Abstract
Homocyst(e)ine (Hcy) inhibits the expression of the antioxidant enzyme cellular glutathione peroxidase (GPx-1) in vitro and in vivo, which can lead to an increase in reactive oxygen species that inactivate NO and promote endothelial dysfunction. In this study, we tested the hypothesis that overexpression of GPx-1 can restore the normal endothelial phenotype in hyperhomocyst(e)inemic states. Heterozygous cystathionine β-synthase-deficient (CBS(−/+)) mice and their wild-type littermates (CBS(+/+)) were crossbred with mice that overexpress GPx-1 [GPx-1(tg+) mice]. GPx-1 activity was 28% lower in CBS(−/+)/GPx-1(tg−) compared with CBS(+/+)/GPx-1(tg−) mice (P < 0.05), and CBS(−/+) and CBS(+/+) mice overexpressing GPx-1 had 1.5-fold higher GPx-1 activity compared with GPx-1 nontransgenic mice (P < 0.05). Mesenteric arterioles of CBS(−/+)/GPx-1(tg−) mice showed vasoconstriction to superfusion with β-methacholine and bradykinin (P < 0.001 vs. all other groups), whereas nonhyperhomocyst(e)inemic mice [CBS(+/+)/GPx-1(tg−) and CBS(+/+)/GPx-1(tg+) mice] demonstrated dose-dependent vasodilation in response to both agonists. Overexpression of GPx-1 in hyperhomocyst(e)inemic mice restored the normal endothelium-dependent vasodilator response. Bovine aortic endothelial cells (BAEC) were transiently transfected with GPx-1 and incubated with dl-homocysteine (HcyH) or l-cysteine. HcyH incubation decreased GPx-1 activity in sham-transfected BAEC (P < 0.005) but not in GPx-1-transfected cells. Nitric oxide release from BAEC was significantly decreased by HcyH but not cysteine, and GPx-1 overexpression attenuated this decrease. These findings demonstrate that overexpression of GPx-1 can compensate for the adverse effects of Hcy on endothelial function and suggest that the adverse vascular effects of Hcy are at least partly mediated by oxidative inactivation of NO.
Mild hyperhomocyst(e)inemia is a risk factor for atherothrombotic vascular disease (reviewed in refs. 1–5). Endothelial dysfunction appears to play a key role in homocyst(e)ine (Hcy)-mediated vascular pathophysiology. Animal models of mild hyperhomocyst(e)inemia manifest impaired endothelium-dependent vasoreactivity and regulation of blood flow, whether induced by vitamin deficiency (6), disruption of the cystathionine β-synthase (CBS) gene (7), or both (8). Impaired endothelium-dependent vasodilator function, but preserved endothelium-independent vasodilator response, is also a common finding in humans with either acutely elevated plasma tHcy levels after a methionine challenge (9–12) or with chronic, mild hyperhomocyst(e)inemia (13, 14). In accordance with these in vivo findings, homocysteine (HcyH) has been shown to decrease the production and/or bioactivity of NO and S-nitrosothiols by cultured endothelial cells (15, 16).
One mechanism proposed to explain the adverse effects of Hcy on endothelial function involves oxidant stress with resulting depletion of bioavailable NO (17). HcyH undergoes autooxidation when added to plasma, leading to the formation of reactive oxygen species, including hydrogen peroxide and superoxide anion (18). Superoxide anion can react with NO to form peroxynitrite, which leads to inactivation of its biological function (19). Hydrogen peroxide decomposes to the toxic oxygen species hydroxyl radical, which is highly reactive and causes lipid peroxidation. Elevated levels of lipid peroxides lead to an increase in peroxyl radicals that can, as well, inactivate NO through the formation of lipid peroxynitrites. Homocysteine-induced vascular oxidant stress may be additionally aggravated by an Hcy-mediated, specific decrease in the expression of the cellular isoform of glutathione peroxidase (GPx-1), as recently shown in vitro and in vivo (15, 20, §). This key enzyme for the cellular defense against oxidant stress uses glutathione to reduce hydrogen peroxide and lipid peroxides to their respective alcohols (22), and may also act as a peroxynitrite reductase (23). In this study, we therefore tested the hypothesis that overexpression of GPx-1 can restore the normal endothelial phenotype in hyperhomocyst(e)inemic states by using a genetic model of hyperhomocyst(e)inemia and cultured endothelial cells exposed to elevated homocysteine concentrations.
Methods
Animal Models.
Mice heterozygous for disruption in the CBS gene [CBS(−/+)] (24) were obtained from The Jackson Laboratory and subsequently bred at Boston University. Genotyping for the targeted CBS allele was performed in each mouse by PCR using genomic DNA obtained from tail biopsies as described (24).
Transgenic mice overexpressing cellular glutathione peroxidase [GPx-1(tg+)] (25) were kindly provided by Y. Ho (Wayne State University, Detroit) and subsequently bred at Boston University. Genotyping for the presence of the transgene was performed by real-time quantitative PCR by using 200 ng of genomic DNA obtained from tail biopsies as templates and molecular beacon technology for quantitation (26) (Stratagene). Offspring were genotyped and used for experiments at 10–12 weeks of age. Correct genotyping was further confirmed by measurement of cardiac GPx-1 activity (see below).
The animals were fed standard chow ad libitum (LabDiet 5001, PMI Feeds, St. Louis) and a diet sufficient in folic acid (0.59 mg of folic acid/100 g of chow), pyridoxine (0.60 mg/100 g of chow), vitamin B12 (0.022 μg/100 g of chow), and selenium (0.027 mg/100 g of chow). The animals were handled following National Institutes of Health guidelines. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Boston University Medical Center.
Plasma Homocyst(e)ine Concentration.
At the time of death, blood was drawn from the inferior vena cava in a syringe containing 1/10 vol CPD (10 mmol/liter citric acid/90 mmol/liter sodium citrate/15 mmol/liter Na2HPO4/142 mmol/liter dextrose, pH 7.35) and immediately centrifuged at 10,000 × g for 10 min. The plasma was separated and aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. Plasma tHcy was measured by a fluorescence polarization immunoassay (27) using an Abbott IMX analyzer.
Cellular GPx-1 Activity.
After blood collection at the time of death, the hearts and aortas were perfused with normal saline via puncture of the left ventricle and then harvested. Heart samples were snap-frozen in liquid nitrogen and stored at −80°C until analysis. Tissue samples were homogenized in an-ice cold buffer containing 50 mmol/liter Tris⋅HCl (pH 7.5), 5 mmol/liter EDTA (pH 8), and 1 mmol/liter DTT. The homogenate was centrifuged at 10,000 × g for 20 min at 4°C. GPx-1 activity was then determined from the supernatant by coupling the reduction of peroxides (t-butylperoxide) and the oxidation of glutathione with the reduction of oxidized glutathione by glutathione peroxidase using NADPH as a cofactor (28). Enzyme activity was normalized to protein concentration measured by the Bradford dye-binding procedure (29) by using a commercially available kit (Bio-Rad).
Mesenteric Microvascular Reactivity Studies.
Vascular reactivity in the mesenteric circulation in response to β-methacholine (MC), bradykinin (BK), and sodium nitroprusside was assessed in vivo using videomicroscopy as described (7, 30).
Aortic Endothelial Nitric Oxide Synthase (NOS) Expression.
Expression of endothelial NOS (eNOS) in aortic tissue was assessed by Western blot analysis.
Cell Culture, Plasmids, and GPx-1 Transfection.
Bovine aortic endothelial cells (BAEC) and bovine aortic vascular smooth muscle cells (BASMC) were obtained from BioWhittaker and maintained in DMEM containing 4,500 mg/liter d-glucose, 10% FBS, and antibiotics (100 units/ml penicillin G sodium and 100 μg/ml streptomycin sulfate; GIBCO/BRL, distributed by Invitrogen). Culture plates were maintained in a humidified incubator at 37°C with a 5% CO2 atmosphere. Cells (passages 6–14) were subcultured after treatment with 0.05% trypsin and 0.53 mmol/liter disodium EDTA.
Expression vectors for human GPx-1 and selenophosphate synthetase (SelD) were generated by reverse transcription–PCR of the respective cDNAs and cloning them into the plasmid pHIHG-Ad2 (generous gift of Richard Mulligan, Harvard University, Boston), in which expression is driven by a cytomegalovirus (CMV) promoter. The human GPx-1 cDNA (31) (GenBank accession no. M83094) and the human SelD cDNA (32) (GenBank accession no. U34044) were amplified by reverse transcription–PCR from RNA from normal human pulmonary artery endothelial cells (BioWhittaker). An expression vector for the Xenopus laevis selenocysteine-specific tRNA (tRNASec) (33) (GenBank accession no. M34507) was generated and kindly provided by Marla Berry (Brigham and Women's Hospital, Boston). An 800-bp EcoRI–HindIII fragment had been cloned into the expression vector pGEM3 (Promega); this vector is denoted pGEM3-tRNASec.
Preliminary experiments had shown that overexpression of GPx-1 in endothelial cells requires cotransfection with SelD and tRNASec (data not shown), two of the known cofactors essential for selenocysteine incorporation into the selenoprotein GPx-1 (32, 34). BAEC were either seeded into 100-mm cell culture dishes (for measurement of GPx-1 activity), 6-well cell culture plates (35-mm diameter/well, for measurement of NO release), or transwell inserts for 6-well plates (24-mm diameter, 0.4-μm pore size; for coculture experiments with BASMC). After cells had reached ≈80% confluency, cells were washed twice with Opti-MEM (GIBCO/BRL) and preincubated in this medium for 30 min at 37°C and 5% CO2 in a humidified incubator. Eight micrograms of pCMV-GPx-1 (1.6 μg for 35-mm plates and 24-mm inserts), and 1 μg (0.2 μg) each of pCMV-SelD and pGEM3-tRNASec were complexed with 26.4 μl (3.3 μl) of the cationic lipid mixture Oligofectin I (Sequitur, Natick, MA) in 3.2 ml (0.8 ml) of Opti-MEM and incubated for 30 min at room temperature. The transfection medium was added to the cells and incubated at 37°C and 5% CO2. Sham-transfected cells were transfected with the same amount of the pGL3 control plasmid (firefly luciferase under the control of an simian virus 40 promoter; Promega). After 6 h of incubation, the medium was changed to DMEM supplemented with 10% FBS, antibiotics, and 100 nmol/liter sodium selenite (GIBCO/BRL), and further incubated for 24 h. Cells were then treated as specified below. In preliminary experiments, a plasmid coding for green fluorescence protein had been transfected under the same conditions and indicated that ≈15% of cells demonstrate green fluorescence (data not shown).
GPx-1 Activity in GPx-1- and Sham-Transfected BAEC Incubated with Homocysteine.
Twenty-four hours after GPx-1 or sham transfection of BAEC grown in 100-mm dishes, cells were washed twice with Dulbecco's PBS (DPBS) (GIBCO/BRL) and incubated with medium containing 0 or 500 μmol/liter dl-homocysteine (Sigma) for 4 h. Cells were then washed twice with ice-cold DPBS, scraped off the plates, and collected in ice-cold buffer (50 mmol/liter Tris⋅HCl, pH 7.5/5 mmol/liter EDTA, pH 8.0/1 mmol/liter DTT). After centrifugation for 5 min at 2,000 × g and 4°C, the pellet was resuspended in the same buffer, snap-frozen in liquid nitrogen, and stored at −80°C until analysis. Cells were then thawed on ice, lysed by sonication (10 pulses at 0.3 s each, 40% output energy, sonicator cell disruptor W-225R, Heat Systems/Ultrasonics), and centrifuged at 10,000 × g and 4°C for 20 min; the supernatant was then aliquoted and kept on ice for analysis. GPx-1 activity was measured as in murine tissue (see above). The specific activity (per mg protein) was calculated after protein determination by the Bradford dye-binding procedure (29).
Diaminofluorescein-2 (DAF-2) Assay.
Nitric oxide release from BAEC was determined by measuring the green fluorescent triazole formed by the reaction of the aromatic vicinal diamines of DAF-2 (Calbiochem) with NO in the presence of molecular oxygen (35). The protein concentration in the wells was determined by the Bradford dye-binding procedure (29).
cGMP Accumulation in BASMC After Short-Term Coculture with GPx-1-Transfected and Homocysteine-Treated BAEC.
BASMC were cultured in 6-well plates and allowed to reach confluence for 24 h. BAEC were cultured in transwell inserts for 6-well plates (24-mm diameter, 0.4-μm pore size, Corning) and transfected with pCMV-GPx-1, pCMV-SelD, and pGEM3-tRNASec, or sham-transfected with pGL3 control, and incubated with sodium selenite for 24 h as described above. BAEC were then washed twice with DPBS and incubated with dl-homocysteine, l-cysteine, or medium for 4 h. After incubation of the BAEC, both BAEC and BASMC were washed twice with DPBS, and the transwell inserts containing BAEC were transferred to the culture plates containing BASMC and incubated with DPBS supplemented with 900 mg/liter d-glucose, 100 μmol/liter l-arginine, and 1 mmol/liter of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (Sigma, prepared from 0.5 mol/liter stock solutions in DMSO) for 15 min at 37°C and 5% CO2. BAEC were then stimulated by adding 10−5 mol/liter BK to the upper well for 60 s. The insert then was removed, the medium was removed, and cGMP was extracted by adding ice-cold ethanol. After 30 min of incubation, the supernatant was collected and dried under a stream of N2. cGMP levels were determined after acetylation of the samples using a commercially available enzyme immunoassay (Cayman Chemicals, Ann Arbor, MI). Preliminary experiments had shown that the cGMP increase in BASMC cocultured with BAEC under these conditions peaks by 60 s of stimulation (data not shown).
Statistical Analysis.
Values are reported as means ± SEM. Differences in the dose–response of microvascular diameter changes to agonists between groups were tested with two-way repeated measures ANOVA with posthoc analysis performed using Scheffé's F test and Bonferroni/Dunn procedures. Other data were analyzed by ANOVA and posthoc comparisons using Fisher's probable least-squares difference. Analyses were performed using the statview software package (Abacus Concepts, Berkeley, CA). Statistical significance was defined as a P value less than 0.05.
Results
Phenotype of CBS(−/+)/GPx-1(tg+) Mice.
There was no obvious phenotypical difference among the four groups of mice studied. Plasma tHcy levels were higher in CBS(−/+)/GPx-1(tg−) and CBS(−/+)/GPx-1(tg+) mice compared with CBS(+/+)/GPx-1(tg−) and CBS(+/+)/GPx-1(tg+) mice (5.15 ± 0.53 vs. 3.38 ± 0.35 μmol/liter, P < 0.05); the presence of the GPx-1 transgene did not influence plasma tHcy levels.
Tissue GPx Activity.
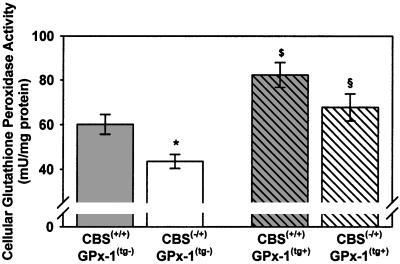
Cellular GPx activity in cardiac tissue was significantly lower in CBS(−/+)/GPx-1(tg−) compared with CBS(+/+)/GPx-1(tg−) mice (43.5 ± 3.1 vs. 60.1 ± 4.4 milliunits/mg protein, P < 0.05), confirming previous findings in hepatic tissue of CBS(−/+) mice.§ CBS(−/+) and CBS(+/+) mice overexpressing GPx-1 had ≈1.5-fold higher cellular GPx activity in cardiac tissue compared with the GPx-1 nontransgenic mice [CBS(−/+)/GPx-1(tg+): 67.7 ± 6.1 milliunits/mg protein, P < 0.05 vs. CBS(−/+)/GPx-1(tg−); CBS(+/+)/GPx-1(tg+): 82.3 ± 5.6 milliunits/mg protein, P < 0.05 vs. CBS(+/+)/GPx-1(tg−)]. Hyperhomocyst(e)inemic GPx-1(tg+) mice tended to have lower GPx-1 activity compared with nonhyperhomocyst(e)inemic GPx-1(tg+) mice, although the difference did not reach statistical significance (Fig. 1).
Figure 1.
Tissue GPx-1 activity in wild-type mice [CBS(+/+)/GPx-1(tg−), n = 6], heterozygous CBS knockout mice [CBS(−/+)/GPx-1(tg−), n = 7], GPx-1 transgenic mice [CBS(+/+)/GPx-1(tg+), n = 5], and GPx-1 transgenic, heterozygous CBS knockout mice [CBS(−/+)/GPx-1(tg+), n = 5]. *, P < 0.05 vs. CBS(+/+)/GPx-1(tg−); $, P < 0.01 vs. CBS(+/+)/GPx-1(tg−); §, P < 0.01 vs. CBS(−/+)/GPx-1tg−.
Microvascular Reactivity and Aortic eNOS Expression.
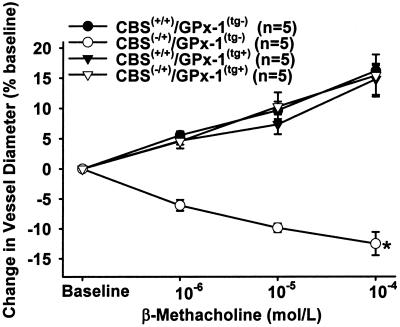
Endothelial function in resistance vessels in response to endothelium-dependent and -independent stimuli was assessed by in vivo videomicroscopy of mesenteric arterioles. Mesenteric arterioles of CBS(−/+)/GPx-1(tg−) mice showed a dose-dependent paradoxical vasoconstriction to superfusion with MC and BK [maximal change in vessel diameter (MVR) −12.5 ± 1.9% in response to MC 10−4 mol/liter and −11.2 ± 2.1% in response to BK 10−5 mol/liter], whereas nonhyperhomocyst(e)inemic mice [CBS(+/+)/GPx-1(tg−) and CBS(+/+)/GPx-1(tg+) mice] demonstrated dose-dependent vasodilation in response to both agonists [MVR to MC: 16.25 ± 0.9% and 14.87 ± 2.6% for GPx-1 nontransgenic and transgenic mice, respectively; MVR to BK: 12.2 ± 1.3% and 16.1 ± 2.3%, respectively; P < 0.001 vs. CBS(−/+)/GPx-1(tg−)] (Fig. 2 for MC data; BK data not shown). These findings are consistent with a decrease in bioavailable NO in hyperhomocyst(e)inemic mice. Overexpression of GPx-1 in hyperhomocyst(e)inemic mice [CBS(−/+)/GPx-1(tg+)] completely restored the microvascular response to both endothelium-dependent agonists [MVR to BMC, 15.4 ± 3.5%; MVR to BK, 13.6 ± 1.9%; P < 0.001 vs. CBS(−/+)/GPx-1(tg−)] (Fig. 2 for MC data; BK data not shown). Superfusion of the mesentery with sodium nitroprusside led to dose-dependent vasodilation in all animals studied (data not shown).
Figure 2.
Mesenteric microvascular response to superfusion with β-methacholine in CBS(+/+)/GPx-1(tg−) mice (●), CBS(−/+)/GPx-1(tg−) mice (○), CBS(+/+)/GPx-1(tg+) mice (▿), and CBS(−/+)/GPx-1(tg+) mice (▾). *, P < 0.001 vs. all other groups.
Western blot analysis for endothelial NOS expression in aortic tissues showed no appreciable difference among the four groups of mice studied, indicating that the effect on microvascular reactivity is independent of enzyme expression (data not shown).
GPx-1 Activity in GPx-1-Transfected and Homocysteine-Incubated BAEC.
Overexpression of GPx-1 in vitro in endothelial cells requires the cotransfection of pCMV-GPx-1 with plasmids coding for genes required for selenocysteine incorporation into the GPx-1 protein (pCMV-SelD and pGEM3-tRNASec) and the supplementation of the cell culture medium with selenium (as selenite). Even under these optimized transfection conditions, GPx-1-transfected and selenium-supplemented BAEC had only 1.4-fold higher GPx-1 activity compared with sham-transfected and selenium-supplemented BAEC, a level of overexpression comparable to the level seen in vivo in GPx-1 transgenic mice. This indicates that other, as yet unknown, cofactors might be necessary for higher levels of GPx-1 expression in vitro.
Incubation of sham-transfected cells with 500 μmol/liter dl-homocysteine for 4 h significantly decreased GPx-1 activity from 136 ± 4.1 to 103 ± 5.4 milliunits/mg protein (P < 0.005). By contrast, HcyH incubation had no effect on the increased GPx-1 activity in GPx-1-transfected cells (190 ± 6.5 and 193 ± 7.8 milliunits/mg protein in control and HcyH-incubated, GPx-1-transfected BAEC, respectively; P < 0.005 vs. no homocysteine control and vs. sham transfection).
Nitric Oxide Release from GPx-1-Transfected and Homocysteine-Treated BAEC.
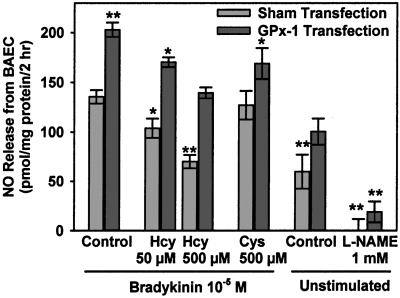
HcyH incubation of sham-transfected cells for 4 h dose-dependently and significantly decreased DAF-2 fluorescence to 76% of control in cells incubated with 50 μmol/liter HcyH (P < 0.05) and to 52% of control in cells incubated with 500 μmol/liter HcyH (P < 0.005) (Fig. 3). Incubation of BAEC with cysteine did not significantly influence DAF-2 fluorescence. GPx-1 transfection led to a 50% increase in DAF-2 fluorescence in control cells (P < 0.005). HcyH incubation of GPx-1-transfected cells also dose-dependently decreased DAF-2 fluorescence. However, NO accumulation in the supernatant of GPx-1-transfected cells incubated with 500 μmol/liter HcyH was still as high as levels in sham-transfected BAEC (P = not significant). Treatment of cells with the NOS inhibitor l-NAME completely suppressed the DAF-2 signal, indicating that the measured signal is specific for NOS-derived NO.
Figure 3.
NO release from BAEC into the medium over 2 h. Cells were transfected with pCMV-GPx-1, pCMV-SelD, and pGEM3-tRNASec or sham-transfected and preincubated with the indicated concentrations of dl-homocysteine or l-cysteine for 4 h. The buffer was then changed to a thiol-free buffer, and DAF-2 fluorescence in the supernatant was measured after stimulation with BK 10−5 mol/liter for 2 h as described in Materials and Methods. n = four experiments. *, P < 0.05 vs. control; **, P < 0.005 vs. control.
cGMP Accumulation in BASMC Cocultured with GPx-1-Transfected and Homocysteine-Treated BAEC.
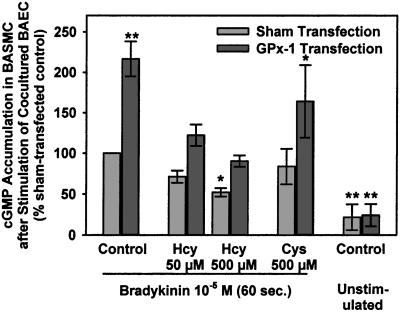
BAEC were grown on semipermeable membranes, sham-transfected or transfected with the GPx-1 cDNA and the essential cofactors, and incubated with control media or media containing HcyH or cysteine for 4 h. BAEC were then transferred to wells containing BASMC and stimulated with BK for 60 s. cGMP accumulation in the cocultured BASMC was measured and paralleled the measured NO release from BAEC (Fig. 4). cGMP levels dose-dependently and significantly decreased in BASMC cocultured with sham-transfected BAEC that had been incubated with increasing concentrations of HcyH (71% control at 50 μmol/liter HcyH; 52% control at 500 μmol/liter HcyH, P < 0.05). Overexpression of GPx-1 in BAEC led to a 217% increase in cGMP levels in cocultured BASMC (P < 0.005) and compensated for the effects of HcyH on BASMC cGMP levels. Cysteine had no significant effect on cGMP levels in BASMC cocultured with sham-transfected BAEC. cGMP levels in than BASMC cocultured with unstimulated BAEC were significantly lower than cGMP levels in BASMC cocultured with BK-stimulated BAEC, indicating that the cGMP levels measured are derived from NO-mediated guanylyl cyclase activation.
Figure 4.
cGMP accumulation in BASMC after short-term coculture with BAEC. BAEC were grown in transwell inserts, transfected with pCMV-GPx-1, pCMV-SelD, and pGEM3-tRNASec or sham-transfected and preincubated with the indicated concentrations of dl-homocysteine or l-cysteine for 4 h. BAEC were then transferred for coincubation with BASMC, incubated with 3-isobutyl-1-methylxanthine for 15 min, and then stimulated with BK 10−5 mol/liter for 60 s. cGMP was measured in the ethanol extract of BASMC by using an enzyme immunoassay. n = three experiments. *, P < 0.01 vs. BK-stimulated control; **, P < 0.001 vs. BK-stimulated control.
Discussion
The major findings of this study are that overexpression of GPx-1 (i) rescues the phenotype of and restores endothelium-dependent vascular reactivity in mildly hyperhomocyst(e)inemic CBS(−/+) mice and (ii) increases bioavailable NO from BAEC, thereby compensating for the adverse effects of Hcy. These data suggest that the vascular pathophysiology of Hcy is at least partly mediated by decreased bioavailable NO owing to oxidative inactivation, as increasing the cellular GPx-1 antioxidant capacity compensates for the effect.
Heterozygous CBS-deficient mice fed a standard rodent chow that is sufficient in folate and vitamin B6 had mildly elevated plasma tHcy levels of about 1.5-fold the concentration found in wild-type mice. Plasma levels of tHcy in mice [3.38 ± 0.35 μmol/liter in CBS(+/+) mice] are generally lower than plasma tHcy levels in humans, where normal total plasma tHcy concentrations range from 5 to 15 μmol/liter in the fasting state (36, 37). Mild hyperhomocyst(e)inemia usually is defined as plasma tHcy levels ranging from 15 to 30 μmol/liter (38), which represents the same proportional increase as seen in CBS(−/+). This increase in plasma tHcy levels due to partial CBS deficiency in mice is sufficient to cause endothelial dysfunction as CBS(−/+) mice have been shown to have attenuated aortic relaxation to the endothelium-dependent agonist acetylcholine (7) and paradoxical vasoconstriction of the mesenteric microcirculation to MC, BK, and A23187, as we have previously shown (7) and confirmed in this study (Fig. 2). As endothelial dysfunction is a commonly observed pathology in mild hyperhomocyst(e)inemia in humans, either with acute elevation of tHcy levels after a methionine challenge (9–12) or chronic hyperhomocyst(e)inemic states (13, 14), this animal model seems to be suitable for the study of vascular pathophysiology of mild hyperhomocyst(e)inemia.
Nitric oxide plays a key role in mediating the endothelium-dependent response to acetylcholine (39). Paradoxical vasoconstriction to cholinergic stimulation is well known to occur in atherosclerotic coronary arteries (40). Chronic exposure to elevated levels of Hcy may also alter the bioactivity of NO. Nitric oxide production from endothelial cells is dose-dependently decreased by HcyH as measured by photolysis-chemiluminescence (15), by an NO-selective electrode (16), or, as in this study, by a recently developed fluorescent dye (Fig. 3).
Decreased cGMP accumulation in BASMC cocultured with endothelial cells that had been pretreated with different concentrations of HcyH (Fig. 4) confirms that HcyH decreases NO bioactivity. This effect observed in endothelial cells is independent of NOS activity or eNOS gene transcription, as shown (15). In vivo, cGMP accumulation has been shown to be diminished in vascular tissue from hyperhomocyst(e)inemic mice stimulated with acetylcholine (7), an effect that seems to be independent of eNOS expression in vivo as previously shown by immunostaining of aortic arch cross sections for eNOS (7) and by Western blot analysis of aortic tissue for eNOS in this study.
Oxidant injury imparted by hyperhomocyst(e)inemia may contribute to impairment of NO action and endothelial function, and a Hcy-specific decrease in the expression of GPx-1 may further contribute to the adverse effect of chronic hyperhomocyst(e)inemia. Several studies have shown previously that HcyH, but not cysteine, decreases the expression of GPx-1 in vitro in cultured endothelial cells (15, 20). This effect can also be observed in vivo in hepatic§ and cardiac tissue (Fig. 1) of CBS(−/+) mice and in hepatic tissue of folate-depleted, hyperhomocyst(e)inemic rats (41).
Hcy thereby seems to exert its effect on the expression of GPx-1 by transcriptional down-regulation as Hcy decreases GPx-1 mRNA levels in endothelial cells (15, 20) and in hepatic tissue of CBS(−/+) mice§ without influencing mRNA stability (15). In accordance with this observation is the finding that GPx-1 activity in hyperhomocyst(e)inemic, GPx-1 transgenic mice is lower than in nonhyperhomocyst(e)inemic GPx-1 transgenic mice (Fig. 1). In these mice, the GPx-1 transgene is under control of its natural promoter. In contrast, HcyH did not decrease GPx-1 activity in BAEC transfected with GPx-1 cDNA under control of a CMV promoter. The exact molecular mechanism by which HcyH controls GPx-1 transcription is still under investigation.
GPx-1 is a key enzyme for the cellular defense against oxidant stress that uses glutathione to reduce hydrogen peroxide and lipid peroxides to their respective alcohols (22), and may also act as a peroxynitrite reductase (23). HcyH, when added to plasma, undergoes autooxidation like other thiol-containing amino acids. This oxidative reaction is accompanied by the generation of reactive oxygen species, such as hydrogen peroxide or superoxide anion (18). Superoxide anion can react with NO to form peroxynitrite, which leads to inactivation of its biological function (19). The role of superoxide formation in Hcy-induced endothelial dysfunction is underscored by the demonstration of greater superoxide production in aortic tissue from CBS(−/+) mice compared with wild-type mice (7), and by the findings that superoxide dismutase can reverse the paradoxical vasoconstriction of mesenteric arterioles of CBS(−/+) mice in response to stimulation with MC (7) and can reverse the decreased cerebrocortical blood flow during superfusion with HcyH-containing buffer (42). Furthermore, the superoxide scavenger 4,5-dihydroxy-1,3-benzene disulfonic acid had been shown to inhibit the effect of HcyH on acetylcholine- and A23187-induced relaxation of rabbit aortic rings (43). Earlier investigations additionally have provided support for a role for hydrogen peroxide in HcyH-induced endothelial toxicity in vitro, as catalase was found to inhibit the HcyH-induced lysis of endothelial cells in the presence of transition metals or ceruloplasmin (44). Furthermore, hydrogen peroxide decomposes to the toxic oxygen species hydroxyl radical, which is highly reactive and causes lipid peroxidation, and hydroxide anion, which promotes alkaline tissue damage. Elevated levels of lipid peroxides lead to an increase in peroxyl radicals that can inactivate NO through the formation of lipid peroxynitrites. Peroxynitrite may further react with cellular tyrosine residues to form nitrosated end products, or with thiols to form S-nitrosothiols. As GPx-1 also acts as a peroxynitrite reductase, GPx-1 deficiency might promote peroxynitrite or lipid peroxynitrite formation. Immunostaining for one such nitrosated end product, 3-nitrotyrosine, has been shown to be positive in aortic tissue from CBS(−/+) compared with CBS(+/+) mice (7). A decrease in GPx-1 activity in chronic hyperhomocyst(e)inemia might, therefore, at least partly explain the observed increased vascular oxidative stress leading to biological inactivation of NO and the increased nitrosative stress under hyperhomocyst(e)inemic conditions that seems to be specific for elevated Hcy concentrations but not for increased concentrations of other low molecular weight thiols.
Overexpression of GPx-1 in BAEC together with selenium supplementation and two of the cofactors essential for selenocysteine insertion increased GPx-1 activity 1.4-fold compared with sham-transfected and selenium-supplemented BAEC. This level of overexpression is comparable with the level observed in the tissue of the transgenic animals. Overexpression of GPx-1 in vascular endothelial cells led to a 1.5-fold increase in NO release from endothelial cells (Fig. 3) and to a 2.1-fold increase in cGMP accumulation in BASMC cocultured with transfected, BK-stimulated endothelial cells (Fig. 4). These results indicate that GPx-1 overexpression increases the bioavailability of NO. Previous studies have shown that addition of purified GPx potentiates S-nitrosothiol-induced inhibition of platelet aggregation and increases S-nitrosothiol-induced cGMP accumulation in platelets in an ex vivo system, and that GPx attenuates the increase in platelet aggregation induced by the addition of lipid peroxides. These effects are consistent with a decrease in the S-nitrosothiol concentration, suggesting that GPx may regulate the availability of NO by two functions: reduction of peroxides, thereby preventing the inactivation of NO; and metabolism of S-nitrosothiols, thereby liberating NO and/or supporting further transnitrosation reactions (45). Our data are in accordance with these previous observations and additionally show that intracellular GPx-1 activity may regulate NO bioavailability in intact endothelial cells.
HcyH, but not cysteine, incubated with endothelial cells, dose-dependently decreases NO release from BAEC and cGMP accumulation in BASMC cocultured with BAEC. GPx-1 overexpression attenuated the effect of HcyH on both parameters of NO bioavailability, shifting the HcyH dose-response curve to higher NO and cGMP levels (Figs. 3 and 4). These effects might be explained by the peroxidase activity of GPx-1, thereby preventing the oxidative inactivation of NO by reactive oxygen species generated during the incubation with HcyH. In addition, this effect may also be explained by the effect of GPx-1 on the metabolism of S-nitrosothiols, thereby increasing the pool of free NO available to overcome oxidative inactivation. An alternative hypothesis that needs to be tested is that the increased cellular Hcy pool under hyperhomocyst(e)inemic conditions facilitates the formation of S-nitrosohomocysteine. S-nitrosohomocysteine is known to have a longer biological half-life than free NO. Most investigators assume that NO traverses cell membranes freely, owing to its relative lipophilicity and modest reactivity, to reach its effector site. It is, therefore, possible that cellular entry and bioactivity of NO may be regulated by transnitrosation reactions. It has been suggested recently that cell-surface protein disulfide isomerase catalyzes transnitrosation reactions and regulates intracellular transfer of nitric oxide from extracellular S-nitrosothiols (46). As GPx is known to liberate NO from S-nitrosothiols, liberation of NO from S-nitrosohomocysteine might explain the effects seen after GPx-1 overexpression in endothelial cells and in transgenic animals.
In summary, the findings presented here demonstrate that elevated Hcy levels induce endothelial dysfunction in transgenic animals and cultured cells, concomitant with a decrease in GPx-1 expression and activity. Overexpression of GPx-1 restores endothelium-dependent vascular function in mildly hyperhomocyst(e)inemic CBS(−/+) mice and increases bioavailable NO in BAEC, thereby compensating for the adverse effects of Hcy. These results suggest that the adverse vascular effects of Hcy are at least partly mediated by oxidative inactivation of NO, as increasing the cellular GPx-1 antioxidant capacity compensates for the effect.
Acknowledgments
We thank Ms. Anne Ward Scribner, Ms. Antoinette Hayes, and Ms. Stephanie Tribuna for expert technical assistance. This work was supported in part by National Institutes of Health Grants HL 55993, HL 58976, and HL 61795 (to J.L.) and by Deutsche Forschungsgemeinschaft Grant WE 1984/2-1 (to N.W.).
Abbreviations
- Hcy
combined pool of homocysteine, homocystine, mixed disulfides involving homocysteine, and homocysteine thiolactone
- tHcy
the total, free, and protein-bound homocyst(e)ine found in plasma
- HcyH
reduced (sulfhydryl) species
- GPx-1
cellular isoform of glutathione peroxidase
- CBS
cystathionine β-synthase
- BAEC
bovine aortic endothelial cells
- MC
β-methacholine
- BK
bradykinin
- NOS
nitric oxide synthase
- BASMC
bovine aortic vascular smooth muscle cells
- SelD
selenophosphate synthetase
- CMV
cytomegalovirus
- DPBS
Dulbecco's PBS
- MVR
maximal change in vessel diameter
- DAF-2
diaminofluorescein-2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Weiss, N., Zhang, Y. & Loscalzo, J. (2000) Circulation 102, II-238 (abstr.).
References
- 1.Boushey C J, Beresford S A A, Omenn G S. J Am Med Assoc. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 2.Malinow M R, Bostom A G, Kraus R M. Circulation. 1999;99:178–182. doi: 10.1161/01.cir.99.1.178. [DOI] [PubMed] [Google Scholar]
- 3.Ueland P M, Refsum H, Beresford S A, Vollset S E. Am J Clin Nutr. 2000;72:324–332. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- 4.Booth G L, Wang E E. Can Med Assoc J. 2000;163:21–29. [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson K. Heart. 2000;83:127–130. doi: 10.1136/heart.83.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentz S R, Sobey C G, Piegors D J, Bhopatkar M Y, Faraci F M, Malinow M R, Heistad D D. J Clin Invest. 1996;98:24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberhardt R T, Forgione M A, Cap A, Leopold J A, Rudd M A, Trolliet M, Heydrick S, Stark R, Klings E S, Moldovan N I, et al. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lentz S R, Erger R A, Dayal S, Maeda N, Malinow M R, Heistad D D, Faraci F M. Am J Physiol. 2000;279:H970–H975. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy M F, McDowell I F, Ramsey M W, Brownlee M, Bones C, Newcombe R G, Lewis M J. Circulation. 1998;98:1848–1852. doi: 10.1161/01.cir.98.18.1848. [DOI] [PubMed] [Google Scholar]
- 10.Hanratty C G, McAuley D F, McGurk C, Young I S, Johnston G D. Lancet. 1998;351:1288–1289. doi: 10.1016/S0140-6736(05)79355-7. [DOI] [PubMed] [Google Scholar]
- 11.Chambers J C, Obeid O A, Kooner J S. Arterioscler Thromb Vasc Biol. 1999;19:2922–2927. doi: 10.1161/01.atv.19.12.2922. [DOI] [PubMed] [Google Scholar]
- 12.Chao C L, Kuo T L, Lee Y T. Circulation. 2000;101:485–490. doi: 10.1161/01.cir.101.5.485. [DOI] [PubMed] [Google Scholar]
- 13.Woo K S, Chook P, Lolin Y I, Cheung A S, Chan L T, Sun Y Y, Sanderson J E, Metreweli C, Celermajer D S. Circulation. 1997;96:2542–2544. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- 14.Tawakol A, Omland T, Gerhard M, Wu J T, Creager M A. Circulation. 1997;95:1119–1121. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 15.Upchurch G R, Jr, Welch G N, Fabian A J, Freedman J E, Johnson J L, Keaney J F, Jr, Loscalzo J. J Biol Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky M S. Am J Physiol. 2000;279:F671–F678. doi: 10.1152/ajprenal.2000.279.4.F671. [DOI] [PubMed] [Google Scholar]
- 17.Loscalzo J. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinecke J W, Rosen H, Suzuki L A, Chait A. J Biol Chem. 1987;262:10098–10103. [PubMed] [Google Scholar]
- 19.Gryglewski R J, Palmer R M, Moncada S. Nature (London) 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 20.Outinen P A, Sood S K, Pfeifer S I, Pamidi S, Podor T J, Li J, Weitz J I, Austin R C. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- 21.Zai A, Rudd M A, Scribner A W, Loscalzo J. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flohé L. In: Glutathione: Chemical, Biochemical and Medical Aspects. Dolphin D, Poulson R, Avramovic O, editors. New York: Wiley; 1989. pp. 644–731. [Google Scholar]
- 23.Sies H, Sharov V S, Klotz L O, Briviba K. J Biol Chem. 1997;272:27812–27817. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow M R, Maeda N. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng W H, Ho Y S, Ross D A, Han Y, Combs G F, Jr, Lei X G. J Nutr. 1997;127:675–680. doi: 10.1093/jn/127.5.675. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi S, Kramer F R. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 27.Schipchandler M T, Moore E G. Clin Chem. 1995;41:991–994. [PubMed] [Google Scholar]
- 28.Paglia D E, Valentine W N. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 29.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Zweifach B W, Schmid-Schonbein G W. Hypertension. 1995;26:397–400. doi: 10.1161/01.hyp.26.3.397. [DOI] [PubMed] [Google Scholar]
- 31.Moscow J A, Morrow C S, He R, Mulenbach G T, Cowan K H. J Biol Chem. 1992;267:5949–5958. [PubMed] [Google Scholar]
- 32.Low S C, Harney J W, Berry M J. J Biol Chem. 1995;270:21659–21664. doi: 10.1074/jbc.270.37.21659. [DOI] [PubMed] [Google Scholar]
- 33.Lee B J, Rajagppalan M, Kim Y S, You K-H, Jacobson K B, Hatfield D. Mol Cell Biol. 1990;10:1940–1949. doi: 10.1128/mcb.10.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low S C, Berry M J. Trends Biochem Sci. 1996;21:203–208. [PubMed] [Google Scholar]
- 35.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- 36.Ueland P M, Refsum H, Stabler S P, Malinow M R, Andersson A, Allen R H. Clin Chem. 1993;39:1764–1779. [PubMed] [Google Scholar]
- 37.Jacobsen D W, Gatautis V J, Green R, Robinson K, Savon S R, Secic M, Ji J, Otto J M, Taylor L M., Jr Clin Chem. 1994;40:873–881. [PubMed] [Google Scholar]
- 38.Kang S S, Wong P W K, Malinow M R. Annu Rev Nutr. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 39.Palmer R M, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 40.Ludmer P L, Selwyn A P, Shook T L, Wayne R R, Mudge G H, Alexander R W, Ganz P. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 41.Huang R-F S, Hsu Y-C, Lin H-L, Yang F L. J Nutr. 2001;131:33–38. doi: 10.1093/jn/131.1.33. [DOI] [PubMed] [Google Scholar]
- 42.Zhang F, Slungaard A, Vercellotti G M, Iadecola C. Am J Physiol. 1998;274:R1704–R1711. doi: 10.1152/ajpregu.1998.274.6.R1704. [DOI] [PubMed] [Google Scholar]
- 43.Lang D, Kredan M B, Moat S J, Hussain S A, Powell C A, Bellamy M F, Powers H J, Lewis M J. Arterioscler Thromb Vasc Biol. 2000;20:422–427. doi: 10.1161/01.atv.20.2.422. [DOI] [PubMed] [Google Scholar]
- 44.Starkebaum G, Harlan J M. J Clin Invest. 1986;77:1370–1376. doi: 10.1172/JCI112442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman J E, Frei B, Welch G N, Loscalzo J. J Clin Invest. 1995;96:394–400. doi: 10.1172/JCI118047. [DOI] [PMC free article] [PubMed] [Google Scholar]