Highlights
-
•
Analysis of complete capsule loci in all 18 serovars of A. pleuropneumoniae.
-
•
Novel insights into evolution of capsule loci in A. pleuropneumoniae.
-
•
Development of two mPCRs for comprehensive capsule typing.
Keywords: Diagnostic, mPCR, Capsule typing, A. pleuropneumoniae, Serovars
Abstract
Problems with serological cross-reactivity have led to development of a number of PCRs (individual and multiplex) for molecular typing of Actinobacillus pleuropneumoniae, the causative agent of porcine pleuropneumonia. Most of these assays were developed for detection of specific amplicons within capsule biosynthetic genes before the availability of complete sequences for the different serovars. Here we describe comparative analysis of the complete capsular loci for all 18 serovars of A. pleuropneumoniae, and development of two multiplex PCRs for comprehensive capsule typing of this important pig pathogen.
1. Introduction
Pleuropneumonia is an economically important disease causing considerable losses in the worldwide swine industry (Sassu et al., 2017). The causative agent, Actinobacillus pleuropneumoniae, can be differentiated into 2 biovars based on the requirement for nicotinamide adenine dinucleotide (NAD-dependent biovar 1; NAD-independent biovar 2); and subsequently into 18 serovars based on surface polysaccharides, mainly capsule (Bossé et al., 2018). The ability to discriminate between serovars is advantageous, as there are differences in geographical distribution that are not static (Gottschalk, 2015; Sassu et al., 2017), as well as differences in levels of virulence (Klitgaard et al., 2010). Thus, accurate typing is essential for diagnosis and for tracking the emergence of serovars rarely, or not previously, reported within a geographical region.
Although a number of serological tests are available for typing A. pleuropneumoniae isolates [see (Gottschalk, 2015) for a recent review], the need for high quality reference antisera limits the number of laboratories able to perform diagnostics, and even then, problems with cross reactivity between certain serovars are unavoidable. Increasingly, laboratories are using molecular typing methods to more accurately and reproducibly identify A. pleuropneumoniae isolates (Gottschalk, 2015; Sassu et al., 2017). PCRs have been developed for detection of specific CPS genes in most of the currently recognized 18 serovars (except 4, 9, 11, 13 and 14), either individually or in multiplex reactions, for detection of predominant serovars in a given geographical region (Angen et al., 2008; Bossé et al., 2014, 2017, 2018; Ito and Sueyoshi, 2015; Jessing et al., 2003; Schuchert et al., 2004; Turni et al., 2014). Some of these PCR assays were developed prior to the availability of whole genome sequences (wgs), and were based on (sometimes incomplete) sequences of the CPS biosynthetic loci.
The aim of this study was to comprehensively analyze the complete CPS loci for all known serovars of A. pleuropneumoniae, and to develop multiplex PCRs capable of their specific identification.
2. Materials and methods
2.1. A. pleuropneumoniae isolates used in this study
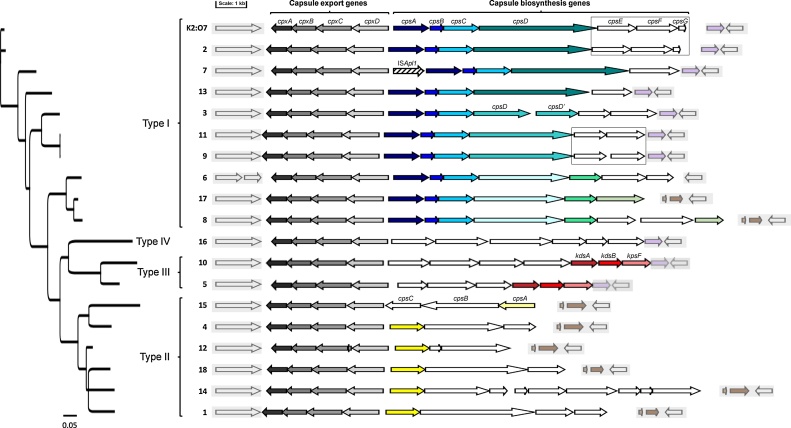
All sequences used in this study are shown in Table 1, with accession numbers shown for previously published whole genomes and for the full CPS loci of serovars lacking full genome sequences in Genbank. In this study, wgs data was generated for the reference strains of serovars 13 (N273), 14 (3906), and 15 (HS143), and for two isolates of K2:O7 (7317/84 and 9712534), as previously described (Bossé et al., 2018). The regions of whole genome sequences containing the complete CPS loci (export and biosynthetic genes) were identified initially by using tBLASTn (http://blast.ncbi.nlm.nih.gov /Blast.cgi) to identify the cpxD gene (accession AIA09380) common to all A. pleuropneumoniae serovars. The complete CPS loci, found between the genes modF and ydeN in all serovars, were extracted for further analysis using BLASTn and BLASTx. Multiple sequence alignments were performed using ClustalW, and a schematic representation of each locus was generated using Gene Graphics (Harrison et al., 2017), with a Neigbour-Joining tree constructed using the Tamura-Nei algorithm with 1000 bootstraps (Fig. 1).
Table 1.
Accession numbers for APP sequences used in this study.
| Serovar | Strain | Accession | Reference |
|---|---|---|---|
| 1 | 4074a | ADOD00000000 | Xu et al. (2010) |
| 1 | KL 16 | CP022715 | Park et al. (2017) |
| 2 | 4222 | ADXN00000000 | Zhan et al. (2010) |
| 2 | S1536 | ADOE00000000 | Xu et al. (2010) |
| K2:07 | 7317/84 | MG868951 | This study |
| K2:07 | 9712534 | MG868952 | This study |
| 3 | JL03 | CP000687 | Xu et al. (2008) |
| 4 | M62 | ADOF00000000 | Xu et al. (2010) |
| 5b | L20 | CP000569 | Foote et al. (2008) |
| 6 | Femø | ADOG00000000 | Xu et al. (2010) |
| 7 | AP76 | CP001091 | |
| 7 | S8 | ALYN00000000 | Li et al. (2012) |
| 8 | MIDG2331 | LN908249 | Bossé et al. (2016) |
| 9 | CVJ13261 | ADOI00000000 | Xu et al. (2010) |
| 10 | D13039 | ADOJ00000000 | Xu et al. (2010) |
| 11 | 56153 | ADOK00000000 | Xu et al. (2010) |
| 12 | 1096 | ADOL00000000 | Xu et al. (2010) |
| 13 | N273 | MG868947 | This study |
| 14 | 3906 | MG868948 | This study |
| 15 | HS143 | MG868949 | This study |
| 16 | A-85/14 | MG868950 | This study |
| 17 | 16287-1 | MG780416 | This study |
| 18 | 7311555 | MG780423 | This study |
The genome listed as serovar 13 str. N273, accession number ADOM00000000, is actually a serovar 7 isolate.
The genome listed as serovar 1 str. 4074, accession number AACK00000000, is actually a serovar 5 isolate.
Fig. 1.
Schematic comparison of the complete capsule loci of A. pleuropneumoniae serovars 1–18 and K2:O7. The capsule loci are arranged according to phylogenetic similarity, as indicated by the tree on the left, and are clustered into their respective CPS types (I–IV) as indicated by the labeled brackets. All loci are flanked by the modF gene at the start (white arrow in shaded grey box; note an internal stop codon is present in the serovar 6 modF sequence), and ydeN at the end, preceded either the by the 552–555 bp hypothetical gene or the 114 bp hypothetical and partial lysA genes (white arrow, preceded by either mauve or brown arrows, respectively, in shaded grey box). The capsule export genes, cpxABCD, are indicated as reverse oriented arrows shaded black to light grey, respectively. The genes of the respective serovar CPS biosynthetic loci are named as follows: cps2ABCDEFG (for both K2:O7 and serovar 2); cps7ABCDE, cps13ABCDE, cps3ABCDD’EF, cps11ABCDEF, cps9ABCDEF, cps6ABCDEFG, cps17ABCDEF, cps8ABCDEFGH, cps16ABCDEF, cps10ABCD kdsAB kpsF, cps5ABC kdsAB kpsF, cps15ABC, cps4ABC, cps12ABB’, cps18ABC,cps14AB1B2B3CDEFG, cps1ABCD. The core cpsABC genes conserved in all of type I CPS loci are indicated as the dark, medium, and light blue arrows, respectively (note the extra gene at the start of the serovar 7 biosynthetic locus, shown as a striped arrow, indicates the ISApl1 insertion present in the AP76 strain, and is not part of the biosynthetic locus). The cpsD genes in the type I loci are indicated in different shades of teal, according to similarity greater than 50% identity (note in serovar 3, an internal stop codon has resulted in two orfs, cps3D and cps3D’). In serovars 6, 17 and 8, the cpsE genes share >80% identity and are shown as bright green arrows; and the last gene in the serovar 8 locus, cps8H, shares 94% identity with the C-terminal half of the serovar 17 cpsF gene, as indicated by the olive shaded arrows in the respective loci. The core genes in the type III CPS loci (kdsAB and kpsF) are indicated by the dark red, bright red, and pink arrows, respectively, at the ends of the serovar 5 and 10 loci. In the type II loci, the conserved core cpsA gene is shown as a yellow arrow. The white arrows in each biosynthetic locus indicate genes unique to each serovar. As expected, the K2:O7 CPS locus shares 96% identity across the entire sequence with that of serovar 2 (with nucleotide differences being mainly found in the cpsABC genes), and the specific cps2EFG genes found at the ends of both of these loci are boxed. Serovars 9 and 11 share 99% identity across their cpsEF genes (also shown boxed), with only a single nucleotide difference resulting in an alternate start codon for the cpsF gene in each locus. The Neighbour-Joining tree shown at the left of the figure was constructed using the Tamura-Nei algorithm with 1000 bootstraps, and the width of the line underneath it shows a 5% nucleotide difference (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
The complete capsule loci (with flanking sequences modF and ydeN) for the reference strains of serovars 13–16, and for the two isolates of K2:O7 (7317/84 and 9712534) have been deposited in GenBank under accession numbers MG868947 to MG868952.
2.2. Diagnostic PCRs
The primer pairs used in APP-mPCR1 for specific detection of serovars 1–12 and 15, along with the common apxIV amplicon used in previous mPCRs for species level detection of A. pleuropneumoniae, are shown in Table 2. Some of the primer pairs were used in our previous mPCR (Bossé et al., 2014), whereas new pairs were designed, either to improve specificity for previously tested serovars (e.g. 3, 6, 7, and 8), or for serovars not previously included in our mPCR. All primers were designed to amplify sequences specific to the relevant serovar, with generation of amplicons of different sizes to allow sufficient separation of all amplicons by gel electrophoresis in 1.5% agarose. The specificity of all primers were initially tested in individual PCRs using genomic DNA from the homologous serovar reference strains, followed by incorporation of all primers into multiplex format, using the Qiagen Multiplex PCR Plus kit as previously described (Bossé et al., 2014). APP-mPCR1 was then tested with all of the 18 A. pleuropneumoniae serovar reference strains (i.e. 4074T, 1536, S1421, M62, K17, L20, Femø, WF83, 405, CVJ13261, D13039, 56153, 8328, N-273, 3906, HS143, A-85/14, 16287-1, and 7311555, respectively), as well as a set of clinical isolates comprising 2–5 isolates of each of the 18 A. pleuropneumoniae serovars, and 31 other porcine-associated bacterial species, used in previous studies (Bossé et al., 2017; 2018). Furthermore, as our previous mPCR (Bossé et al., 2014) was found to detect both serovar 2 and 8 amplicons using DNA from the K2:O7 isolates 7317/84 and 9712534, we specifically compared our new APP-mPCR1 to our previous mPCR for detection of specific amplicons from these two isolates.
Table 2.
APP-mPCR1 primers for detection of serovars 1–12 and 15.
| Primer name | Sequence | Target genea | Amplicon size | Reference or source |
|---|---|---|---|---|
| AP1F | CTGGAGTAATTACGGCGACTATTCC | cps1B | 959 | Bossé et al. (2014) |
| AP1R | AGGAGAAGCTAGTAGTACTTGCATTTTC | cps1B | ||
| AP2F | GAGTGTGATGATGATGCTCTGGTTC | cps2E | 247 | Bossé et al. (2014) |
| AP2R | TACCAATAACTGTTGCAACTAACGC | cps2E | ||
| AP3F | TTGTAGAGCCCGCCAGATTTACG | cps3F | 500 | This study |
| AP3R | CATTCGCACCAGCAATCACC | cps3F | ||
| AP4F | CAGCATGGGTTTGGTCCTGTTG | cps4B | 204 | This study |
| AP4R | GGCTTTCTCCGTGTATGAATAAAGTG | cps4B | ||
| AP5F | AGCCACAAGACCCGAATGGTATAATG | cps5B | 825 | Bossé et al. (2014) |
| AP5R | CCATCAAATGCAGCTTCAAGGAGC | cps5B | ||
| AP6F | TGACTGGCTTCGTGAAAATGAG | cps6F | 718 | This study |
| AP6R | GTCTGAAGTTTTATTCGCAGCTCC | cps6F | ||
| AP7F | TTGGAATGGATTCATGATTGGGC | cps7E | 601 | Bossé et al. (2014) |
| AP7R | CGGAAATGGCCTATTGAAAAACG | cps7E | ||
| AP8F | ACATCCAAGCCGTTCTCCAG | cps8F | 1126 | This study |
| AP8R | CATCCATGAGCCAATGAGGG | cps8G | ||
| AP9F | GTAGGACGTGGTAACATTGAGGC | cps9E | 2105 | This study |
| AP9R | ACGGGTGCAATTTCTAAAGCTG | cps9F | ||
| AP10F | GGTGGTGATGGAACAAGGTTATGG | cps10A | 183 | Bossé et al. (2014) |
| AP10R | CTGTAATTGATGCGAAATAGTAGATTGGTGC | cps10A | ||
| AP12F | TAAAGGTATTATAACGCCGGCTCT | cps12A | 347 | Bossé et al. (2014) |
| AP12R | CTCCCATCTGTTGTCTAAGTAGTAG | cps12A | ||
| AP15F | GCAACTTGGAGAACATGGTTAAATCAAG | cps15B | 1595 | This study |
| AP15R | CAACCCTCCAATGTAAGCGAAGG | cps15C | ||
| apxIVA1 | TTATCCGAACTTTGGTTTAGCC | apxIV | 418 | Zhou et al. (2008) |
| apxIVA3 | CATATTTGATAAAACCATCCGTC | Intergenicb |
For each serovar capsule locus, the biosynthetic genes are labeled alphabetically starting from A in each case.
Intergenic region immediately downstream of apxIVA.
The primer pairs used in APP-mPCR2 for specific detection of A. pleuropneumoniae serovars 13–14, and 16–18, along with the species specific apxIV amplicon and the full length nadV gene found in biovar 2 isolates, are shown in Table 3. The specificity of these primers was evaluated as for the mPCR1 primers above.
Table 3.
APP-mPCR2 for detection of serovars 13, 14, and 16–18.
| Primer name | Sequence | Target genea | Amplicon size | Reference or source |
|---|---|---|---|---|
| AP13F | GTTGTGTATCGAGGTTGGCATTTC | cps13E | 665 | This study |
| AP13R | ATGTAAAGGATCTAAGCCGTGTG | cps13E | ||
| AP14F | TGCATTACGCTTATATTCTGAATGG | cps14G | 1911 | This study |
| AP14R | TTGTCGATCGAGAGGGAGTAACG | ydeN | ||
| AP16F | TTACTCACTTGGGCTAGGGATAG | cps16C | 212 | Bossé et al. (2017) |
| AP16R | ACCAGCAATATGATTACGCCC | cps16D | ||
| AP17F | TTGTAATGGCGGTGTAATGCTAC | cps17F | 302 | Bosse et al. (2018) |
| AP17R | CATAAGTGCAGCCATCTCTTTCAG | cps17F | ||
| AP18F | CGGAGTTTGGCAGCATAAAGG | cps18B | 514 | Bosse et al. (2018) |
| AP18R | CCATAATCGGTGCTCAACTAAGAATG | cps18B | ||
| nadVF | CTCACTAAACAAAACTCTGCGTTC | nadV | 1339 | This study |
| nadVR | TTCGGATGACAGAACTTTTACCCG | nadV | ||
| apxIVA1 | TTATCCGAACTTTGGTTTAGCC | apxIV | 418 | Zhou et al. (2008) |
| apxIVA3 | CATATTTGATAAAACCATCCGTC | Intergenicb |
For each serovar capsule locus, the biosynthetic genes are labelled alphabetically starting from A in each case.
Intergenic region immediately downstream of apxIVA.
3. Results and discussion
Initial analyses of the CPS loci in publically available genomes of A. pleuropneumoniae (Table 1) revealed that sequences previously deposited in Genbank as Shope 4074 (accession AACK00000000) and N273 (accession ADOM00000000) appear to be incorrect, having CPS loci matching serovars 5 and 7, respectively. The serovar 1 CPS locus is found in the genome of Shope 4074 (accession ADOD00000000), as well as in the recently closed genome of strain KL 16 (accession CP022715) (Xu et al., 2010; Park et al., 2017). The correct serovar 13 CPS locus (accession number MG868947) was identified in the draft genome of N273 that was generated as part of this study, and although similar to the serovar 7 locus (Fig. 1), the cps13D gene encodes a predicted protein that shares only 43% identity with that of the Cps7D CDP-glycerol-glycerophosphate glycerophosphotransferase protein. Furthermore, the cps13E gene shows no homology to any other sequence in Genbank at the nucleotide level, and the encoded protein shares limited identity (<35%) with hypothetical proteins from various Gram-positive species (e.g. accession number WP_010632822).
Although sequences for the CPS loci of serovars 14, 15, and 16 have previously been deposited in Genbank (accession numbers AB810251, AB701753, KX907602), we have extended these sequences (accession numbers MG868948, MG868949, MG868950) to encompass the complete CPS loci in order to allow a more thorough comparison with the other serovars. Additionally, we have generated draft genomes of two serovar K2:O7 isolates in order to compare their CPS loci (accession numbers MG868951 and MG868952) to those in the two published serovar 2 genomes (accession numbers ADXN00000000 and ADOE00000000) (Xu et al., 2010; Zhan et al., 2010). Although the genes in the K2:O7 CPS loci encode the same proteins as in the serovar 2 CPS loci, there are differences at the nucleotide level that explain amplification of both serovar 2 and 8 amplicons for K2:O7 isolates using our previously designed mPCR (Bossé et al., 2014). In that assay, the serovar 8 primers were designed to amplify an 1106 bp fragment spanning parts of the cpsAB genes, and were specific when tested using a large number of clinical isolates, including 45 serovar 2 and 115 serovar 8 isolates (Bossé et al., 2014), however no K2:O7 isolates were tested at that time. Furthermore, we recently found that these serovar 8 primers also produced amplicons from some, but not all, serovar 17 isolates (Bossé et al., 2018). Before redesigning new primers for specific detection of serovar 8, as well as designing primers for detection of specific capsule genes in serovars 4, 9, 11, and 14 (for which there are currently no molecular diagnostics), we thoroughly analysed the complete CPS loci of all known A. pleuropneumoniae serovars.
In each serovar, the complete CPS locus is found between the genes modF, encoding a putative molybdenum transport ATP-binding protein, and ydeN, encoding a predicted serine hydrolase (Fig. 1). The CPS export genes, cpxABCD, are transcribed divergently from the CPS biosynthetic genes for all but serovar 15, where the export genes are immediately downstream of the biosynthetic genes, in the same orientation (Ito and Sueyoshi, 2014). The organization of the CPS export genes next to the biosynthetic genes is similar in other members of the Pasteurellaceae, as well as in other Gram-negative bacteria, such as Escherichia coli and Neisseria meningitidis, suggesting a common molecular origin for these loci (Boulnoisl and Jann, 1989; Frosch et al., 1991; Lâm et al., 2011). In many of these bacteria, a third region containing genes involved in post-polymerization modifications/transport is found on the other side of the biosynthetic locus, such that the central biosynthetic genes (region II) are variable, whilst the flanking regions I and III are constant in a given species (Boulnoisl and Jann, 1989; Frosch et al., 1991). In A. pleuropneumoniae, only regions I and II are contiguous, whereas genes encoding proteins sharing 63% and 66% identity, respectively, with HcsA and HcsB (encoded by region III of Haemophilus influenzae) are found elsewhere in the chromosome (accession numbers WP_039709373 and WP_005610771).
The CPS biosynthetic loci of the different A. pleuropneumoniae serovars can be grouped into four types (Fig. 1), with common core genes identified in each for types I-III, and type IV only found in serovar 16 (Xu et al., 2010; Bossé et al., 2017). The most common are the type I loci, found in serovars 2, 3, 6, 7, 8, 9, 11, 13, and 17 (Xu et al., 2010; Bossé et al., 2018), which produce teichoic acid-type polymers with phosphodiester linkages joining repeating glycosyl-glycitol units (Perry et al., 1990; MacLean et al., 2004). The core cpsABC genes of the type I loci are well conserved, sharing a minimum of 88% identity, and encode a CDP-glycerol glycerophosphotransferase, a glycerol-3-phosphate cytidylyltransferase, and a protein of unknown function, respectively. Although less well conserved, the type I cpsD genes all encode proteins with a glycosyl transferase domain (pfam04464) and a glycosyl transferase group 1 domain (either pfam00534 or pfam13692), that are, along with the products of the cpsABC genes, predicted to be involved in synthesis of the teichoic acid-type polymers characteristic of type I CPSs. In serovar 3, the cpsD gene has been split into two open reading frames due to an internal stop codon. It is not known if the second orf (cpsD’) is translated, as there is no obvious ribosomal binding site preceding the start. The sequences spanning cpsD and cpsD’ in serovar 3 share 86% identity with the cpsD genes found in serovars 9 and 11; the cpsD gene from serovar 2 shares 76% identity with that of serovar 7, but only 56% with that of serovar 13; and the cpsD gene from serovar 8 shares 96% and 98% identity with those from serovars 6 and 17, respectively. Serovars 6, 8 and 17 further share a common cpsE gene (84% identity for the serovar 6 gene compared to the serovar 8 and 17 genes, which share 99% identity). Additionally, the last gene of the serovar 8 locus (cps8H) shares 90% identity with the final 911 bases of the 1548 bp cps17F gene, as previously noted (2).
Type II loci are found in serovars 1, 4, 12, 14, 15, and 18 (Xu et al., 2010; Ito and Sueyoshi, 2014; Ito, 2015; Bossé et al., 2018), and produce repeating oligosaccharide polymers with phosphate linkages, as shown for serovars 1, 4, 12, and 15 (Perry et al., 1990; 2011). The conserved cpsA genes in these loci (99% identical for all but cps15A, which shares only 65% identity) all encode a capsular polysaccharide phosphotransferase which likely mediates the phosphate linkages characteristic of type II CPSs. Serovars 5 and 10 have type III loci and produce glycosidically linked sugar polymers (Perry et al., 1990), with the common core genes (kdsA, kdsB and kpsF, found at the 3′ end of theses loci) encoding 2-dehydro-3-deoxyphosphooctonate aldolase, 3-deoxy-manno-octulosonate cytidylyltransferase, and arabinose 5-phosphate isomerase, respectively. The serovar 16 CPS locus (type IV) is entirely unique (Bossé et al., 2017), and the structure of this CPS has not yet been determined.
All of the type II loci have a 114 bp orf followed by a partial lysA gene (of varying length) upstream of ydeN (Fig. 1) that are not likely involved in CPS synthesis. These sequences are also found in the type I loci of serovars 8 and 17; whereas all the remaining serovars with type I, III and IV loci, have a 552–555 bp gene encoding a hypothetical protein of unknown function (though again, not likely involved in CPS synthesis) immediately upstream of ydeN, except for the serovar 6 reference strain Femø, where ydeN immediately follows the last cps gene (Fig. 1). These genes preceding ydeN delineate the 3′ boundaries of the different CPS biosynthetic loci in A. pleuropneumoniae, and give an indication of possible evolutionary origins.
There are terminal inverted repeats (IRs) of 35–46 bp, containing a central IR sequence, flanking all of the A. pleuropneumoniae capsule loci that end with the 114 bp orf and partial lysA gene. Along with the atypically low GC content of the A. pleuropneumoniae CPS loci (especially the biosynthetic genes that have GC contents around 10% lower than respective genomes), the presence of IRs at the boundaries are indicative of the CPS loci being insertion elements acquired by horizontal transfer (Darmon and Leach, 2014). All A. pleuropneumoniae serovars, regardless of capsule type, have at least part of the 46 bp sequence TAAAGGAAATCCCCc/tTCTTTAGTAAAGAGGGg/aTTAGGGGAGATTTG
(lower case letters indicating differences in some serovars at these bases; IR sequence underlined) downstream of modF, along with an almost perfect repeat of the central IR (CCCCTCTTTGCTAAAGAGGGG) close to the 3′ end of cpxA, suggesting it may be a rho-independent terminator for this gene. In serovars 1, 9, 11, and 18, there may have been recombination between the two copies of the IR such that only a single copy is found, with 35 of the 46 bp sequence conserved (TAAAGGAAATCCCCCTCTTTAGTAAAGAGGGGGGAT) upstream of cpxA in these serovars. An almost identical, but inverted, 46 bp sequence (CAAATCTCCCCTATCc/aCCTCTTTACTAAAGAGGGGg/aATTTCCTTTA) is found downstream of the final CPS biosynthetic gene in all loci that are followed by the 114 bp orf and partial lysA gene, whereas in serovars with CPS loci followed by a 552–555 bp gene, no evidence of this sequence is seen. These data suggest that, in A. pleuropneumoniae, a type II CPS locus was the first to be acquired by horizontal transfer, with subsequent diversification of the other biosynthetic loci via gene deletion and/or acquisition of other capsule genes by horizontal transfer and homologous recombination via conserved flanking sequences.
The A. pleuropneumoniae serovar 14 type II CPS locus was previously shown to be almost identical to that found in Actinobacillus suis K1 strains such as ATCC 33415 (accession CP009159) (Ito, 2015). As in A. pleuropneumoniae, the CPS export genes in A. suis are downstream of modF. The biosynthetic genes in A. suis are followed by the same 114 bp orf as in the A. pleuropneumoniae type II CPS loci. However, in A. suis, this orf is followed by a complete lysA gene (1251 bp), and three further genes (gst, hemN, and a 600 bp gene encoding a putative nucleoside-diphosphate-sugarepimerase), prior to the ydeN gene. This variation in gene arrangement allows differentiation of these two species by diagnostic PCR (see below). In A. pleuropneumoniae, the five genes found upstream of ydeN in A. suis are found elsewhere in the genome (downstream of frdD). These data suggest that A. pleuropneumoniae serovar 14 may have acquired the A. suis CPS locus en bloc, with the resulting duplication of the 114 bp orf and part of the lysA gene. The other A. pleuropneumoniae serovars with type II CPS loci have reduced complexity of CPS genes compared to serovar 14, with only the cpsA gene conserved, and the other genes specific for each serovar.
Previously, the most complex mPCR for typing of A. pleuropneumoniae contained primers for detection of serovars 1–3, 5–8, 10 and 12, with the addition of primers for apxIV to allow detection of other serovars not included (Bossé et al., 2014). Now, with a total of 18 serovars, plus the species-specific apxIV amplicon, it was technically challenging to accurately resolve all of the products in a single mPCR. We therefore developed two mPCRs (APP mPCR1 and APP mPCR2), with the first capable of detecting serovars 1–12 and 15, and the second for detection of serovars 13, 14, and 16–18. All primer sequences for APP mPCR1 and APP mPCR2 are shown in Table 2, Table 3. To reduce the risk of non-specific priming, all primers were designed to have a Tm of 58–63 °C.
We kept some of the primer pairs from our previous 9-serovar mPCR (Bossé et al., 2014), including those for detection of serovars 1, 2, 5, 10 and 12, but revised our selection of primers for serovars 3, 6, 7, and 8 in order to target the more serovar-specific genes towards the end of these biosynthetic loci. To maintain good size separation of products in the revised mPCR, the new serovar 3, 6, 7, and 8 primers were designed to produce similar sized amplicons as the previous primer pairs. To this new mPCR (APP mPCR1), we added primers for detection of a 1595 bp fragment spanning the cpsBC genes of serovar 15. As there have not previously been diagnostic PCRs for the capsule genes of serovars 4, 9, or 11, we analyzed their biosynthetic loci in order to find appropriate specific priming sites. For serovar 4, primers were designed to amplify a unique 204 bp sequence at the 3′ end of the cpsB gene. Alignments of the complete CPS loci (including the export genes and flanking sequences to modF and the 555 bp gene) of the serovar 9 and 11 reference strains revealed the only difference is a single base deletion in the final cps gene in serovar 11, such that the reading frame ahead of the deletion shifted to use an alternate start codon. In serovar 9, the cpsF gene is 1146 bp, and that of serovar 11 is 1242 bp. The encoded proteins have the same C-terminal 349 AAs. It is possible that the altered N-terminal AAs are responsible for the slight differences in the CPS structures of these serovars reported by Perry et al. (Perry et al., 1990). These serovars also share an almost identical LPS O-antigen (Perry et al., 1990), and serologically, it is difficult to distinguish serovars 9 and 11. As these serovars also produce the same complement of Apx toxins (Frey, 1995), there may be little value in distinguishing them. The primers we have designed for combined detection of serovars 9/11 amplify a 2105 bp fragment spanning their cpsEF genes.
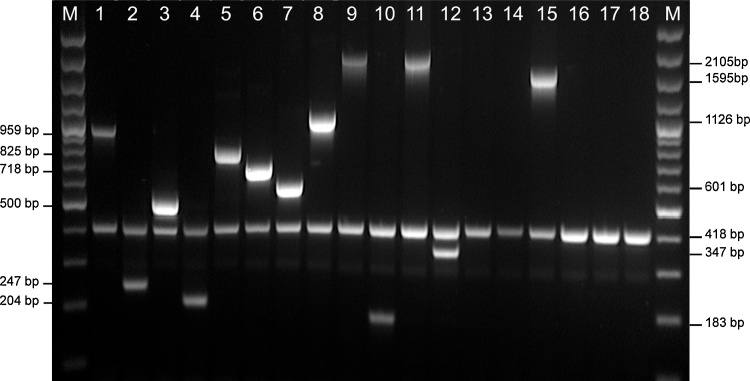
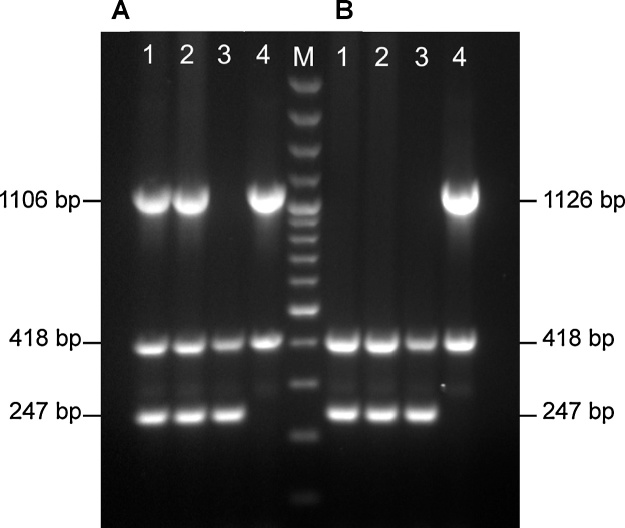
The new APP-mPCR1 detects amplicons from serovars 1–12 and 15, ranging in size from 204 to 2105 bp, plus the 418 bp apxIV amplicon as an internal control for detection of all A. pleuropneumoniae serovars, as shown for each of the A. pleuropneumoniae reference strains tested (Fig. 2). The specificity of this mPCR was further demonstrated using DNA prepared from our collection of clinical A. pleuropneumoniae isolates, other Actinobacillus species, other Pasteurellaceae, and other major pathogens of pigs, in addition to virtual PCRs using all available genomes of the species investigated, where available, as previously described (Bossé et al., 2017). All A. pleuropneumoniae isolates produced the 418 bp apxIV amplicon in addition to the appropriate serovar-specific bands for each of serovars 1–12 and 15. Furthermore, DNA from the K2:O7 isolates 7317/84 and 9712534, that produced amplicons for both serovars 2 and 8 (and apxIV) with our previous 9-serovar mPCR, produced only the serovar 2 and apxIV amplicons in the new APP-mPCR1 (Fig. 3). All non-A. pleuropneumoniae isolates were negative for all amplicons (data not shown).
Fig. 2.
Serovar-specific detection of amplicons from A. pleuropneumoniae serovars 1–12 and 15 by APP mPCR1. An apxIV (418-bp) amplicon is detected in all 18 serovar reference strains. Lane M contains molecular size markers (100-bp Plus DNA Ladder; Invitogen). Lanes 1 to 18 contain the following strains: 1, 4074 T; 2, S1536; 3, S1421; 4, M62; 5, L20; 6, Femø; 7, WF83; 8, 405; 9, CVJ13261; 10, D13039; 11, 56153; 12, 8329; 13, N-273; 14, 3906; 15, HS143; 16, A-85/14; 17, 16287-1; 18, 7311555.
Fig. 3.
Comparison of previous mPCR (8), and new APP mPCR1 for detection of A. pleuropneumoniae serovars 2, 8, and K2:O7. The K2:O7 isolates (7317/84 in lane 1; 9712534 in lane 2) produced amplicons indicative of both serovars 2 and 8 with the previous mPCR (A), but only the correct serovar 2 amplicon with APP mPCR1 (B). Lanes 3 and 4 contain serovar 2 strain S1536 and serovar 8 strain 405, respectively. Lane M contains molecular size markers (100-bp Plus DNA Ladder; Invitogen).
For development of the new APP-mPCR2, we combined the same apxIV primers used in APP-mPCR1 with our previously designed primers for detection of the most recently identified serovars 16, 17 and 18 (212, 302, and 514 bp amplicons, respectively) (Bossé et al., 2017; 2018). To this mPCR, we added primers for detection of serovars 13 and 14. As mentioned at the beginning of this discussion, the serovar 13 CPS locus contains a unique gene, cps13E, not seen in any other sequenced bacterium, so we designed primers to detect a 665 bp fragment of this gene. The analysis of the serovar 14 CPS locus, as discussed above, indicated that primers designed to detect any of the cps genes would also detect the related swine pathogen, A. suis. However, by designing primers to amplify a 1911 bp fragment from cps14G to ydeN, it is possible to differentiate this sequence from A. suis, where the ydeN gene is almost 6 kb downstream of the final cps gene. Since serovars 13, 14, and 17 are predominantly biovar 2, with biovar 1 isolates also reported for serovars 13 and 17 (Bossé et al., 2018; Gottschalk, 2015; Sassu et al., 2017), we added primers for detection of a 1339 bp fragment of the nadV gene, which confers NAD-independence.
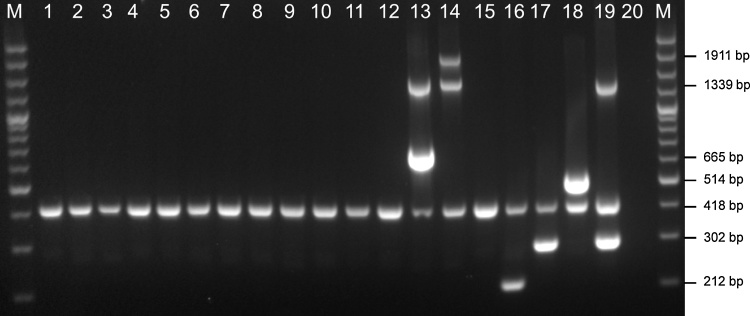
Thus, the new APP-mPCR2 detects amplicons for apxIV, nadV, and serovars 13–14 and 16–18, ranging in size from 212 to 1911 bp. Specific detection of these amplicons was demonstrated using DNA from each of the A. pleuropneumoniae reference strains (Fig. 4), as well as the same set of samples used above for validation of the APP-mPCR1. Again, all non-A. pleuropneumoniae isolates were negative for all amplicons (data not shown). Comparison of biovar 1 and 2 isolates of serovar 17 show that the inclusion of the nadV primers in this mPCR facilitate differentiation of these isolates (Fig. 4). Lack of detection of a nadV amplicon in NAD-independent non-A. pleuropneumoniae isolates, such as A. suis, indicates that the primer sequences used are specific for the A. pleuropneumoniae nadV gene.
Fig. 4.
Serovar-specific detection of amplicons from A. pleuropneumoniae serovars 13–14 and 16–18 by APP mPCR2. An apxIV (418-bp) amplicon is detected in all 18 serovar reference strains, and an additional 1339 bp nadV amplicon is detected only in the biovar 2 strains. Lane M contains molecular size markers (100-bp Plus DNA Ladder; Invitogen). Lanes 1 to 18 contain the following strains: 1, 4074 T; 2, S1536; 3, S1421; 4, M62; 5, L20; 6, Femø; 7, WF83; 8, 405; 9, CVJ13261; 10, D13039; 11, 56153; 12, 8329; 13, N-273; 14, 3906; 15, HS143; 16, A-85/14; 17, 16287-1; 18, 7311555. Lane 19 contains the biovar 2 serovar 17 isolate, 14-022, and Lane 20 contains A. suis CCM 5586T.
In conclusion, we have developed two mPCRs, APP-mPCR1 and APP-mPCR2, for specific detection of all 18 known serovars of A. pleuropneumoniae. Inclusion of primers for detection of apxIV in both mPCRs provides an internal control for species-specific detection of all A. pleuropneumoniae serovars. In general, we would recommend testing samples with APP-mPCR1 first. Any samples producing a positive apxIV band, but none of the amplicons for serovars 1–12 or 15, should then be tested using APP-mPCR2.
Funding
This work was supported by a Longer and Larger (LoLa) grant from the Biotechnology and Biological Sciences Research Council (grant numbers BB/G020744/1, BB/G019177/1, BB/G019274/1 and BB/G018553/1), the UK Department for Environment, Food and Rural Affairs, and Zoetis awarded to the Bacterial Respiratory Diseases of Pigs-1 Technology (BRaDP1T) consortium. MTGH was supported by the Wellcome Trust (grant number 098051). RS and LF were supported by the Hungarian Scientific Research Fund (OTKA 112826). Genome sequencing provided by MicrobesNG (www.microbesng.uk) was supported by the BBSRC (grant number BB/L024209/1).
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgements
The BRaDP1T Consortium comprises: Duncan J. Maskell, Alexander W. (Dan) Tucker, Sarah E. Peters, Lucy A. Weinert, Jinhong (Tracy) Wang, Shi-Lu Luan, Roy R. Chaudhuri (University of Cambridge); Andrew N. Rycroft, Gareth A. Maglennon, Jessica Beddow (Royal Veterinary College); Brendan W. Wren, Jon Cuccui, Vanessa S. Terra (London School of Hygiene and Tropical Medicine); and Janine T. Bossé, Yanwen Li and Paul R. Langford (Imperial College London).
References
- Angen Ø., Ahrens P., Jessing S.G. Development of a multiplex PCR test for identification of Actinobacillus pleuropneumoniae serovars 1, 7, and 12. Vet. Microbiol. 2008;132:312–318. doi: 10.1016/j.vetmic.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Bossé J.T., Li Y., Sárközi R., Gottschalk M., Angen Ø., Nedbalcova K., Rycroft A.N., Fodor L., Langford P.R. BRaDP1T consortium, Proposal of serovars 17 and 18 of Actinobacillus pleuropneumoniae based on serological and genotypic analyses. Vet. Microbiol. 2018;217:1–6. doi: 10.1016/j.vetmic.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé J.T., Chaudhuri R.R., Li Y., Leanse L.G., Fernandez Crespo R., Coupland P., Holden M.T.G., Bazzolli D.M., Maskell D.J., Tucker A.W., Wren B.W., Rycroft A.N., Langford P.R., BRaDP1T consortium Complete genome sequence of MIDG2331, a genetically tractable serovar 8 clinical isolate of Actinobacillus pleuropneumoniae. Genome Announc. 2016;4:e01667–15. doi: 10.1128/genomeA.01667-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé J.T., Li Y., Angen Ø., Weinert L.A., Chaudhuri R.R., Holden M.T., Williamson S.M., Maskell D.J., Tucker A.W., Wren B.W., Rycroft A.N., Langford P.R., BRaDP1T consortium Multiplex PCR assay for unequivocal differentiation of Actinobacillus pleuropneumoniae serovars 1 to 3, 5 to 8, 10, and 12. J. Clin. Microbiol. 2014;52:2380–2385. doi: 10.1128/JCM.00685-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossé J.T., Li Y., Sárközi R., Gottschalk M., Angen Ø., Nedbalcova K., Rycroft A.N., Fodor L., Langford P.R. A unique capsule locus in the newly designated Actinobacillus pleuropneumoniae serovar 16 and development of a diagnostic PCR. J. Clin. Microbiol. 2017;55:902–907. doi: 10.1128/JCM.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnoisl G.J., Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol. Microbiol. 1989;3:1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Darmon E., Leach D.R.F. Bacterial genome instability. Microbiol. Mol. Biol. Rev. 2014;78:1–39. doi: 10.1128/MMBR.00035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S.J., Bossé J.T., Bouevitch A.B., Langford P.R., Young N.M., Nash J.H.E. The complete genome sequence of Actinobacillus pleuropneumoniae L20 (serotype 5b) J. Bacteriol. 2008;190:1495–1496. doi: 10.1128/JB.01845-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995;3:257–261. doi: 10.1016/s0966-842x(00)88939-8. [DOI] [PubMed] [Google Scholar]
- Frosch M., Edwards U., Bousset K., Krauße B., Weisgerber C. Evidence for a common molecular origin of the capsule gene loci in Gram-negative bacteria expressing group II capsular polysaccharides. Mol. Microbiol. 1991;5:1251–1263. doi: 10.1111/j.1365-2958.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Gottschalk M. The challenge of detecting herds sub-clinically infected with Actinobacillus pleuropneumoniae. Vet. J. 2015;206:30–38. doi: 10.1016/j.tvjl.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Harrison K.J., de Crécy-Lagard V., Zallot R. Gene Graphics: a genomic neighborhood data visualization web application. Bioinformatics. 2017 doi: 10.1093/bioinformatics/btx793. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H. The genetic organization of the capsular polysaccharide biosynthesis region of Actinobacillus pleuropneumoniae serotype 14. J. Vet. Med. Sci. 2015;77:583–586. doi: 10.1292/jvms.14-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Sueyoshi M. Development of a multiplex polymerase chain reaction method for cps typing of Actinobacillus pleuropneumoniae serovars 1, 2, 5, 7, and 15. JARQ. 2015;49:277–280. [Google Scholar]
- Ito H., Sueyoshi M. The genetic organization of the capsular polysaccharide biosynthesis region of Actinobacillus pleuropneumoniae serotype 15. J. Vet. Med. Sci. 2014;77:483–486. doi: 10.1292/jvms.14-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessing S.G., Angen Ø., Inzana T.J. Evaluation of a multiplex PCR test for simultaneous identification and serotyping of Actinobacillus pleuropneumoniae serotypes 2, 5, and 6. J. Clin. Microbiol. 2003;41:4095–4100. doi: 10.1128/JCM.41.9.4095-4100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard K., Friis C., Angen Ø., Boye M. Comparative profiling of the transcriptional response to iron restriction in six serotypes of Actinobacillus pleuropneumoniae with different virulence potential. BMC Genomics. 2010;11:698. doi: 10.1186/1471-2164-11-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lâm T.-T., Claus H., Frosch M., Vogel U. Sequence analysis of serotype-specific synthesis regions II of Haemophilus influenzae serotypes c and d: evidence for common ancestry of capsule synthesis in Pasteurellaceae and Neisseria meningitidis. Res. Microbiol. 2011;162:483–487. doi: 10.1016/j.resmic.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Li G., Xie F., Zhang Y., Wang C. Draft genome sequence of Actinobacillus pleuropneumoniae serotype 7 strain S-8. J. Bacteriol. 2012;194:6606–6607. doi: 10.1128/JB.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean L.L., Perry M.B., Vinogradov E. Characterization of the antigenic lipopolysaccharide O chain and the capsular polysaccharide produced by Actinobacillus pleuropneumoniae serotype 13. Infect. Immun. 2004;72:5925–5930. doi: 10.1128/IAI.72.10.5925-5930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.-S., Han J., Shin D.-J., Jeong Y.-J., Lee N. Complete genome sequence of Actinobacillus pleuropneumoniae strain KL 16 (serotype 1) Genome Announc. 2017;5:e01025–17. doi: 10.1128/genomeA.01025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M.B., Altman E., Brisson J.-R., Beynon L.M., Richards J.C. Structural characteristics of the antigenic capsular polysaccharides and lipopolysaccharides involved in the serological classification of Actinobacillus (Haemophilus) pleuropneumoniae strains. Serodiag. Immunother. Infect. Dis. 1990;4:299–308. [Google Scholar]
- Perry M.B., MacLean L.L., Vinogradov E. Structural characterization of the antigenic capsular polysaccharide and lipopolysaccharide O-chain produced by Actinobacillus pleuropneumoniae serotype 15. Biochem. Cell Biol. 2011;83:61–69. doi: 10.1139/o04-112. [DOI] [PubMed] [Google Scholar]
- Sassu E.L., Bossé J.T., Tobias T.J., Gottschalk M., Langford P.R., Hennig-Pauka I. Update on Actinobacillus pleuropneumoniae - knowledge, gaps and challenges. Transbound. Emerg. Dis. 2017 doi: 10.1111/tbed.12739. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Schuchert J.A., Inzana T.J., Angen Ø., Jessing S. Detection and identification of Actinobacillus pleuropneumoniae serotypes 1, 2, and 8 by multiplex PCR. J. Clin. Microbiol. 2004;42:4344–4348. doi: 10.1128/JCM.42.9.4344-4348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turni C., Singh R., Schembri M.A., Blackall P.J. Evaluation of a multiplex PCR to identify and serotype Actinobacillus pleuropneumoniae serovars 1, 5, 7, 12 and 15. Lett. Appl. Microbiol. 2014;59:362–369. doi: 10.1111/lam.12287. [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen X., Li L., Li T., Wang S., Chen H., Zhou R. Comparative genomic characterization of Actinobacillus pleuropneumoniae. J. Bacteriol. 2010;192:5625–5636. doi: 10.1128/JB.00535-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Zhou Y., Li L., Zhou R., Xiao S., Wan Y., Zhang S., Wang K., Li W., Li L., Jin H., Kang M., Dalai B., Li T., Liu L., Cheng Y., Zhang L., Xu T., Zheng H., Pu S., Wang B., Gu W., Zhang X.-L., Zhu G.-F., Wang S., Zhao G.-P., Chen H. Genome biology of Actinobacillus pleuropneumoniae JL03, an isolate of serotype 3 prevalent in China. PLoS One. 2008;3:e1450. doi: 10.1371/journal.pone.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan B., Angen Ø., Hedegaard J., Bendixen C., Panitz F. Draft genome sequences of Actinobacillus pleuropneumoniae serotypes 2 and 6. J. Bacteriol. 2010;192:5846–5847. doi: 10.1128/JB.00867-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Jones S.C.P., Angen Ø., Bossé J.T., Nash J.H.E., Frey J., Zhou R., Chen H.C., Kroll J.S., Rycroft A.N., Langford P.R. Multiplex PCR that can distinguish between immunologically cross-reactive serovar 3, 6, and 8 Actinobacillus pleuropneumoniae strains. J. Clin. Microbiol. 2008;46:800–803. doi: 10.1128/JCM.01787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]