Abstract
The disruption of key epigenetic processes during critical periods of brain development can increase an individual’s vulnerability to psychopathology later in life. For instance, DNA methylation in the glucocorticoid receptor gene (NR3C1) in adulthood is known to be associated with early-life adversities and has been suggested to mediate the development of stress-related disorders. However, the association between NR3C1 methylation and the emergence of internalizing symptoms in childhood and adolescence has not been studied extensively. In the present report, we used saliva DNA from a cohort of Swedish adolescents (13–14 years old; N = 1149) to measure NR3C1 methylation in the exon 1F region. Internalizing psychopathological symptoms were assessed using the Center for Epidemiologic Studies Depression Scale for Children (CES-DC). We found that NR3C1 hypermethylation was cross-sectionally associated with high score for internalizing symptoms in the whole group as well as among the female participants. In addition, an analysis of social environmental stressors revealed that reports of bullied or lacking friends were significantly associated with NR3C1 hypermethylation. This cross-sectional association of NR3C1 exon 1F hypermethylation with internalizing psychopathology in adolescents, as well as with bullying and lack of friends are novel results in this field. Longitudinal studies are needed to address whether NR3C1 methylation mediates the link between social stressors and psychopathology in adolescence.
Introduction
Adolescence represents a transitional state between childhood and adulthood that is accompanied both by marked endogenous changes, e.g., pubertal increases in hormonal levels, and by increased demand from the surrounding environment, e.g., social and intellectual performances. Adolescence also constitutes a critical period for brain maturation and is characterized by heightened responsiveness to incentives and socioemotional stimuli1. Anxiety and affective disorders often have their onset or prodromal phase in adolescence2.
Chronic stress is a risk factor for anxiety and affective disorders. Stress refers to the innate response of an organism to environmental threats that allows for physiological and behavioral adaptations in order to maintain homeostasis. In mammals, this response is mainly mediated through the activation of the hypothalamic–pituitary axis in the brain, which results in the secretion of cortisol from the adrenal glands; collectively forming the hypothalamic–pituitary–adrenal (HPA) axis stress response system. Although this cascade of events initially increases awareness and behavioral flexibility3, disturbances in the regulation of the HPA axis during stress can result in the development of psychopathology4. For example, internalizing disorders, including anxiety and depression, have been associated with increased cortisol secretion and a hyper-active HPA axis5,6
On the cellular level, cortisol acts by binding to the glucocorticoid receptor (GR), a pleiotropic transcription factor that is encoded by the NR3C1 gene. Upon binding to GR, cortisol induces a number of stress-related responses to acute and chronic stress. Yet, one of GR’s critical roles is to mediate negative feedback regulation of the HPA axis to terminate the stress response. Various mechanisms can reduce the sensitivity of this negative feedback loop resulting in sustained high cortisol levels. One such mechanism is the decrease in NR3C1 expression by DNA methylation. DNA methylation is an epigenetic mechanism occurring mainly at cytosine residues at CpG sites. NR3C1 contains nine untranslated alternative exon 1 variants7 that are independently controlled by unique upstream promoters8. Most of these alternative first exons, including their promoters, are found within a sequence region rich of CpGs. Upon methylation, CpG-containing sequence sites can no longer serve as binding sites for their transcription factors and, consequently, the mRNA and protein expression of NR3C1 is reduced9.
Preclinical studies have demonstrated that early environmental stressors, e.g., lack of maternal care, can cause increased methylation (hypermethylation) of specific CpG regions and alter the HPA axis responses to stress10. Early-life traumas in humans, in the form of childhood abuse or parental loss, were also found in adults to be associated with NR3C1 hypermethylation in CpGs of the exon 1F region, which includes a binding site of the transcription factor NGFI-A11–15. Hypermethylation of the same exon was also found in infants of depressed mothers16,17. Furthermore, there are studies showing that childhood abuse is associated with DNA hypermethylation in adults in other exon 1 variants of NR3C1, including exons 1B, 1C, and 1H9. On the other hand, a recent human study reported hypomethylation of NR3C1 exon 1F region in the case of early adversity and the presence of substance abuse, depressive or anxiety disorders18; these findings suggest complexity in the interaction between adversity exposure and the DNA methylation of this gene.
Despite the extensive literature on NR3C1 methylation as mediator of the effects of retrospective early adversities on the development of psychopathology later in life19,20, less is known about how NR3C1 methylation is associated with recent or current adversities and psychopathology, especially during adolescence. In the few available studies, NR3C1 exon 1F hypermethylation was reported to be associated with internalizing symptoms in children at age 4–16 years21, and in another study, NR3C1 exon 1H and 1F hypermethylation was reported to be associated with traumatic youth experiences and milder stressful life events in adolescents aged 16–18 years22. Given the limited literature focusing on adolescents, we measured DNA methylation levels of NR3C1’s exon 1F in a sample of adolescent individuals, aged 13–14 years old, and investigated the cross-sectional associations between methylation and internalizing psychopathological symptoms on the one side; and between methylation and exposure to environmental stressors on the other side.
The study hypotheses were as follows: NR3C1 exon 1F methylation would be higher among adolescents with internalizing symptomatology than among those without these symptoms. Since exon 1F methylation level was previously reported to be positively associated with total stress-induced cortisol output in adult healthy women but not in men23, and peripheral blood cortisol level is positively associated with internalizing disorder24, sex stratified analyses were performed. In line with the previous evidence of increased NR3C1 exon 1F methylation in those exposed to early maltreatment19 we hypothesized that NR3C1 exon 1F hypermethylation would be higher among adolescents reporting social stressors (e.g., bullying that was previously reported to be associated with depressive psychopathology25) than among those without this experience.
Materials and methods
Study participants
Study participants consisted of children attending the seventh grade (13–14 years old) of compulsory school in both urban and rural areas of southern and central Sweden. The participant recruitment took place during the school years fall 2013–spring 2014 and fall 2014–spring 2015 as part of the KUPOL project26. For this project, a total of 3959 children, from 101 schools, and their legal guardians gave informed consent and answered the study questionnaires. Saliva samples were collected from a random subsample of 1315 consenting students, of which 1149 were successfully analyzed (Supplementary figure). Complete demographics, and detailed information on the KUPOL project, have already been published elsewhere26. The study was approved by the Stockholm Ethics Review Board, and written informed consent was obtained from all participants.
Assessment of internalizing symptoms
Internalizing symptoms were assessed by means of the Center for Epidemiologic Studies Depression Scale for Children (CES-DC), which evaluates symptoms of depression and anxiety (referred as internalizing symptoms), and was self-reported by the students. The score threshold for presence (yes/no) of symptoms was CES-DC ≥ 30, which has demonstrated high specificity in Swedish population samples27.
Psycho-social stressors
We considered some psycho-social events or conditions as predictors of epigenetic changes (NR3C1 hypermethylation). All information on these factors was self-reported in questionnaires either by the students (being bullied, lack of friends, alcohol and tobacco use, and cohabitation with parents) or by their parents (parental education, employment status, and birth country).
DNA sample preparation
Saliva samples (4 ml) were collected from the participants using a whole-saliva collection device (Oragene•DNA; DNA Genotek Inc., Ottawa, Canada). DNA was successfully extracted from 1304 saliva samples using a protocol that is based on magnetic bead separation according to chemagen technology (PerkinElmer) on a ChemagicStar®-robot (Hamilton), and was dissolved in 500 µl 10 mM Tris-HCl buffer, pH 8.0. DNA concentration and purity were measured by ultraviolet absorbance.
DNA methylation analysis
Bisulfite conversion of the DNA was performed using EZ-96 DNA Methylation-Gold™ MagPrep kit (Zymo Research Corporation; Irvine; USA) according to the manufacturer’s protocol and the processed DNA was stored at −20 °C until further analysis. A 162-base pair (bp) fragment, corresponding to the NR3C1 exon 1F region (NCBI reference sequence: NG_009062.1 (+36 416 to +36 577) from the transcriptional start site), was amplified using the PyroMark PCR Kit (Qiagen, Hilden, Germany) and the following primers: forward 5′-AGTTTTAGAGTGGGTTTGGAG-3′, reverse biotin-5′-CCCCCAACTCCCCAAAAA-3′. PCR conditions were as follows: 94 °C for 15 min, followed by 45 cycles of 94 °C for 30 s, 60 °C for 60 s and 72 °C for 30 s with a final extension of 10 min at 72 °C. For pyrosequencing the sequencing primer, 5′-GAGTGGGTTTGGAGT-3′, was used to sequence a 50 bp region, of the 162 bp PCR fragment, containing five CpG sites, including the NGFI-A-binding site at CpG3 (Fig. 1). Pyrosequencing was performed on a Pyromark Q96 device (Qiagen, Hilden, Germany) and data were analyzed using the Pyromark Q96 5.2.8 software. DNA methylation data that passed quality control for all CpGs were obtained from 1184 out of the 1304 samples, and used for subsequent analyses. The average between-plate coefficient of variation for CpG1–5 was estimated at 10.3% (range 4.5–16.2%) based on the following control samples prepared in duplicates: 0, 6, 9, and 12% methylated DNA controls, prepared by combining different dilutions of commercially available fully methylated and non-methylated genomic human HCT116 DKO DNA (Zymo Research Corporation). Ninety-eight percent of the samples were within 0–12% methylation at any of the CpG sites studied. The analyses were performed blinded to the phenotypes.
Fig. 1. The NR3C1 exon 1F sequence showing the CpG sites CpG1–5 (bold) whose DNA methylation levels were quantified in the present study.
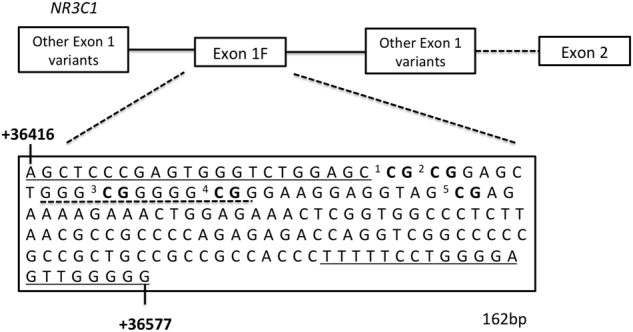
The CpG sites CpG1–5 correspond to the CpGs 35–39 analyzed in McGowan et al.11 and Melas et al.12. The broken underlined sequence corresponds to nerve growth factor-induced protein A (NGFI-A) binding site, as reported by McGowan et al.11. The solid underlined sequences correspond to the primers
Statistical analyses
The association between NR3C1 methylation and internalizing symptoms was assessed on a univariate level for all CpG sites separately. For all models the outcome was a binary indicator (yes/no) of symptoms on the individual level of the participant and the explanatory covariate was one of the CpGs. Due to the high number of participants having 0% methylation at a CpG site, data were categorized for each CpG into three groups, as follows: (1) unmethylated group: participants with no detectable methylation (0%); (2) low methylation group: methylation levels below the median of detectable methylation; and (3) high methylation group: methylation levels above the aforementioned median. Logistic regression analyses, with or without sex stratification, were performed with regard to the presence of internalizing symptoms according to the CES-DC scale, dichotomized at a cutoff of ≥30. In all analyses the unmethylated group constituted the reference. A sensitivity analysis was performed to estimate the probability of false positive CES-DC score vs methylation level score associations. We performed 10 000 permutations of random rearrangement of the links between CES-DC score and methylation level score. For each of the 10 000 simulations we calculated the association of methylation on depression, where after we compared the distribution of the simulated outcomes vs the actual outcomes from our data. In order to examine associations between NR3C1 methylation and potential stressors in the social environment, we performed regression analyses where the following covariates constituted the predictors of methylation level: experience of being bullied; lack of friends in school; current use of tobacco; use of alcohol during the past year; both parents with education below university level; at least one parent unemployed; at least one parent born outside Sweden; and parents not living together. In this analysis the outcome was NR3C1 methylation (low or high methylated vs unmethylated). Sex-specific analyses were not performed due to the limited sample size. All data management and statistical analyses were performed using Stata 14.2. All test assumptions were fulfilled. All statistical tests are two-sided.
Results
Prevalence of psychopathological symptoms
The analytical sample comprised 1149 grade 7 school children from 101 schools, i.e., those children recruited in the cohort who provided DNA samples that passed quality control and answered the CES-DC scale for the assessment of the psychopathological internalizing symptoms (Supplementary Figure). The prevalence of symptoms above the chosen cutoff was 13.8% in females and 2.5% in males (Table 1). A comparison of symptom prevalence between all participating adolescents and those in the sample included in this study showed very similar estimates (Table 1).
Table 1.
Prevalence of depressive psychopathological symptoms, by sex
| All males N = 1933 |
All females N = 2026 |
Males with DNA samples N = 524 |
Females with DNA samples N = 625 |
|
|---|---|---|---|---|
| CES-DC (depression-anxiety) | ||||
| Above threshold % | 2.5 | 14.4 | 2.5 | 13.8 |
| Below threshold % | 86.5 | 77.1 | 97.5 | 86.2 |
| Missing % | 11.0 | 8.5 | — | — |
The Kupol Study, Stockholm 2013–2015
CES-DC Center for Epidemiologic Studies Depression Scale for Children
Association between NR3C1 methylation and CES-DC internalizing symptoms
Eight percent to 51% of the sample showed methylation above 0% at the five CpG sites studied. The level of methylation (percent of DNA being methylated) in these samples was for the majority low, with medians spanning between 3 and 5% (Table 2). Since the distribution of methylation levels in our sample was very skewed toward zero we categorized it into three groups, the unmethylated group, the low methylation group with values between 0% and the median, and the high methylation group with values above the median. The mean and range of methylation level for each group and CpG site is presented in Supplementary Table S1. The NR3C1 high methylation group was significantly associated with internalizing symptoms, for CpG sites 1, 2, and 3 (odds ratio (OR) range: 1.93–2.46; Table 3). A separate analysis by sex showed that these associations were detected only in females (point-wise OR range: 2.06–2.70; Table 3). Any methylation at the CpG5 site had a tendency for association with depression symptoms among females, but the estimates were not formally statistically significant. The prevalence of internalizing psychopathological symptoms in boys was too low to detect possible sex differences in association to NR3C1 methylation.
Table 2.
NR3C1 methylation prevalence and group cutoffs
| CpG site | Number (%) of saliva samples with methylation > 0% for each CpG | Median methylation level (% DNA being methylated) among the samples with methylation > 0%a | ||
|---|---|---|---|---|
| Males N = 524 |
Females N = 625 |
Males | Females | |
| CpG1 | 87 (16.6) | 118 (18.9) | 3.1 | 3.9 |
| CpG2 | 64 (12.2) | 79 (12.6) | 3.4 | 3.7 |
| CpG3 | 261 (49.8) | 319 (51.0) | 3.6 | 3.4 |
| CpG4 | 107 (20.4) | 109 (17.4) | 3.2 | 3.7 |
| CpG5 | 40 (7.6) | 56 (9.0) | 4.3 | 4.8 |
The Kupol Study, Stockholm 2013–2015
aMedians used for categorizing an individual into low methylation group or high methylation group
Table 3.
Cross-sectional odd ratios (OR) and corresponding 95% confidence interval (CI) of CES-DC score above the threshold, indicating depression symptoms, according to NR3C1 methylation
| Methylation site, category | All N = 1149 |
Males N = 524 |
Females N = 625 |
|||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| CpG1 low | 1.47 | 0.75–2.87 | 0.72 | 0.09–5.68 | 1.77 | 0.84–3.70 |
| CpG1 high | 2.46 | 1.38–4.37 | ND | ND | 2.70 | 1.45–4.98 |
| CpG2 low | 1.23 | 0.55–2.77 | 1.10 | 0.34–8.70 | 1.34 | 0.54–3.32 |
| CpG2 high | 2.09 | 1.06–4.14 | ND | ND | 2.45 | 1.78–5.10 |
| CpG3 low | 1.09 | 0.63–1.87 | 0.69 | 0.14–3.47 | 1.12 | 0.62–2.01 |
| CpG3 high | 1.93 | 1.20–3.09 | 1.65 | 0.49–5.50 | 2.06 | 1.22–3.50 |
| CpG4 low | 1.45 | 0.57–2.29 | 1.45 | 0.31–6.81 | 1.31 | 0.59–2.91 |
| CpG4 high | 1.38 | 0.72–2.62 | 0.85 | 0.11–6.77 | 1.50 | 0.75–3.04 |
| CpG5 low | 1.94 | 0.85–4.47 | ND | ND | 2.42 | 0.99–5.92 |
| CpG5 high | 1.94 | 0.85–4.47 | ND | ND | 2.20 | 0.91–5.33 |
The Kupol Study, Stockholm 2013–2015
Reference category = unmethylated
ND not determined due to too few observations
In order to rule out the probability that we pick up false positive associations in our dataset, and that separation into low and high methylation groups was relevant for the type of analysis we did, we performed a sensitivity analysis with 10 000 permutations of random rearrangement of the links between CES-DC score and methylation level score. For each of the 10 000 simulations we calculated the association of methylation on development of internalizing symptoms. Next, we compared the distribution of the simulated outcomes vs the actual outcomes from our data. The results clearly showed that the associations of high methylation in CpG1 and CpG3 with CES-DC were very unlikely to be false positive, with estimates lying well above the 95th percentile of the distribution of the simulated estimates. Also the high methylation for CpG2 had results above the 95th percentile, albeit not as strong as the other two.
NR3C1 hypermethylation and potential social stressors
In order to explore the associations between NR3C1 methylation level and potential childhood environmental stressors we analyzed level of methylation at CpG1–5 and selected covariates indicative of childhood stress measured in the KUPOL study. The mean and range of methylation level for each group and CpG site are presented in Table S1. The analysis of NR3C1 methylation and putative environmental stressors showed that high methylation for CpG2 was significantly cross-sectionally associated with having been bullied or lack of friends (bullying: OR = 1.89, 95% confidence interval (CI) = 1.08–3.32; lack of friends: OR = 2.30, 95% CI = 1.09–4.86; Table 4). Although not statistically significant, the CpG sites 1, 4, and 5, also showed high methylation among those having been bullied (CpG1: OR = 1.26, 95% CI = 0.74–2.15; CpG4: OR = 1.24, 95% CI = 0.75–2.07; CpG5: OR = 1.79, 95% CI = 0.91–3.52; Table S4) and the CpG1 site showed high methylation among those lacking friends (CpG1: OR = 1.25, 95% CI = 0.58–2.70; Table S4). Means and range of methylation level for each CpG for bullied/not bullied as well as lacking friends/having friends are presented in Tables S2 and S3. None of the other putative stressors was associated with NR3C1 methylation at any site.
Table 4.
Cross-sectional odds ratio (OR) and corresponding 95% confidence interval of NR3C1 CpG site 2 methylation according to potential stressors
| Potential stressors | Proportion Non-methylated (%) | Proportion Low methylated (%) | ORlow vs zero, 95% CI | Proportion High methylated (%) | ORhigh vs zero, 95% CI |
|---|---|---|---|---|---|
| Bullying | |||||
| Yes N = 180 |
83.3 | 6.7 | 1.11, 0.59–2.12 |
10.0 | 1.89, 1.08–3.32 |
| No N = 949 |
88.1 | 6.3 | Ref | 5.6 | Ref |
| Friends in school | |||||
| No N = 74 |
78.7 | 9.3 | 1.76, 0.77–4.02 |
12.0 | 2.30, 1.09–4.86 |
| Yes N = 1061 |
88.2 | 5.9 | Ref | 5.9 | Ref |
| Currently using tobacco | |||||
| Yes N = 15 |
93.3 | 6.7 | 0.99, 0.13–7.70 |
0.0 | ND |
| No N = 1134 |
87.5 | 6.3 | Ref | 6.2 | Ref |
| Alcohol the past year | |||||
| Yes N = 21 |
85.7 | 14.3 | 2.39, 0.69–8.34 |
0.0 | ND |
| No N = 1117 |
87.6 | 6.1 | Ref | 6.3 | Ref |
| Both parents’ education below university | |||||
| Yes N = 315 |
87.0 | 5.7 | 0.92, 0.53–1.60 |
7.3 | 1.30, 0.77–2.18 |
| No N = 827 |
88.0 | 6.3 | Ref | 5.7 | Ref |
| At least one parent unemployed | |||||
| Yes N = 196 |
88.8 | 4.1 | 0.62, 0.29–1.31 |
7.1 | 1.20, 0.65–2.21 |
| No N = 936 |
87.6 | 6.5 | Ref | 5.9 | Ref |
| At least one parent born outside Sweden | |||||
| Yes N = 196 |
88.3 | 5.1 | 0.80, 0.40–1.60 |
6.6 | 1.08, 0.58–2.02 |
| No N = 903 |
87.6 | 6.3 | Ref | 6.1 | Ref |
| Parents not cohabiting | |||||
| Yes N = 199 |
87.5 | 6.5 | 1.08, 0.58–2.02 |
6.0 | 1.00, 0.52–1.90 |
| No N = 923 |
87.9 | 6.1 | Ref | 6.0 | Ref |
The Kupol Study, Stockholm 2013–2015
Discussion
The DNA methylation status of the GR gene, NR3C1, specifically hypermethylation of the human exon 1F, has in adults proved to be associated with retrospective traumatic events in childhood, including emotional, physical, or sexual abuse; neglect; early parental death; and other traumatic events11–13,15, reviewed in refs. 19,20. Methylation levels and effect sizes are mostly consistent between these studies also when results from brain tissue and peripheral tissue such as leukocytes and lymphocytes were compared. The latter was proposed to possibly reflect a stress response signal acting coordinately on multiple cell types19. NR3C1 exon 1F hypermethylation has been reported to also mediate the association between traumatic events and internalizing symptoms in preschoolers28 and to mediate an association between early adversity and borderline personality disorder in adults29. However, few studies have examined if NR3C1 methylation is associated with recent or current adversities or with psychopathology during adolescence.
In the present report, we investigated the association of NR3C1 methylation with depressive symptoms using a large sample of 13- to 14-year-old students in Sweden. The CpG sites that we analyzed are part of the NR3C1 exon 1F and two are within the canonical binding site of the transcription factor NGFI-A (CpG3 and CpG4). We found that hypermethylation of NR3C1, at CpG1, 2, and 3, was associated with self-reported internalizing symptoms in adolescents. Interestingly, in preschoolers hypermethylation at exon 1F CpG3 represented the strongest association with internalizing symptoms28 and hypermethylation at a CpG of a neighboring NGFI-A-binding site associated with internalizing symptoms in 4- to 16-year-old kids21. In previous studies of adults, hypermethylated exon 1F CpGs associated with retrospective early adversity has also commonly been located in a putative NGFI-A-binding site, reviewed by Palma-Gudiel et al.20. The low methylation levels reported for the exon 1F CpGs is in agreement with them being located in a strong promoter region. The associations in our study remained significant when we analyzed only the females. The prevalence of internalizing psychopathological symptoms in boys was too low to detect possible sex differences in association to NR3C1 methylation. However, sex differences in biological stress response pathways and cellular environment (e.g., sex hormones) may account for possible differences in NR3C1 hypermethylation as well as the different prevalence of depressive psychopathology23,30,31.
Finally, in order to generate new hypotheses on factors in the social environment that could induce the methylation of NR3C1, we performed an analysis of potential social and behavioral stressors. Adolescents reporting lack of friends and those reporting having been bullied were significantly more likely to harbor NR3C1 hypermethylation in CpG2, while the observed increase in methylation at CpG1, 4, and 5 was not statistically significant. Previously it was reported from adolescents aged 16–18 years that sexual abuse was associated with hypermethylation in exon 1F and 1H, whereas other life-threatening childhood or adolescence traumatic events associated with hypermethylation in exon 1H only, and milder stressful life events less strongly so22. A very recent study of genome-wide DNA methylation in relation to youth victimization in 1669 young persons, that is 50% more individuals than in our study, analyzed bullying but detected no genome-wide significant signal for bullying. Further, none of the 42 CpGs in NR3C1 studied passed gene-wide significance threshold for victimization32, indicating complexity and limited effect size in the influence of adversity exposure on the NR3C1 methylation, and possibly technical difficulties in analysis of low methylation levels.
This study had a number of limitations, the major one being the assessment of NR3C1 methylation using DNA from a peripheral tissue (saliva DNA). Still, previous studies on NR3C1 exon 1F methylation and early trauma have generated similar results by utilizing DNA from brain10,11, saliva12,18,28, and whole blood leukocytes13,14,16,22. In addition, the cross-sectional design prevented the study of the timing of methylation in relation to the timing of the stressful events and of the onset of symptoms. Therefore, the possibility that NR3C1 methylation preceded these events cannot be ruled out. We also lacked information about traumatic events occurring earlier in the lives of these individuals, some of which may have caused symptoms, rather than later occurring events as it was suggested from an analysis of maltreated children at age 528.To this end, longitudinal studies are needed in order to assess causal effects of stressful life events during adolescence on development of psychopathology later in life, as well as the possible mediator role of NR3C1 methylation in this process.
In summary, this study reports the novel finding that hypermethylation of NR3C1’s exon 1F is cross-sectionally associated with internalizing symptoms in a large population of early adolescent females. Moreover, bullying and lack of friends during early adolescence was associated with higher NR3C1 methylation levels, which supports the hypothesis of a molecular link between these types of stressors and internalizing psychopathological symptoms.
Electronic supplementary material
Acknowledgements
We thank all participating students and parents as well as the teachers, and the administrative and health-care staff of the participating school. We wish to acknowledge the hard and relentless work of the Kupol study’s operative staff, in particular the field coordinator Elin Arnö, the administrator Jon Edlund, and the research assistants Fanny Engman and Johanna Lindman without whom the study would have never been possible. The Kupol study is financially supported by a unique grant (nr 259-2012-48), which includes funds from the Swedish Research Council Formas, The Swedish Research Council for Health, Working Life and Welfare, The Swedish Research Council-Vetenskapsrådet, and VINNOVA.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-018-0169-8).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamin HS, Kertes DA. Cortisol and DHEA in development and psychopathology. Horm. Behav. 2017;89:69–85. doi: 10.1016/j.yhbeh.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 6.Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav. Genet. 2002;32:301–314. doi: 10.1023/A:1020258104318. [DOI] [PubMed] [Google Scholar]
- 7.Presul E, Schmidt S, Kofler R, Helmberg A. Identification, tissue expression, and glucocorticoid responsiveness of alternative first exons of the human glucocorticoid receptor. J. Mol. Endocrinol. 2007;38:79–90. doi: 10.1677/jme.1.02183. [DOI] [PubMed] [Google Scholar]
- 8.Cao-Lei L, et al. Transcriptional control of the human glucocorticoid receptor: identification and analysis of alternative promoter regions. Hum. Genet. 2011;129:533–543. doi: 10.1007/s00439-011-0949-1. [DOI] [PubMed] [Google Scholar]
- 9.Labonte B, et al. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol. Psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 11.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melas PA, et al. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. Int. J. Neuropsychopharmacol. 2013;16:1513–1528. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- 13.Perroud N, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl. Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields AE, et al. Childhood abuse, promoter methylation of leukocyte NR3C1 and the potential modifying effect of emotional support. Epigenomics. 2016;8:1507–1517. doi: 10.2217/epi-2016-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrka AR, et al. Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor gene promoter and exposure to adversity in preschool-aged children. Dev. Psychopathol. 2015;27:577–585. doi: 10.1017/S0954579415000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberlander TF, et al. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 17.Murgatroyd C, Quinn JP, Sharp HM, Pickles A, Hill J. Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Transl. Psychiatry. 2015;5:e560. doi: 10.1038/tp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyrka AR, et al. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: associations with early adversity and depressive, anxiety and substance-use disorders. Transl. Psychiatry. 2016;6:e848. doi: 10.1038/tp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol. Psychiatry. 2016;79:87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palma-Gudiel H, Cordova-Palomera A, Leza JC, Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: a critical review. Neurosci. Biobehav. Rev. 2015;55:520–535. doi: 10.1016/j.neubiorev.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Dadds MR, Moul C, Hawes DJ, Mendoza Diaz A, Brennan J. Individual differences in childhood behavior disorders associated with epigenetic modulation of the cortisol receptor gene. Child Dev. 2015;86:1311–1320. doi: 10.1111/cdev.12391. [DOI] [PubMed] [Google Scholar]
- 22.van der Knaap LJ, et al. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl. Psychiatry. 2014;4:e381. doi: 10.1038/tp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelman S, et al. Epigenetic and genetic factors predict women’s salivary cortisol following a threat to the social self. PLoS ONE. 2012;7:e48597. doi: 10.1371/journal.pone.0048597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thase, M. E. in Handbook of depression (eds Gotlib, I. H. & Hammen, C. L.) 187–217 (Guilford Press, New York, 2009).
- 25.Rettew DC, Pawlowski S. Bullying. Child Adolesc. Psychiatr. Clin. N. Am. 2016;25:235–242. doi: 10.1016/j.chc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Galanti MR, et al. School environment and mental health in early adolescence - a longitudinal study in Sweden (KUPOL) BMC Psychiatry. 2016;16:243. doi: 10.1186/s12888-016-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson G, von Knorring AL. Depression among Swedish adolescents measured by the self-rating scale Center for Epidemiology Studies-Depression Child (CES-DC) Eur. Child Adolesc. Psychiatry. 1997;6:81–87. doi: 10.1007/BF00566670. [DOI] [PubMed] [Google Scholar]
- 28.Parade SH, et al. Methylation of the glucocorticoid receptor gene promoter in preschoolers: links with internalizing behavior problems. Child Dev. 2016;87:86–97. doi: 10.1111/cdev.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Blanco A, et al. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J. Psychiatr. Res. 2014;57:34–40. doi: 10.1016/j.jpsychires.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu. Rev. Clin. Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 32.Marzi SJ. Analysis of DNA methylation in young people: limited evidence for an association between victimization stress and epigenetic variation in blood. Am. J. Psychiatry. 2018;175:517–529. doi: 10.1176/appi.ajp.2017.17060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


