Abstract
The incidence of differentiated thyroid cancer has been increasing. Nevertheless, its molecular mechanisms are not well understood. In recent years, extracellular nucleotides and nucleosides have emerged as important modulators of tumor microenvironment. Extracellular ATP is mainly hydrolyzed by NTPDase1/CD39 and NTPDase2/CD39L1, generating AMP, which is hydrolyzed by ecto-5′-nucleotidase (CD73) to adenosine, a possible promoter of tumor growth and metastasis. There are no studies evaluating the expression and functionality of these ectonucleotidases on normal or tumor-derived thyroid cells. Thus, we investigated the ability of thyroid cancer cells to hydrolyze extracellular ATP generating adenosine, and the expression of ecto-enzymes, as compared to normal cells. We found that normal thyroid derived cells presented a higher ability to hydrolyze ATP and higher mRNA levels for ENTDP1–2, when compared to papillary thyroid carcinoma (PTC) derived cells, which had a higher ability to hydrolyze AMP and expressed CD73 mRNA and protein at higher levels. In addition, adenosine induced an increase in proliferation and migration in PTC derived cells, whose effect was blocked by APCP, a non-hydrolysable ADP analogue, which is an inhibitor of CD73. Taken together, these results showed that thyroid follicular cells have a functional purinergic signaling. The higher expression of CD73 in PTC derived cells might favor the accumulation of extracellular adenosine in the tumor microenvironment, which could promote tumor progression. Therefore, as already shown for other tumors, the purinergic signaling should be considered a potential target for thyroid cancer management and treatment.
Keywords: Extracellular ATP, Adenosine, NTPDases, CD73, Thyroid papillary carcinoma, Purinergic signaling
Introduction
The incidence of papillary thyroid carcinoma (PTC) has been increasing [1]; nevertheless, its molecular mechanisms are not well understood. In recent years, research focused on the niche within tumors microenvironment, in an attempt to make possible target-directed therapeutic approaches [2], and extracellular nucleotides (e.g. ATP) and nucleosides (e.g. adenosine) have emerged as important modulators of tumor microenvironment [3–8]. The signaling events induced by these molecules are controlled by ectonucleotidases, a group of ectoenzymes which regulate the metabolism of ubiquitous nucleosides and nucleotides in extracellular space. Their concentrations are low in physiological conditions, when compared to tumor microenvironment, where they might contribute to tumor development and progression [9].
The major members of ectonucleotidases are ectonucleoside triphosphate diphosphohydrolase (NTPDase; E.C.3.6.1.5) and ecto-5′-nucleotidase (e’NT/CD73; E.C.3.1.3.5) [10]. The molecular characterization and distribution of eight different NTPDases have already been described: NTPDase1, 2, 3 and 8 are cell surface-located enzymes with an extracellular facing catalytic site, while NTPDase4, 5, 6 and 7 are intracellular and only NTPDase5 and 6 have been shown to be secreted after expression. Extracellular ATP is hydrolyzed by NTPDase1–3 and 8 and the resulting AMP is hydrolyzed to adenosine by ecto-5′-nucleotidase (CD73). There are no studies evaluating ectonucleotidase activities or gene expression on thyroid cells, although CD73 expression and activity has shown to be upregulated in thyroid tissues with PTC [11].
In this study, we analyzed the ATP, ADP and AMP catabolism on the surface of thyroid cell lines, as well as, the ectonucleotidase gene expression in cells derived from normal thyroid and PTC. Based on the different, but complementary ectoenzyme status of normal and tumor thyroid cells, we showed that upregulation of CD73 can contribute to the accumulation of adenosine, which can be one of the molecular mechanisms involved in the regulation of thyroid cancer cells.
Material and Methods
Ethics Statement
This project was submitted and approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil (Number 15–0950).
Chemicals
The following chemicals were obtained commercially in analytical grade: cell culture supplies, nucleotides/nucleoside (ATP, ADP, AMP and adenosine), potassium dihydrogen phosphate (KH2PO4) (Sigma-Aldrich, St. Louis, MO, USA), tetrabutylammoniumchloride (C16H36ClN) (Sigma-Aldrich, Steinheim, Switzerland), HPLC grade methanol (Panreac ITW Companies, Barcelona, Spain). All solutions were filtered through a 0.22 μm pore membrane (Millipore, Bedford, USA) before HPLC analysis.
Thyroid Cell Cultures
Human thyroid cell lines Nthy-1, TPC-1 [12] and K1 [13] derived, respectively, from normal thyroid, papillary thyroid carcinoma (PTC), and from metastasis of a well-differentiated PTC, were grown in DMEM containing 5% fetal bovine serum (FBS), and 50 mg/mL ampicillin/streptomycin in a 5% CO2 atmosphere. PCCL-3 [14] and FRTL-5 cell lines [15], which were derived from rat normal thyroid, were cultured in Ham’s F-12 Coon’s modification medium supplemented with 10% FBS, 10 μg/mL insulin, 5 μg/mL transferrin, 1 mU/mL TSH, 100 U/mL kanamycin at 37 °C with 5% CO2. TPC-1 and K1 cell lines were kindly supplied by Dr. Ana Luiza Silva Maia (Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil), and Nthy-1, PCCL3 and FRTL-5 cell lines were kindly supplied by Dr. Denise Pires de Carvalho (Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil).
RNA Isolation, cDNA Synthesis and qPCR
Cellular total RNA was extracted with Trizol® Reagent (Macherey Nagel, Düren, Germany) and reverse transcribed with the SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). mRNA was detected by real-time quantitative PCR using Fast SYBRGreen Master Mix (Applied Biosystems, Foster City, CA, USA) for human gene were described previously by our group [16] and for RT-qPCR analyses of rat genes were described in Table 1. Ectonucleotidase mRNA levels were calculated with the standard curve method using a serial five-fold dilution of samples and ACTB mRNA levels were used as control.
Table 1.
Primer sequences used for gene amplification of rat thyroid cell lines
| Gene | Primer sequence | Product length (bp) |
|---|---|---|
| Entpd1 | Forward: 5’-CTGCCCTTACTCCCAGTGTG-3’ | 124 |
| Reverse: 5’-GACACTGTCGTTCGCCATCT-3’ | ||
| Entpd2 | Forward: 5’-TCTGCTACTTTGCGTCCCTAC-3’ | 131 |
| Reverse: 5’-TGTCATTCTCCTTGTCCGCTG-3’ | ||
| Entpd3 | Forward: 5’-GGAGTGGTCAGCCAAACCTT-3’ | 140 |
| Reverse: 5’-TCGTGGAGATGCTTTGGGAC-3’ | ||
| Entpd5 | Forward: 5’-TGCCGGCACCTTTTATGGAA-3’ | 141 |
| Reverse: 5’-GAAAGTCCCGGCTTCACAGA-3’ | ||
| Entpd6 | Forward: 5’-CACCTTGACCCACGAAACCT-3’ | 120 |
| Reverse: 5’-GGAATGTGTTGCTTGGCGAC-3’ | ||
| Reverse: 5’-CCCAATCCAAGCGACACGAT-3’ | ||
| Nt5e | Forward: 5’-TTTGGATGCTGGCGATCAGT-3’ | 147 |
| Reverse: 5’-TCAATCAGTCCTTCCACACCG-3’ | ||
| Actb | Forward: 5’-CAGGATGCAGAAGGAGATTAC-3’ | 115 |
| Reverse: 5’-CAGTGAGGCCAGGATAGA-3’ |
Ectonucleotidase Assays
Ectonucleotidase enzymatic activities of thyroid cells were evaluated using ATP and AMP as substrates, as described previously [5]. Briefly, 5 × 103 cells/well were seeded in 24 multi-well plates and cultivated until reaching 90–95% confluence. ATPase and ADPase activities were measured by adding 1 mM ATP or ADP to the reaction medium (2 mM CaCl2, 120 mM NaCl, 5 mM KCl, 10 mM glucose, 20 mM Hepes – pH 7.4) at 37°Cfor 10, 20, 30 or 60 min. For AMP hydrolysis, the same incubation conditions were used, substituting CaCl2by 2 mM MgCl2 in the reaction medium. A chemical competitive inhibitor of CD73, a non-hydrolysable ADP analog, adenosine 5′-(α,β-methylene) diphosphate (APCP), was used to confirm AMP hydrolysis via CD73: cells were preincubated with 10 μM APCP for 15 min, 1 mM AMP was added to the reaction medium containing APCP, for 10, 20, 30 or 60 min. The reaction was stopped by removing an aliquot of the incubation medium, which was transferred to a pre-chilled tube containing 5% w/v trichloro-acetic acid. The release of inorganic phosphate (Pi) was measured by the Malachite Green method [13] using KH2PO4 as a Pi standard. Specific activity was expressed as nmol of Pi released per min per mg of protein. Protein concentration of samples was measured by the Bradford protein assay [14].
Chromatographic Separation and Analysis of Extracellular ATP Metabolism
FRTL-5, TPC-1 and Nthy-1 cells were seeded in 24 multi-well plates (5 × 103 cells/well) and confluent cells were exposed to 100 μM ATP or 100 μM AMP, after preincubation with 10 μM APCP or not, as described above. After 0, 10, 20, 30, 60, 90 and 120 min, there actions were stopped by removing an aliquot of the incubation media and transferring it to pre-chilled tubes. Samples were centrifuged at 12,000 g for 15 min and stored at −80 °C until analysis. HPLC system used was a Shimadzu Prominence (Shimadzu, Kyoto, Japan) equipped with degasser DGU-20A5, quaternary gradient solvent delivery unit LC-20AT, autosampler SIL-20A, column oven CTO-20A and diode array detector SPD-M20A.The nucleotide/nucleoside separation was performed on a Shimadzu column Shim-pack CLC (M) C18 (150 × 4.6 mm × 5 μm) attached to a guard column Shimadzu Shim-pack GVP-ODS (4.6 × 10 mm). The flow rate was 1.200 mL.min−1 and 10 μL of sample was injected onto the column, held at 32 °C. Data were acquired and processed using the LC Solution Software (Shimadzu). The mobile phase was constituted by solvent A, a buffer solution prepared with 60 mM KH2PO4, 5.0 mM C16H36ClN (pH 5.9); and solvent B, with 60 mM KH2PO4, 5.0 mM C16H36ClN, 30% methanol (pH 5.9). A linear gradient program was setup with 0–25 min 100% A, 25–26 min 100% B and 26–45 min 100% A. The analyte was detected at 254 nm and compared with the nucleotide/nucleoside standard solutions concentration of 100 μM. Data were expressed as μM.
Western Blot Analysis
Protein was extracted from Nthy-1 and TPC-1 cells line. Thyroid cells were homogenized in RIPA buffer containing 20 mM (pH 8.0) Tris-HCl, 150 mM NaCl, 5 mM EDTA, 10% glycerol, plus 1μg/ml aprotinin, 1μg/ml leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were centrifuged at 12.000 x g for 30 min at 4 °C, and supernatants were used for the assays. Protein content was measured by the Bradford method and 33 micrograms of protein were separated in a 12% SDS-polyacrylamide gel with a standard molecular weight marker (Spectra™ Multicolor Broad, Thermo Fisher Scientific Inc., Rockford, IL, USA). Proteins were transferred to Immobilon-P polyvinylidene difluoride (0.45 μm PVDF) blotting membrane with a semi-dry transfer cell (Bio-Rad Trans-Blot® SD, Hercules, CA, USA). Afterwards, membranes were blocked by incubation with Tris-buffered saline containing 0.1% Tween 20 and 3% BSA for 2 h at room temperature and then incubated overnight at +4 °C with rabbit monoclonal anti-human CD73 antibody (dilution 1:1000; D7F9A - Cell Signaling, Massachusetts, USA). Primary antibodies were detected by secondary antibody (1:2000, for 2 h) followed by ECL and X-ray film exposition (Kodak-Xmat).
Cell Proliferation Assays
The cell proliferation assays were conducted by cell counting in a hemocytometer and quantification of KI-67 expression. TPC-1 cells were seeded at 1.0 × 103 cells per well in 24-well plates in DMEM/ 5% SBF for 24 h. Then, cells were deprived of SBF for 24 h and treated with DMEM/5%SBF, or DMEM/5%SBF plus100μM ATP or 100 μM ADP or 100 μM AMP or 100 μM AMP + 10 μM APCP or 100 μM ADO. After 24 and 48 h, the medium was removed, cells were washed with 1X PBS, digested with 0.25% Trypsin-EDTA and counted in a hemocytometer.
Also, total RNA was extracted 24 h or 48 h after these treatment as described above and the relative quantification of KI-67 expression was calculated using the delta–delta CT method.
Migration Assay
For the wound-healing migration assay, TPC-1 cells were grown to confluence in 6-well plates in DMEM/ 5% SBF medium. Cells were then scratched with a P200 pipette tip, as described previously [17] and the culture medium was replaced with treatment media: serum free-DMEM, serum free-DMEM with 100 μM ATP or 100 μM ADP or 100 μM AMP, or 100 μM AMP plus 10 μM APCP or 100 μM ADO. Photographs were obtained at 0, 3, 6 and 24 h. The wound gap was quantified by National Institutes of Health ImageJ software.
Statistical Analysis
Differences between cell proliferation and migration for the treatment groups were evaluated using the Generalized Estimating Equations method with Bonferroni adjustment. Statistical analyses were performed using SPSS for Windows version 20.0 (IBM, USA) and analyses were performed at the 0.05 significance level.
Results
Extracellular Nucleotide Hydrolysis by Thyroid Cell Lines
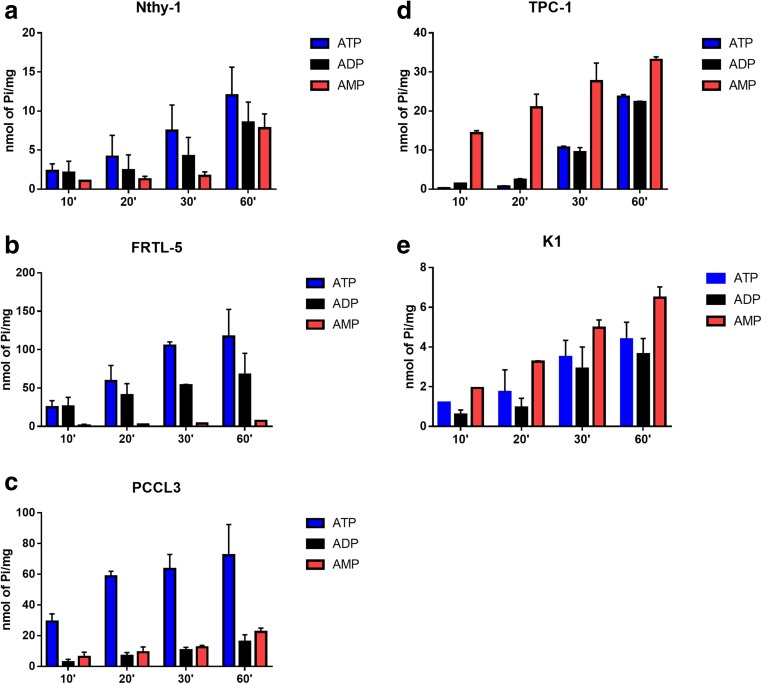
Firstly, we tested the ability of thyroid cells to hydrolyze extracellular ATP, ADP and AMP. All cell lines were able to hydrolyze these nucleotides, but different amounts of inorganic phosphate were released, according to the cell line (Fig. 1 and Table 2). Importantly the ratio of ATP/AMP hydrolysis was at least 7-fold higher in normal thyroid cells Nthy-1, FRTL-5 and PCCL3 cells, as compared to PTC-derived cells (K1 and TPC-1 cells). Inversely, the ratio of AMP/ATP hydrolysis was higher in PTC-derived cells than normal thyroid cells. ADP and ATP hydrolysis were similar in Nthy-1, FRTL-5, K1 and TPC-1 cells, whereas in PCCL3 cells, ADP and AMP hydrolysis were similar (Table 2).
Fig. 1.
ATP, ADP and AMP hydrolysis on the surface of thyroid cells. Thyroid cells were incubated in phosphate-free buffer containing 1 mM of nucleotides at 37 °C for 10, 20, 30 and 60 min, as described in material and methods. In normal thyroid-derived cells, a Nthy-1, b FRTL-5 and c PCCL3, ATP hydrolysis was higher than AMP, while in papillary thyroid carcinoma derived cells, d TPC-1 and e K1, AMP hydrolysis was higher than ATP and ADP. Data are expressed as mean ± SD of three experiments, performed in duplicates
Table 2.
Specific activities for ATP, ADP and AMP hydrolysis and their rates in normal thyroid, FRTL-5, PCCL3 and Nthy-1, and papillary thyroid carcinoma (PTC), TPC-1 and K1derived cell lines
| Cell Line | ATP | ADP | AMP | ATP/ADP | ATP/AMP | AMP/ATP |
|---|---|---|---|---|---|---|
| Nthy-1 | 0.25 ± 0.075 | 0.14 ± 0.06 | 0.06 ± 0.01 | 1.76 | 4.36 | 0.23 |
| FRTL-5 | 2.62 ± 0.41 | 1.94 ± 0.79 | 0.14 ± 0.01 | 1.26 | 17.87 | 0.06 |
| PCCL3 | 2.66 ± 0.82 | 0.32 ± 0.05 | 0.52 ± 0.11 | 8.40 | 5.32 | 0.19 |
| TPC-1 | 0.35 ± 0.03 | 0.32 ± 0.04 | 1.15 ± 0.38 | 1.12 | 0.31 | 3.24 |
| K1 | 0.11 ± 0.02 | 0.07 ± 0.02 | 0.17 ± 0.03 | 1.59 | 0.62 | 1.62 |
Ectonucleotidase activities of intact cells were determined at 37 °C, following 30 min incubation with 1 mM ATP, ADP or AMP. Nucleotide hydrolysis is expressed as nmol of Pi released/min/mg of protein and represents mean ± SD of three experiments
Extracellular ATP Metabolism and NTPDases Expression
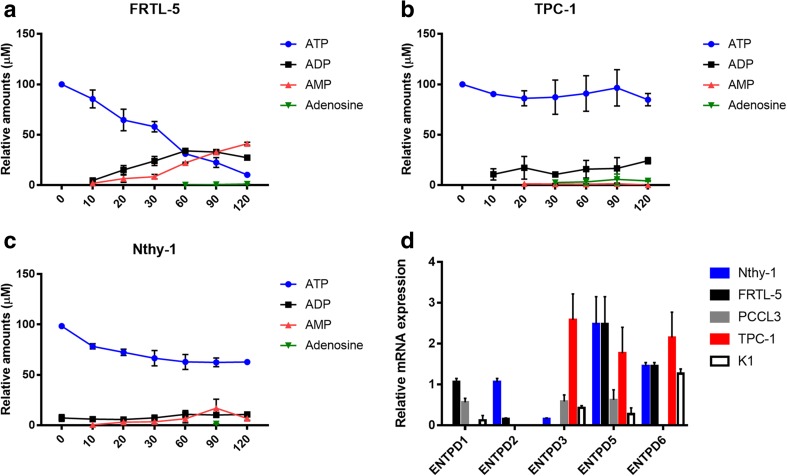
To better investigate the pattern of extracellular ATP hydrolysis, a time course analysis was performed, comparing normal (Nthy-1 and FRTL-5) and cancer (TPC-1) thyroid cells. Thyroid cell cultures were exposed to medium containing 100 μM ATP (0–120 min), and ATP degradation metabolites were measured by HPLC (Fig. 2). FRTL-5 cells (Fig. 2a) metabolized ATP gradually along 120 min of incubation with subsequent production of ADP and AMP, which were the main products accumulated at the end of the reactions (27.32 and 41.13 μM, respectively). In opposite, ATP was poorly metabolized by TPC-1 cells (Fig. 2b) and small amounts of ADP and AMP were detected in the extracellular medium. After 120 min of incubation, the amount of ATP remaining in FRTL-5 and TPC-1 cells was, respectively, 10.36 ± 0.25 and 84.86 ± 4.3 μM. The incubation with ATP led to an accumulation of AMP in FRTL5 cells but not in TPC-1cells, confirming the increased CD73 activity in cancer cells (Fig. 1). In FRTL-5 cells, ATP seemed not to be directly dephosphorylated to AMP, since ADP levels were detected in the extracellular medium, which were smaller inTPC-1 cells. Nthy-1 cells had lower ability to hydrolyze ATP and generate ADP, AMP and adenosine (Fig. 2c).
Fig. 2.
Metabolism of extracellular ATP and expression of NTPDases by normal and thyroid cancer cells. The hydrolysis of extracellular ATP and formation of degradation product were analyzed by HPLC in comparison with reference standards. a FRTL-5, b TPC-1 and c Nthy-1 cells were incubated with 100 μM ATP and supernatant aliquots were collected after 0, 10, 20, 30, 60, 90 and 120 min. ATP, ADP, AMP and adenosine (ADO) were quantified by HPLC. Data are shown as mean ± SD of triplicates. d The expression of NTPDases in normal and thyroid cancer cell lines was analyzed by RT-qPCR. mRNA levels were normalized by ACTB gene expression. ENTPD1 and ENTPD2 mRNA were more strongly expressed in cells derived from normal thyroid, while ENTPD3 mRNA was identified only in tumor-derived thyroid cells. mRNA levels of ENTPD5 and ENTPD6 were similar in normal and tumor cells. NTPDases expression is shown as mean ± SD of at least three experiments, performed in duplicates
After, to analyze the NTPDases expressed by cancer and normal cells, we performed RT-qPCR to evaluate mRNA expression of ENTPD 1, 2, 3, 5, 6, 8 in thyroid cell lines (Fig. 2d). Notably, ENTPD1 and ENTPD2 were expressed at very low or undetectable levels in tumor derived cells, suggesting that their expression is lost during cancer progression. The absence of mRNA levels of ENTPD1 in Nthy-1 (Fig. 2d) corroborated the lower ATP hydrolysis rate, as measured by HPLC analysis (Fig. 2c). ENTPD3 could not be detected in FRTL-5 and PCCL-3 cells, and was weakly expressed in Nthy-1, while its expression was higher in tumor derived cells (TPC-1 and K1). ENTPD6 was moderately expressed in both, normal and tumor derived cells, but not detected in PCCL3. ENTPD8 mRNA could not be detected in any of the five thyroid cell lines studied.
Inhibition of AMPase Activity by APCP in TPC-1 Cells and CD73 Expression
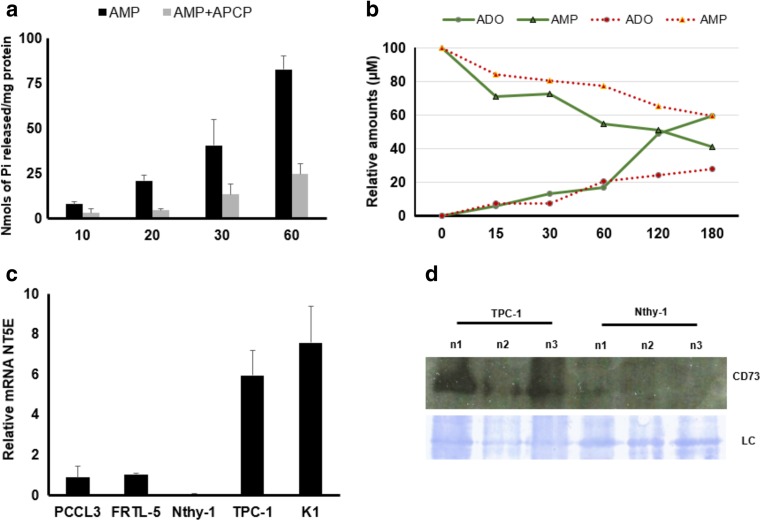
In order to confirm the enzymatic participation of CD73 on AMP metabolism in TPC-1 cells, these cells were incubated with AMP in the presence of APCP, and AMP hydrolysis was evaluated by malachite green and HPLC assays. As shown in Fig. 3 (panel A), APCP (10 μM) inhibited AMPase activity in ~30%, when compared to control. HPLC experiments (Fig. 3b; triangle “solid line”; green) confirmed that TPC-1 cells were able to hydrolyze extracellular AMP to adenosine. Indeed, ~60% of AMP was metabolized by cancer cells after 180 min. APCP (10 μM) decreased AMP metabolism (Fig. 3b; “dotted line”; red), decreasing extracellular adenosine generation ~20%, after 180 min. These findings suggest that APCP, which prevents the binding of AMP to CD73, decreased the ability of cells to hydrolyze AMP, adding evidence to CD73 role in converting AMP to adenosine in these cells. In accordance with these results the expression of CD73 mRNA was about 5 times higher in TPC-1 and K1 cells (Fig. 3c), leading to a higher CD73 imunocontent in a TPC-1 (Fig. 4d).
Fig. 3.
APCP inhibits AMP hydrolysis in thyroid cancer TPC-1 cells. a TPC-1 cells were incubated with 1 mM AMP without (black bars) or with (gray bars) 10 μM APCP. AMPase activity was determined by the Malachite Green assay. Data are expressed as nmol Pi released/mg of protein and represent the mean ± SD of three experiments in triplicates. b HPLC analysis showing extracellular AMP metabolism in TPC-1cells, after incubation with 100 μM of AMP without (solid line; green) or with (dotted line; red) 1 μM APCP. APCP decreased adenosine (ADO) formation. AMP and ADO were quantified by HPLC in comparison with reference standards. c The expression of CD73 was analyzed by RT-qPCR and mRNA level was normalized with ACTB. The ectonucleotidase expression was higher (~10 fold) in tumor derived thyroid cells when compared to cells derived from normal thyroid. Data are shown as mean ± SD of three experiments, performed in duplicates. d Western blot of CD73 in normal thyroid cell line (Nthy-1) and tumoral thyroid cell (PTC-1) obtained from three independent cell cultures (n1, n2 and n3). The loading control (LC) shows representative bands of the Coomassie-stained membrane
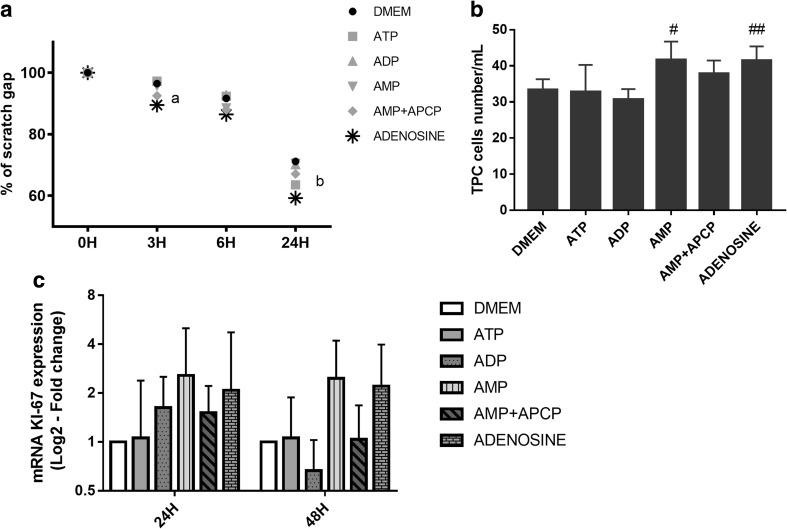
Fig. 4.
Effect of nucleotides on TPC-1 migration and proliferation in vitro. a TPC-1 cells were scratch-wounded with a micropipette tip (200 μl) and treated with serum free DMEM; or serum free DMEM plus 100 μM ATP; or 100 μM AMP; or 100 μM AMP plus 10 μM APCP; or 100 μM ADO. The wound gap was evaluated at 0, 3, 6, and 24 h post-scratching. Mean relative quantification of the fraction of the wound that remains uncovered by the migratory cells as a function of time for each treatment are shown. Bars represent means ± SEM; n = 3. ª adenosine vs. control p < 0.0001; b ATP vs. control p < 0.0001 and, adenosine vs. control p < 0.00001. b Determination of ATP, ADP, AMP and, adenosine effect by cell counting in a Neubauer cell chamber. After 48 h of exposure with AMP and adenosine, the percentage of TPC-1 cells was significantly elevated as compared to the control group (# AMP vs. control p=0.001; ## adenosine vs. control p=0.001). c The RT-qPCR analysis showed that Ki-67 mRNA level was increased, but not significantly, in TPC-1 cells treated with AMP or adenosine. The stimulatory effect on TPC-1 migration and proliferation caused by AMP was reverted in co-treatment with APCP (Adenosine 5′-(α,β-methylene)- diphosphate)
Adenosine Promotes Migration and Proliferation on TPC-1 Cells In Vitro
The scratch wound healing assay was performed in order to evaluate the effects of ATP, ADP, AMP and adenosine on TPC-1 migration in vitro. As shown in Fig. 4, panels A and B, treatment for 3 h with adenosine (p < 0.0001; n = 3), 6 h with ADP (p < 0.0001; n = 3), and 24 h with ATP (p < 0.0001; n = 3) or adenosine (p < 0.00001; n = 3) increased migration of TPC-1 cells after scratch wound.
For investigation of the effect of ATP, ADP, AMP and adenosine, in tumor thyroid cells proliferation, TPC-1 cells were counted in a hemocytometer and KI-67 mRNA levels, a proliferation marker, were analyzed by RT-qPCR. As shown in Fig. 4, panel B, AMP or adenosine increased non-significantly Ki-67 gene expression. Cell number increased after treatment with AMP (p = 0.001; n = 3) or adenosine (p = 0.001; n = 3) for 48 h; this effect was reversed by APCP (p = 0.079; n = 3).
Discussion
The present study demonstrates that genes encoding NTPDases are expressed in thyroid cell lines derived from normal thyroid and PTC. The ecto-nucleotidases profiles differed according to the origin of the cell lines in two aspects: (i) the ability to hydrolyze ATP and AMP and (ii) the mRNA levels for these enzymes. Cells derived from normal thyroid showed a higher ability to hydrolyze ATP and, as expected, expressed higher mRNA levels for ENTPD1 and 2. Inversely, tumor cells had a higher ability to hydrolyze AMP and showed the highest levels of CD73 mRNA.
It is well-known that high levels of extracellular ATP and adenosine are detected in the tumor microenvironment of solid tumors [18]. Among thyroid cancers, Solini et al. showed that ATP concentration in the supernatants of PTC derived cells, FB1 and FB2, were about 3-fold higher than in the supernatant of thyrocytes isolated from nodular goiter [19]. Cell death, hypoxia and the presence of inflammatory cells probably mediated this increase. Once established, these microenvironment changes could favor tumor growth and inhibit local immune response.
Our results showing higher CD73 mRNA levels in two human PTC derived cells (K1 and TPC-1), as compared to cells derived from normal thyroid (Nthy-1, FRTL-5 and PCCL3) are in line with a previous study which described the overexpression of CD73 in slices of PTC, in comparison to normal or benign thyroid [11]. The same authors measured the CD73 activity in thyroid tissues using Wachstein and Meisel’s methods, showing that its activity was faint in normal thyroid cells and strong in PTC cells [11]. Similarly, we observed that more than 60% of AMP was hydrolyzed by TPC-1 cells after 180 min, with the expected increase in adenosine accumulation.
On the other hand, FRTL-5 cells showed a progressive accumulation of AMP and very low adenosine production, when ATP was given as substrate. The profile of low ATPase and high AMPase activities in PTC cells could be explained by the low to absent levels of ENTPD1 and ENTPD2 and high CD73 gene expression in these cells. Previously, our group showed a similar profile of high ATP and low AMP degradation in normal glial cells, and low ATP and high AMP degradation in glioma cancer cells [3–5].
In addition, our group has shown that a proportional increase in CD73 activity and mRNA expression occurred in a dose-response relationship after triiodothyronine (T3) treatment in C6 rat glioma cells [20] and in smooth muscle cells [21], suggesting that its status could be affected by thyroid follicular cell hyperfunction. In this line, it was observed that rats submitted to chronic stimulation by low-iodine diet or treated with propylthiouracil had an increase in the enzymatic activity of CD73 [22].
In the present report, we were able to demonstrate increased cell proliferation and migration rates in TPC-1 cells, after treatment with AMP and adenosine. These stimulatory effects caused by AMP were reverted by co-treatment with APCP, a non-hydrolysable ADP analogue which inhibits CD73. Our data suggests that adenosine mediates its effect through the P1 receptors subtype. Consistent with this, the expression of adenosine receptor A1 gene (P1A1) was higher in patients with PTC than in normal thyroid tissue [23]. Also, adenosine receptor A3 (P1A3) protein was detected in PTCs, but not in normal human thyroid tissue [24].
The importance of P1 receptors signaling is shown by a study that promoted a chronic cAMP stimulation in the thyroid. The authors developed a transgenic mouse expressing the adenosine receptor A2a under the control of the thyroglobulin promoter (Tg-A2aR). Their results showed that 360 genes were modulated in the thyroid, when compared with normal mice. The transgenic animals developed huge goiters and died prematurely by cardiac failure caused by hyperthyroidism [25]. In addition, the stimulation of FRTL-5 cells with CGS21680, an adenosine A2a-specific agonist, increased the expression of vascular endothelial growth factor (VEGF) [26]. Taken together, our results and other researchers data suggest that adenosine, via P1 receptors, could be a player in thyroid cancer progression and metastatic dissemination.
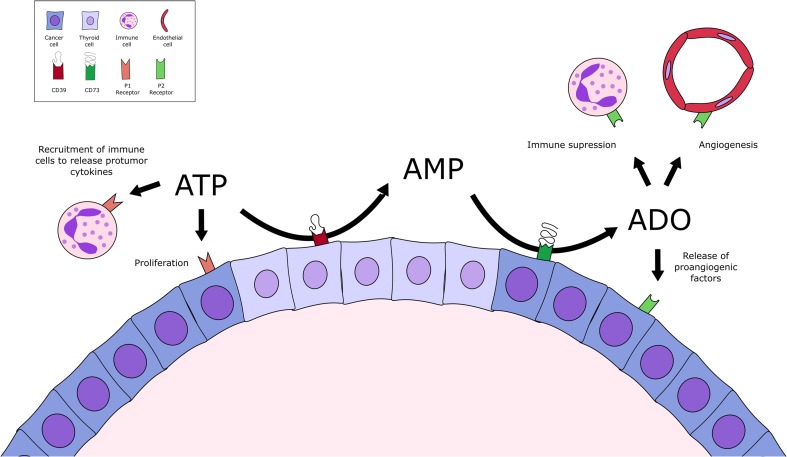
We hypothesize that within thyroid lesions, where normal and cancer follicular cells co-exist, these ‘normal’ thyroid cells hydrolyze ATP and ADP to AMP, while tumor thyroid cells hydrolyze AMP, producing adenosine (Fig. 5). Because of low ATP/ADP metabolism by cancer cells, ATP accumulation within and in surrounding tumor might sensitize P2 receptors both in tumor cells, inducing cell proliferation, as well as in immune cells and normal thyroid cells, promoting its recruitment to tumor area with consequent release of protumor cytokines. Moreover, the high ATP/ADP metabolism of normal cells might provide AMP, the substrate of CD73, highly expressed by tumor cells. Adenosine by activating P1 receptor plays an important role in angiogenesis and in immune suppression [27]. Therefore, the orchestrated extracellular adenine nucleotide metabolism by normal and cancer cells might promote differential P1/P2R sensitization on both, normal and tumor cells, generating a “proliferative” advantage to cancer cells. Since both, ATP and adenosine accumulation, are described in tumor microenvironment, we suggest that normal and tumor cells crosstalk might favor niches of ATP and/or adenosine accumulation, which might promote a favorable microenvironment for tumor progression. Thus, purinergic signaling could be considered as a potential target for thyroid cancer management/treatment in the future.
Fig. 5.
Schematic illustration summarizing nucleotide metabolism and ectonucleotidases expression profile in normal and papillary thyroid cancer (PTC) cells. Normal thyroid cells express higher levels of ENTPD1 and 2, when compared to PTC cells, leading to increased extracellular ATP hydrolysis with consequent accumulation of ADP and AMP. In the other hand, PTC cells express ENTPD3, while ENTPD1 and 2 are absent, decreasing ATP metabolism, and its accumulation in tumor microenvironment. As CD73 is highly expressed in TPC-1 and K1 cells in comparison with normal cells, AMP is immediately metabolized to adenosine. Therefore, in the thyroid tumor microenvironment, a crosstalk between normal cells, which are more efficient to hydrolyze ATP and ADP, could provide AMP to be dephosphorylated to adenosine by tumor cells. Adenosine could be involved in several pro-tumorigenic features, as immunosuppression and angiogenesis, in the tumor microenvironment
Acknowledgements
We thank Dr. Ana Luiza Silva Maia (UFRGS-HCPA, Porto Alegre, Brazil) for providing the K1 and TPC-1 cells lines, and Dr. Denise Pires de Carvalho (UFRJ, Rio de Janeiro, Brazil) for providing Nthy-1, FRTL-5 and PCCL3 cells.
Funding
APSB was supported by a post doc fellowship from CAPES/PNPD (Programa Nacional de Pós-Doutorado); EB, MRW and TWF are recipients of research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This study was supported by CNPq, Novas Terapias Portadoras de Futuro (457394/2013–7); CAPES-PROBITEC (004/2012) and PROCAD (158819); ICGEB (405231/2015–6 MCTI/CNPq-ICGEB); FIPE/HCPA (N° 15–0590); and Fundação de Amparo à Pesquisa do Rio Grande do Sul (Pronex FAPERGS/CNPq 16/2551–0000473-0).
Compliance with Ethical Standards
Conflict of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Omry-Orbach G. Risk stratification in differentiated thyroid cancer: an ongoing process. Rambam Maimonides Med J. 2016;7(1):e0003. doi: 10.5041/RMMJ.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braganhol E, Morrone FB, Bernardi A, Huppes D, Meurer L, Edelweiss MI, Lenz G, Wink MR, Robson SC, Battastini AM. Selective NTPDase2 expression modulates in vivo rat glioma growth. Cancer Sci. 2009;100(8):1434–1442. doi: 10.1111/j.1349-7006.2009.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrone FB, Oliveira DL, Gamermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198(2):211–218. doi: 10.1016/S0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 6.Buffon A, Ribeiro VB, Wink MR, Casali EA, Sarkis JJ. Nucleotide metabolizing ecto-enzymes in Walker 256 tumor cells: molecular identification, kinetic characterization and biochemical properties. Life Sci. 2007;80(10):950–958. doi: 10.1016/j.lfs.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Buffon A, Wink MR, Ribeiro BV, Casali EA, Libermann TA, Zerbini LF, Robson SC, Sarkis JJ. NTPDase and 5′ ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the Walker 256 tumor. Biochim Biophys Acta. 2007;1770(8):1259–1265. doi: 10.1016/j.bbagen.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Tamajusuku AS, Villodre ES, Paulus R, Coutinho-Silva R, Battasstini AM, Wink MR, Lenz G. Characterization of ATP-induced cell death in the GL261 mouse glioma. J Cell Biochem. 2010;109(5):983–991. doi: 10.1002/jcb.22478. [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2016;36:293–303. doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo T, Nakazawa T, Murata SI, Katoh R. Expression of CD73 and its ecto-5′-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48(5):612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka J, Ogura T, Sato H, Hatano M. Establishment and biological characterization of an in vitro human cytomegalovirus latency model. Virology. 1987;161(1):62–72. doi: 10.1016/0042-6822(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 13.Challeton C, Branea F, Schlumberger M, Gaillard N, de Vathaire F, Badie C, Antonini P, Parmentier C. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37(1):163–169. doi: 10.1016/S0360-3016(96)00449-X. [DOI] [PubMed] [Google Scholar]
- 14.Fusco A, Berlingieri MT, Di Fiore PP, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7(9):3365–3370. doi: 10.1128/MCB.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambesi-Impiombato FS, Parks LA, Coon HG. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naasani LI, Rodrigues C, de Campos RP, Beckenkamp LR, Iser IC, Bertoni AP, Wink MR. Extracellular nucleotide hydrolysis in dermal and Limbal mesenchymal stem cells: a source of adenosine production. J Cell Biochem. 2017;118:2430–2442. doi: 10.1002/jcb.25909. [DOI] [PubMed] [Google Scholar]
- 17.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Zhang Y, Lin X, Gao Y, Zhu Y. Prognositic value of CD73-adenosinergic pathway in solid tumor: a meta-analysis and systematic review. Oncotarget. 2017;8(34):57327–57336. doi: 10.18632/oncotarget.16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149(1):389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 20.Wink MR, Tamajusuku AS, Braganhol E, Casali EA, Barreto-Chaves ML, Sarkis JJ, Battastini AM. Thyroid hormone upregulates ecto-5′-nucleotidase/CD73 in C6 rat glioma cells. Mol Cell Endocrinol. 2003;205(1–2):107–114. doi: 10.1016/S0303-7207(03)00197-7. [DOI] [PubMed] [Google Scholar]
- 21.Tamajusuku AS, Carrillo-Sepulveda MA, Braganhol E, Wink MR, Sarkis JJ, Barreto-Chaves ML, Battastini AM. Activity and expression of ecto-5′-nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Mol Cell Biochem. 2006;289(1–2):65–72. doi: 10.1007/s11010-006-9148-0. [DOI] [PubMed] [Google Scholar]
- 22.Bastomsky CH, Zakarija M, McKenzie JM. Thyroid hydrolysis of cyclic AMP as influenced by thyroid gland activity. Biochim Biophys Acta. 1971;230(2):286–295. doi: 10.1016/0304-4165(71)90215-7. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, Pellegata NS, de la Chapelle A. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci U S A. 2001;98(26):15044–15049. doi: 10.1073/pnas.251547398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morello S, Petrella A, Festa M, Popolo A, Monaco M, Vuttariello E, Chiappetta G, Parente L, Pinto A. Cl-IB-MECA inhibits human thyroid cancer cell proliferation independently of A3 adenosine receptor activation. Cancer Biol Ther. 2008;7(2):278–284. doi: 10.4161/cbt.7.2.5301. [DOI] [PubMed] [Google Scholar]
- 25.Goffard JC, Jin L, Mircescu H, Van Hummelen P, Ledent C, Dumont JE, Corvilain B. Gene expression profile in thyroid of transgenic mice overexpressing the adenosine receptor 2a. Mol Endocrinol. 2004;18(1):194–213. doi: 10.1210/me.2003-0249. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Xu J, Sun N, Cai H, Ren M, Zhang J, Yu C, Wang Z, Gao L, Zhao J. The presence of adenosine A2a receptor in thyrocytes and its involvement in Graves’ IgG-induced VEGF expression. Endocrinology. 2013;154(12):4927–4938. doi: 10.1210/en.2012-2258. [DOI] [PubMed] [Google Scholar]
- 27.Zeiser R, Robson SC, Vaikunthanathan T, Dworak M, Burnstock G. Unlocking the potential of purinergic signaling in transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2016;16(10):2781–2794. doi: 10.1111/ajt.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]