Abstract
People are frequently and unintentionally exposed to many chemical compounds, such as environmental pollutants and endocrine-disrupting chemicals (EDCs), in food and from the atmosphere. In particular, endocrine-disrupting TBBPA and dioxins are found in human breast milk and in the body. Conventional studies evaluate toxicity by administering a single substance to cells or animals, but evaluation of the toxicity of mixtures of these ingested compounds is essential for “true” toxicological assessment. We evaluated toxic effects in vitro using human mesenchymal stem cells (hMSCs). TBBPA increased the number of lipid droplets, and upregulated the expression of adipocyte-related mRNA, aP2 and LPL, through a PPARγ-dependent mechanism. TCDD suppressed lipid droplets and adipocyte-related mRNA levels. Adipocyte differentiation was stimulated by TBBPA and inhibited by TCDD in a dose-dependent manner. TBBPA did not influence osteoblast differentiation, but TCDD suppressed ALP staining and activity, calcium deposition, and osteoblast-related mRNA levels. In a mixture of TBBPA and TCDD, TBBPA inhibited TCDD suppression of adipocyte and osteoblast differentiation in a dose-dependent manner. Interestingly, we observed lipid droplets in TBBPA-treated cells differentiated into osteoblasts. These results suggest that TBBPA and TCDD disrupted differentiation into adipocytes and osteoblasts and contributes to a more complete toxicological understanding of exposure to these chemical substances.
Abbreviations: ALP, alkaline phosphatase; aP2, adipocyte-specific protein 2; BFRs, brominated flame retardants; C/EBPα, CCAAT-enhancer-binding protein alpha; DOHaD, developmental origins of health and disease; EDCs, endocrine-disrupting chemicals; LPL, lipoprotein lipase; MSC, mesenchymal stem cell; PCDDs/DFs, polychlorinated dibenzo-p-dioxins and dibenzofurans; PPARγ, peroxisome proliferator activated receptor gamma; RUNX2, runt-related transcription factor 2; TBBPA, tetrabromobisphenol A; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin
Keywords: Tetrabromobisphenol A; 2,3,7,8-tetrachlorodibenzo-p-dioxin; Human mesenchymal stem cell; Adipocyte differentiation; Osteoblast differentiation
1. Introduction
Society has developed many synthetic chemical compounds to increase our well-being and comfort. However, some of these chemical compounds pollute the environment, contaminate our food, and are absorbed directly into our body, which may affect our health by disrupting physiological functions such as endocrine systems. In particular, there are several known endocrine-disrupting chemicals (EDCs) including dioxins, diethylstilbestrol (DES), bisphenol A (BPA), tetrabromobisphenol A (TBBPA), dichlorodiphenyltrichloroethane (DDT), tributyltin (TBT), perfluorooctanoate (PFOA), phthalateas and polybrominated diphenylethers (PBDEs) [[1], [2], [3], [4], [5], [6], [7], [8]]. We are frequently and unintentionally exposed to EDCs in food and from the atmosphere, which can be revealed through cohort studies. However, cohort studies involve a huge amount of time and expense, a large study population, and should control for a number of factors, such as race and age, which makes them unsuitable for evaluating mixtures of chemical substances. In addition, conventional studies evaluate toxicity by administering a single substance to cells or animals, but evaluation of a mixture of chemical compounds is needed for a more complete understanding or complex toxicological assessment. We evaluated toxic effects in vitro using stem cells to examine in vivo differentiation.
Human mesenchymal stem cells (hMSCs) are multipotent cells, which can be isolated from bone and adipose tissue [9,10]. MSC plasticity can be used for an in vitro model of differentiation because MSCs can differentiate into several tissue-forming cells such as bone, cartilage, fat, muscle, tendon, liver, kidney, heart, and even brain cells in vitro. In particular, the method of MSC differentiation into adipocytes and osteoblasts is well known [[11], [12], [13]]. An increase in adipose deposits in bone marrow decreases bone density and increases the risk of obesity [14,15]. Thus, there is close relationship of osteoporosis to obesity, which suggests that disruption of MSC differentiation into adipocytes and osteoblasts by EDCs may exacerbate both osteoporosis and obesity.
Barker et al. proposed the developmental origins of health and disease (DOHaD) hypothesis, which suggests that environmental factors, such as nutrition and exposure to chemicals during pregnancy and postnatal development, influence adolescent and adult health, and the risk of various diseases [16]. Moreover, there is high sensitivity to EDCs at critical developmental points such as the embryonic and neonatal periods [17]. The effect of EDCs seems to be higher in the fetus and in infants than in adults. In addition, we previously observed contamination of EDCs, such as TBBPA and dioxins, in human breast milk and in the body; the average concentration of TBBPA in a Japanese mother’s breast milk was 1.9 ng/g lipid, and polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDDs/DFs: 17 congeners) and coplanar polychlorinated biphenyls (Co-PCBs: 14 congeners) were 3.6 and 3.5 pg TEQ/g lipid, respectively [[18], [19], [20], [21]]. The contamination levels in human milk have been widely studied to provide a good indicator of overall human exposure, especially for infants. Thus, the exposure of EDCs such as TBBPA and TCDD in the fetus and infants cause the failure to thrive.
In 2015, 18,000 tons of TBBPA were used in Japan [22], and TBBPA pollutes the environment during manufacture, use, and disposal. TBBPA bioaccumulates in the food chain [23,24], and is considered an EDC because it activates peroxisome proliferator activated receptor (PPAR) γ in mice [25,26]. In addition, TBBPA facilitates adipocyte differentiation of 3T3-L1 cells via PPARγ activation [18]. On the other hand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a persistent organic pollutant generated from waste incinerators as an undesired by-product. TCDD contaminates the body because of a long half-life and bioaccumulates in the food chain [27]. Exposure of pregnant rats to TCDD results in altered bone geometry, mineral density, and mechanical strength in the offspring [28]. TCDD interferes with osteoblast and osteoclast differentiation in bone marrow stem cells [29], inhibits adipogenesis, and attenuates insulin-induced glucose uptake in 3T3-L1 cells [30].
The unintentional intake of BFRs and dioxins via food and breast milk, and the toxic effects of individual molecules have been studied, but the effects of mixtures of these toxicant have not been previously examined. Here, we investigated the toxic effects of mixtures of TBBPA and TCDD on adipocyte and osteoblast differentiation in hMSCs.
2. Material and methods
2.1. Chemicals
TBBPA and TCDD were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Chemicals were dissolved in dimethyl sulfoxide (DMSO) to a final concentration in culture medium of 0.1% v/v. StemBeads fibroblast growth factor 2 (FGF2) was purchased from Stem Culture (Rensselaer, NY). All other reagents were the highest quality commercially available and obtained from Sigma-Aldrich (St. Louis, MO) and Nacalai Tesque (Kyoto, Japan).
2.2. Cell cultures
After Institutional Review Board approval, human MSC (MSC-R14) isolated from bone marrow of ilium was provided by RIKEN BRC (Ibaraki, Japan). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin and 3 ng/mL StemBeads FGF2 at 37 °C [31]. The two chemicals and DMSO were uesd at a concentration not showing cytotocity determined by a tetrazolium-based colorimetric assay, the WST-8 kit (Nacalai Tesque), according to the manufacturer’s protocol (data not shown).
2.3. Adipocyte differentiation and oil red O staining
hMSCs were seeded on a 24-well plate. Two days after confluence (designated as Day 0), cells were treated with adipocyte differentiation medium containing 1 μM dexamethasone, 0.5 mM 3-isobuthyl-1-methylxanthine (IBMX), 200 μM indomethacin and 10 μg/mL insulin with and without TBBPA and/or TCDD. After three days (Day 3), the media was replaced and maintained with and without TBBPA and/or TCDD containing 10 μg/mL insulin alone to Day 21.
Differentiated cells were stained with oil red O to detect lipid droplets in adipocytes. After washing twice with phosphate buffered saline (PBS), cells were fixed with 4% paraformaldehyde at room temperature, and then stained with 3.3 mg/mL oil red O in 60% isopropanol for one hour. Cells were washed with PBS, and observed under an IX71 microscope (Olympus, Tokyo, Japan). Stained oil red O was eluted with isopropanol and the absorbance was measured at a wavelength of 550 nm using a SPECTRA FLUOR (TECAN, Männedorf, Switzerland) for quantitative analysis and normalized to the cell protein contents, determined using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA).
2.4. Osteoblast differentiation and alizarin red S staining
At Day 0, cells were treated with osteoblast differentiation medium containing 0.1 μM dexamethasone, 10 mM β-glycerophosphate and 50 μg/mL L-ascorbic acid with and without TBBPA and/or TCDD, and maintained for 21 days.
Differentiated cells were examined by alizarin red S staining for the presence of calcium deposits. Briefly, cells were fixed with ice cold 70% ethanol, rinsed with distilled water, and then stained with 40 mM alizarin red S dissolved in distilled water (pH 4.2; adjusted with 10% ammonium hydroxide) for 5 min. Cells were washed with distilled water, and observed under an IX71 microscope. After imaging, the dye was eluted with 10% acetic acid, and the absorbance was measured at 450 nm using a TriStar LB 941 microplate reader (Berthold, Bad Wildbad, Germany) and normalized to the cell protein contents, determined using a BCA protein assay kit.
2.5. ALP activity assay and cell matrix ALP staining
Cells were washed with PBS and then lysed with lysis buffer (50 mM Tris-HCl, pH 7.5, and 1% TrironX-100). ALP activity was determined colorimetrically by incubating protein lysates with the substrate p-nitrophenyl phosphate in a 96-well plate at 37 °C for 60 min. The reaction was stopped by adding 0.2 M NaOH. Absorbance was measured at 415 nm using a TriStar LB 941 microplate reader, and normalized against the corresponding protein concentrations determined with a BCA protein assay kit using bovine serum albumin as a standard.
For ALP staining, cells were washed with PBS, and fixed with 4% paraformaldehyde. Cells were stained with a mixture of 0.1 mg/mL naphthol AS-MX phosphate, 0.6 mg/mL fast-blue BB salt, 2 mM MgCl2, 5 μL/mL N,N-dimethylformamide, and 100 mM Tris-HCl (pH 8.8) buffer at 37 °C for 20 min. When the cells turned blue, they were washed and visualized using an IX71 microscope.
2.6. Adipocyte and osteoblast mRNA level quantification by real-time reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA from hMSCs was extracted with ISOGEN (Nippongene, Toyama, Japan) and reverse transcribed with PrimeScript RT Master Mix (TaKaRa, Kyoto, Japan). PCR reactions were performed using a KAPA SYBR FAST Universal qPCR kit (Kapa Biosystems, Boston, USA) and assayed using a Thermal Cycler Dice (TaKaRa). The oligonucleotides used for RT-qPCR included β-actin (forward: 5′-AGATCAAGATCATTGCTCCTCCTG-3′, reverse: 5′-CAAGAAAGGGTGTAACGCAACTAAG-3′), aP2 (forward: 5′-AGGAAAGTCAAGAGCACCATA-3′, reverse: 5′-CACCAGTTTATCATCCTCTCG-3′), LPL (forward: 5′-AGAGGACTTGGAGATGTGGA-3′, reverse: 5′-TCATAGCCCAGATTGTTGC-3′), PPARγ (forward: 5′-GCGATTCCTTCACTGATAC-3′, reverse: 5′-CTTCCATTACGGAGAGATCC-3′), C/EBPα (forward: 5′-TGGACAAGAACAGCAACGAGTA-3′, reverse: 5′-ATTGTCACTGGTCAGCTCCAG-3′), GAPDH (forward: 5′-TCTCTGCTCCTCCTGTTC-3′, reverse: 5′-CTCCGACCTTCACCTTCC-3′), osteocalcin (forward: 5′-AGGGCAGCGAGGTAGTGAAGA-3′, reverse: 5′-AAGGGCAAGGGGAAGAGGAAAGAA-3′), osteopontin (forward: 5′-GTACCCTGATGCTACAGACGAG-3′, reverse: 5′-CATAACTGTCCTTCCCACG-3′), RUNX2 (forward: 5′-AACCCAGAAGGCACAGACA-3′, reverse: 5′-GGACACCTACTCTCATACTGGGAT-3′), osterix (forward: 5′-TTGAGGAGGAAGTTCACTATGG-3′, reverse: 5′-CTTTGCCCAGAGTTGTTGAG-3′). Because the expression of β-actin and GAPDH mRNA were not significantly different at any stage, each sample was normalized on the basis of its β-actin (adipocyte) or GAPDH (osteoblast) content.
2.7. Statistical analysis
The statistical significance of differences in means was determined using Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. P values < 0.05 were considered significant. All data were statistically analyzed using Prism software (Graph Pad Software).
3. Results
3.1. TBBPA facilitated and TCDD suppressed adipocyte differentiation in hMSCs
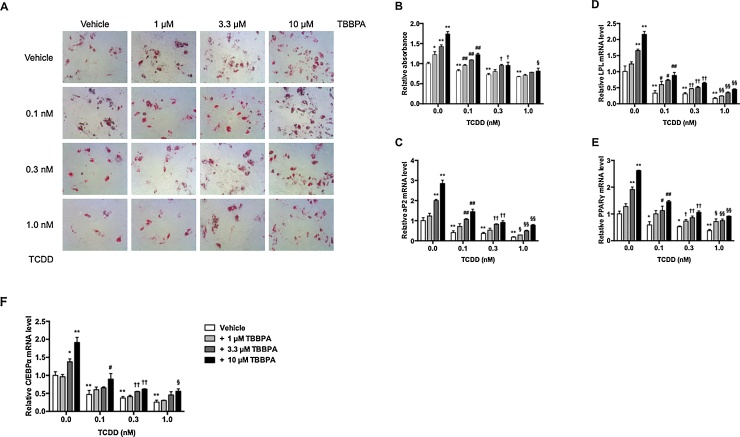
To evaluate the effects of TBBPA and TCDD alone or combination on hMSC differentiation into adipocytes, we used oil red O staining and quantified lipid droplet staining. Lipid droplet number increased in a dose-dependent manner in the presence of TBBPA (Fig. 1A). The relative absorbance at 550 nm of 1, 3.3, and 10 μM TBBPA increased 1.2-fold, 1.4-fold, and 1.7-fold over vehicle-treated cells, respectively (Fig. 1B). On the other hand, the number of lipid droplets decreased in TCDD-treated cells. The relative oil red O absorbance at 550 nm extracted from lipid droplets decreased in a dose-dependent manner in the presence of 0.1, 0.3, and 1.0 nM TCDD to 0.82-fold, 0.73-fold, and 0.67-fold lower compared with that of the vehicle-treated cells, respectively. For a mixture of TBBPA and TCDD, the numbers of lipid droplets were inhibited by TCDD and upregulated by TBBPA in a dose-dependent manner.
Fig. 1.
Effects of TBBPA and TCDD on adipocyte differentiation in hMSCs.
hMSCs were incubated with 1, 3.3, and 10 μM TBBPA and/or 0.0, 0.1, 0.3, and 1 nM TCDD for 21 days. (A) Lipid droplets were stained with oil red O. Morphological changes were observed under a microscope at 40× magnification. (B) Quantitative measurement of lipid droplets stained with oil red O. Oil red O in lipid droplets was eluted with isopropanol. The relative absorbance at 550 nm was expressed as the fold induction as compared with the vehicle. After incubation, aP2 (C), LPL (D), PPARγ (E), and C/EBPα (F) mRNA levels were measured by quantitative real-time PCR. The relative mRNA level was expressed as the fold induction as compared with the vehicle. The data are presented as means ± SD (n = 5). *P < 0.05, **P < 0.01, compared with vehicle-treated cells in 0.0 nM TCDD. #P < 0.05, ##P < 0.01, compared with vehicle-treated cells in 0.1 nM TCDD. †P < 0.05, ††P < 0.01, compared with vehicle-treated cells in 0.3 nM TCDD. §P < 0.05, §§P < 0.01, compared with vehicle-treated cells in 1.0 nM TCDD.
To determine whether TBBPA and TCDD induced the expression of adipocyte-related genes, adipocyte-specific protein 2 (aP2), lipoprotein lipase (LPL), and adipocyte-related transcription factors, peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα), were quantitatively analyzed by real-time PCR assay (Fig. 1C–F). TBBPA elevated mRNA expression of both adipocyte-related genes and transcription factors in a dose-dependent manner. In particular, 10 μM TBBPA increased aP2, LPL, PPARγ, and C/EBPα levels 2.9-fold, 2.2-fold, 2.6-fold, and 1.9-fold compared with those of vehicle-treated cells, respectively. By contrast, 1.0 nM TCDD downregulated aP2, LPL, PPARγ, and C/EBPα mRNA levels by 0.19-fold, 0.17-fold, 0.38-fold, and 0.26-fold compared with those of vehicle-treated cells, respectively. For the mixture of TCDD and TBBPA, mRNA levels were similar to that of oil red O staining, and a significant difference was observed at 0.1 nM TCDD and 1–10 μM TBBPA. These results indicated that the presence of TBBPA facilitated and TCDD suppressed adipocyte differentiation in hMSCs. In addition, the effect of a combination of TBBPA and TCDD on adipocyte differentiation was weaker than that of TBBPA and TCDD alone.
3.2. TCDD suppressed osteoblast differentiation in hMSCs
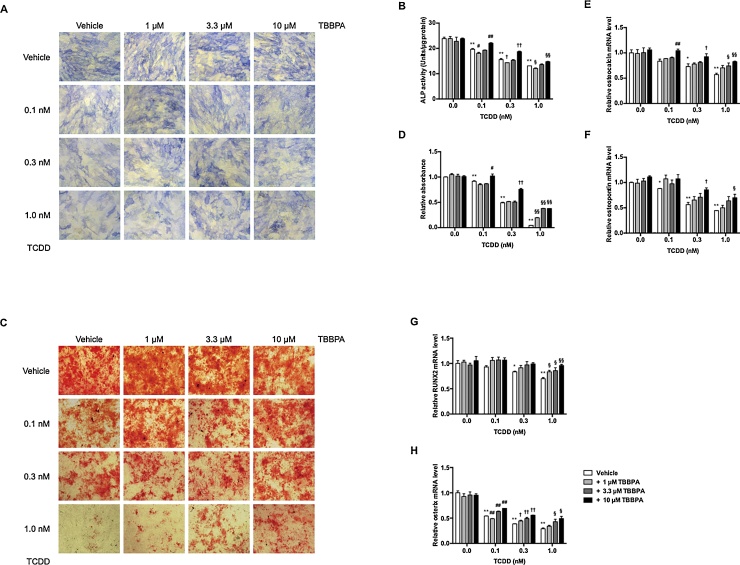
To determine the effects of TBBPA and TCDD on hMSC differentiation into osteoblasts, we examined the early osteoblastic marker alkaline phosphatase (ALP) (Fig. 2A and B). TBBPA did not influence ALP expression, but TCDD decreased osteoblast differentiation in a dose-dependent manner. Addition of 0.1, 0.3, and 1.0 nM TCDD decreased ALP activity to 19.6, 15.6, and 12.1 units/μg protein, respectively. Moreover, 0.1, 0.3, and 1.0 nM TCDD decreased alizarin red staining in a dose-dependent manner by 0.92-fold, 0.49-fold, and 0.05-fold, respectively, compared with that of vehicle-treated cells (Fig. 2C and D). Furthermore, we detected expression of osteoblast-related genes, osteocalcin and osteopontin, and osteoblast-related transcription factors, runt-related transcription factor 2 (RUNX2) and osterix (Fig. 2E–H). The mRNA levels of osteocalcin, osteopontin, RUNX2, and osterix decreased 0.57-fold, 0.45-fold, 0.70-fold, and 0.29-fold, respectively, after addition of 1.0 nM TCDD compared with that of vehicle-treated cells. In a mixture of TBBPA and TCDD, TBBPA, which did not influence osteoblast differentiation, inhibited TCDD suppression of differentiation in a dose-dependent manner. These results indicated that TCDD suppressed osteoblast differentiation in hMSCs. In addition, TBBPA inhibited TCDD suppression of osteoblast differentiation.
Fig. 2.
Effects of TBBPA and TCDD on osteoblast differentiation in hMSCs.
hMSCs were incubated with 1, 3.3, and 10 μM TBBPA and/or 0.0, 0.1, 0.3, and 1 nM TCDD for 14 or 21 days. At Day 14, ALP staining (A) and activity (B) were measured. At Day 21, calcium deposits were stained with alizarin red S (C). Morphological changes were observed under a microscope at 40× magnification. (D) Quantitative measurement of calcium deposites stained with alizarin red S. Alizarin red S in calcium deposites was eluted with 10% acetic acid. The relative absorbance at 450 nm was expressed as the fold induction as compared with the vehicle. At Day 21, osteocalcin (E), osteopontin (F), RUNX2 (G), and osterix (H) mRNA levels were measured by quantitative real-time PCR. The relative mRNA level was expressed as the fold induction as compared with the vehicle. The data are presented as means ± SD (n = 5). *P < 0.05, **P < 0.01, compared with vehicle-treated cells in 0.0 nM TCDD. #P < 0.05, ##P < 0.01, compared with vehicle-treated cells in 0.1 nM TCDD. †P < 0.05, ††P < 0.01, compared with vehicle-treated cells in 0.3 nM TCDD. §P < 0.05, §§P < 0.01, compared with vehicle-treated cells in 1.0 nM TCDD.
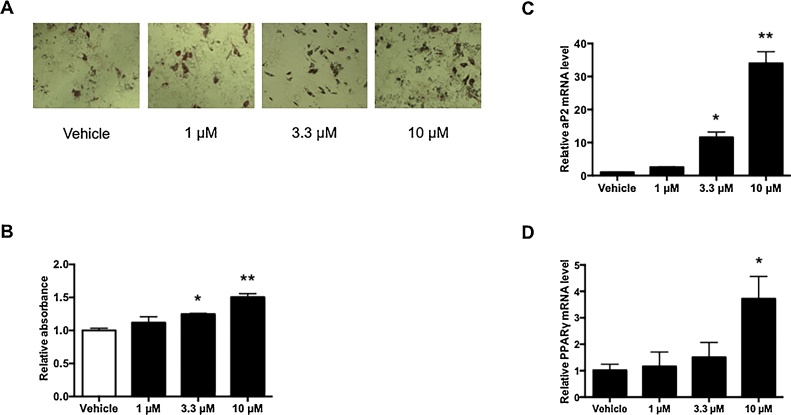
3.3. TBBPA induced lipogenesis in osteoblast differentiation
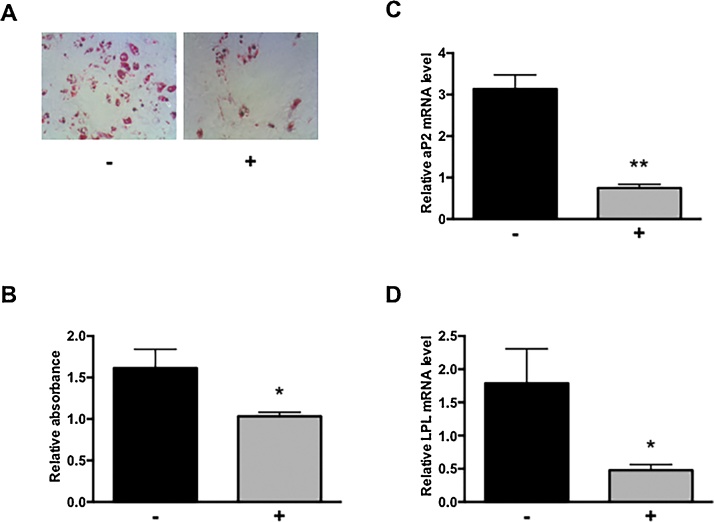
Although TBBPA did not affect osteoblast differentiation (Fig. 2), as shown in Fig. 3A, the number of lipid droplets increased in a TBBPA dose-dependent manner. The absorbance at 550 nm of 1 μM TBBPA was equal to that of vehicle-treated cells, whereas that of 3.3 and 10 μM TBBPA was 1.2-fold and 1.5-fold higher, respectively (Fig. 3B). In addition, aP2 mRNA expression at 1, 3.3, and 10 μM TBBPA increased 2.6-fold, 11.6-fold, and 34.0-fold over the vehicle-treated cells, respectively (Fig. 3C). Similarly, 1, 3.3, and 10 μM TBBPA treatment increased PPARγ mRNA expression 1.16-fold, 1.51-fold, and 3.71-fold, respectively, compared with that of vehicle-treated cells (Fig. 3D). Compared with adipocyte differentiation (Fig. 1), althought adipocyte formation in osteoblast differentiation is slight, TBBPA facilitated lipid droplets formation in both adipocyte and osteoblast differentiation. To clarify the formation of lipid droplets by TBBPA, we used the TBBPA antagonist, GW9662. GW9662 treatment inhibited formation of lipid droplets compared with that of TBBPA alone (Fig. 4A and B). Furthermore, we observed that GW9662 dramatically suppressed TBBPA-induced expression of adipocyte markers such as aP2, LPL, and PPARγ (Fig. 4C–E). Similar results were obtained in adipocyte differentiation. These results suggest that TBBPA did not influence osteoblast differentiation, but promoted adipocyte differentiation in hMSCs differentiating into osteoblasts through a PPARγ-dependent mechanism.
Fig. 3.
TBBPA-induced lipogenesis in hMSC osteoblast differentiation.
hMSCs were incubated with 1, 3.3, and 10 μM TBBPA in osteoblast differentiation medium for 21 days. (A) Lipid droplets were stained with oil red O. Morphological changes were observed under a microscope at 40× magnification. (B) Quantitative measurement of lipid droplets stained with oil red O. Oil red O in lipid droplets was eluted with isopropanol. The relative absorbance at 550 nm was expressed as the fold induction as compared with the vehicle. aP2 (C) and PPARγ (D) mRNA levels were measured by quantitative real-time PCR. The relative mRNA level was expressed as the fold induction as compared with the vehicle. The data are presented as means ± SD (n = 5). *P < 0.05, **P < 0.01, significantly different from that in vehicle-treated cells.
Fig. 4.
Involvement of PPARγ in TBBPA-induced lipogenesis.
hMSCs differentiated into osteoblast with 10 μM TBBPA in the absence (-) or presence (+) of 1 μM GW9662 for 21 days. (A) Lipid droplets were stained with oil red O. Morphological changes were observed under a microscope at 40× magnification. (B) Quantitative measurement of lipid droplets stained with oil red O. Oil red O in lipid droplets was eluted with isopropanol. The relative absorbance at 550 nm was expressed as the fold induction as compared with the vehicle. aP2 (C), LPL (D), and PPARγ (E) mRNA levels were measured by quantitative real-time PCR. The relative mRNA level was expressed as the fold induction as compared with the vehicle. The data are presented as means ± SD (n = 5). *P < 0.05, **P < 0.01, significantly different from that in the absence of GW9662.
4. Discussion
We examined the behavior of differentiated hMSCs after exposure to TBBPA and TCDD. Adipocyte differentiation was upregulated by TBBPA, and both adipocyte and osteoblast differentiation were downregulated by TCDD. In a mixture of TBBPA and TCDD, TBBPA inhibited TCDD suppression of adipocyte and osteoblast differentiation. Thus, TBBPA and TCDD have opposing activity in adipocyte differentiation.
MSC differentiation to adipocytes is controlled by the interaction of PPARγ and C/EBPα [[32], [33], [34]]. PPARγ activated by ligand induces the expression of C/EBPα. Through a positive feedback loop, C/EBPα maintains the expression of PPARγ. Our results indicated that the mRNA expression level of PPARγ by TBBPA is higher than that of C/EBPα. Therefore, C/EBPα may be expressed under the influence of PPARγ on TBBPA-induced adipocyte differentiation of hMSCs. Wakabayashi et al. reported that histone H4K20 monomethylation (H4K20me1) by Setd8, a PPARγ target gene, was involved in adipocyte differentiation [35]. Setd8 expression and upregulation of H4K20me1 was observed in mouse 3T3-L1 cells exposed to TBBPA (our unpublished data). Thus, TBBPA may facilitate adipocyte differentiation in hMSCs through epigenetic changes such as H4K20me1.
We showed that adipocyte differentiation was suppressed by TCDD in a dose-dependent manner. TCDD decreased the expression of both PPARγ and C/EBPα. Inhibition of differentiation by TCDD was dependent on the expression of both transcriptional factors. Pohjanvirta et al. and Phillips et al. reported that the body weight of mice exposed to TCDD decreased, and that TCDD suppressed adipocyte differentiation in mouse 3T3-L1 cells [36,37]. TCDD is a known aryl hydrocarbon receptor (AhR) ligand [38]. The ligand-AhR complex regulates the transcription of specific genes involved in xenobiotic metabolism. AhR negatively regulates lipid synthesis and is involved in cell differentiation [39,40]. The AhR signaling pathway may be involved in TCDD suppression of differentiation via PPARγ and C/EBPα downregulation in hMSCs.
RUNX2 and osterix are osteoblast-specific transcriptional factors, and osterix acts downstream of RUNX2 [41,42]. TCDD inhibited the expression of both transcriptional factors, and strongly inhibited the expression of osterix more than that of RUNX2. These results suggest that inhibition of differentiation by TCDD depends on the expression of RUNX2, and especially osterix. Previously, Korkalainen et al. showed that TCDD suppressed osteoblast differentiation in rodent MSCs [29] and Watt et al. showed that TBBPA suppressed osteoblast differentiation in mouse MSCs [26]. However, our results showed that TBBPA did not affect osteoblast differentiation in human MSCs, which might be because of differences in osteoblast differentiation medium or species specificity.
MSC differentiation to adipocytes or osteoblasts is controlled by the balance of PPARγ and RUNX2 expression and transcriptional activation. The activation of PPARγ in osteoblasts suppresses osteoblast differentiation and inhibits osteocalcin expression both by suppressing the expression of RUNX2 and by interfering with the transactivation ability of RUNX2 [[43], [44], [45]]. Previously, Riu et al. and Akiyama et al. showed that TBBPA was a partial ligand of PPARγ [18,25]. Interestingly, we observed that TBBPA induced lipogenesis in osteoblast differentiation, which may be dependent on increased PPARγ expression. TBBPA did not change the expression of RUNX2, but facilitated PPARγ expression in osteoblast differentiation. Therefore, PPARγ is a key factor in adipocyte and osteoblast differentiation under the mixed condition of TBBPA and TCDD.
In conclusion, we evaluated the effects of in vitro exposure to individual chemicals and mixtures in hMSCs. Further studies are needed to clarify the molecular mechanisms by which pluripotency is disrupted by BFRs and dioxins. Although TBBPA and TCDD were used as model compounds, it is possible to evaluate other chemical mixtures using this system. Moreover, our study contributes to a complex toxicological understanding of exposure to a mixture chemical substances.
Acknowledgements
This study was supported by the Health and Labour Sciences Research Grant of Japan (H23-Food-Young Scientist-017) and by a Grant-in-Aid for Scientific Research (B) (Grant No. 25281032) from the Japan Society for the Promotion of Science. We thank Erika Shimizu, Tasuku Itakura, Kotoe Furutsuka and Asami Hiromoto for excellent technical support and Dr. Yukio Kato (Hiroshima University) for providing hMSC.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2018.06.007.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Buchanan D.L., Sato T., Peterson R.E., Cooke P.S. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol. Sci. 2000;57:302–311. doi: 10.1093/toxsci/57.2.302. [DOI] [PubMed] [Google Scholar]
- 2.Meerts I.A., Letcher R.J., Hoving S., Marsh G., Bergman A., Lemmen J.G. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamorro-Garcia R., Sahu M., Abbey R.J., Laude J., Pham N., Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ. Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS One. 2013;8 doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newbold R.R., Padilla-Banks E., Jefferson W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009;304:84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin B.S. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Skinner M.K., Manikkam M., Tracey R., Guerrero-Bosagna C., Haque M., Nilsson E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11(228) doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang-Peronard J.L., Andersen H.R., Jensen T.K., Heitmann B.L. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes. Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science (New York, N.Y.) 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 2015;35 doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhadlaq A., Mao J.J. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 12.Marion N.W., Mao J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science (New York, N.Y.) 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 14.Kawai M., de Paula F.J.A., Rosen C.J. New insights into osteoporosis: the bone-fat connection. J. Intern. Med. 2012;272:317–329. doi: 10.1111/j.1365-2796.2012.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy B., Curtis M.E., Fears L.S., Nahashon S.N., Fentress H.M. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front. Physiol. 2016;7:439. doi: 10.3389/fphys.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osmond C., Barker D.J. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ. Health Perspect. 2000;108(Suppl. 3):545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida M., Takenaka A., Katsuda S., Kurokawa Y., Maekawa A. Neonatal exposure to p-tert-octylphenol causes abnormal expression of estrogen receptor alpha and subsequent alteration of cell proliferating activity in the developing Donryu rat uterus. Toxicol. Pathol. 2002;30:357–364. doi: 10.1080/01926230252929936. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama E., Kakutani H., Nakao T., Motomura Y., Takano Y., Sorakubo R. Facilitation of adipocyte differentiation of 3T3-L1 cells by debrominated tetrabromobisphenol A compounds detected in Japanese breast milk. Environ. Res. 2015;140:157–164. doi: 10.1016/j.envres.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Nakao T., Akiyama E., Kakutani H., Mizuno A., Aozasa O., Akai Y. Levels of tetrabromobisphenol a, tribromobisphenol a, dibromobisphenol a, monobromobisphenol a, and bisphenol a in Japanese breast milk. Chem. Res. Toxicol. 2015;28:722–728. doi: 10.1021/tx500495j. [DOI] [PubMed] [Google Scholar]
- 20.Aozasa O., Mochizuki A., Ohta S., Nakao T., Miyata H., Nomura T. Optimization of the purification method for dioxin analysis in human serum and temporal changes in background dioxin levels in the general population. Chemosphere. 2003;50:1157–1165. doi: 10.1016/s0045-6535(02)00631-8. [DOI] [PubMed] [Google Scholar]
- 21.Kakutani H., Aozasa O., Mizuno A., Akiyama E., Nakao T., Ohta S. In vitro and in vivo induction of cytochrome P450 by coplanar polychlorinated/brominated biphenyls (Co-PXBs) providing high TEQ in mother’s milk in Japan. Toxicology. 2014;324C:68–75. doi: 10.1016/j.tox.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 22.2016. Ministry of the Environment of Japan, Annual Report of Ministry of the Environment of Japanese Government: “polybrominated dibenzo-p-dioxins and dibenzofurans”.www.env.go.jp/chemi/dioxin/chosa/result_h27.pdf March 2015. Available at (in Japanese) [Google Scholar]
- 23.Johnson-Restrepo B., Adams D.H., Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70:1935–1944. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Morris S., Allchin C.R., Zegers B.N., Haftka J.J.H., Boon J.P., Belpaire C. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ. Sci. Technol. 2004;38:5497–5504. doi: 10.1021/es049640i. [DOI] [PubMed] [Google Scholar]
- 25.Riu A., Grimaldi M., le Maire A., Bey G., Phillips K., Boulahtouf A. Peroxisome proliferator-activated receptor gamma is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watt J., Schlezinger J.J. Structurally-diverse, PPARgamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology. 2015;331:66–77. doi: 10.1016/j.tox.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leeuwen F.X., Feeley M., Schrenk D., Larsen J.C., Farland W., Younes M. Dioxins: WHO’s tolerable daily intake (TDI) revisited. Chemosphere. 2000;40:1095–1101. doi: 10.1016/s0045-6535(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen H.M., Pulkkinen P., Jamsa T., Koistinen J., Simanainen U., Tuomisto J. Effects of in utero and lactational TCDD exposure on bone development in differentially sensitive rat lines. Toxicol. Sci. 2005;85:1003–1012. doi: 10.1093/toxsci/kfi136. [DOI] [PubMed] [Google Scholar]
- 29.Korkalainen M., Kallio E., Olkku A., Nelo K., Ilvesaro J., Tuukkanen J. Dioxins interfere with differentiation of osteoblasts and osteoclasts. Bone. 2009;44:1134–1142. doi: 10.1016/j.bone.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Hsu H.-F., Tsou T.-C., Chao H.-R., Kuo Y.-T., Tsai F.-Y., Yeh S.-C. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J. Hazard. Mater. 2010;182:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsumi S., Shimazu A., Miyazaki K., Pan H., Koike C., Yoshida E. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 32.Wu Z., Rosen E.D., Brun R., Hauser S., Adelmant G., Troy A.E. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 33.Siersbaek R., Nielsen R., Mandrup S. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–3249. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Rosen E.D., Hsu C.H., Wang X., Sakai S., Freeman M.W., Gonzalez F.J. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi K.-I., Okamura M., Tsutsumi S., Nishikawa N.S., Tanaka T., Sakakibara I. The peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha heterodimer targets the histone modification enzyme PR-Set7/Setd8 gene and regulates adipogenesis through a positive feedback loop. Mol. Cell. Biol. 2009;29:3544–3555. doi: 10.1128/MCB.01856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pohjanvirta R., Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol. Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- 37.Phillips M., Enan E., Liu P.C., Matsumura F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Cell Sci. 1995;108(Pt 1):395–402. doi: 10.1242/jcs.108.1.395. [DOI] [PubMed] [Google Scholar]
- 38.Whitlock J.P.J. Mechanistic aspects of dioxin action. Chem. Res. Toxicol. 1993;6:754–763. doi: 10.1021/tx00036a003. [DOI] [PubMed] [Google Scholar]
- 39.Alexander D.L., Ganem L.G., Fernandez-Salguero P., Gonzalez F., Jefcoate C.R. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J. Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- 40.Hu T., Wang D., Yu Q., Li L., Mo X., Pan Z. Aryl hydrocarbon receptor negatively regulates lipid synthesis and involves in cell differentiation of SZ95 sebocytes in vitro. Chem.-Biol. Interact. 2016;258:52–58. doi: 10.1016/j.cbi.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Komori T. Mechanism of transcriptional regulation by Runx2 in osteoblasts. Clin. Calcium. 2006;16:801–807. [PubMed] [Google Scholar]
- 42.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 43.Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell. Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 44.Jeon M.J., Kim J.A., Kwon S.H., Kim S.W., Park K.S., Park S.-W. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J. Biol. Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 45.Liu L.-F., Shen W.-J., Zhang Z.H., Wang L.J., Kraemer F.B. Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARgamma and adiponectin. J. Cell. Physiol. 2010;225:837–845. doi: 10.1002/jcp.22291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.