Abstract
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy, and the lack of chemoresistance biomarkers contributes to the poor prognosis. Cancer stem cells (CSC) have been investigated in EOC to understand its relationship with chemoresistance and recurrence. In this context, in vitro cultivation-models are important tools for CSC studies. MicroRNAs (miRNAs) play key roles in cancer, CSC regulation and apoptosis. Thus, this study aims to evaluate the tumorsphere model as CSC-enrichment method in EOC studies and investigate apoptosis-related miRNAs in tumorspheres-derived EOC cell lines. TOV-21G and SKOV-3 were cultured in monolayer and tumorspheres. Genetic profiles of cell lines were obtained using COSMIC database. CD24/CD44/CD146/CD177 and ALDH1 markers were evaluated in cell lines and tumorspheres-derived by flow cytometry. Eleven miRNAs were selected by in silico analysis for qPCR analysis. According to COSMIC, TOV-21G and SKOV-3 have eight and nine cancer-related mutations, respectively. TOV-21G showed a CD44+/high/CD24−/low/CD117−/low/CD146−/low/ALDH1low profile in both culture models; thus, no significant difference between cultivation models was identified. SKOV-3 showed a CD44+/high/CD24+/high/ CD117−/low/CD146−/low/ALDH1low profile in both culture models, although the tumorsphere model showed a significant increase in CD24+/high subpopulation (ovarian CSC-like). Among eleven miRNAs, we observed differences in miRNA expression between culture models. MiR-26a was overexpressed in TOV-21G tumorspheres, albeit downregulated in SKOV-3 tumorspheres. MiR-125b-5p, miR-17-5p and miR-221 was downregulated in tumorsphere model in both cell lines. Given that tumorsphere-derived SKOV-3 had a higher ratio of CD24+/high cells, we suggest that miR-26a, miR-125b-5p, miR-17-5p and miR-221 downregulation could be related to poor EOC prognosis.
Keywords: Epithelial ovarian cancer, Cancer stem cells, microRNAs, Apoptosis, In vitro cultivation-models
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy, and its mortality rate is higher than 60% [1]. Late-stage diagnosis, associated with the lack of effective molecular markers to detect chemotherapy resistance contributes to high mortality rates for EOC [2].
Accordingly, cancer stem cells (CSC) have been increasingly investigated to understand the biology of cancer, and several studies have shown that CSC may be responsible for the high rate of chemoresistance and EOC recurrence [3]. CSC are found in specialized niches or microenvironments and consist of several cell types that interact with each other, including fibroblasts, endothelial and perivascular cells, adipose cells, immune cells, and macrophages [4]. Furthermore, this microenvironment provide the requirements for the maintenance of CSC characteristics such as self-renewal, chemoresistance, anchorage-independent growth, apoptosis resistance, invasion and metastasis [4]. Ponti et al. [5] showed that cultivating cells onto an ultra-low attachment surface and into a serum-free medium enriched in growth factors was efficient in maintaining breast CSC. In such conditions, cells grew as multicellular three-dimensional clones termed “tumorspheres” [6]. This cell culture model is widely used to analyze the self-renewal capability of CSC to enrich these cells from bulk cancer and established cancer cell lines [6].
Mechanisms of CSC regulation, drug resistance, progression and EOC recurrence have been associated with MicroRNAs (miRNAs) signatures [7, 8]. miRNAs are small non-coding RNAs that regulate posttranscriptional gene expression and play a key role in cancer progression and expression [8]. Therefore, several studies have investigated the potential use of miRNA to provide information for EOC identification, classification, treatment and/or prognosis [9, 10].
Previous studies from our group described different apoptosis-related genes as EOC biomarkers associated with disease prognosis [11–13]. Considering that aberrant miRNAs expression plays a key pathogenic role in different stages of the carcinogenesis process and is involved in the deregulation of several biological processes, including apoptosis, miRNA profile analysis may reveal new biomarkers that can be used for EOC prognosis and chemorresistance [14]. Thus, this study aims to evaluate the tumorsphere model as in vitro CSC enrichment method in EOC studies and investigate apoptosis-related miRNAs in tumorspheres-derived EOC cell lines.
Methods
Cell Culture
TOV-21G, (Cat. #CRL-11730™) and SKOV-3 (Cat. #HTB-77™) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and propagated in monolayer (culture adherent), respectively, in DMEM (Sigma, St. Louis, MO, USA, cat. # M2279) supplemented with 15% fetal bovine serum (FBS) (Sigma, cat. # F6178) and McCoy’s 5A Medium (Sigma, cat. # M4892) supplemented with 10% FBS. Tumorspheres were obtained from TOV-21G and SKOV-3. The cell lines were trypsinized, and cell suspensions were plated (2.5 × 104 cells/well) in 96-well plates for non-adherent cells (Corning, Cat. # CLS3474), using 200 μL per well of DMEM/F-12 medium (Sigma, Cat. # D0547) supplemented with 20 ng/mL epidermal growth factor (EGF) (Sigma, cat. # E1257), 10 ng/mL basic fibroblast growth factor (FGF-b) (ImmnunoTools, Friesoythe, Germany, cat. # 11343625) and 10 μg/mL bovine insulin (Sigma, cat. # I1882) and incubated for seven days at 37 °C in 5% CO2 to obtain the tumorspheres [15].
Analysis of Genetic Differences between SKOV-3 and TOV-21G Cell Lines
Genetic differences between TOV-21G and SKOV-3 cells were analyzed in the Catalog of Somatic Mutations in Cancer (COSMIC - http://cancer.sanger.ac.uk/cell_lines) to investigate cancer-related mutations in each cell line [16].
Flow Cytometry Analysis
CD44 (Mouse Anti-Human CD44 APC, Immunotools, cat. # 21850446,), CD24 (Human CD24 FITC conjugate, Invitrogen, Carlsbad, CA, USA, cat. #MHCD2401,), CD117 (Mouse Anti-Human CD117 PerCP-Cy™5.5, BD Pharmingen, San Diego, CA, USA, cat. #562094) and CD146 (Mouse Anti-Human CD146 PE, cat. #550315, BD Pharmingen) antibodies and ALDEFLUOR Stem Cell Identification kit (StemCell Technologies, Vancouver, Canada, cat. #01700,) were used for all flow cytometry experiments. SKOV-3 and TOV-21G cells and the tumorspheres derived from them were cultured as previously mentioned and dissociated by trypsinization and/or mechanically into single-cell suspensions. Cells were resuspended in wash buffer (PBS 1X with BSA 0.25% and azide 1 mM). Extracellular staining was performed with fluorochrome-conjugated monoclonal antibodies human or their respective isotype controls at the concentrations recommended by the manufacturer and incubated at 4 °C in the dark for 15 min. ALDEFLUOR Stem Cell Identification kit was used to assess ALDH activity in the EOC cell lines. Cells were suspended in PBS or ALDEFLUOR Stem Cell Identification kit buffer and analyzed on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA), and data analysis was performed in FACSDiva 6.1.3 software.
In Silico Selection of Apoptosis-Related miRNA Targets
miRNA targets were selected for qRT-PCR and validated using prediction (TargetScan, miRanda, MirTarget2) and validation (TarBase/DianaTools, miRTarBase) databases. miRNAs indicated in more than one prediction and/or validation databases and those that met the prediction parameters were preferred [17].
Total RNA Extraction and Quality Analysis
SKOV-3 and TOV-21G cells lines and tumorsphere-derived total RNA extraction was performed using mirVana kit (Invitrogen, cat # AM1560), according to the manufacturer’s recommendations. Concentrations of total RNA and the 260/280 absorbance ratio were measured using a NanoVue spectrophotometer (GE Healthcare, Little Chalfont, UK). The quality of the RNA samples was assessed in a 2100 Bioanalyzer using the Pico LabChip Kit (Agilent Technologies, Santa Clara, CA, USA, cat. # 5067–1514), according to manufacturer’s instructions.
Analysis of miRNAs Gene Expression
miRNA expression, selected by in silico analysis, was validated for qRT-PCR. The cDNA was synthesized using the TaqMan® MicroRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA, cat # 4366597). Inventoried assays (TaqMan® MicroRNA Assays - Applied Biosystems) were used according to manufacturer’s recommendation. The small nucleolar SNORD43 and U6 small nuclear 6 (RNU6-6P) were used as endogenous controls. Relative expression levels of miRNAs were calculated using the 2(−ΔΔCq) method as described by LIVAK and SCHMITTGEN, 2001 [18].
Statistical Analysis
Data were analyzed using the REST 2009 software (Qiagen, Hilden, Germany) to evaluate differential gene expression between groups, in addition to the SPSS 18.0 software package (IBM SPSS Inc., Chicago, IL, USA) and Bionumerics 7.0 (Applied Maths, Sint-Martens-Latem, Belgium).
Results
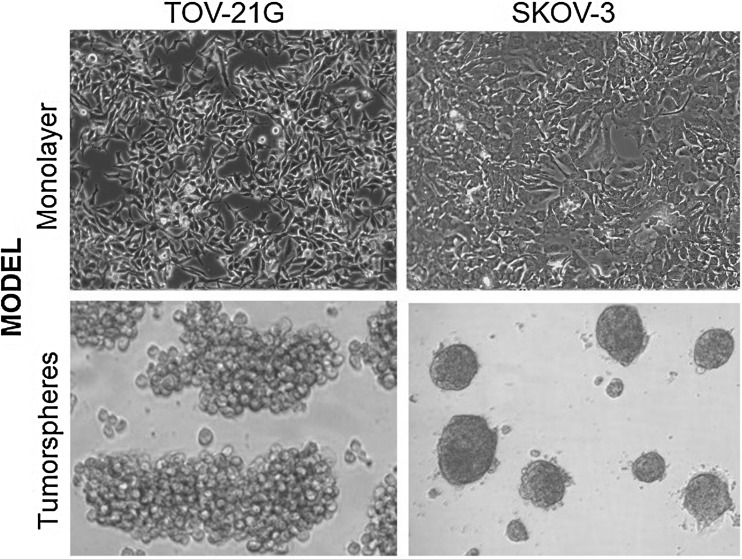
According to information available at the COSMIC cell line project, TOV-21G and SKOV-3 have eight and nine cancer-related mutations, respectively (Table 1). The morphology of TOV-21G and SKOV-3 cell lines, cultured in monolayer and tumorsphere models is shown in Fig. 1. TOV-21G-derived tumorspheres grew in loose grape-like clusters, whereas SKOV-3-derived tumorspheres were tightly packed and difficult to separate into single-cell suspensions.
Table 1.
Cancer-related genes mutations in TOV-21G and SKOV-3 cell lines
| Cell line | Cancer-related genes mutations |
|---|---|
| TOV-21G | ATR, DDX3X, HIF1A, HOOK3, KRAS, NOTCH1, PIK3CA, RNF43 |
| SKOV-3 | ARID1A, BCL11B, EP300, ERC1, FBXW7, MYH11, NF1, PIK3CA, TP53 |
COSMIC cell line project (http://cancer.sanger.ac.uk/cell_lines)
Fig. 1.
TOV-21G and SKOV-3 cell lines and tumorsphere-derived cells
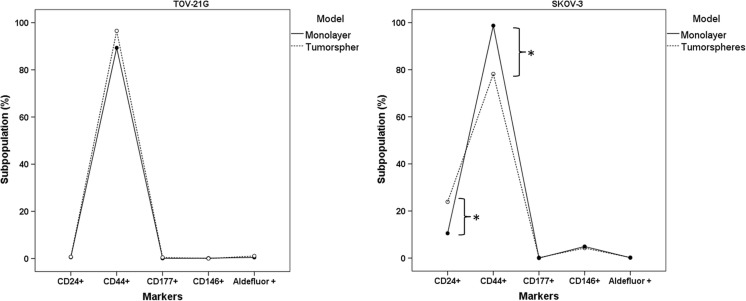
Flow cytometry analysis showed that TOV-21G cell lines have a CD44+/high/CD24−/low/CD117−/low/CD146−/low and ALDHlow phenotype profile in both culture models (monolayer and tumorspheres) with no significant differences in subpopulation profile between the cell culture models (Fig. 2a). SKOV-3 cell lines cultured in monolayer and tumorsphere models showed a CD44+/high/CD24+/high/CD117−/low/CD146−/low and ALDHlow profile, albeit with a significant increase in CD24+/high subpopulation and decrease in CD44+/high subpopulation in the tumorsphere model (Fig. 2b).
Fig. 2.
TOV-21G (a) and SKOV-3 (b) Cell population characterization in monolayer and in tumorsphere models Software: SPSS 18.0; Two-way ANOVA, post hoc, DMS method, n = 3. *Significantly different (α = 5%), comparing the monolayer cultures with the tumorsphere model
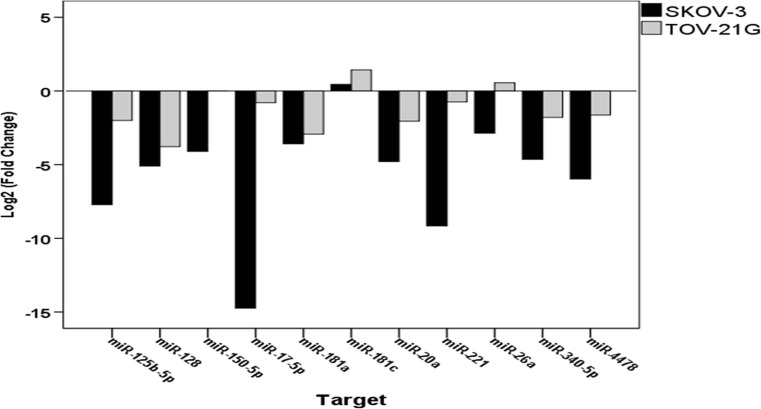
Eleven miRNAs (miR-125b-5p, miR-128, miR-150-5p, miR-17-5p, miR-181a, miR-181c, miR-20a, miR-221, miR-26a, miR-340 and miR-4478) were selected by in silico analysis of miRNA targets using the relationship with apoptotic pathways as criterion (Table 2) for qPCR gene expression analysis. MiR-125b-5p, miR-340-5p, miR-128, miR-150-5p, hsa-miR-17-5p, miR-20a, miR-340 and miR-4478 was significantly downregulated in the tumorsphere model compared with the monolayer model, in both cell lines (Table 1). Conversely, TOV-21G cells showed miR-26a overexpression in the tumorsphere model compared with the monolayer model, whereas miR-26a was downregulated in SKOV-3-derived tumorspheres. SKOV-3 also showed miR-181c overexpression in the tumorsphere model, with significant differences in miRNAs expression profile between the study cell lines (Fig. 3).
Table 2.
Apoptosis-related miRNA target selection and expression analysis
| Cell line | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOV-21G | SKOV-3 | |||||||
| miRNA selected | Predicted targets related to apoptosis | Prediction Database | Validation Database | Taqman Assay ID | Log2(FC)** | Interpretation | Log2(FC) | Interpretation |
| hsa-miR-150-5p | TP53, AIFM2 | TargetScan, miRanda | miRTarBase | 000473 | −0.003 | *Sub expressed (p < 0.01) |
−4.094 | *Sub expressed (p < 0.01) |
| hsa-miR-181a | DDX3X, BCL2 | TargetScan, miRanda | TARBASE (DianaTools) | 002317 | −2.932 | p = 0.498 | −3.580 | *Sub expressed (p < 0.01) |
| hsa-miR-340-5p | DDX5, BCL2L2 | TargetScan | TARBASE (DianaTools) | 002258 | −1.797 | *Sub expressed (p < 0.01) |
−4.645 | *Sub expressed (p < 0.01) |
| hsa-miR-128 | ACIN1, AFF1 | TargetScan, | miRTarBase | 002216 | −3.777 | *Sub expressed (p < 0.01) |
−5.090 | *Sub expressed (p < 0.01) |
| hsa-miR-125b-5p | BAK1, BCL2 | TargetScan | TARBASE (DianaTools) | 000449 | −1.999 | *Sub expressed (p < 0.01) |
−7.722 | *Sub expressed (p < 0.01) |
| hsa-miR-26a | BID, THAP5 | TargetScan | TARBASE (DianaTools) | 002443 | 0.558 | p = 0.502 | −2.866 | *Sub expressed (p < 0.01) |
| hsa-miR-181c | BCL2, BCL2L11 | TargetScan, miRanda | miRTarBase | 000482 | 1.426 | p = 0.502 | 0.445 | *Superexpressed (p < 0.01) |
| hsa-miR-20a | BCL2, BCL2L11, THAP1 | TargetScan | TARBASE (DianaTools) | 000580 | −2.056 | *Sub expressed (p < 0.01) |
−4.792 | *Sub expressed (p < 0.01) |
| hsa-miR-221 | BMF, APAF1 | TargetScan | miRTarBase | 000524 | −0.746 | p = 0.498 | −9.155 | *Sub expressed (p < 0.01) |
| hsa-miR-17-5p | CAAP1, CASP8, AREL1 | MirTarget2 | – | 002308 | −0.795 | *Sub expressed (p < 0.01) |
−14.744 | *Sub expressed (p < 0.01) |
| hsa-miR-4478 | APAF1, TNFRSF10C, TNFRSF10B | MirTarget2 | – | 465302_mat | −1.636 | *Sub expressed (p < 0.01) |
−5.974 | *Sub expressed (p < 0.01) |
Software: REST 2009 (Qiagen). *Significantly different (α = 5%), comparing tumorspheres to monolayer model, n = 2
**Fold Change (FC) = (2–ΔΔCq)
Fig. 3.
miRNAs expression profile between EOC cell lines (TOV-21G and SKOV-3) comparing tumorspheres with the monolayer model
Discussion
EOC is a diverse and genomically complex disease, and the identification of mutated genes is a key step in the diagnosis and treatment of these malignancies [19, 20]. EOC classification as type I and type II tumors, proposed by Kurman and Shih [21], considers the genetic profile of each histological type. Type I tumors have specific mutations in the KRAS, BRAF and ERBB2 genes, commonly found in low grade serous carcinomas, whereas TP53 mutations are rare in this type of tumors [21]. High-grade serous carcinomas, classic type II tumors, are characterized by a high frequency of TP53 mutations (> 80% of cases) and amplification in CCNE1 [21]. Type I tumors usually show slow growth, are confined to the ovary at diagnosis and are relatively genetically stable, compared with Type II tumors, comprising low-grade serous, low-grade endometrioid, clear cell, mucinous and transitional (Brenner) carcinomas [21]. Type II tumors are highly aggressive, rapidly progressing carcinomas and are almost always identified at an advanced stage at diagnosis and include conventional high-grade serous carcinoma, undifferentiated carcinoma and malignant mixed mesodermal tumors (carcinosarcoma) [21]. Based on information available at COSMIC (Table 1), TOV-21G cells show a typical profile of type I tumors, which frequently have mutations in KRAS and PIK3CA. SKOV-3, in contrast, shares mutations characteristic of type I (PIK3CA mutation) and type II (TP53 mutation) tumors. Thus, TOV-21G and SKOV-3 are selected as two different and representative types of EOC in both histological and molecular characteristics.
Tumorspheres are a model of in vitro cancer stem cell expansion, established in a serum-free medium supplemented with growth factors widely used for enriching and maintaining CSC from several cell lines [6, 22]. In recent years, the CSC model has been highlighted for further research towards developing therapeutic strategies for cancer treatment [23]. Cellular and molecular heterogeneity among tumors complicate the identification of CSC markers unique to different tumor types [24]. However, some surface markers, such as CD44, CD24, CD133, CD146, EpCAM and the expression of aldehyde dehydrogenase (ALDH) enzyme are described in the literature as CSC markers in different tumors, including breast, lung, pancreas, prostate, colorectal, renal and ovarian tumors [24–26]. TOV-21G cultured in the tumorsphere model formed loosely adhered grape-like cell clusters that can be easily dispersed by pipetting. A loose morphology can be related to the mesenchymal phenotype, a property that is crucial for tumor cells to become metastatic, and affects their ability to grow as spheroids [27]. Considering the markers analyzed in this study, no significant increase of any cell subpopulation was detected in TOV-21G cultures, regardless of culture model, suggesting that the tumorsphere-enrichment model used was unable to promote the CSC-like growth and proliferation of this cell line. However, the SKOV-3 cell line had a compact spherical morphology that required disaggregation by enzymatic and mechanical treatment and showed a significant increase in the CD24+/high subpopulation when cultured in the tumorsphere model. Experiments in vitro showed that the CD24+/high fraction has higher chemoresistance, quiescence and self-renewal capacity than the CD24−/low fraction [28]. Furthermore, CD24 can be related to epithelial-mesenchymal-transition (EMT) induction in ovarian cancer, a process involved in cell invasion, resistance to chemotherapy and the formation of CSC side populations [29]. Ishiguro et al. [30] have cataloged antineoplastic agents according to their effectiveness against platinum-resistant and platinum-sensitive ovarian carcinoma cell lines and concluded that SKOV-3 is the most chemoresistant cell line for platinum derivatives. Thus, the CD24+/high subpopulation increase observed in SKOV-3 tumorspheres suggests CSC-like enrichment and may explain the higher chemoresistance observed in this cell line.
Several miRNAs involved in various biological processes, such as developmental timing, differentiation and apoptosis, are aberrantly expressed in various cancer types and some act as tumor suppressors, whereas others act as oncogenes (oncomiRs), depending on which genes or pathways they regulate [31]. Each tumor has a characteristic miRNAs combination; this key information can be used, with the advent of personalized medicine, to direct therapeutic decisions by analyzing patient-specific molecular biomarkers [32]. Among the miRNAs analyzed, miR-125b-5p, miR-340-5p, miR-128, miR-150-5p, hsa-miR-17-5p, miR-20a, miR-340 and miR-4478 were significantly underexpressed in TOV-21G-derived tumorspheres compared with their levels in TOV-21G monolayers (Table 2). The difference in expression of these miRNAs between the culture systems used is apparently more related to the three-dimensional profile (Fig. 1) than to the CSC phenotype (Fig. 2), which was shown to be the same in the TOV-21G line in both culture models. In contrast, SKOV-3 showed changes in the three-dimensional cell profile (Fig. 1), in the ratio of cell subpopulations (Fig. 2), and in the differential expression of the miRNAs evaluated between the two culture models (Table 2).
One of the major cell regulatory pathways that is affected in cancer is apoptosis [33]. Although several studies show that one miRNA targets a several mRNAs, plays either anti- or pro-apoptotic roles in different tumors, and is important for apoptosis regulation during cancer development [34, 35]. Therefore, the investigation of miRNAs involved in apoptotic pathways may provide new information about the mechanisms of tumorigenesis and chemoresistance [34]. Then, the in vitro expression profile of miR-181c, miR-125b-5p, miR-17-5p, miR-221 and miR-26a evaluated in this study may provide important information for EOC prognosis (Fig. 3). MiR-181c was overexpressed in the tumorsphere model, compared with the monolayer model, in both cell lines, although no significant difference was found in TOV-21G cell lines. MiR-181c inhibited the anti-apoptotic factors BCL2 and MCL1, and is related to apoptosis induction [36]. However, the function of miR-181c depends on tumor type and cellular context because miR-181c is downregulated in glioblastoma multiforme, yet is overexpressed in gastric cancer, skin basal cell carcinoma and in osteosarcomas [37]. MiR-125b-5p is downregulated in both TOV-21G and SKOV-3-derived tumorspheres and is more strongly downregulated in SKOV-3-derived tumorspheres. Yang et al. [38] showed that miR-125b-5p miR-125b-5p directly suppresses BCL2 expression and increases the sensitivity of gallbladder cancer cells and mouse models to cisplatin treatment. Furthermore, the combination of miR-125b-5p low expression and BCL2 high expression is highly correlated with poor prognosis in gallbladder cancer patients [38]. Given that SKOV-3 is a platinum-resistant cell line and that miR-125b-5p is strongly downregulated in the tumorsphere model, miR-125b-5p may be a platinum chemoresistance marker. Another strongly miRNA downregulated in SKOV-3-derived tumorspheres is miR-17-5-p. Among the predicted targets of miR-17-5-p (Table 2), CAAP1 was very interesting. CAAP1 is an anti-apoptotic protein and its knockdown expression induces apoptosis in different cancer cell lines that proceeds independently of the caspase-8-dependent death receptor pathway [39]. Li et al. [40] showed that miR-17-5p overexpression plays a regulatory role in the malignant progression of non-small cell lung cancer (NSCLC), promoting significantly inhibited proliferation while inducing the apoptosis of NSCLC H460 cells. Thus, strategies of co-transfection can be elucidating the relationship of miR-17-5p and CAAP1 expression in apoptosis resistance. In addition, miR-17-5p overexpression is also associated to significantly inhibited cell proliferation, migration and invasion in triple-negative breast cancer (TNBC) cells and miR-17-5p down-regulated in TNBC samples were significantly correlated with shorter overall survival, lager tumors size and advanced stages of disease [41]. MiR-221 is also downregulated in both TOV-21G and SKOV-3 tumorspheres-derived, but more strongly downregulated in SKOV-3. However, Li et al. [42] showed, for the first time that APAF1 is a direct target of miR-221 in human EOC cells, and APAF1 overexpression, through miR-221 inhibition, suppressed EOC cell lines proliferation and induced cell apoptosis in vitro. MiR-26a was overexpressed in TOV-21G-derived tumorspheres compared with monolayer TOV-21G cells and downregulated in SKOV-3-derived tumorspheres. MiR-26a expression is frequently abnormal in tumors, indicating that it may play key roles in tumor formation [43]. Deng et al. [44] showed that miR-26a levels were associated with the gastric cancer clinical stage and presence of lymph node metastases and analysis revealed that patients whose primary tumors displayed low expression of miR-26a had shorter survival and relapse-free survival. Moreover, miR-26a overexpression enhanced the sensitivity to doxorubicin, inducing apoptosis in hepatocellular carcinoma cells [45] and significantly inhibit the proliferation, promoting apoptosis in ovarian cancer cells [46].
Conclusion
Considering that miR-26a, miR-125b-5p, miR-17-5p and miR-221 expression was lower in SKOV-3-derived tumorspheres, which had a higher ratio of CD24+/high cells (ovarian CSC-like), we suggest the need to investigate the relationship of the downregulation of these miRNAs towards understanding apoptosis resistance and poor prognosis in EOC. Moreover, co-transfection strategy can be help to elucidate the relationship of miR-17-5p and CAAP1 expression in apoptosis resistance mechanisms.
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for CDS - PPM-00383-14 and CDS - BPV-00277-16 grants and Pró-Reitoria de Pesquisa from Universidade Federal de Minas Gerais (PRPq –UFMG) for support.
Compliance with Ethical Standards
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Garson K, Vanderhyden BC. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149:R59–R70. doi: 10.1530/REP-14-0234. [DOI] [PubMed] [Google Scholar]
- 2.KK A, Josahkian JA, Francis J-A, et al. Current state of biomarkers in ovarian cancer prognosis. Future Oncol. 2015;11:3187–3195. doi: 10.2217/fon.15.251. [DOI] [PubMed] [Google Scholar]
- 3.Kwon MJ, Shin YK. Regulation of ovarian cancer stem cells or tumor-initiating cells. Int J Mol Sci. 2013;14:6624–6648. doi: 10.3390/ijms14046624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivos DJ, Mayo LD. Emerging non-canonical functions and regulation by p53: p53 and stemness. Int J Mol Sci. 2016;17:1–30. doi: 10.3390/ijms17121982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem / progenitor cell properties. Cancer Res. 2005;65:5506–5512. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 6.Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. doi: 10.1016/j.neo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen D, Zhang Y, Wang J, et al. MicroRNA-200c overexpression inhibits tumorigenicity and metastasis of CD117+CD44+ ovarian cancer stem cells by regulating epithelial-mesenchymal transition. J Ovarian Res. 2013;6:50. doi: 10.1186/1757-2215-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi R-U, Miyazaki H, Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laios A, O’Toole S, Flavin R, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilming Elgaaen B, Olstad OK, Haug KBF, et al. Global miRNA expression analysis of serous and clear cell ovarian carcinomas identifies differentially expressed miRNAs including miR-200c-3p as a prognostic marker. BMC Cancer. 2014;14:80. doi: 10.1186/1471-2407-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga LC, Ramos APAS, Traiman P, et al. TRAIL-R3-related apoptosis: epigenetic and expression analyses in women with ovarian neoplasia. Gynecol Oncol. 2012;126:268–273. doi: 10.1016/j.ygyno.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Braga LC, Silva LM, Piedade JB, et al. Epigenetic and expression analysis of TRAIL-R2 and BCL2: on the TRAIL to knowledge of apoptosis in ovarian tumors. Arch Gynecol Obstet. 2014;289:1061–1069. doi: 10.1007/s00404-013-3060-0. [DOI] [PubMed] [Google Scholar]
- 13.Braga LC, Silva LM, Ramos APÁ d S, et al. Single CpG island methylation is not sufficient to maintain the silenced expression of CASPASE-8 apoptosis-related gene among women with epithelial ovarian cancer. Biomed Pharmacother. 2014;68:87–91. doi: 10.1016/j.biopha.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4:143–159. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.House CD, Hernandez L, Annunziata CM (2015) In vitro enrichment of ovarian cancer tumor-initiating cells. J Vis Exp:1–8. 10.3791/52446 [DOI] [PMC free article] [PubMed]
- 16.Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths-Jones S, Grocock RJ, van Dongen S, et al. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and clinical treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toss A, Tomasello C, Razzaboni E, et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed Res Int. 2015;2015:341723. doi: 10.1155/2015/341723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurman R, Shih I. The origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvet CY, André FM, Mir LM. The culture of cancer cell lines as tumorspheres does not systematically result in cancer stem cell enrichment. PLoS One. 2014;9:e89644. doi: 10.1371/journal.pone.0089644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharmacol Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luna JI, Grossenbacher SK, Murphy WJ, Canter RJ (2016) Targeting cancer stem cells with natural killer cell immunotherapy. Expert Opin Biol Ther 17:313–324. 10.1080/14712598.2017.1271874 [DOI] [PMC free article] [PubMed]
- 25.Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. doi: 10.1155/2012/708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L, Lv W, Zhao X. CD24 as a molecular marker in ovarian cancer: a literature review. Cancer Transl Med. 2016;2:29. doi: 10.4103/2395-3977.177563. [DOI] [Google Scholar]
- 27.Iglesias JM, Beloqui I, Garcia-Garcia F, et al. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0077281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao MQ, Choi YP, Kang S, et al. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Terai Y, Tanabe A, et al. CD24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the Akt and ERK pathways. Oncol Rep. 2017;37:3189–3200. doi: 10.3892/or.2017.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishiguro K, Zhu Y-L, Lin ZP et al (2016) Cataloging antineoplastic agents according to their effectiveness against platinum-resistant and platinum-sensitive ovarian carcinoma cell lines. J Transl Sci 2:117–124. 10.15761/JTS.1000127 [DOI] [PMC free article] [PubMed]
- 31.Mulrane L, McGee SF, Gallagher WM, O’Connor DP. miRNA dysregulation in breast cancer. Cancer Res. 2013;73:6554–6562. doi: 10.1158/0008-5472.CAN-13-1841. [DOI] [PubMed] [Google Scholar]
- 32.Sethi S, Ali S, Sethi S, Sarkar FH. MicroRNAs in personalized cancer therapy. Clin Genet. 2014;86:68–73. doi: 10.1111/cge.12362. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Steer CJ. MicroRNAs as gatekeepers of apoptosis. J Cell Physiol. 2010;223:289–298. doi: 10.1002/jcp.22066. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Hashimi SM, Good DA, et al. Apoptosis and microRNA aberrations in cancer. Clin Exp Pharmacol Physiol. 2012;39:739–746. doi: 10.1111/j.1440-1681.2012.05700.x. [DOI] [PubMed] [Google Scholar]
- 35.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayala-Ortega E, Arzate-Mejía R, Pérez-Molina R, et al. Epigenetic silencing of miR-181c by DNA methylation in glioblastoma cell lines. BMC Cancer. 2016;16:226. doi: 10.1186/s12885-016-2273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Zhan M, Chen T, et al. miR-125b-5p enhances chemotherapy sensitivity to cisplatin by down-regulating Bcl2 in gallbladder cancer. Sci Rep. 2017;7:43109. doi: 10.1038/srep43109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Johansson E, Miller ML, et al. Identification of a conserved anti-apoptotic protein that modulates the mitochondrial apoptosis pathway. PLoS One. 2011;6:e25284. doi: 10.1371/journal.pone.0025284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Zhou H, Luo J, Huang J. MicroRNA-17-5p inhibits proliferation and triggers apoptosis in non-small cell lung cancer by targeting transforming growth factor β receptor 2. Exp Ther Med. 2017;13:2715–2722. doi: 10.3892/etm.2017.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Lai Y, Ma J, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17:745. doi: 10.1186/s12885-017-3674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Li Q, Huang H, et al. Overexpression of miRNA-221 promotes cell proliferation by targeting the apoptotic protease activating factor-1 and indicates a poor prognosis in ovarian cancer. Int J Oncol. 2017;50:1087–1096. doi: 10.3892/ijo.2017.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Liu QG. The role of miR-26 in tumors and normal tissues (review) Oncol Lett. 2011;2:1019–1023. doi: 10.3892/ol.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng M, lin TH, X hong L, et al. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One. 2013;8:1–10. doi: 10.1371/annotation/61b7e0d5-6062-49b7-a270-2c115dd3cb8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin F, Wang Y, Li M, et al. MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis. 2017;8:e2540. doi: 10.1038/cddis.2016.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun TY, Xie HJ, He H, et al. miR-26a inhibits the proliferation of ovarian cancer cells via regulating CDC6 expression. Am J Transl Res. 2016;8:1037–1046. [PMC free article] [PubMed] [Google Scholar]