Abstract
Involvement of matrix metalloproteinases (MMPs) in the pathogenesis of urothelial carcinoma elects them to be sensitive marker for clinical and prognostic implications. MMPs regulate tumor growth and invasion by inducing epithelial-to-mesenchymal transition (EMT) which is characterized by the complex reprogramming of epithelial cells and ultimately bring about major changes in the structural organization of bladder urothelium. The present study has been undertaken to evaluate the clinical relevance of MMPs in two distinct types of bladder cancer disease. Expression analysis of MMPs namely MMP-2, MMP-7, MMP-9 and EMT markers including epithelial marker, E-cadherin; mesenchymal markers, N-cadherin and Vimentin; and EMT-activating transcriptional factors (EMT-ATFs), Snail, Slug, Twist and Zeb was done in 64 cases of bladder tumor tissues [{Non-muscle invasive bladder cancer (NMIBC): 35 cases} and {Muscle invasive bladder cancer (MIBC): 29 cases}] by real-time quantitative polymerase chain reaction (RT-qPCR). Immunohistochemistry (IHC) staining was done in matched bladder tumor tissues to evaluate the protein expression and localization of E-cadherin, N-cadherin, Vimentin, Snail, and Slug. Our data showed overexpression of MMP-2, MMP-7 and MMP-9 at transcriptome level in 32.8%, 25% and 37.5% bladder tumor cases respectively. These tumor tissues were examined for higher expression of mesenchymal markers (N-cadherin and Vimentin) at mRNA and protein level and exhibited statistical association with tumor stage and tumor grade (p = 0.02, p = 0.04, Mann-Whitney test). Significant statistical correlation in tumor tissues with overexpressed MMPs has also been observed between gain of transcriptional factors and weak expression of E-cadherin with tumor stage, grade, gender, presence of hematuria and smoking history of the patients. Gene expression patterns of EMT markers in bladder tumors with overexpressed MMPs and their significant association with clinical profile validate the important role of MMPs in the pathogenesis of urothelial carcinoma of bladder (UCB). Increased expression of specific MMPs may affect several downstream EMT programs and thus may improve its diagnostic and prognostic utility in clinical setting.
Keywords: Matrix metalloproteinases, Epithelial-mesenchymal transition, Diagnosis and prognosis, Clinicopathological parameters, Urinary bladder cancer
Introduction
Urinary bladder cancer is the second most common cause of genitourinary cancer-related deaths worldwide [1]. Surgical resection, adjuvant and neo-adjuvant therapies are commonly employed strategies to fight cancer. These approaches have been successfully accepted in patients with low risk non muscle invasive cancer and locally advanced cancer. Nevertheless, patients with high risk muscle invasive cancers fail to achieve complete pathologic response rates following various treatment modalities [2]. Owing to heterogeneous clinical outcomes and lack of novel strategies that can overcome therapeutic resistance, intense investigation of tumors by their molecular profile and their functions in tumor progression is required.
Protein degrading enzymes/ proteases are extremely important signaling molecules and their dysregulated protease activity leads to not only cancer pathologies but also osteoporosis; cardiovascular and inflammatory diseases; and neurological disorders [3]. Matrix metalloproteinases (MMPs)/ proteases consist of Zn2+ dependent endopeptidases, participate in the degradation of extracellular matrix (ECM) and thereby create disturbances in ECM regulation which is a prerequisite for unregulated tumor growth, local invasion and metastasis [4]. Expression, secretion of MMP-2 by the stromal cells like fibroblasts and its binding to specific docking sites of nearby tumor cells mark cancer cells for ECM degradation and enable invasive bladder tumor growth [5]. Despite series of encouraging preclinical trials in different cancer models, several small-molecule drugs targeting proteases have been found to be inefficient against advanced stage tumors [3].
Higher expression of MMPs with ECM-degrading functions is detected in bladder cancer tissue and is associated with high risk of disease progression and recurrence [6]. Their involvement in early stages of bladder carcinogenesis by regulating differentiation, angiogenesis, apoptosis and tumor growth has been reported [7]. Increased proteolytic activity of MMPs including MMP-7 and MMP-9, classified as matrilysins and gelatinases respectively, cleaves, liberates biologically active basement membrane fragments including extracellular domain of E-cadherin from the cell surface, impairs epithelial cell adhesion, reorganizes surrounding ECM, enhances cell motility and promotes tumor cell invasion via aberrant induction of Epithelial-to-mesenchymal transition (EMT) [8–10].
EMT is characterized by the complex reprogramming of epithelial cells which includes loss of epithelial cell properties, gain of cell motility and mesenchymal phenotype, increased invasion of tumor cells to surrounding tissues and their metastatic dissemination [11]. Loss of epithelial marker (E-cadherin); gain of mesenchymal markers including N-cadherin and Vimentin; and high expression of EMT-activating transcription factors (EMT-ATFs) define the EMT state of tumors of epithelial origin including urinary bladder cancer [12, 13]. Involvement of EMT and Neutrophil gelatinase-associated lipocalin (NGAL)/ MMP-9 complex as a part of tumor microenvironment has been examined in bladder cancer development and its progression. Identification of conserved, 23–25 nucleotides long, non-coding specific microRNAs (miRNAs) associated with EMT pathway and NGAL/ MMP-9 complex to predict the development of bladder cancer at early stage further validates its important role as diagnostic markers in bladder cancer [14, 15]. In extension to this, another study by Rao et al. examines an alternative new therapeutic approach which employs the use of either estrogen receptor β (ERβ)-siRNA, ERβ antagonist PHTPP, or CCR2 antagonist to interrupt ERβ induced CCL2/CCR2/EMT/MMP-9 pathway in order to reverse the mast cell-enhanced bladder cancer cells’ invasion [16].
Recent studies document MMPs as the biomarkers for predicting outcomes of bladder cancer. Meta-analysis has been done to evaluate prognostic significance of high MMPs expression; nevertheless, correlation between bladder tumor progression/ metastasis and overexpression of MMPs is still debatable [17]. Enhanced migratory and invasive capacity of MMPs and their involvement in neoplastic transformation, probably through aberrant downstream activation of EMT, makes them an ideal candidate to evaluate their diagnostic and prognostic relevance in two distinct types of bladder cancer disease [18]. The objective of the current study is to examine the downstream activation of EMT programs as a consequence of increased expression of specific MMPs and their clinical utility in early stages of bladder carcinogenesis and tumor invasion.
Materials and Methods
Patients and Tissue Specimens
A total of 64 frozen tissue samples or formalin fixed and paraffin embedded tissue samples of urothelial carcinoma of bladder (UCB) were obtained from Department of Urology, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, India during 2014–2016. Ethical clearance was obtained from Bioethics Cell, Institutional Ethics Committee (IEC), SGPGIMS, Lucknow (Reference no. 2014–166-CP-80) and research was performed on humans in compliance with the Helsinki Declaration. All bladder tumor tissue specimens were of TCC (Transitional cell carcinoma) and included 35 NMIBC (stage pTa-pT1) and 29 MIBC (stage pT2-pT4) types. Cases included tumor samples from patients who underwent transurethral resection of the bladder (TURB, 54 cases) or radical cystectomy (10 cases). Ten tissue specimens as normal tissues were collected from patients with BPH (benign prostate hyperplasia) who had undergone cold cup biopsy of normal bladder mucosa. A small fraction of the excised tumor or normal tissue was surgically resected, immersed in RNA later solution (Ambion; Thermo Fisher Scientific Life Sciences, Waltham, MA), and stored at -80 °C until further use. The histological diagnoses of the tumor tissues were done independently by pathologists according to WHO-ISUP, 2004 classification criteria [19]. The clinicopathological profiles of the patients included in the study are summarized in Table 1.
Table 1.
Clinicopathological profile of the patients with urinary bladder cancer
| Clinical variables | Number:n (%) |
|---|---|
| Total no. of patients | 64(100) |
| Age (years) median, range | 60, 38–82 |
| ➢ n < 60 | 26(40.6) |
| ➢ n ≥ 60 | 38(59.4) |
| ➢ Males | 60(93.75) |
| ➢Females | 4(6.25) |
| Hematuria | |
| ➢Present | 53(82.8) |
| ➢Absent | 11(17.2) |
| ➢No information | 0(0) |
| Smoking status | |
| ➢Smokers | 33(51.5) |
| ➢Non-smokers | 19(29.7) |
| ➢No information | 12(18.8) |
| Tumor type | |
| ➢UCC | 64(100) |
| ➢SCC/ others | 0(0) |
| Tumor grade (Total) | |
| ➢Low | 18(28.2) |
| ➢High | 46(71.8) |
| Tumor stage | |
| ➢Ta-T1 (Low) | 35(54.6) |
| •Low grade | 18(51.4) |
| •High grade | 17(48.6) |
| ➢T2-T4 (High) | 29(45.4) |
| •Low grade | 0(0) |
| •High grade | 29(100) |
| ➢N0 | 57 (89.1) |
| ➢N1 | 7 (10.9) |
| Metastasis | |
| ➢M0 | 57 (89.1) |
| ➢M1 | 7 (10.9) |
| Recurrence | |
| ➢Primer | 34(53.1) |
| ➢Recurrent | Total:30(46.9) |
| ➢Recurrent low stage; low grade | 5 (16.67) |
| ➢Recurrent low stage; high grade | 10 (33.33) |
| ➢Recurrent high stage; high grade | 15 (50) |
| Surgical procedure | |
| ➢TURB | 54(84.4) |
| ➢Radical cystectomy | 10(15.6) |
RNA Extraction and Real Time –Quantitative Polymerase Chain Reaction (RT-PCR) for Quantitative Evaluation of MMP and EMT Markers’ Expression at Transcriptome Level
Total cellular RNA extraction was done from frozen tumor and control tissue samples using 1 ml Trizol reagent (Ambion; Thermo Fisher Scientific Life Sciences) and quantified by measuring the absorbance at 260 nm and purity at absorbance of 260/280.cDNA synthesis was done with 1 μg of RNA using the verso cDNA synthesis kit (Thermo Fisher Scientific Life Sciences) according to the manufacturer’s instructions. Subsequently, 10 ng of cDNA was subjected to real-time quantitative polymerase chain reaction (RT-qPCR) in 5 μl reaction mix. RT-qPCR was performed in a Quant Studio 7 Flex Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific Life Sciences) using Light Cycler 480 Syber green I master mix (TaKaRa, Clontech) with gene-specific primers mentioned in Table 2. The cycling conditions for RT-qPCR were initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 1 min, and final extension at 72 °C for 10 min. The relative levels of mRNA expression were quantified as ∆Ct values,by comparing it with the mean Ct values of β-actin taken as reference/endogenous control gene (∆Ct = Cttarget- Ctreference)to normalize the possible differences in the amount of total RNA. Furthermore, ∆∆Ct for the target gene in tumor sample was calculated using ∆Ct of normal tissue (∆∆Ct = ∆Cttumor-∆Ctnormal). Average fold change expression was then calculated by 2^-∆∆Ct. Ct values against all the genes were analyzed in triplicates.
Table 2.
Primer sequences of the genes amplified for expression analysis
| Name of marker | Primer sequences (Forward and Reverse) |
|---|---|
| E-cadherin | forward: 5’ACGTCGTAATCACCACACTGA3’ reverse:5’TTCGTCACTGCTACGTGTAGAA3’ |
| N-cadherin | forward:5’ACAGTGGCCACCTACAAAGG3’ reverse:5’CCGAGATGGGGTTGATAATG3’ |
| Vimentin | forward:5’CTTCGCCAACTACATCGACA3’ reverse:5’GCTTCAACGGCAAAGTTCTC3’ |
| Slug | forward:5’TCGGACCCACACATTACC3’ reverse:5’CCGAGATGGGGTTGATAATG3’ |
| Snail | forward:5’TCGTCCTTCTCCTCTACTTC3’ reverse:5’TTCCTTGTTGCAGTATTTGC3’ |
| Zeb | forward:5’CTGAAGAGGACCAGAGGCAG3’ reverse:5’CCCAGAACTGCGTCACATGTC3’ |
| Twist | forward: 5’AGCTGAGCAAGATTCAGACCCTC3’ reverse: 5’CCGTCTGGGAATCACTGT3’ |
| MMP-2 | forward:5’GGATGATGCCTTTGCTCG3’ reverse:5’ATAGGATGTGCCCTGGAA3’ |
| MMP-7 | forward:5’GTGGTCACCTACAGGATCGT3’ reverse:5’ACCATCCGTCCAGCGTTCAT3’ |
| MMP-9 | forward: 5’AGGACGGCAATGCTGATC3’ reverse: 5’TCGTAGTTGGCGGTGGTG 3’ |
| β-actin | forward:5’GGACTTCGAGCAAGAGATGG3’ reverse:5’AGCACTGTGTTGGCGTACAG3’ |
Immunohistochemical (IHC) Staining
The immunohistochemical staining for EMT markers was performed using Ultra Vision Quanto detection system, horseradish peroxidase diaminobenzidine (Thermo Fisher Scientific Life Sciences), according to the manufacturer’s instructions.Primary antibodies used in the study include antibodies to E-cadherin (EP700Y; 246R-15; Cell Marque, Rocklin, CA) (1:100 dilution), N-cadherin (6G11; M3613; Dako, Carpinteria, CA) (1:50 dilution), Vimentin (SP20; 347R-15; Cell Marque) (1:50 dilution), Snail (Ab180714; Abcam, Cambridge, CA) (1:100 dilution), and Slug (H-140; sc-15,391; Santa Cruz Biotechnology, Santa Cruz, CA) (1:50 dilution). Three micron thick formalin fixed and paraffin embedded tumor tissue was taken on poly-L-lysine coated glass slides and fixed at 70 °C for 3 h. The tissue sections were dewaxed using xylene and then washed with 100%, 70%, 50%, and 20% alcohol for 5 min each followed by washing in running water for 5 min. The slides were then subjected to antigen retrieval with citrate buffer [low pH (citrate) buffer or high pH (Tris ethylene diamine tetra acetic acid) buffer] in antigen retrieval system for 2 cycles of 15 min at 98 °C depending on the type of antibody. Sections were washed after blocking the activity of endogenous peroxidase by 3% hydrogen peroxide in methanol for 30 min. Following the treatment of sections with 2–3 drops of skimmed milk, the tissue sections were incubated with the primary antibodies for either 1 h at room temperature or refrigerated temperature for overnight and then incubated with a secondary antibody (amplifier) for 10 min at room temperature. The signals were detected by adding hydrogen peroxide and diaminobenzidine as substrate and chromogen, respectively, followed by counterstaining with Mayers’ hematoxylin for 1 min. The slides were mounted in dibutyl phthalate xylene (DPX) medium and visualized for the appearance of brown reaction product. Evaluation of immunohistochemical markers for their localization and expression was done using a Nikon Eclipse 50i light microscope (Nikon, Tokyo, Japan). A total of 1000 cells were counted in consecutive fields at a magnification of 400X. Immunoreactivity of the tumor tissue was analyzed as strong, weak, novel, or no expression. Marker protein was studied for their membranous, nuclear, or cytoplasmic localization. The staining intensity was also assessed in the scale of 0 as no expression, 1 as weak expression, 2 as moderate expression and 3 as strong expression. The overall IHC score was calculated by multiplying the % positive cells and its staining intensity.
IHC score of 201–300 was taken as strong expression and below 200 was taken as weak expression in the case of E-cadherin, Snail and Slug. However, IHC score of 0 was taken as no expression and IHC score of >1 was considered as novel expression in case of N-cadherin and Vimentin.
Statistical Tests
Quantitative expression of MMPs and EMT markers at transcriptome level was statistically correlated with pathological features including tumor stage, tumor grade, recurrence, clinical features like smoking and presence of hematuria and non-clinical parameters including gender and age using non-parametric Mann-Whitney U test and one sample t-test. Immunohistochemical evaluation of EMT marker expression and their correlation studies with clinicopathological profile was done using Fisher’s exact probability test or chi square test (SPSS version 16.0 software, Microsoft, Redmond, WA). Quantitative data are presented as the mean ± standard error of the mean. The p values less than 0.05 were considered statistically significant.
Results
Clinicopathological Characteristics
Total of 64 patients with UCB and a cohort of 10 cases of normal bladder were included in the current study (Table 1). Among 64 patients, 93.7% (60/64,) were male patients. A total of 82.8% patients (53/64) were presented with gross total hematuria as the primary clinical symptom during diagnosis. Smoking history was known in 52 patients; out of which 63.4% (33/52) were smokers and 36.5% (19/52) were non-smokers. Of the total patients involved in study, 84.4% (54/64) underwent TURB procedure while 15.6% (10/64) underwent radical cystectomy due to high-risk tumor progression. Histological tumor staging and grading was determined on the biopsies or resected tumor tissues according to WHO-ISUP, 2004 criterion. Of 64 cases, 54.6% (35/64) were classified as NMIBC (stage pTa-pT1) and 45.4% (29/64) were staged as MIBC (stage pT2-pT4). Further, 28.1% (18/64) tumors were classified as low grade and 71.9% (46/64) were presented as high grade. Among 64 cases studied, 47% (30/64) exhibited recurrence over a mean period of fourteen months after initial diagnosis. Metastasis and lymph node involvement was observed in 10.9% (7/64) patients over a mean period of 42 months since their initial diagnosis of bladder tumor.
Expression Analysis of MMPs in Tumor Tissues of Urothelial Carcinoma and their Involvement with EMT Cascade
The mRNA expression of MMPs including, MMP-2, MMP-7 and MMP-9 and EMT markers including epithelial marker, E-cadherin; mesenchymal markers, N-cadherin and Vimentin; and EMT-activating transcriptional factors, Snail, Slug, Twist and Zeb was examined in 64 bladder tumor specimens. Ten normal bladder tissue specimens were also examined for gene expression by RT-qPCR and treated as control to calculate the fold change in expression of the given markers in tumor tissues (Fig. 1a–f). IHC was done to check the protein expression and localization of E-cadherin, N-cadherin and Vimentin, Snail and Slug in NMIBC and MIBC bladder tumor tissues. Focal loss of E-cadherin expression in membranous regions, novel expression of membranous N-cadherin and Vimentin in epithelial region, and strong nuclear expression of Snail and Slug in tumor areas of NMIBC and MIBC were examined. Tumors were examined for reduced and higher expression of MMPs and other markers, compared to control bladder tissue. Expression results were then analyzed with non-clinical, clinical and pathological parameters to examine the oncological association of MMPs through regulating EMT in bladder cancer pathogenesis.
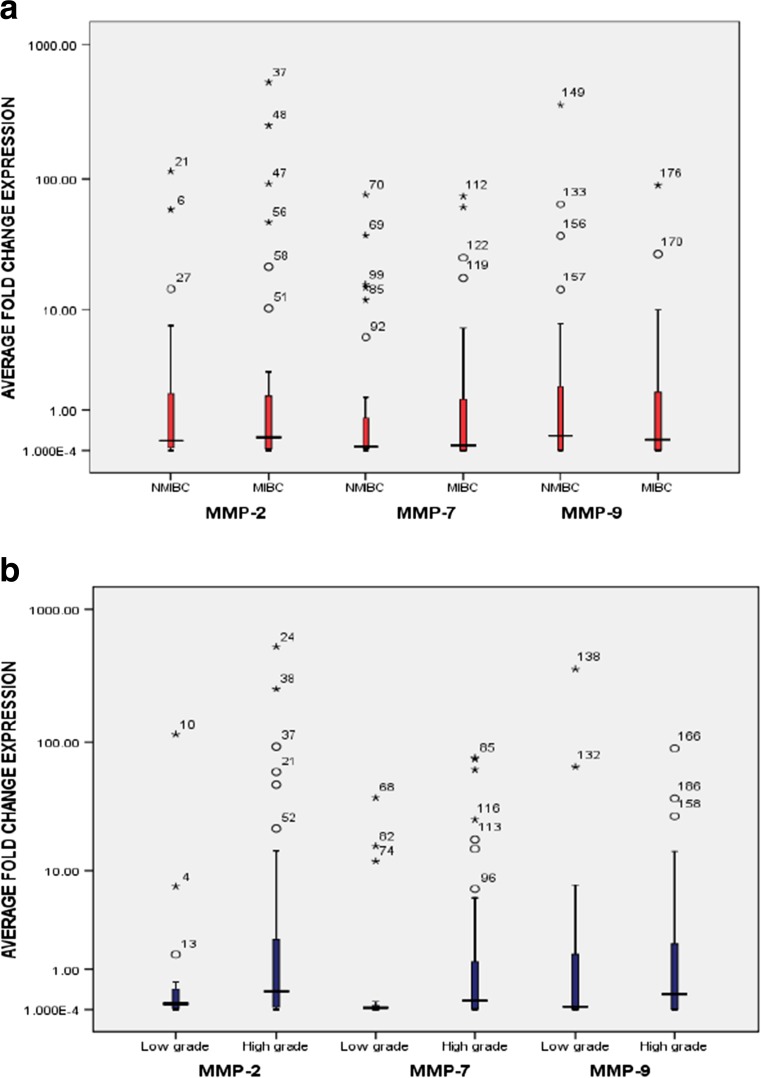
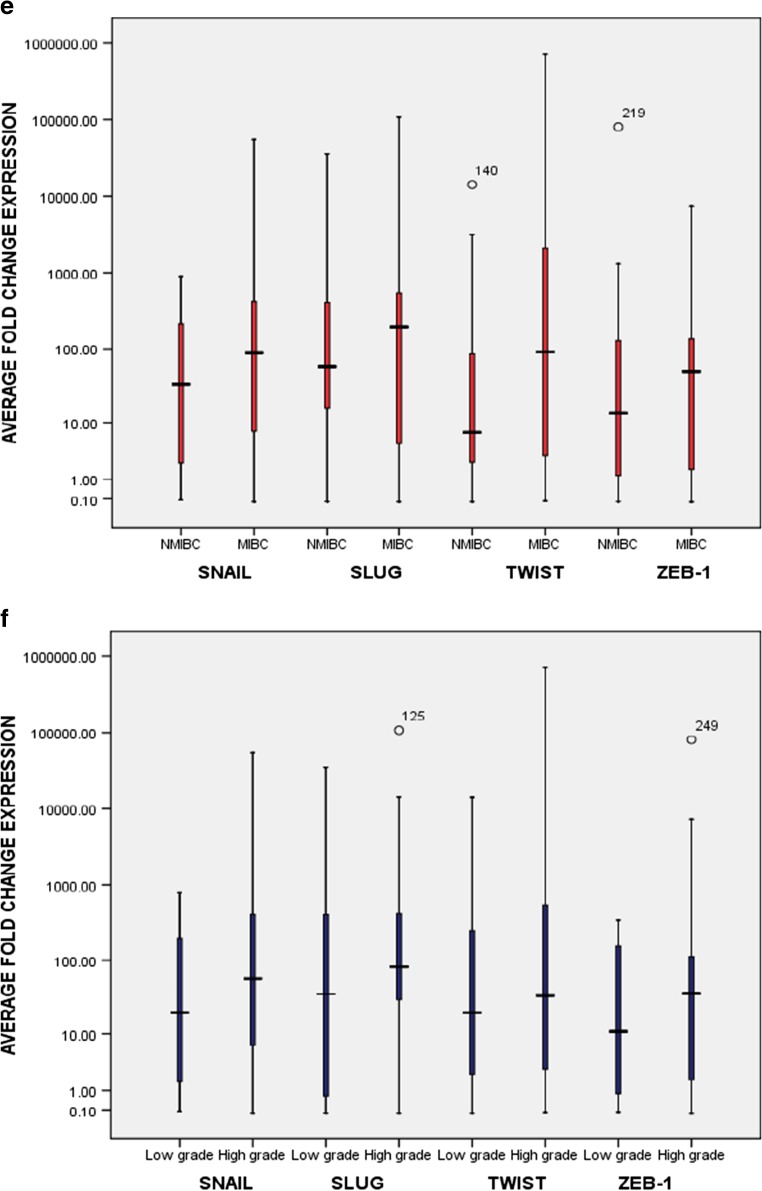
Fig. 1.
Graphical box-plot representation. Expression profile of MMP-2, MMP-7, MMP-9 at transcriptome level in 64 bladder tumor specimens: a with tumor stage [34.5% (10/29), 24.1% (7/29), 34.5% (10/29) MIBC showed high expression of MMP-2, MMP-7, and MMP-9 respectively]; and (b) with tumor grade [34.8% (16/46), 26.1% (12/46), 39.1% (18/46) high grade tumors showed high expression of MMP-2, MMP-7, and MMP-9 respectively]. Expression profile of EMT marker proteins (E-cadherin, N-cadherin and Vimentin) in 64 bladder tumor specimens at transcriptome level: c with tumor stage [24.1% (7/29); 75.8% (22/29); and 72.4%(21/29) MIBC exhibited weak expression of E-cadherin, strong expression of N-cadherin and Vimentin respectively]; (d) with tumor grade [19.6% (9/46); 82.6% (38/46); and 69.6% (32/46) high grade bladder tumors showed weak expression of E-cadherin, strong expression of N-cadherin and Vimentin respectively]. Expression profile of EMT transcription factors (Zeb, Twist, Snail and Slug) at transcriptome level: e with tumor stage [79.3% (23/29), 72.4% (21/29), 79.3% (23/29) and 72.4% (21/29) MIBC showed high expression of Snail, Slug, Twist and Zeb-1 respectively]; (f) with tumor grade [82.6% (38/46), 80.4%(37/46), 80.4% (37/46) and 76.1% (35/46) high grade tumors showed high expression of Snail, Slug, Twist and Zeb-1 respectively]. Expression level in tumor tissues was examined by comparing the fold change values obtained in normal urinary bladder tissues. Y axis is logarithmically scaled, box-plots represent the 25th to 75th percentile while median values are shown by horizontal lines
Clinical Relevance of MMP-2 and EMT Cascade
Among 64 cases analyzed, increased expression of MMP-2 was reported in 32.8% (21/64) tumors, out of which 85.7% (18/21) were of high grade and 47.6% (10/21) were of high stage (Fig. 1a, b). Elevated expression of MMP-2 was observed in 76.19% (16/21) hematuric patients, 61.9% (13/21) patients with positive smoking status and 52.4% (11/21) patients who developed tumor recurrence over a period of time.Among 21 cases with overexpressed MMP-2 at transcriptome level, 19% (4/21) showed weak expression of E-cadherin, 85.7% (18/21) showed increased expression of mesenchymal markers and 17/21 (80.9%) showed an increased expression of EMT-ATFs. Among tumors with overexpressed MMP-2, 16.6% (3/18) and 11.7% (2/17) metastatic tumors showed increased expression of mesenchymal markers and transcriptional factors respectively.Significant statistical correlation was observed between the expression of MMP-2 and gain of mesenchymal markers (N-cadherin + Vimentin) with low stage (p = 0.02, Mann-Whitney test). Significant association was also observed between increased MMP-2 expression and gain of EMT-ATFs (Snail + Slug + Twist + Zeb) with the presence of hematuria (p = 0.04, Mann-Whitney test) (Table 3). IHC results in tumors with higher MMP-2 levels showed weak expression of E-cadherin in 23.8% (5/21) tumors, however, 19% (4/21) showed novel expression of mesenchymal markersand 33.3% (7/21) showed an increased expression of Snail and Slug (Table 3, Figs. 2 and 3). Statistical relevance was determined for loss of E-cadherin with tumor high stage (p = 0.016, One sample T-test), tumor high grade (p = 0.016, One sample T-test) and presence of hematuria (p = 0.04, Mann-Whitney test). Tumors showing increased expression of EMT-ATFs (Snail and Slug) were assessed for statistical significance with tumor high stage (p = 0.04, Mann-Whitney test) and smoking (p = 0.001, One sample T-test) (Table 3).
Table 3.
Expression analysis of EMT marker genes in tumors exhibiting increased expression of MMP-2 at transcriptome level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | Reduced expression than normal bladder tissue (n = 4/21) | Increased expression than normal bladder tissue (n = 18/21) | Increased expression than normal bladder tissue (n = 17/21) | |||
| Parameters | Percentage | p | percentage | p | percentage | p |
| Age 58 (38–78) in years | 0.23* | 0.5* | 0.14* | |||
| < 60 | 1/4(25%) | 8/18(44.4%) | 9/17(52.9%) | |||
| ≥ 60 | 3/4(75%) | 10/18(55.6%) | 8/17(47.1%) | |||
| Gender | 0.105** | 0.22* | 0.02** | |||
| Male | 4/4(100%) | 17/18(94.4%) | 17/17(100%) | |||
| female | 0/4(0%) | 1/18(5.6%) | 0/17(0%) | |||
| Tumor stage | 0.66* | 0.02* | 0.16* | |||
| NMIBC | 2/4(50%) | 11/18(61.1%) | 10/17(58.8%) | |||
| MIBC | 2/4(50%) | 7/18(38.9%) | 7/17(41.2%) | |||
| Tumor grade | 0.1** | 0.22* | 0.53* | |||
| Low grade | 0/4(0%) | 3/18(16.6%) | 2/17(11.7%) | |||
| High grade | 4/4(100%) | 15/18(83.4%) | 15/17(88.3%) | |||
| Primer tumor | 1/4(25%) | 0.5* | 9/18(50%) | 0.25* | 6/17(35.3%) | 0.4* |
| Recurrent tumor | 3/4(75%) | 9/18(50%) | 11/17(64.7%) | |||
| Smoking | 0.1** | 0.58* | 0.3* | |||
| Yes | 4/4(100%) | 10/18(55.6%) | 10/17(58.8%) | |||
| No | 0/4(0%) | 5/18(27.7%) | 4/17(23.5%) | |||
| Unknown | 0/4(0%) | 3/18(16.7%) | 3/17(17.7%) | |||
| Hematuria | 0.5* | 0.53* | 0.04* | |||
| Present | 3/4(75%) | 14/18(77.7%) | 14/17(82.3%) | |||
| Absent | 1/4(25%) | 4/18(22.3%) | 3/17(17.7%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma
**p values in bold are significant
Fig. 2.
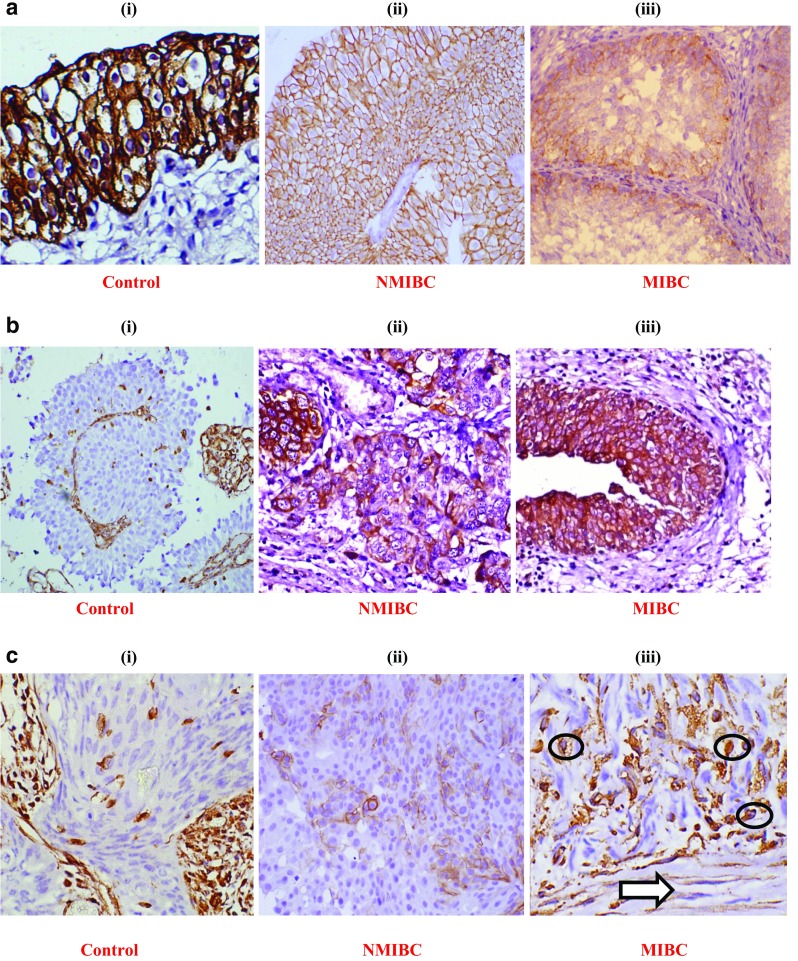
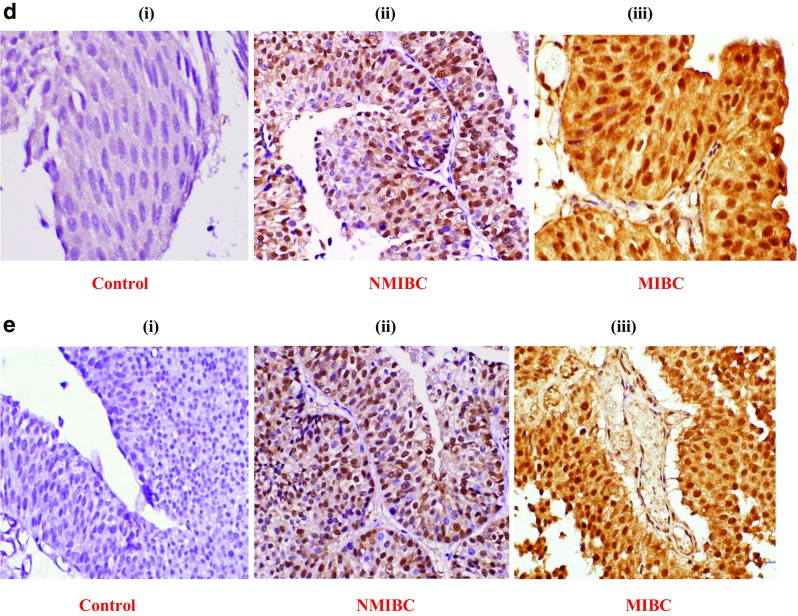
Representative pictures of immunohistochemical (IHC) staining: (a) E-cadherin expression: a (i) normal urothelium, a (ii) NMIBC (low grade Low stage) showing 100% positivity, a (iii) focal loss of membranous expression in MIBC (high grade and high stage tumor) showing <100% positivity; (b) N-cadherin expression: b (i) internal control showing stromal positivity and negative epithelial tumor region; b (ii) novel membranous expression in NMIBC, b (iii) membranous expression in MIBC showing >50% positivity; (c) Vimentin expression: c (i) internal control showing stromal positivity and negative epithelial tumor region, c (ii) novel membranous expression in NMIBC, c (iii) membranous expression in MIBC showing distorted spindle shaped tumor cells (circled) infiltrating towards muscle layer (arrow used); (d) Snail expression: d (i) negative control, d (ii) 80% tumor cells showing nuclear expression in NMIBC, d (iii) tumor cells showing 100% positivity with nuclear and cytoplasmic expression in MIBC (only nuclear expression accounted for positive expression); (e) Slug expression: e (i) negative control, e (ii) tumor cells showing >50% positivity with nuclear and cytoplasmic expression in NMIBC, e (iii) tumor cells showing 100% positivity with nuclear and cytoplasmic expression of in MIBC (only nuclear expression accounted for positive expression). Images were captured at 400X magnification. NMIBC: non muscle invasive bladder cancer; MIBC: muscle invasive bladder cancer
Fig. 3.
Immunohistochemical (IHC) expression analysis of EMT marker genes evaluated for increased expression of MMP-2, MMP-7 and MMP-9 in bladder tumors of NMIBC and MIBC type. (a) E-cadherin, an epithelial marker protein analysed for weak or strong expression; (b) N-cadherin and Vimentin, mesenchymal marker proteins analysed for novel or no expression; (c) Snail and Slug, EMT-ATFs, analysed for strong or weak expression. NMIBC: non muscle invasive bladder cancer; MIBC: muscle invasive bladder cancer; EMT: epithelial-to-mesenchymal transition; EMT-ATFs: epithelial-to--mesenchymal transition-activating transcription factors
Clinical Relevance of MMP-7 and EMT Cascade
Higher expression of MMP-7 at transcriptome level was reported in 25% (16/64) bladder tumor cases, out of which 81.25% (13/16) were identified as high grade and 43.75% (7/16) were examined as high stage (Fig. 1a, b). Increased expression of MMP-7 has been found to be correlated with tumor recurrence [43.8%; (7/16)]; hematuria [100%; (16/16)] and smoking status [62.5%; 10/16)]. Out of 16 cases with increased expression of MMP-7, 25% (4/16) showed weak expression of E-cadherin while 87.5% (14/16) cases exhibited increased expression of mesenchymal markers (N-cadherin + Vimentin) and EMT-ATFs. Among tumors with higher levels of MMP-7 at transcriptome level, 14.2% (2/14) and 7.1% (1/14) metastatic tumors exhibited increased expression of mesenchymal markers and transcriptional factors respectively.Significant statistical association has been found between the expression of MMP-7 and gain of mesenchymal markers with tumor low stage (p = 0.02, Mann-Whitney test) and tumor high grade (p = 0.02, Mann-Whitney test) (Table 4).
Table 4.
Expression analysis of EMT marker genes in tumors exhibiting increased expression of MMP-7 at transcriptome level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | Reduced expression than normal bladder tissue (n = 4/16) | Increased expression than normal bladder tissue (n = 14/16) | Increased expression than normal bladder tissue (n = 14/16) | |||
| parameters | percentage | p | percentage | p | percentage | p |
| AGE 58(38–78) in years | 0.689* | 0.66* | 0.03* | |||
| < 60 | 1/4(25%) | 6/14(42.9%) | 7/14(50%) | |||
| ≥ 60 | 3/4(75%) | 8/14(57.1%) | 7/14(50%) | |||
| Gender | 0.105** | 0.216** | 0.133** | |||
| Male | 4/4(100%) | 14/14(100%) | 14/14(100%) | |||
| Female | 0/4(0%) | 0/14(0%) | 0/14(0%) | |||
| Tumor stage | 0.27* | 0.02* | 0.05* | |||
| NMIBC | 2/4(50%) | 9/14(64.3%) | 8/14(57.1%) | |||
| MIBC | 2/4(50%) | 5/14(35.7%) | 6/14(42.9%) | |||
| Tumor grade | 0.105** | 0.02* | 0.06* | |||
| Low grade | 0/4(0%) | 3/14(21.4%) | 2/14(14.3%) | |||
| High grade | 4/4(100%) | 11/14(78.6%) | 12/14(85.7%) | |||
| Primer tumor | 0/4(0%) | 0.105** | 9/14(64.3%) | 0.274* | 7/14(50%) | 0.56* |
| Recurrent tumor | 4/4(100%) | 5/14(35.7%) | 7/14(50%) | |||
| Smoking | 0.357* | 0.5* | 0.37* | |||
| Yes | 3/4(75%) | 8/14(57.1%) | 9/14(64.3%) | |||
| No | 1/4(25%) | 4/14(28.6%) | 3/14(21.4%) | |||
| Unknown | 0/4(0%) | 2/14(14.3%) | 2/14(14.3%) | |||
| Hematuria | 0.105** | 0.216** | 0.13** | |||
| Present | 4/4(100%) | 14/14(100%) | 14/14(100%) | |||
| Absent | 0/4(0%) | 0/14(0%) | 0/14(0%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma
**p values in bold are significant
Immunohistochemical analysis revealed weak expression of E-cadherin in 18.8% (3/16) cases, novel expression of mesenchymal markers (N-cadherin + Vimentin) in 18.8% (3/16) cases while upregulated expression of EMT-ATFs (Snail + Slug) in 31.2% (5/16) tumors (Figs. 2 and 3). These tumor tissues were examined for statistical significance for gain of EMT-ATFs with tumor high grade (p = 0.001, One sample T-test), smoking (p = 0.001, Mann-Whitney test) and presence of hematuria (p = 0.001, One sample T-test) (Table 7).
Table 7.
Immunohistochemical (IHC) analysis of EMT marker genes evaluated for increased expression of MMP-7 at protein level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | IHC score ≤ 200(n = 3/16) | IHC score > 200(n = 3/16) | IHC score > 200(n = 5/16) | |||
| parameters | percentage | P | percentage | P | Percentage | P |
| Age 58(38–78) | 1.00* | 0.22* | 0.55* | |||
| < 60 | 2/3(66.7%) | 1/3(33.3%) | 3/5(60%) | |||
| ≥ 60 | 1/3(33.3%) | 2/3(66.7%) | 2/5(40%) | |||
| Gender | 1.00* | 0.18* | 0.001** | |||
| Male | 3/3(100%) | 3/3(100%) | 5/5(100%) | |||
| Female | 0/3(0%) | 0/3(0%) | 0/5(0%) | |||
| Tumor stage | 1.00* | 0.22* | 0.76* | |||
| NMIBC | 3/3(100%) | 2/3(66.7%) | 2/5(40%) | |||
| MIBC | 0/3(0%) | 1/3(33.3%) | 3/5(60%) | |||
| Tumor grade | 1.00* | 0.22* | 0.001** | |||
| Low grade | 3/3(100%) | 2/3(66.7%) | 5/5(100%) | |||
| High grade | 0/3(0%) | 1/3(33.3%) | (0%) | |||
| Primer tumor | 1/3(33.3%) | 1.00* | 3/3(100%) | 0.18* | 3/5(60%) | 0.76* |
| Recurrent tumor | 2/3(66.7%) | 0/3(0%) | 2/5(40%) | |||
| Smoking | 1.00* | 0.22* | 0.001* | |||
| Yes | 2/3(66.7%) | 1/3(33.3%) | 4/5(80%) | |||
| No | 1/3(33.4%) | 2/3(66.7%) | 0/5(0%) | |||
| Unknown | 0/3(0%) | 0/3(0%) | 1/5(20%) | |||
| Hematuria | 1.00* | 0.18* | 0.001** | |||
| Present | 3/3(100%) | 3/3(100%) | 5/5(100%) | |||
| Absent | 0/3(0%) | 0/3(0%) | 0/5(0%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma; IHC- Immunohistochemical score calculated as the product of percent positive cells and staining intensity
**p values in bold are significant
Clinical Relevance of MMP-9 and EMT Cascade
Higher expression of MMP-9 through RT-qPCR at transcriptome level was reported in 37.5% (24/64) cases, out of which 75% (18/24) were of high grade and 41.7% (10/24) were examined as high stage (Fig. 1 a and b). Higher expression levels of MMP-9 have been examined for its association with presence of hematuria [75% (18/24)]; smoking status [58.3% (14/24)] and tumor recurrence [45.8% (11/24)]. Among 24 cases with upregulated expression of MMP-9, 20.8% (5/24) showed weak expression of E-cadherin while 83.3% (20/24) and 87.5% (21/24) exhibited increased expression of mesenchymal markers (N-cadherin + Vimentin) and EMT-ATFs(Snail + Slug + Twist + Zeb) respectively. Statistical relevance has been found between the expression of MMP-9 and loss of epithelial marker with tumor high grade (p = 0.04, One sample T-test). Gain of mesenchymal markers and increased expression of MMP-9 showed significant correlation with tumor low stage (p = 0.02, Mann-Whitney test) and gain of EMT-ATFs with tumor high grade (p = 0.04, Mann-Whitney test) (Table 5). Among tumors with higher levels of MMP-9, 15% (3/20) and 14.2% (3/21) metastatic tumors showed increased expression of mesenchymal markers and transcriptional factors respectively.
Table 5.
Expression analysis of EMT marker genes in tumors exhibiting increased expression of MMP-9 at transcriptome level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | Reduced expression than normal bladder tissue (n = 5/24) | Increased expression than normal bladder tissue (n = 20/24) | Increased expression than normal bladder tissue (n = 21/24) | |||
| parameters | percentage | P | percentage | P | percentage | P |
| AGE 58(38–78) | 0.48* | 0.25* | 0.23* | |||
| < 60 | 1/5(20%) | 10/20(50%) | 11/21(52.3%) | |||
| ≥ 60 | 4/5(80%) | 10/20(50%) | 10/21(47.7%) | |||
| Gender | 0.04** | 0.9* | 0.18* | |||
| Male | 5/5(100%) | 18/20(90%) | 19/21(90.5%) | |||
| Female | 0/5(0%) | 2/20(10%) | 2/21(9.5%) | |||
| Tumor stage | 0.24* | 0.02* | 0.12* | |||
| NMIBC | 2/5(40%) | 14/20(70%) | 13/21(61.9%) | |||
| MIBC | 3/5(60%) | 6/20(30%) | 8/21(38.1%) | |||
| Tumorgrade | 0.04** | 0.09* | 0.04* | |||
| Low grade | 0/5(0%) | 6/20(30%) | 5/21(23.8%) | |||
| High grade | 5/5(100%) | 14/20(70%) | 16/21(76.2%) | |||
| Primer tumor | 2/5(40%) | 0.56* | 11/20(55%) | 0.76* | 10/21(47.7%) | 0.36* |
| Recurrent tumor | 3/5(60%) | 9/20(45%) | 11/21(52.3%) | |||
| Smoking | 0.15* | 0.9* | 0.9* | |||
| Yes | 4/5(80%) | 11/20(55%) | 12/21(57.1%) | |||
| No | 1/5(20%) | 7/20(35%) | 7/21(33.3%) | |||
| Unknown | 0/5(0%) | 2/20(10%) | 2/21(9.6%) | |||
| Hematuria | 0.48 | 0.45* | 0.74* | |||
| Present | 4/5(80%) | 15/20(75%) | 16/21(76.2%) | |||
| Absent | 1/5(20%) | 5/20(25%) | 5/21(23.8%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma
**p values in bold are significant
Immunohistochemical analysis was done in 24 cases which were observed for increased expression of MMP-9. IHC analysis in 24 cases with higher levels of MMP-9 revealed focal loss of membranous E-cadherin in 25% (6/24) cases, while 16.7% (4/24) and 29.2% (7/24) cases showed novel membranous expression of mesenchymal markers (N-cadherin + Vimentin)and nuclear expression of EMT-ATFs (Snail + Slug) respectively in invasive front of budding tumor areas (Figs. 2 and 3). These tumor cases were statistically analyzed for loss of epithelial marker with presence of hematuria (p = 0.04, Mann Whitney test) (Tables 6, 7 and 8). Tumors exhibiting lower expression of MMPs were examined for the expression of EMT markers at both transcriptome and protein level to check their clinical relevance. Lower expression of MMP-2, MMP-7 and MMP-9 was examined in 67% (43/64), 75% (48/64) and 62.5% (40/64) tumors respectively. Tumors with lower MMP-2 levels showed focal loss of E-cadherin in [11.6% (5/43) at transcriptome level; 13.9% (6/43) at protein level]; increased expression of mesenchymal markers in [95.3% (41/43) at transcriptome level; 32.5% (14/43) at protein level]; and increased expression of EMT-ATFs in [100% (43/43) at transcriptome level; 44.1% (19/43) at protein level]. Tumors exhibiting lower levels of MMP-7 revealed focal loss of E-cadherin in [10.4% (5/48) at transcriptome level; 14.6% (7/48) at protein level]; increased expression of mesenchymal markers in [93.7% (45/48) at transcriptome level; 29.0% (14/48) at protein level]; and increased expression of EMT-ATFs in [95.8% (46/48) at transcriptome level; 43.7% (21/48) at protein level]. Tumors analysed for lower MMP-9 levels showed weak E-cadherin in [10% (4/40) at transcriptome level; 7.5% (3/40) at protein level]; increased expression of mesenchymal markers in [97.5% (39/40) at transcriptome level; 32.5% (13/40) at protein level]; and increased expression of EMT-ATFs in [97.5% (39/40) at transcriptome level; 47.5% (19/40) at protein level]. Despite the fact that tumors with downregulated levels of MMP-2, MMP-7 and MMP-9 exhibited focal loss of epithelial marker, gain of mesenchymal and EMT-ATFs in large number of cases, nevertheless none of the markers examined in such tumors showed statistical association with any of the non-clinical, clinical and pathological parameters. These results point towards the downstream activation of several EMT programs as a consequence of enhanced expression of specific MMPs and their positive correlation with clinical outcome in a subset of bladder tumors.
Table 6.
Immunohistochemical (IHC) analysis of EMT marker genes evaluated for increased expression of MMP-2 at protein level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | IHC score ≤ 200(n = 5/21) | IHC score > 1(n = 4/21) | IHC score > 200(n = 7/21) | |||
| parameters | percentage | P | percentage | P | Percentage | P |
| Age 58(38–78) | 0.41* | 0.43* | 0.85* | |||
| < 60 | 3/5(60%) | 2/4(50%) | 3/7(42.9%) | |||
| ≥ 60 | 2/5(40%) | 2/4(50%) | 4/7(57.1%) | |||
| Gender | 0.016** | 0.65* | 0.001** | |||
| Male | 5/5(100%) | 3/4(75%) | 7/7(100%) | |||
| Female | 0/5(0%) | 1/4(25%) | 0/7(0%) | |||
| Tumor stage | 0.016** | 0.22* | 0.04* | |||
| NMIBC | 0/5(0%) | 2/4(50%) | 3/7(42.9%) | |||
| MIBC | 5/5(100%) | 2/4(50%) | 4/7(57.1%) | |||
| Tumorgrade | 0.016** | 0.65* | 0.19* | |||
| Low grade | 0/5(0%) | 1/4(25%) | 1/7(14.3%) | |||
| High grade | 5/5(100%) | 3/4(75%) | 6/7(85.7%) | |||
| Primer tumor | 1/5(20%) | 0.61* | 3/4(75%) | 0.18* | 3/7(42.9%) | 0.58* |
| Recurrent tumor | 4/5(80%) | 1/4(25%) | 4/7(57.1%) | |||
| Smoking | 0.56* | 0.48* | 0.001** | |||
| Yes | 3/5(60%) | 1/4(25%) | 5/7(71.4%) | |||
| No | 1/5(20%) | 2/4(50%) | 0/7(0%) | |||
| Unknown | 1/5(20%) | 1/4(25%) | 2/7(28.6%) | |||
| Hematuria | 0.04* | 1.00* | 0.23* | |||
| Present | 4/5(80%) | 2/4(50%) | 5/7(71.4%) | |||
| Absent | 1/5(20%) | 2/4(50%) | 2/7(28.6%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma; IHC- Immunohistochemical score calculated as the product of percent positive cells and staining intensity
**p values in bold are significant
Table 8.
Immunohistochemical (IHC) analysis of EMT marker genes evaluated for increased expression of MMP-9 at protein level
| Epithelial marker | Mesenchymal markers | Transcriptional factors | ||||
|---|---|---|---|---|---|---|
| Clinicopathological | IHC score ≤ 200(n = 6/24) | IHC score > 200(n = 4/24) | IHC score > 200(n = 7/24) | |||
| parameters | percentage | P | percentage | P | Percentage | P |
| Age 58(38–78) | 0.62* | 0.43* | 0.11* | |||
| < 60 | 4/6(66.7%) | 2/4(50%) | 6/7(85.7%) | |||
| ≥ 60 | 2/6(33.3%) | 2/4(50%) | 1/7(14.3%) | |||
| Gender | 0.01** | 0.124** | 0.11* | |||
| Male | 6/6(100%) | 4/4(100%) | 6/7(85.7%) | |||
| Female | 0/6(05) | 0/4(0%) | 1/7(14.3%) | |||
| Tumor stage | 0.32* | 0.18* | 0.19* | |||
| NMIBC | 2/6(33.3%) | 3/4(75%) | 4/7(57.1%) | |||
| MIBC | 4/6(66.7%) | 1/4(25%) | 3/7(42.9%) | |||
| Tumor grade | 0.35* | 0.18* | 0.35* | |||
| Low grade | 1/6(16.7%) | 3/4(75%) | 3/7(42.9%) | |||
| High grade | 5/6(83.3%) | 1/4(25%) | 4/7(57.1%) | |||
| Primer tumor | 3/6(50%) | 0.81* | 4/4(100%) | 0.124* | 4/7(57.1%) | 0.35* |
| Recurrent tumor | 3/6(50%) | 0/4(0%) | 3/7(42.9%) | |||
| Smoking | 0.56* | 0.18* | 0.11* | |||
| Yes | 3/6(50%) | 1/4(25%) | 6/7(85.7%) | |||
| No | 1/6(16.7%) | 3/4(75%) | 1/7(14.3%) | |||
| Unknown | 2/6(33.3%) | 0/4(0%) | 0/7(0%) | |||
| Hematuria | 0.04* | 0.18* | 1.00* | |||
| Present | 4/6(66.7%) | 3/4(75%) | 4/7(57.1%) | |||
| Absent | 2/6(33.3%) | 1/4(25%) | 3/7(42.9%) | |||
Statistical tests: *Mann-Whitney test, **One sample T-test; p values ≤0.05 taken as statistically significant values; NMIBC- Non-muscle invasive carcinoma, MIBC- Muscle invasive carcinoma; IHC- Immunohistochemical score calculated as the product of percent positive cells and staining intensity
**p values in bold are significant
Discussion
Critical involvement of MMPs in tumor-supporting cellular processes including loss of cell adhesion, ECM degradation and EMT reveal their potential role as markers of clinical significance in different stages of disease course. EMT is characterized by loss of cell adhesion, enhanced cell motility and invasive tumor growth. Differences in EMT related programming could be one of the possible reasons for the observed differences in disease progression of two distinct bladder cancer types [13, 20]. The present study has been undertaken to examine the downstream activation of EMT programs as a consequence of enhanced expression of specific MMPs and their clinical relevance in a subset of bladder tumors. The current study involves assessment of levels of MMPs (MMP-2, MMP-7 and MMP-9) and panel of EMT markers (E-cadherin, N-cadherin, Vimentin and EMT-ATFs) by RT-qPCR at transcriptome level. IHC was done to quantitatively localize the protein expression of EMT markers (E-cadherin, N-cadherin, Vimentin, Snail and Slug). The results of the experiments/ expression of marker genes were statistically correlated with clinical and pathological features in two distinct types of bladder cancer disease namely NMIBC and MIBC.
Growth factors and cytokines regulate the expression of MMP-2. High levels of MMP-2 facilitate its binding to the docking sites of nearby tumor cells, mark them for the degradation of ECM thus enable invasive tumor growth and correlate with metastatic bladder cancer phenotype. Research studies report the predominant localization of MMP-2, its high activity, enhanced expressions and correlation with poor prognosis (high tumor stage and grade) in bladder tumor cells and clearly indicate its role in metastatic bladder cancer phenotype [5, 21–24]. Increased migration ability and tumor invasion has been associated with higher expression of MMP-2 and EMT phenotype in bladder cancer cell lines [25, 26]. Comparative analysis of different studies reports conflicting results with respect to the clinical/ prognostic utility of MMP-2 in bladder tumor tissues. Studies by Hara et al. and Grignon et al. showed no correlation between the prognosis and MMP-2 levels, nevertheless studies by Vasala et al. and Kanamaya et al. found clear association between high expression of MMP-2 and poor disease-specific survival. Variations in patient selection with respect to tumor stage (both MIBC and NMIBC or either of it); type of clinical sample (frozen tissue/ paraffin embedded tissue/ blood plasma/ serum/ urine); analytical methods employed (IHC/ northern blot/ RT-qPCR/ western blot analysis); interpretation of the results; and statistical methods used are the probable reasons for the stated variations in validating the true prognostic relevance of MMP-2 in bladder cancer [5, 21, 23, 27]. In the current study, high expression of MMPs examined by RT-qPCR demonstrates its relationship with focal loss of E-cadherin, gain of mesenchymal markers and increased levels of EMT-ATFs in tumor tissues in concordance with earlier studies indicate important role of MMP-2 in impairing epithelial cell adhesion, and promoting tumor cell invasion via activating EMT program [8, 26, 28]. Significant correlation of EMT-associated markers in MMP-2 overexpressed tumors with tumor stage, grade, age gender, smoking and hematuria confirm the diagnostic and prognostic utility of markers.
MMP-7, smallest member of MMP family with wide substrate specificity, has been known to be involved in E-cadherin and Fas receptor cleavage thus disrupts cell-cell interaction, inhibits apoptosis, induces EMT and plays versatile role in tumor metastasis [8, 29]. Its involvement in the development of chemotherapy resistance further enhances its utility as a marker of therapeutic importance. Despite the differences in sample collection methods, type of biological specimen (tissue, urine, serum) and method of analysis in independent patient cohort, elevated MMP-7 levels has been observed to be significantly related with lymph node metastasis and clearly proved to be an unfavorable prognostic factor for metastasis free bladder cancer disease [30]. Study by Svatek et al. determined the clinical relevance of high levels of circulating MMP-2 using a particle-based flow cytometric assay in plasma samples from patients with bladder cancer [31]. Present study analyses the reduced expression of membranous E-cadherin; novel membranous expression of N-cadherin and Vimentin; enhanced nuclear expression of Snail and Slug through IHC in in tumors with overexpressed MMP-7 (identified by RT-qPCR); their clinical association with tumor stage, grade, gender, smoking status and presence of hematuria. Suggest the possible involvement of MMP-7 in downstream activation of EMT and bladder carcinogenesis.
Several growth factors, cytokines (interferon-γ, epidermal growth factor, tumor necrosis factor), embryonic stem cell markers (Oct-3/4) are shown to increase MMP-9 expression and thus enhanced invasion and metastatic potential in urinary bladder cancer cells in experimental setting [32–35]. Further, central role of MMP-9 in decreased cell adhesion capacity and enhanced cell motility via inducing EMT has been linked with bladder cancer progression [7, 36]. Current study demonstrates the clinical association of MMP-9 with high tumor stage, grade, sex, presence of hematuria and smoking status. EMT- associated markers in tumors with over expressed MMP-9 showed significant correlation with tumor high grade, gender and hematuria. Apparent differences in validating the prognostic value of MMP-9 are noted. Studies by Durkan et al., Hara et al., Vasala et al., McConkey et al., and Liu et al. report higher expression levels of MMP-9 as favorable prognostic factor while Slaton et al. establish no correlation between high levels of MMP-9 and survival [5, 26, 27, 36–38]. Use of MMP-9 as a complementary and a valuable prognostic tool requires validation in large number of independent cohorts of patients. Given the clear association of MMPs with oncological outcomes in patients with bladder cancer, MMPs hold promise in substantial risk stratification and form the basis of markers of therapeutic response.
Absence of significant association of either weak expression of E-cadherin, increased / novel expression of mesenchymal markers or high levels of EMT-TFs with any of the clinicopathological parameters in tumors exhibiting low expression of MMPs further hypothesizes the downstream activation of EMT program as a consequence of high levels of MMPs and their clinical relevance in a subset of patients with urothelial carcinoma of bladder. Clinical investigations in more number of clinical samples in two types of bladder cancer disease and multiple experimental methods for expression analysis are required to better understand the complex role of MMPs in EMT cascade.
Conclusions
Complex functions of distinct MMPs in various tumor supporting cellular processes mark them an important clinical tool in experimental setting. Enhanced expression of MMPs and their correlation with EMT-associated phenotype of bladder tumors further validate their functions in the pathogenesis of urothelial carcinoma. Extensive expression analysis of specific MMPs at transcriptome level by RT-qPCR; EMT marker genes at both transcriptome and protein levels by RT-qPCR and IHC respectively; and their statistical association with clinicopathological variables in a cohort of bladder cancer patients reveal their potential roles as biomarkers of diagnostic and prognostic significance at different stages of disease progression. Correlation of expression levels of EMT markers in tumors with overexpressed MMPs with their clinical and biological behavior might help to improve the risk classification thereby forms the basis of development of novel risk-adapted therapeutic procedures in the treatment of bladder cancer.
Acknowledgements
Authors thank Department of Science and Technology, Govt. of India for providing financial support to carry out research work (research grant no. SR/SO/HS-0113/2010). One of the authors, RS is thankful to University grant commission (UGC), Govt. of India for providing research fellowship.
Compliance with Ethical Standards
Conflict of Interest
Authors declare no potential conflict of interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Garg M. Urothelial cancer stem cells and epithelial plasticity: current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015;34(4):691–701. doi: 10.1007/s10555-015-9589-6. [DOI] [PubMed] [Google Scholar]
- 3.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 4.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasala K, Pääkkö P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 immunoreactive protein as a prognostic marker in bladder cancer. Urology. 2003;62:952–957. doi: 10.1016/S0090-4295(03)00660-5. [DOI] [PubMed] [Google Scholar]
- 6.Džombeta T, Krušlin B (2017) High grade T1 papillary urothelial bladder cancer shows prominent peritumoral retraction clefting. Pathol Oncol Res.10.1007/s12253-017-0279-2 [DOI] [PubMed]
- 7.Szarvas T, vom Dorp F, Ergün S, Rübben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol. 2011;8(5):241–254. doi: 10.1038/nrurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 8.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horejs CM. Basement membrane fragments in the context of the epithelial-to-mesenchymal transition. Eur J Cell Biol. 2016;95:427–440. doi: 10.1016/j.ejcb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 12.Garg M. Epithelial-mesenchymal transition–activating transcription factors – multifunctional regulators in cancer. World Journal of Stem Cells. 2013;5(4):188–195. doi: 10.4252/wjsc.v5.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Ansari JA, Maurya N, Mandhani A, Agrawal V, Garg M (2017) Epithelial-to-mesenchymal transition and its correlation with clinicopathologic features in patients with urothelial carcinoma of the bladder. Clin Genitourin Cancer 15(2):e187–e197 [DOI] [PubMed]
- 14.Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, et al. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7(45):72758–72766. doi: 10.18632/oncotarget.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candido S, Di Maso M, Serraino D, McCubrey JA, Bortolus R, Zanin M, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin/matrix metalloproteinase-9 pathway in transitional cell carcinoma of the bladder. Tumour Biol. 2016;37(7):9855–9863. doi: 10.1007/s13277-016-4872-x. [DOI] [PubMed] [Google Scholar]
- 16.Rao Q, Chen Y, Yeh CR, Ding J, Li L, Chang C, et al. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget. 2016;7(7):7842–7855. doi: 10.18632/oncotarget.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao C, Liang C, Zhu J, Xu A, Zhao K, Hua Y, et al. Prognostic role of matrix metalloproteinases in bladder carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(19):32309–32321. doi: 10.18632/oncotarget.15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wieczorek E, Jablonowski Z, Tomasik B, Konecki T, Jablonska E, Gromadzinska J, et al. MMP, VEGF and TIMP as prognostic factors in recurring bladder cancer. Clin Biochem. 2015;48(18):1235–1240. doi: 10.1016/j.clinbiochem.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Eble JN, Sauter G, Epstein JE, Sesterhenn IA. World Health Organization classification of tumours, pathology and genetics of male genital organs. Lyon: IARC Press; 2004. [Google Scholar]
- 20.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of pooroutcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 21.Grignon DJ, Sakr W, Toth M, Ravery V, Angulo J, Shamsa F, et al. High levels of tissue inhibitor of metalloproteinase-2 (TIMP-2) expression are associated with poor outcome in invasive bladder cancer. Cancer Res. 1996;56:1654–1659. [PubMed] [Google Scholar]
- 22.Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, et al. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365–5369. [PubMed] [Google Scholar]
- 23.Kanayama H, Yokota K, Kurokawa Y, Murakami Y, Nishitani M, Kagawa S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer. 1998;82:1359–1366. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1359::AID-CNCR20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Wallard MJ, Pennington CJ, Veerakumarasivam A, Burtt G, Mills IG, Warren A, et al. Comprehensive profiling and localisation of the matrix metalloproteinases in urothelial carcinoma. Br J Cancer. 2006;94:569–577. doi: 10.1038/sj.bjc.6602931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Qi L, Lv H, Zu X, Chen M, Wang J, et al. MiRNA-141 and miRNA-200b are closely related to invasive ability and considered as decision-making biomarkers for the extent of PLND during cystectomy. BMC Cancer. 2015;15:92. doi: 10.1186/s12885-015-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Miyake H, Nishikawa M, Fujisawa M. Expression profile of epithelial-mesenchymal transition markers in non-muscle-invasive urothelial carcinoma of the bladder: correlation with intravesical recurrence following transurethral resection. Urol Oncol. 2015;3(3):110.e11–110.e18. doi: 10.1016/j.urolonc.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Hara I, Miyake H, Hara S, Arakawa S, Kamidono S. Significance of matrix metalloproteinases and tissue inhibitors of metalloproteinase expression in the recurrence of superficial transitional cell carcinoma of the bladder. J Urol. 2001;165:1769–1772. doi: 10.1016/S0022-5347(05)66411-7. [DOI] [PubMed] [Google Scholar]
- 28.CL W, Ho JY, Chou SC, Yu DS. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7(18):26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szarvas T, Becker M, VomDorp F, Gethmann C, Tötsch M, Bánkfalvi A, et al. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010;101:1300–1308. doi: 10.1111/j.1349-7006.2010.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szarvas T, Jäger T, Becker M, Tschirdewahn S, Niedworok C, Kovalszky I, et al. Validation of circulating MMP-7 level as an independent prognostic marker of poor survival in urinary bladder cancer. Pathol Oncol Res. 2011;17(2):325–332. doi: 10.1007/s12253-010-9320-4. [DOI] [PubMed] [Google Scholar]
- 31.Svatek RS, Shah JB, Xing J, Chang D, Lin J, McConkey DJ, et al. A multiplexed, particle-based flow cytometric assay identified plasma matrix metalloproteinase-7 to be associated with cancer-related death among patients with bladder cancer. Cancer. 2010;116(19):4513–4519. doi: 10.1002/cncr.25401. [DOI] [PubMed] [Google Scholar]
- 32.Nutt JE, Durkan GC, Mellon JK, Lunec J. Matrix metalloproteinases (MMPs) in bladder cancer: the induction of MMP9 by epidermal growth factor and its detection in urine. BJU Int. 2003;91:99–104. doi: 10.1046/j.1464-410X.2003.04020.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Park SS, Cho YH, Park K, Kim EJ, Jung KH, et al. Activation of matrix metalloproteinase-9 by TNF-α in human urinary bladder cancer HT1376 cells: the role of MAP kinase signaling pathways. Oncol Rep. 2008;19:1007–1013. [PubMed] [Google Scholar]
- 34.Shin KY, Moon HS, Park HY, Lee TY, Woo YN, Kim HJ, et al. Effects of tumor necrosis factor-α and interferon-γ on expressions of matrix metalloproteinase-2 and -9 in human bladder cancer cells. Cancer Lett. 2000;159:127–134. doi: 10.1016/S0304-3835(00)00522-X. [DOI] [PubMed] [Google Scholar]
- 35.Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL, Wu CL. Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 2008;68:6281–6291. doi: 10.1158/0008-5472.CAN-08-0094. [DOI] [PubMed] [Google Scholar]
- 36.Durkan GC, Nutt JE, Marsh C, Rajjayabun PH, Robinson MC, Neal DE, et al. Alteration in urinary matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio predicts recurrence in nonmuscle-invasive bladder cancer. Clin Cancer Res. 2003;9:2576–2582. [PubMed] [Google Scholar]
- 37.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28(3–4):335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slaton JW, Millikan R, Inoue K, Karashima T, Czerniak B, Shen Y, et al. Correlation of metastasis related gene expression and relapse-free survival in patients with locally advanced bladder cancer treated with cystectomy and chemotherapy. J Urol. 2004;171:570–574. doi: 10.1097/01.ju.0000108845.91485.20. [DOI] [PubMed] [Google Scholar]