously showed that the inhibition of cancer cell growth in vivo was directly correlated to the efficiency and degree of the gastrin down-regulation by RNA interference.7 For example, BxPC-3 gastrin-knockout stable clones with 75% gastrin mRNA down-regulation had slower growth rates, whereas those with 90% or more gastrin mRNA knockdown failed to grow tumors at all in mice. In the present investigation, some of the mice bearing BxPC-3 tumors treated with the CCK-B receptor targeted scrambled siRNA polyplex appeared to have smaller tumors, although this was not significant. Our rationale for this observation is that perhaps the target-specific polyplex may bind to the CCK-B receptors on the tumor cells and block endogenous gastrin binding, reducing signal transduction from gastrin produced in an autocrine fashion.
To confirm tumor uptake of the polyplex in vivo, we imaged BxPC-3 mice bearing pancreatic cancer orthotopic tumors after intraperitoneal injection of targeted or untargeted NPs loaded with Cy3-labeled gastrin siRNA using an IVIS Lumina III in Vivo Optical Imaging System. Imaging showed fluorescence localized only in the tumors after 5 hours of mice treated with the CCK-receptor–targeted polyplex NP and not the untargeted polyplex NP (Figure 4C). These data confirm that the polyplex NPs that were constructed to selectively target the CCK-B receptor were taken up and concentrated in the tumors that overexpress this receptor. Measurement of gastrin mRNA by qRT-PCR in the excised tumors confirmed that there was decreased gastrin gene expression only in the tumors of mice treated with targeted siRNA to gastrin (Figure 4D). Gastrin peptide was decreased significantly as confirmed by immunohistochemistry in tumors of animals treated with the targeted siRNA polyplex, thus supporting efficient gastrin siRNA delivery and subsequent protein down-regulation (Figures 4E1–E5). Analysis of the immunohistochemical staining by densitometry confirmed that only tumors of mice treated with targeted gastrin siRNA polyplex had decreased peptide expression (Figure 4E6). The proliferative index, as determined by Ki67 immunohistochemical staining, also significantly was reduced only in the tumors of mice treated with the targeted siRNA polyplex (Figures 4F1–I3) compared with controls, which correlates with the diminished gastrin expression observed in this treatment group. These in vivo results confirm that treatment with the CCK-receptor–targeted specific polyplex NP was an effective vehicle to deliver gastrin siRNA therapy to human pancreatic cancer and inhibit growth by down-regulating the proliferative growth peptide gastrin.
Treatment With CCK-B–Receptor–Targeted Gastrin siRNA Polyplex Alters Metastasis Genes
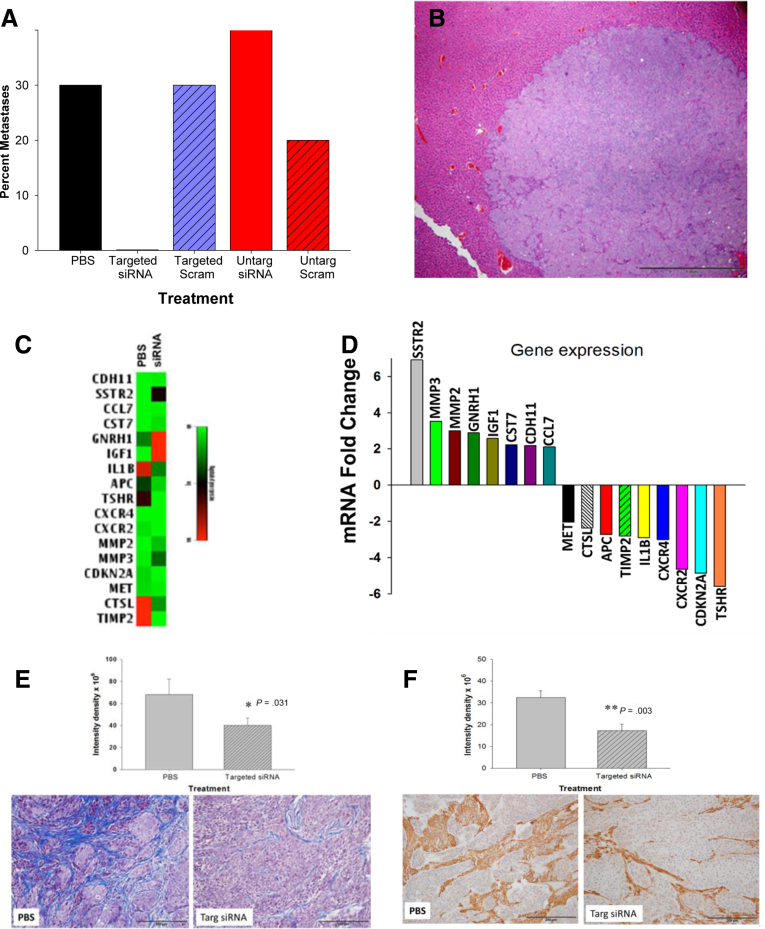
One remarkable finding of this investigation was that none of the mice bearing BxPC-3 or PANC-1 orthotopic tumors, and treated with the targeted gastrin siRNA polyplex, had evidence of metastases. In both animal experiments, we found that metastases occurred in control mice treated with PBS, untargeted or targeted scrambled RNA, and untargeted siRNA, but no metastases were found in mice treated with the receptor-targeted polyplex NPs carrying the gastrin siRNA (Figure 5A). Control mice had histologically confirmed metastases to liver (Figure 5B), and mesenteric lymph nodes. To identify which metastatic genes showed altered expression with gastrin siRNA therapy and understand the potential mechanisms for the inhibition of metastasis with this NP platform, we performed an 84-gene tumor metastases PCR array on the excised primary tumors from mice treated with CCK-receptor–targeted gastrin siRNA and tumors of the control mice. Figure 5C shows a heat map of genes up- or down-regulated in tumors of mice treated with gastrin siRNA compared with control tumors. Table 1 shows the number and location of the metastases that were identified in the control mice. The fold change of specific metastatic genes that were significantly increased or decreased are shown in Figure 5D. The metastatic PCR array performed from tumors of control mice and mice treated with the target-specific polyplex carrying gastrin siRNA confirm that the polyplex had accumulated in the tumors and altered genes specifically related to decreased gastrin expression. The specific genes altered with targeted gastrin siRNA treatment are shown in Table 2. The somatostatin-receptor gene, for example, was up-regulated nearly 7-fold in tumors of mice treated with the targeted gastrin siRNA, and this finding supports the fact that gastrin peptide expression was undeniably down-regulated in the tumors because the feedback loop for gastrin production is controlled through somatostatin. Several matrix metalloprotease (MMP) genes also were affected by the intratumoral reduction in gastrin peptide, such as MMP2 and MMP3, as well as the chemokine genes for chemokine receptor-2 and chemokine receptor-4. Pancreatic cancer is characterized by its dense fibrotic microenvironment, with macrophages and other immune cells that enable the cancer to evade immune surveillance.13, 14, 34 CCK-B receptors also are localized on the pancreatic stellate cells11, 12 (fibroblasts), and when stimulated by gastrin they are responsible for the collagen and fibrosis production in the pancreatic cancer microenvironment.13, 14 Therefore, a reduction in gastrin would lead to increased metalloprotease activity and less fibrosis, which has been associated with decreased metastasis.35 It is possible that our CCK-receptor–targeted NP also blocked activation of the cancer-associated fibroblasts and that may be one reason for the decreased metastases. We previously showed that if gastrin signaling at the CCK-B receptor was blocked with a CCK-receptor antagonist, that fibrosis and inflammation in the tumor microenvironment is decreased significantly.9, 36 in part by interference with the CCK-B receptor on the pancreatic stellate cells or cancer-associated fibroblasts. Therefore, it is not surprising to find that the polyplex carrying gastrin siRNA to tumors also can alter genes associated with tumor fibrosis and inflammation.
Figure 5.
CCK-receptor–targeted polyplex gastrin siRNA blocks metastases in a pancreatic cancer orthotopic model. (A) Percentage of mice with metastases in various treatment groups is shown. No metastases were found in mice treated with targeted gastrin siRNA polyplex. (B) Representative H&E image of a metastatic lesion in the liver of a PBS-treated control mouse. Scale bar: 200 μm. (C) Heat map showing absolute expression levels of metastatic genes altered with targeted siRNA treatment. (D) Fold change in genes from tumors treated with targeted siRNA compare to control tumors. Names of genes from excised tumors that were increased (left) or decreased (right) with targeted siRNA therapy and the fold change. (E) Graphic representation of differentially expressed genes from tumors of mice treated with targeted siRNA treatment. Masson’s trichrome stain of representative PBS-treated control tumor (left) showing extensive intratumoral fibrosis. Tumor on right shows marked decreased fibrosis in tumors of mice treated with targeted siRNA nanoparticles. Quantitative analysis with of fibrosis shows significantly less fibrosis (∗P = .031) in tumors of mice treated with the NPs. (F) IHC stain of tumors with anti-SMAa staining shows statistically less fibrosis (∗∗P = .003) in the tumors of mice treated with the NP (right) compared to tumors of PBS-treated control mice (left).
Table 1.
Number of Metastases and Location From Orthotopic Tumor Models
| Treatment | PBS | Targeted siRNA | Targeted scrambled | Untargeted siRNA | Untargeted scrambled |
|---|---|---|---|---|---|
| Lymph nodes | 3 | 1 | 1 | 1 | |
| Liver | 1 | 1 | 1 | 1 | |
| Spleen | 1 | 1 | 1 | ||
| Peritoneal | 1 | 1 | 2 |
Table 2.
Metastatic Genes Altered by Gastrin siRNA Polyplex Therapy
| Gene | Name | Fold change |
|---|---|---|
| SSTR | Somatostatin receptor | +6.9 |
| MMP3 | Matrix metallopeptidase-3 | +3.92 |
| MMP2 | Matrix metallopeptidase-2 | +3 |
| GNRH1 | Gonadotropin releasing hormone | +2.88 |
| IGF1 | Insulin growth factor | +2.57 |
| CST7 | Cystatin | +2.53 |
| CDH11 | Cadherin-11 | +2.18 |
| CCL7 | C-C motif chemokine ligand 7 | +2.11 |
| TSHR | TSH receptor | -5.59 |
| CDKN2A | Cyclin dep kinase inhibitor | -4.87 |
| CXCR2 | CXC chemokine receptor-2 | -4.65 |
| CXCR4 | CXC chemokine receptor-4 | -3.01 |
| IL1β | Interleukin 1β | -2.91 |
| TIMP2 | TIMP metallopeptidase inhibitor | -2.81 |
| APC | Adenomatous polyposis coli | -2.72 |
| CTSL | Cathepsin | -2.37 |
| MET | Tyrosine kinase | -2.05 |
TIMP2, tissue inhibitor of metalloproteinases 2; TSH: thyroid stimulating hormone receptor.
We also found that the expression of the tumor-suppressor gene CDKN2A, cyclin-dependent kinase inhibitor 2A, was down-regulated significantly approximately 5-fold by treatment with polyplex delivering gastrin siRNA. Mutations in CDKN2A frequently are found in pancreatic cancer and their presence interferes with in the normal cell-cycle regulation. We previously showed that knockdown of the CCK-B receptor resulted in cell-cycle arrest at the G0/G1 stage, perhaps one of the mechanisms by which gastrin promotes tumor growth is mediated through CDKN2A. These changes in known metastatic genes confirm that the targeted siRNA polyplex had selective antimetastatic properties, and also may help us understand the molecular interactions of gastrin-involved cancer growth and metastases. Tumor fibrosis was evaluated with Masson’s trichrome staining (Figure 5E). A significant reduction in fibrosis was found in the tumors of mice treated with the targeted siRNA NP compared to control mice. Additional confirmation of the reduction in intratumoral fibrosis was confirmed by IHC to anti-SMAa (Figure 5F). Tumors of mice treated with the targeted gastrin siRNA exhibited less anti-SMAa immunoreactivity compared to PBS treated control mice.
Discussion
Cancer therapy can be markedly improved when cancer-specific receptors are identified and used to enhance on-target delivery, reduce off-target exposure, and decrease systemic toxicity. Of all the solid tumors, pancreatic cancer has the poorest survival rate; one reason is that specific targets have not been identified. Our approach to this aggressive deadly cancer is novel in that we have developed a highly selective nanomedicine platform to deliver gene therapy specifically to the tumor to down-regulate a trophic gene: gastrin. These in vivo studies confirm that only the polyplex nanoparticles designed to selectively target the CCK-B receptor and deliver the gastrin siRNA were effective in decreasing the primary tumor size, and, remarkably, metastasis. Because the gastrin mRNA and peptide were decreased only in the tumors of mice treated with the CCK-B–receptor–targeted gastrin siRNA construct, these data confirm that the mechanism by which the polyplex NP inhibited growth was via selective targeting and uptake by the CCK-B receptor, and down-regulation of the growth peptide gastrin by specific (not scrambled) siRNA.
One of the extraordinary findings from this investigation was that the polyplex NPs completely prevented metastasis in both pancreatic cancer models. Most patients with pancreatic cancer die of metastatic disease; therefore, a compound that can halt metastasis could improve survival.
Although CCK-B receptors are found in some normal tissues at considerably lower density, such as gastric parietal cells, the cells possessing these receptors do not make the peptide gastrin, and hence no pharmacologic effect would be expected by uptake of gastrin siRNA. The ability to deliver siRNA to pancreatic cancer cells through CCK-B–receptor targeting with this NP platform suggests that this platform also may have utility for knockdown of other key stimulatory proteins by substituting, or even combining, with other siRNAs to increase efficacy. The CCK-B receptor also is overexpressed on other gastrointestinal malignancies, such as gastric and colon cancer, and other cancers including some lung cancers and medullary thyroid cancers, further increasing the potential utility of this construct.
Although very reliable methods have been developed to efficiently deliver siRNAs through lipid bilayers of eukaryotic cells in cell culture, there continues to be limitations to the in vivo use of these molecules.37 Recent advances in cancer therapeutics have come with the evolution of nanoparticle formulations that serve as delivery platforms for gene therapy (eg, siRNAs).38 Obstacles impairing the advancement of nanomedicine, and the efficient site-specific delivery of siRNAs by NPs, include degradation of the RNA payload by nucleases of the peripheral blood,39, 40 nonspecific tissue uptake (off-target exposure),41 lack of effective endosomal escape,42 and rapid clearance by the mononuclear phagocytic system.43 We have clearly shown that our polyplex NP can overcome all of these obstacles.
Several nanomedicines have been developed for cancer therapy, such as pegylated liposomal doxorubicin44 (Doxil, Janssen, Titusville, NJ), which is used in breast cancer, ovarian cancer, myeloma, and acquired immune deficiency syndrome–related Kaposi’s sarcoma. Liposomal encapsulation of chemotherapeutic agents such as doxorubicin44 and gemcitabine45 has been shown to decrease systemic-free drug exposure and associated cardiac toxicity. Cancer-associated receptors are ideal targets for therapeutics,46 and other NPs recently have been developed that selectively target cancer receptors such as Her-2/neu,47 epidermal growth factor receptors,48 and transferrin receptors.49
Some nanomedicine chemotherapies have been used to treat advanced pancreatic cancer. Abraxane (Celgene, Summit, NJ) is a 130-nm, albumin-bound, nanoparticle formulation of the legacy drug paclitaxel.50 Combination therapy with gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer showed improved survival compared with gemcitabine alone51; although the mean survival was still only 8.5 months. Another nanomedicine formulation, Onivyde (Ipsen Biopharmaceuticals, Inc, Basking Ridge, NJ), is a nanoliposomal irinotecan (a topoisomerase-I inhibitor) formulation that also has been tested in patients with advanced pancreatic cancer.52 However, 65% of those treated with this therapy in a clinical study experienced at least 1 treatment-emergent adverse event categorized as grade 3 or higher,52 and survival was not improved markedly. Unfortunately, none of these aforementioned chemotherapeutic nanomedicine preparations were actively targeted to receptors on pancreatic cancer, and none represented a substantial improvement compared with existing therapies with regard to efficacy or toxicity.
Actively targeted nanomedicines also currently are undergoing development for imaging and treatment of pancreatic cancer. For example, monoclonal antibody targeted quantum dots are being evaluated for pancreatic cancer localization.53 Because the transferrin receptor is overexpressed in several cancers, including pancreatic cancer, transferrin has been used as a target of silica nanorods54 and liposomal-based nanoparticles55 for possible imaging. Other pancreatic cancer receptors under development for nanomedicine targeting are the apoferritin (an iron transport protein)56 and insulin growth factor-1 receptors.57 Because we can fluorescently label the polyplex CCK-B–receptor–targeted NPs, this strategy also could be used as a noninvasive means for imaging or earlier diagnosis.
Our polyplex nanoparticle platform establishes a strong foundation for the development of CCK-receptor–targeted therapeutics and for further exploration of this strategy in other types of cancer. The NP formulation we used in this investigation can be modified to selectively target other tumor cell surface receptors to deliver siRNA or even therapeutics. In addition, more than one siRNA can be reacted to form the micelle polyplex NP to perhaps target other driver genes such as the untargetable mutant KRAS. Rarely is monotherapy used in cancer therapeutics today, therefore, one could consider combination therapy using this NP platform with standard chemotherapeutic regimens such as folinic acid, fluorouracil, irinotecan, and oxaliplatin or gemcitabine nab-paclitaxel to improve efficacy and survival. Although CCK receptors also are overexpressed in some lung cancers and other gastrointestinal malignancies, we focused our work on pancreatic cancer in this investigation because this disease has such a dismal prognosis and therapeutic options are limited. Having a theronostic NP that can be fluorescently labeled to localize small tumors (diagnostic) and also treat cancer (therapeutic) by decreasing primary tumor growth and metastasis could change the outcome of this devastating disease.
Footnotes
Author contributions Jill P. Smith and Stephan T. Stern conceived the idea; Jill P. Smith secured funding for the project and obtained Institutional Animal Care and Use Committee approval for the in vivo experiments; Abdullah Malmud was responsible for the design and development of the polyplex; Jong-In Hahm, Abdullah Malmud, and Stephan T. Stern characterized the properties of the nanoparticles; Julian Burks and Sandeep Nadella performed most of the in vitro studies, immunofluorescence, quqntitative reverse-transcription polymerase chain reaction assays, and immunoanalysis; Julian Burks, Sandeep Nadella, Charoen Mankongpaisarnrung, Juan Wang, Robin D. Tucker, and Jill P. Smith performed the animal surgical procedures, imaging, treatments, and dissections of experimental animals; Narayan Shivapurkar and Julian Burks performed the metastatic polymerase chain reaction arrays and analysis; Jill P. Smith wrote the manuscript draft; and all authors contributed to the editing and approval of the final manuscript.
Conflicts of interest These authors disclose the following: Georgetown University jointly owns with the National Institutes of Health a patent application related to the medical technology described in this article on which Jill P. Smith is an inventor from Georgetown University and Abdullah Mahmud and Stephen Stern are the inventors from the National Institutes of Health.
Funding Supported by the Ruesch Center for the Cure of Gastrointestinal Cancers, Georgetown University Medical Center. These studies were conducted in part at the Georgetown Lombardi Comprehensive Cancer Center Histopathology and Tissue Shared Resource and in the Preclinical Imaging Research Laboratory, which is supported in part by National Institutes of Health/National Cancer Institute grant P30-CA051008. This work was supported in part with federal funds from the National Institutes of Health National Cancer Institute grant HHSN261200800e001E. Leidos Biomedical Research is a subcontractor of the National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 4.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Ma W.W., Adjei A.A. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 6.Maitland M.L., Schilsky R.L. Clinical trials in the era of personalized oncology. CA Cancer J Clin. 2011;61:365–381. doi: 10.3322/caac.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.P., Liu G., Soundararajan V., McLaughlin P.J., Zagon I.S. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am J Physiol. 1994;266:R277–R283. doi: 10.1152/ajpregu.1994.266.1.R277. [DOI] [PubMed] [Google Scholar]
- 8.Smith J.P., Solomon T.E. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol Gastrointest Liver Physiol. 2014;306:G91–G101. doi: 10.1152/ajpgi.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J.P., Cooper T.K., McGovern C.O., Gilius E.L., Zhong Q., Liao J., Molinolo A.A., Gutkind J.S., Matters G.L. Cholecystokinin receptor antagonist halts progression of pancreatic cancer precursor lesions and fibrosis in mice. Pancreas. 2014;43:1050–1059. doi: 10.1097/MPA.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fino K.K., Matters G.L., McGovern C.O., Gilius E.L., Smith J.P. Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1244–G1252. doi: 10.1152/ajpgi.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berna M.J., Seiz O., Nast J.F., Benten D., Blaker M., Koch J., Lohse A.W., Pace A. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285:38905–38914. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips P.A., Yang L., Shulkes A., Vonlaufen A., Poljak A., Bustamante S., Warren A., Xu Z., Guilhaus M., Pirola R., Apte M.V., Wilson J.S. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apte M.V., Park S., Phillips P.A., Santucci N., Goldstein D., Kumar R.K., Ramm G.A., Buchler M., Friess H., McCarroll J.A., Keogh G., Merrett N., Pirola R., Wilson J.S. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Apte M.V., Wilson J.S., Lugea A., Pandol S.J. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith J.P., Fantaskey A.P., Liu G., Zagon I.S. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995;268:R135–R141. doi: 10.1152/ajpregu.1995.268.1.R135. [DOI] [PubMed] [Google Scholar]
- 16.Smith J.P., Shih A., Wu Y., McLaughlin P.J., Zagon I.S. Gastrin regulates growth of human pancreatic cancer in a tonic and autocrine fashion. Am J Physiol. 1996;270:R1078–R1084. doi: 10.1152/ajpregu.1996.270.5.R1078. [DOI] [PubMed] [Google Scholar]
- 17.Brand S.J., Fuller P.J. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem. 1988;263:5341–5347. [PubMed] [Google Scholar]
- 18.Bardram L., Hilsted L., Rehfeld J.F. Progastrin expression in mammalian pancreas. Proc Natl Acad Sci U S A. 1990;87:298–302. doi: 10.1073/pnas.87.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suissa Y., Magenheim J., Stolovich-Rain M., Hija A., Collombat P., Mansouri A., Sussel L., Sosa-Pineda B., McCracken K., Wells J.M., Heller R.S., Dor Y., Glaser B. Gastrin: a distinct fate of neurogenin3 positive progenitor cells in the embryonic pancreas. PLoS One. 2013;8:e70397. doi: 10.1371/journal.pone.0070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J.P., Hamory M.W., Verderame M.F., Zagon I.S. Quantitative analysis of gastrin mRNA and peptide in normal and cancerous human pancreas. Int J Mol Med. 1998;2:309–315. doi: 10.3892/ijmm.2.3.309. [DOI] [PubMed] [Google Scholar]
- 21.Prasad N.B., Biankin A.V., Fukushima N., Maitra A., Dhara S., Elkahloun A.G., Hruban R.H., Goggins M., Leach S.D. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 22.Matters G.L., Harms J.F., McGovern C.O., Jayakumar C., Crepin K., Smith Z.P., Nelson M.C., Stock H., Fenn C.W., Kaiser J., Kester M., Smith J.P. Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas. 2009;38:e151–e161. doi: 10.1097/MPA.0b013e3181a66fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith J.P., Verderame M.F., Ballard E.N., Zagon I.S. Functional significance of gastrin gene expression in human cancer cells. Regul Pept. 2004;117:167–173. doi: 10.1016/j.regpep.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Aloj L., Caraco C., Panico M., Zannetti A., Del V.S., Tesauro D., De L.S., Arra C., Pedone C., Morelli G., Salvatore M. In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging. J Nucl Med. 2004;45:485–494. [PubMed] [Google Scholar]
- 25.Brom M., Joosten L., Laverman P., Oyen W.J., Behe M., Gotthardt M., Boerman O.C. Preclinical evaluation of 68Ga-DOTA-minigastrin for the detection of cholecystokinin-2/gastrin receptor-positive tumors. Mol Imaging. 2011;10:144–152. [PMC free article] [PubMed] [Google Scholar]
- 26.Roosenburg S., Laverman P., Joosten L., Cooper M.S., Kolenc-Peitl P.K., Foster J.M., Hudson C., Leyton J., Burnet J., Oyen W.J., Blower P.J., Mather S.J., Boerman O.C., Sosabowski J.K. PET and SPECT imaging of a radiolabeled minigastrin analogue conjugated with DOTA, NOTA, and NODAGA and labeled with (64)Cu, (68)Ga, and (111)In. Mol Pharm. 2014;11:3930–3937. doi: 10.1021/mp500283k. [DOI] [PubMed] [Google Scholar]
- 27.Sosabowski J., Lee M., Dekker B., Simmons B., Singh S., Bereford H., Hagan S., McKenzie A., Mather S., Watson S. Formulation development and manufacturing of a gastrin/CCK-2 targeting peptide as an intermediate drug product for clinical imaging study. Eur J Pharm Sci. 2007;31:102–111. doi: 10.1016/j.ejps.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Webb M.S., Saxon D., Wong F.M., Lim H.J., Wang Z., Bally M.B., Choi L.S., Cullis P.R., Mayer L.D. Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): effects on the pharmacokinetics of liposomal vincristine. Biochim Biophys Acta. 1998;1372:272–282. doi: 10.1016/s0005-2736(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 30.Altinoglu E.I., Russin T.J., Kaiser J.M., Barth B.M., Eklund P.C., Kester M., Adair J.H. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano. 2008;2:2075–2084. doi: 10.1021/nn800448r. [DOI] [PubMed] [Google Scholar]
- 31.Barth B.M., Sharma R., Altinoglu E.I., Morgan T.T., Shanmugavelandy S.S., Kaiser J.M., McGovern C., Matters G.L., Smith J.P., Kester M., Adair J.H. Bioconjugation of calcium phosphosilicate composite nanoparticles for selective targeting of human breast and pancreatic cancers in vivo. ACS Nano. 2010;4:1279–1287. doi: 10.1021/nn901297q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feig C., Gopinathan A., Neesse A., Chan D.S., Cook N., Tuveson D.A. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matters G.L., McGovern C., Harms J.F., Markovic K., Anson K., Jayakumar C., Martenis M., Awad C., Smith J.P. Role of endogenous cholecystokinin on growth of human pancreatic cancer. Int J Oncol. 2011;38:593–601. doi: 10.3892/ijo.2010.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quante M., Varga J., Wang T.C., Greten F.R. The gastrointestinal tumor microenvironment. Gastroenterology. 2013;145:63–78. doi: 10.1053/j.gastro.2013.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo A., Wang L.C., Scholler J., Monslow J., Avery D., Newick K., O'Brien S., Evans R.A., Bajor D.J., Clendenin C., Durham A.C., Buza E.L., Vonderheide R.H., June C.H., Albelda S.M., Pure E. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith J.P., Wang S., Nadella S., Jablonski S.A., Weiner L.M. Cholecystokinin receptor antagonist alters pancreatic cancer microenvironment and increases efficacy of immune checkpoint antibody therapy in mice. Cancer Immunol Immunother. 2018;67:195–207. doi: 10.1007/s00262-017-2077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salzano G., Costa D.F., Torchilin V.P. siRNA delivery by stimuli-sensitive nanocarriers. Curr Pharm Des. 2015;21:4566–4573. doi: 10.2174/138161282131151013190410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengupta S. Cancer nanomedicine: lessons for immuno-oncology. Trends Cancer. 2017;3:551–560. doi: 10.1016/j.trecan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzer C., Dirin M., Winkler A.M., Baumann V., Winkler J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15. doi: 10.1016/j.jconrel.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Wang J., Lu Z., Wientjes M.G., Au J.L. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler J., Stessl M., Amartey J., Noe C.R. Off-target effects related to the phosphorothioate modification of nucleic acids. ChemMedChem. 2010;5:1344–1352. doi: 10.1002/cmdc.201000156. [DOI] [PubMed] [Google Scholar]
- 42.Juliano R.L., Carver K., Cao C., Ming X. Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides. J Drug Target. 2013;21:27–43. doi: 10.3109/1061186X.2012.740674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White P.J., Anastasopoulos F., Pouton C.W., Boyd B.J. Overcoming biological barriers to in vivo efficacy of antisense oligonucleotides. Expert Rev Mol Med. 2009;11:e10. doi: 10.1017/S1462399409001021. [DOI] [PubMed] [Google Scholar]
- 44.Duggan S.T., Keating G.M. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi's sarcoma. Drugs. 2011;71:2531–2558. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Birhanu G., Javar H.A., Seyedjafari E., Zandi-Karimi A. Nanotechnology for delivery of gemcitabine to treat pancreatic cancer. Biomed Pharmacother. 2017;88:635–643. doi: 10.1016/j.biopha.2017.01.071. [DOI] [PubMed] [Google Scholar]
- 46.Bae Y.H., Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan W.B., Jiang S., Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 2007;28:1565–1571. doi: 10.1016/j.biomaterials.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Nascimento A.V., Singh A., Bousbaa H., Ferreira D., Sarmento B., Amiji M.M. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol Pharm. 2014;11:3515–3527. doi: 10.1021/mp5002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pirollo K.F., Nemunaitis J., Leung P.K., Nunan R., Adams J., Chang E.H. Safety and efficacy in advanced solid tumors of a targeted nanocomplex carrying the p53 gene used in combination with docetaxel: a phase 1b study. Mol Ther. 2016;24:1697–1706. doi: 10.1038/mt.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai N., Trieu V., Yao Z., Louie L., Ci S., Yang A., Tao C., De T., Beals B., Dykes D., Noker P., Yao R., Labao E., Hawkins M., Soon-Shiong P. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 51.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., Harris M., Reni M., Dowden S., Laheru D., Bahary N., Ramanathan R.K., Tabernero J., Hidalgo M., Goldstein D., Van C.E., Wei X., Iglesias J., Renschler M.F. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko A.H., Tempero M.A., Shan Y.S., Su W.C., Lin Y.L., Dito E., Ong A., Wang Y.W., Yeh C.G., Chen L.T. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer. 2013;109:920–925. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yong K.T., Ding H., Roy I., Law W.C., Bergey E.J., Maitra A., Prasad P.N. Imaging pancreatic cancer using bioconjugated InP quantum dots. ACS Nano. 2009;3:502–510. doi: 10.1021/nn8008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao R., Han X., Li Y., Wang H., Ji T., Zhao Y., Nie G. Photothermal effect enhanced cascade-targeting strategy for improved pancreatic cancer therapy by gold nanoshell@mesoporous silica nanorod. ACS Nano. 2017;11:8103–8113. doi: 10.1021/acsnano.7b02918. [DOI] [PubMed] [Google Scholar]
- 55.Camp E.R., Wang C., Little E.C., Watson P.M., Pirollo K.F., Rait A., Cole D.J., Chang E.H., Watson D.K. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013;20:222–228. doi: 10.1038/cgt.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang M.P., Lee J.W., Lee K.E., Lee K.H. Think modular: a simple apoferritin-based platform for the multifaceted detection of pancreatic cancer. ACS Nano. 2013;7:8167–8174. doi: 10.1021/nn403465a. [DOI] [PubMed] [Google Scholar]
- 57.Zhou H., Qian W., Uckun F.M., Wang L., Wang Y.A., Chen H., Kooby D., Yu Q., Lipowska M., Staley C.A., Mao H., Yang L. IGF1 receptor targeted theranostic nanoparticles for targeted and image-guided therapy of pancreatic cancer. ACS Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]