Highlights
-
•
Debates persist between the superiority of perioperative chemotherapy or adjuvant chemoradiotherapy in the management of gastric adenocarcinoma.
-
•
This review is to synthesize current knowledge about adjuvant chemoradiotherapy.
-
•
It will also present ongoing trials.
Abbreviations: 5FU, 5-fluorouracil; 5FU-LV, 5-fluorouracil leucovorin; CRT, chemoradiotherapy; CT, chemotherapy; DCF, Doxorubicin Cisplatin 5-fluorouracil; ECF, Epirubicin Cisplatin 5-fluorouracil; ECX, Epirubicin Cisplatin Capecitabin; FOLFOX, 5-fluorouracil oxaliplatin; FUFOL, bolus 5-fluorouracil followed by leucovorin over 15 minutes; LV, leucovorin; IMRT, intensity modulated radiation therapy; RT, radiation therapy; XELOX, capecitabin oxaliplatine
Keywords: Gastric cancer, Adjuvant therapy, Chemoradiotherapy, IMRT, Adenocarcinoma
Abstract
An estimated 990,000 new cases of gastric cancer are diagnosed worldwide each year. Surgical excision, the only chance for prolonged survival, is feasible in about 20% of cases. Even after surgery, the median survival is limited to 12 to 20 months due to the frequency of locoregional and/or metastatic recurrences. This led to clinical trials associating surgery with neoadjuvant or adjuvant treatments to improve tumor control and patient survival. The most studied modalities are perioperative chemotherapy and adjuvant chemoradiotherapy. To date, evidence has shown a survival benefit for postoperative chemoradiotherapy and for perioperative chemotherapy. Phase III trials are ongoing to compare these two modalities. The aim of this review is to synthesize current knowledge about adjuvant chemoradiotherapy in the management of gastric adenocarcinoma, and to consider its prospects by integrating modern radiotherapy techniques.
Background
Despite a worldwide decline in incidence, gastric cancer remains the 4th most common cancer (incidence of approximately 1,000,000/year) and the 2nd most common cause of cancer death worldwide (approximately 750,000 per year) [1]. In 90% of cases, its histology is adenocarcinoma, either of the intestinal type (predominant in the elderly, decreasing incidence) or of diffuse type (mainly in elderly women, increasing incidence). Stromal, endocrine, or lymphoma tumors are not included in this review.
There is a 10-fold variation in incidence between the highest and lowest risk populations [2]. The highest incidence rates are observed in East Asia, East Europe, and South America, while the lowest rates are found in North America and most parts of Africa [1]. Helicobacter pylori infection, smoking, salt and nitrate-rich foods are the most important risk factors [2]. The interactions between dietary factors, environmental conditions, and the development of gastric cancer are also well described, with a number of clearly identifiable dietary exposures strongly associated with gastric cancer induction and prevention [2].A family history is found in 10% to 30% of cases. However, hereditary factors are rare and affect only 1% to 3% of patients (Lynch syndrome, hereditary diffuse cancer secondary to a mutation of E-cadherin, mutation of BRCA) [1]. Finally, a history of partial gastrectomy also increases the risk of developing gastric cancer, usually 10–15 years after the surgery [1].
Early publications on the use of adjuvant chemoradiotherapy (CRT) for gastric cancer date back to the early 1980s. They reported series of patients treated postoperatively with radiation therapy from 20 Gy to 50 Gy with concurrent 5-fluorouracil (5FU) [3], [4], [5]. In Intergroup 0116 phase III trial published in 2001 by MacDonald et al., 556 patients with gastric or gastroesophageal junction cancers were randomized after complete resection between observation and postoperative CRT [6]. The radiation therapy dose was 45 Gy in 25 fractions and 5 weeks. This treatment provided a significant benefit in terms of overall survival (36 months versus 27 months, HR = 1.4, 95%CI = 1.1–2.0, p = 0.005) and relapse-free survival (30 months versus 19 months, HR = 1.5, 95%CI = 1.2–1.9, p < 0.001). This benefit was confirmed after a follow-up of more than 10 years [7]. Results of controlled prospective studies of adjuvant radiotherapy for gastric cancer are summarized in Table 1. Gastroesophageal junction (GEJ) adenocarcinomas will not be specifically addressed in this review, as these tumors are sometimes treated as gastric cancer and other times as oesophageal cancer. For example, patients with GEJ tumors were included in Intergroup 0116 and TOPGEAR, but not in ARTIST studies.
Table 1.
Summary of randomized controlled prospective studies of adjuvant radiotherapy for gastric cancer.
| Refs. | Study | n pts | RT dose & technique | Concurrent chemotherapy | Overall survival | Progression free survival | Grade 3–4 toxicity |
||
|---|---|---|---|---|---|---|---|---|---|
| Haematologic | Digestive | All | |||||||
| [4] | Dent, Cancer 1979 | 35 adj CRT 31 observation |
20 Gy 2D |
5FU | 5-year: favor CRT HR 0.80 (95%CI: 0.37–1.70) |
NA | 77% | NA | NA |
| [3] | Moertel CG, J Clin Oncol 1984 | 39 adj CRT 23 observation |
37,5 Gy 2D |
5FU | 5-year: favor CRT HR 0.39 (95%CI: 0.17–0.88) |
5-year: favor CRT HR 0.48 (95%CI0.25–0.91) |
56% | 56% | NA |
| [5] | Allum WH, Br J Cancer 1989 | 153 adj RT 145 adj CT |
45 Gy + 5 Gy 2D |
5FU + adriamycin + mitomycin C | Median OS 15 months not significant difference between adj-RT and adj-CT |
NA | 1% | 27% | NA |
| [6],[7] | Macdonald JS, N Engl J Med 2001 Smalley SR, J Clin Oncol 2012 |
281 adj CRT 275 observation |
45 Gy 2D |
FUFOL | 5-year favor CRT HR 1.32 (95%CI:1.10–1.60) |
5-year: favor CRT HR 1.51 (95%CI: 1.25–1.83) |
54% | 33% | 64% |
| [49] | Bamias A, Cancer Chemother Pharmacol 2010 | 72 adj CRT 71 adj CT |
45 Gy 3D |
Docetaxel + Cisplatin (45 pts) Docetaxel + Carboplatin (98 pts) |
3 -year: not significant HR 1.2 (95%CI: 0.75–1.91) |
3-year: not significant HR 1.04 (95%CI: 0.66–1.63) |
25% | 4% | NA |
| [44] | Kwon H-C, Asia Pac J Clin Oncol 2010 | 31 adj CRT 30 adj CT |
45 Gy IMRT |
Capecitabin | 5-years: not significant p = 0.814 |
5-year: not significant p = 0.887 |
61% | 10% | 74% |
| [35] | Yu C, J Cancer Res Clin Oncol 2012 | 34 adj CRT 34 adj CT |
45 Gy IMRT |
FUFOL | 3-year: favor CRT p = 0.037 |
3-year: favor CRT p = 0.021 |
50% | 24% | NA |
| [38] | Zhu W, Radiother Oncol J Eur Soc Ther Radiol Oncol 2012 | 186 adj CRT 165 adj CT |
45 Gy IMRT |
FUFOL | 5-year: not significant p = 0.122 |
5-year: favor CRT p = 0.029 |
8% | 4% | 91% |
| [54] | Kim TH, J Radiat Oncol Biol Phys 2012 | 46 adj CRT 44 adj CT |
45 Gy 3D |
FUFOL | 5-ys OS: not significant p = 0.67 |
5-year: not significant HR 0.69 (CI 0.37–1.29) p = 0.246 |
20% | 17% | NA |
| [9],[10] | Lee J, ARTIST Trial. J Clin Oncol 2012 Park, J Clin Oncol 2015 |
230 adj CRT 228 adj CT |
45 Gy 3D |
Capecitabin | 7-year: not significant HR 1.13 (95%CI: 0.78–1.65) |
7-year: not significant HR 0.74 (95%CI: 0.52–1.05) |
48% | 16% | 82% |
Abbreviations: adj, adjuvant; CRT, chemoradiotherapy; CT, chemotherapy; RT, radiation therapy; IMRT, intensity modulated radiation therapy; 5FU, 5-fluorouracil; HR, hazard ratio; CI, confidence interval; NA, not available.
Post-operative CRT had been the standard adjuvant treatment of resected gastric adenocarcinoma until the publication of the MAGIC trial [8]. In this UK phase III trial, 503 patients were randomized between surgery alone versus perioperative chemotherapy with three cycles of ECF (epirubicin, cisplatin, 5FU) pre-surgery and post-surgery. Patients who received perioperative chemotherapy had a significantly higher 5-year overall survival rate than those who did not (36% versus 23%, HR = 0.75, 95% CI = 0.60–0.93, p = 0.009). Since then, this regimen has become the standard of care in Europe, eclipsing adjuvant CRT.
To date, except for HER2, there are no established evidence-based biomarkers predictive of tumour response to targeted agents, and the majority of patients do not yet benefit from molecularly directed therapies [9]. Classic biomarkers for gastric cancer diagnosis include carcinoembryonic antigen and cancer antigen 19-9, while microRNA and DNA hypomethylation are proposed as novel biomarkers. Modern biomedical research has explored many potential gastric cancer biomarker genes by utilising serum protein antigens, oncogenic genes or gene families through improving molecular biological technologies, such as microarray, RNA-Seq and the like [10]. Excluding classical biomarkers, those determining prognosis and the progression of gastric cancer focus on targeting microRNAs, epigenetic alterations and genetic polymorphisms [11]. Recently, the small noncoding microRNAs (miRNAs) have been suggested to be critical regulators in the oncogenesis pathways and to serve as useful clinical biomarkers [10].
We aim to summarize current knowledge and practices concerning adjuvant CRT for gastric cancer (excluding GEJ cancer patients) and to propose recommendations concerning the technical modalities of radiation therapy and the choice of concurrent chemotherapy.
Radiation therapy: technique, modalities
Dose
Historically, the radiation therapy dose used in clinical studies, including Intergroup 0116, was 45 Gy delivered in 25 fractions of 1.8 Gy in 5 weeks [6]. Subsequent trials have mainly used this dose of 45 Gy until the development of new radiation therapy techniques such as 3-dimensional conformal radiation therapy (3D-CRT) and intensity modulated radiation therapy (IMRT).
In the Korean phase III ARTIST trial, 458 patients were randomized between adjuvant chemotherapy with six cycles of cisplatin and capecitabine (XP) versus two cycles of XP followed by postoperative CRT (45 Gy with concurrent capecitabine) followed by two cycles of XP [12], [13]. Radiation therapy was delivered using two antero-posterior beams. The 3-year disease-free survival rate was 74% in the XP arm versus 78% in the XP-CRT-XP arm (p = 0.09) [12], [14]. However, a post hoc analysis including the 396 patients with pathological lymph node involvement showed a benefit of CRT in this population with a 3-year disease-free survival rate of 72% in the XP arm versus 78% in the XP-CRT-XP (p = 0.04) [12], [14].
Several dosimetric studies have shown that IMRT can lower the dose to organs at risk (liver and kidneys), suggesting the possibility of dose escalation [15], [16]. In a retrospective study, a dose of 50.4 Gy delivered by IMRT was compared to a dose of 45 Gy by 3D-CRT treatment in 24 patients in combination with concurrent chemotherapy [17]. A similar safety profile was observed in this small series. A prospective phase II study including 110 patients assessed a 50.4 Gy (28 fractions of 1.8 Gy) IMRT with concurrent FOLFOX [18]. The tolerance was acceptable [18]. Its efficacy was not compared to the same treatment at a dose of 45 Gy. However, there is no clinical study comparing prospectively a dose escalation to the standard treatment of 45 Gy.
Neoadjuvant radiation therapy trials for unresectable locally advanced gastric cancer using doses of 50.4 Gy resulted in significant tumor responses [19], [20]. One study reported 42% of pathological complete responses, still 4% of patients had grade 5 toxicity [19]. The second reported 75% and 38% overall radiological and pathological response rates, respectively, with 5% of grade 4 toxicity [20].
In patients with R1 or R2 resection, a phase I trial evaluated the tolerance of adjuvant radiation therapy at a dose of 45 Gy followed by a 10.8 Gy boost with an IMRT technique and concurrent capecitabine [21]. Out of the six patients included, only two completed the boost dose prescribed, because of issues in delineating the boost area in the absence of surgical clips [21]. The two patients who received a total dose of 55.8 Gy had good tolerance [21]. Recently, in a retrospective cohort of 67 patients treated between 2003 and 2008 according to the MacDonald regimen, Soyfer et al. showed an influence of the overall treatment time of radiation therapy on the prognosis of patients, with an increase of 10% of the risk of locoregional relapse per extra day of radiation therapy beyond 36 days (HR = 1.1, 95%CI = 1.0–1.2, p = 0.001) [22].
Proposal
There is a consensus on the dose of radiation therapy in gastric cancer adjuvant therapy, i.e. 45 Gy in 25 fractions, 5 fractions a week. Irradiation techniques using IMRT are recommanded to reduce the dose delivered to organs at risk. Limited data suggest the feasibility of a dose escalation to 50.4 Gy in 28 fractions, or even 54 Gy without increasing toxicity.
Target volumes
In surgical and autopsic series, it has been shown that local and locoregional relapses of gastric tumors are very common [23]. In the Intergroup 0116 trial, the local relapse rate was 29% and the regional relapse rate was 75% without CRT versus 19% and 65% after adjuvant CRT, respectively [6]. Therefore, radiation therapy can be estimated to reduce the risk of local relapse by 10%, decreasing tumor spreading to the peritoneum, small omentum or large omentum, pancreas, and duodenum, as well as lymphatic or hematogenic dissemination, especially to the liver.
During the preparation of radiation therapy, the acquisition of the CT scan images with slices of 3–5 mm thickness is necessary, with the patient in supine position and arms above the head. The injection of contrast enhancer facilitates the delineation of lymph nodes [24]. Clips placed during the dissection allow for tumor bed identification.
In the trial published by MacDonald et al., patients had postoperative tumor bed irradiation, defined as the surgical anastomosis with proximal and distal margins of 2 cm including the lymph node areas [6]. The tumor bed delineation benefit from either a scanner, a barium transit or clips placed during the surgery [6]. Treatment of the left abutment of the diaphragm was proposed in the case of a locally advanced proximal tumor (T3). The definition of lymph node target volumes was based on the recommendations of the Japanese Society of Gastroenterology (JCGA) [25]. These included the perigastric, para-aortic, splenic, hepato-duodenal, duodenopancreatic, and hepatoportal areas. In addition, in case of cardia tumor, the para-esophageal and para-cardial areas were included, while the duodeno-pancreatic area might be excluded. Similarly, a tumor in the antrum may not receive irradiation of the splenic area to avoid harming kidney function.
The literature has confirmed the utility of 18-fluorodeoxy-glucose positron emission tomography-computed tomography (18-FDG-PET/CT) diagnostics in the staging of gastric cancer [26], the detection of recurrence [27] and its prognostic and predictive value [28]. PET-CT is standard in esophageal and gastro-esophageal cancer staging and can identify distant metastases in 15–20% of patients with newly diagnosed esophageal cancer not identified by CT [29]. A study described a way to use PET during delineation of the target, performing individualized interpretations of the regions with increased FDG uptake and manually creating contours in the context of other clinical data [30]. From this perspective, 18-FDG-PET/CT in gastric cancer radiotherapy planning may affect the GTV delineation [30]. The 4D gated PET/CT examination may also help to establish the tumor boundaries and sparing of normal tissue that is of similar density to the tumor [31]. Unfortunately, few prospective studies have evaluated the impact of changes in the GTV introduced after the application of PET for further RT results [30]. Also, the use of Magnetic Resonance Imaging (MRI) in Radiotherapy (RT) planning is rapidly expanding and could help with more precise delineation, in order to deliver appropriate treatment while reducing toxicity to healthy organs [32].
Currently, the definition and delineation of target volumes is not consensual.
In the ARTIST trial, target volumes included the anastomosis with proximal and distal margins of 2 cm, duodenal stump, and regional lymph nodes [12].
In a study published in 2001, the authors proposed a definition of the tumor residue in collaboration with the surgeon and a clinical target volume (CTV) including three elements:
-
(i)
A volume of tumor extension: preoperative tumor zone, gastric and transverse colon spaces;
-
(ii)
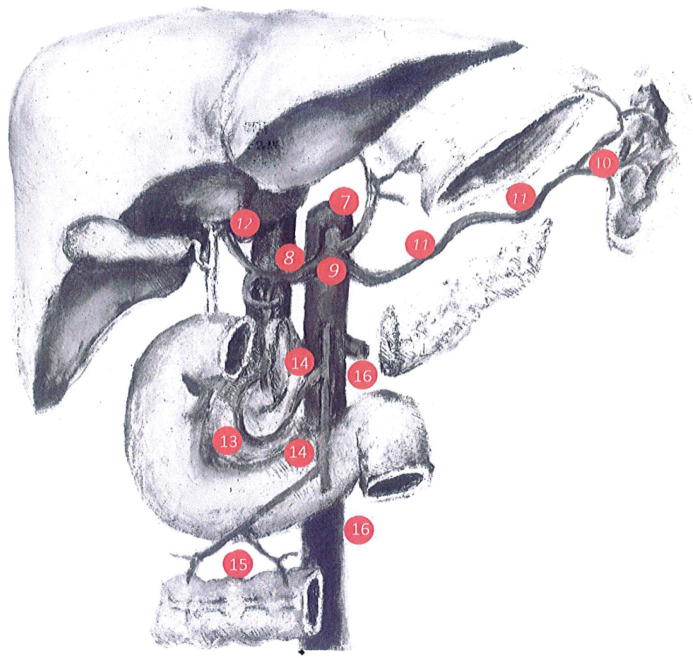
A volume of lymphatic extension including areas 1 to 16 of the Japanese JCGA classification [25] (Fig. 1);
-
(iii)
A volume of peritoneal extension (peritoneal cavity containing the tumor and, if possible, the parietal peritoneum with respect to the laparotomy), widening to include the hepatic pedicle and the splenic hilum [33].
Fig. 1.
Gastric lymph nodes groups localization, according to the Japanese gastric cancer treatment guidelines.
Recommendations were then published in 2002 for gastric cancer adjuvant radiation therapy, including volumes and modalities [34]. Still, these referred to a two-dimensional irradiation technique using two antero-posterior beams, a technique which should be considered currently as obsolete [34].
Knowledge of the surgical technique of gastrectomy is necessary, mainly total gastrectomy versus partial distal including the different means of restoring continuity (esophagojejunal anastomosis, Billroth…). Surgical series have identified the 3 and 4 nodal areas at risk which should always be included in the target volume definitions, while the paracardial 1 and 2 proximal areas could be excluded in case of distal tumor [35]. Similarly, in the case of a proximal tumor, the intrapyloric 5 and 6 zones might be excluded.
Even if D2 surgical dissection is currently the reference in the surgical treatment of gastric cancer [23], in Western countries, the recommended practices are D1 extensive or “D1+” lymphadenectomy, corresponding to a D2 dissection without pancreatectomy or splenectomy. Current recommendations state that D1 or D2 dissections should allow for the pathological analysis of at least 15 or 25 lymph nodes, respectively. It consists of excision of the N1 group (1, 2, 3, 4, 5, 6) and the N2 group (7, 8, 9, 10, 11) of lymph nodes. According to the Japanese classification, tumor-positive lymph nodes of the N3 (12, 13, 14) or N4 [15], [16] groups are considered as distant metastatic disease [35].
Extent of lymphadenectomy is a critical issue in addressing adjuvant treatments for gastric cancer. Despite increased morbidity and mortality in the Dutch gastric cancer group trial [36] compared to asian series where the procedure is routinely performed (10% vs <1%), D2 dissection was correlated with increased locoregional control and cancer-specific survival [37]. It has been argued that inconsistent RT benefit observed in different trials may result from a “salvage” effect of radiotherapy in rescuing an inadequate surgery. In particular INT 0016 showed no difference in outcome after stratification for dissection level, but was probably underpowered to detect differences since <10% of patients received D2 dissection [6]. Conversely, the Dutch trial reported improved local control after chemoradiotherapy only in patients receiving D1 dissection [38]. Future trials should take into account this information to avoid bias.
A French group proposed in 2008 an irradiation of the nodal areas 1 to 12 in body or antral tumors, 1 to 4 and 7 to 11 in gastroesophageal junction tumors and 1 to 9 in the other situations [39]. On the other hand, the authors recommended that group 12 should not be systematically irradiated due to rare metastatic involvement and increased volume of irradiated healthy hepatic parenchyma when treated. In the RecoRad publication (guidelines for optimization, harmonization and homogenization of practices in external radiation therapy in France), authors recommended an irradiation of the following nodal area [40]:
-
–
3, 4sa, 4sb, 7, 9, 10, 11p, 11d, 19, 20, 110, 111, and 112 for proximal tumors
-
–
1, 2, 3, 4sa, 4sb, 4sd, 5, 6, 8a, 8p, 9, 10, 11p, 11d, 18, 19, and 20 for central tumors
-
–
3, 4sd, 5, 6, 8a, 8p, 9, 10, 11p, 12, 13, 17, and 18 for distal tumors.
But even if a delineation guide is provided, interobserver variability in delineation of the CTV is observed. In a retrospective review of 3D-CRT treatment plans in ten centers participating in CRITICS, there were changes in CTV volume from 240 to 821 cm3 and in PTV from 634 to 1677 cm3, mainly in the cranio-caudal direction [41].
Proposal
The post-operative CTV must include the residual tumor volume (GTV) for incomplete resection (in absence of salvage surgery), the tumor bed (its delineation may benefit from the surgeon's assistance), the surgical anastomosis (duodenal for partial gastrectomy or distal tumor) and nodal areas. The definition of nodal risk areas depends on the tumor localization according to the Japanese recommendations. However, an irradiation of 3 and 4 areas, corresponding to the small curvature and the great omentum, should be systematic (Table 2). The development of a contouring atlas as proposed by the RTOG for pancreatic cancer is of great interest [42].
Table 2.
Recommendation for nodal areas delineation according to the tumor localization.
| Station n° | Definition | Cardia tumor | Proximal tumor | Middle tumor | Distal tumor |
|---|---|---|---|---|---|
| 1 | Right paracardial LNs, including those along the first branch of the ascending limb of the left gastric artery. | X | X | X | |
| 2 | Left paracardial LNs including those along the esophagocardiac branch of the left subphrenic artery. | X | X | X | |
| 3a | Lesser curvature LNs along the branches of the left gastric artery. | X | X | X | X |
| 3b | Lesser curvature LNs along the 2nd branch and distal part of the right gastric artery. | X | X | X | X |
| 4sa | Left greater curvature LNs along the short gastric arteries (perigastric area). | X | X | X | X |
| 4sb | Left greater curvature LNs along the left gastroepiploic artery (perigastric area). | X | X | X | X |
| 4d | Right greater curvature LNs along the 2nd branch and distal part of the right gastroepiploic artery. | X | X | X | X |
| 5 | Suprapyloric LNs along the 1st branch and proximal part of the right gastric artery. | X | X | X | X |
| 6 | Infrapyloric LNs along the first branch and proximal part of the right gastroepiploic artery down to the confluence of the right gastroepiploic vein and the anterior superior pancreatoduodenal vein. | X | X | X | X |
| 7 | LNs along the trunk of left gastric artery between its root and the origin of its ascending branch. | X | X | X | X |
| 8a | Anterosuperior LNs along the common hepatic artery. | X | X | X | X |
| 8p | Posterior LNs along the common hepatic artery. | X | X | X | X |
| 9 | Celiac artery LNs | X | X | X | X |
| 10 | Splenic hilar LNs including those adjacent to the splenic artery distal to the pancreatic tail, and those on the roots of the short gastric arteries and those along the left gastroepiploic artery proximal to its 1st gastric branch. | X | X | X | |
| 11p | Proximal splenic artery LNs from its origin to halfway between its origin and the pancreatic tail end. | X | X | X | X |
| 11d | Distal splenic artery LNs from halfway between its origin and the pancreatic tail end to the end of the pancreatic tail. | X | X | X | |
| 12a | Hepatoduodenal ligament LNs along the proper hepatic artery, in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas. | X | X | X | |
| 12b | Hepatoduodenal ligament LNs along the bile duct, in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas. | X | X | ||
| 12p | Hepatoduodenal ligament LNs along the portal vein in the caudal half between the confluence of the right and left hepatic ducts and the upper border of the pancreas. | X | X | ||
| 13 | LNs on the posterior surface of the pancreatic head cranial to the duodenal papilla. | X | |||
| 14v | LNs along the superior mesenteric vein. | ||||
| 15 | LNs along the middle colic vessels. | ||||
| 16a1 | Paraaortic LNs in the diaphragmatic aortic hiatus. | X | X | ||
| 16a2 | Paraaortic LNs between the upper margin of the origin of the celiac artery and the lower border of the left renal vein. | X | X | X | |
| 16b1 | Paraaortic LNs between the lower border of the left renal vein and the upper border of the origin of the inferior mesenteric artery. | X | X | ||
| 16b2 | Paraaortic LNs between the upper border of the origin of the inferior mesenteric artery and the aortic bifurcation. | ||||
| 17 | LNs on the anterior surface of the pancreatic head beneath the pancreatic sheath. | X | |||
| 18 | LNs along the inferior border of the pancreatic body. | X | X | ||
| 19 | Infradiaphragmatic LNs predominantly along the subphrenic artery. | X | X | ||
| 20 | Paraesophageal LNs in the diaphragmatic esophageal hiatus. | X | X | ||
| 110 | Paraesophageal LNs in the lower thorax. | X | X | ||
| 111 | Supradiaphragmatic LNs separate from the esophagus. | X | X | ||
| 112 | Posterior mediastinal LNs separate from the esophagus and the esophageal hiatus. | X | X |
Reference: Créhange G, Huguet F, Quero L, N’Guyen TV, Mirabel X, Lacornerie T. Radiothérapie des cancers de l’œsophage, du cardia et de l’estomac. Cancer/Radiothérapie. 2016 Sep; 20: S161–S168.
Irradiation technique
In the princeps study published by MacDonald et al. in 2001, a two-dimensional irradiation with two anterior and posterior beams was recommended. Consequently, 17% of patients were unable to complete the planned irradiation due to significant toxicities (6). Grade 3–4 haematological toxicity rate was 54% and grade 3–4 gastrointestinal toxicity was 33%. Since then, the advent of new radiation therapy techniques has made this treatment technique obsolete. The first evolution of radiation therapy in gastric cancer was 3D-CRT with direct planning. In a dosimetric study comparing 2D and 3D treatment plans, there was an advantage with 3D-CRT both in terms of target volume coverage (99% of PTV receiving more than 95% of the prescribed dose for a 3D plan with 6 beams, compared with 93% with 2 anteroposterior beams) and a reduction of the dose received by the kidneys [43]. However, a higher dose to the liver with 3D-CRT was noted, although the latter remained below the maximum recommended dose. The ARTIST phase III trial did not show any benefit in terms of overall survival of adjuvant radiation therapy, but it demonstrated a good tolerance of this technique in a large cohort of patients [12]. The main adverse reaction requiring a change of treatment was neutropenia. Grade 3–4 toxicities rates were similar in both arms. Grade 3–4 haematological toxicity in the CRT arm was 48% and the gastrointestinal toxicity rate was 17%. Therefore, this trial was the first large-scale trial demonstrating the feasibility of 3D-CRT after extensive D2 surgical lymph node dissection.
In a dosimetric study, the authors compared the treatment plans of 20 patients in 3D-CRT (5 beams) with IMRT plans (7 or 9 beams) [44]. For 90% of the patients, the two radiation oncologists who were asked to blindly designate the most appropriate plan from the dose-volume histograms (DVH) chose an IMRT treatment plan because of better coverage of the target volume while better sparing organs at risk. Subsequently, many dosimetric studies had compared 3D-CRT treatment plans with IMRT treatment plans, showing a reduction in the dose received by the organs at risk [16], [45]. One retrospective study reported a decrease in the dose received by the liver and kidneys by comparing the treatments received by 57 patients. Indeed, 26 patients were treated using 3D-CRT and 31 with IMRT, with a total dose of 45 Gy in both groups [46]. The rate of gastrointestinal toxicity was comparable in both groups. However, more treatment interruptions were reported in the 3D-CRT arm than in the IMRT arm (3 versus 0, respectively) as well as a higher elevation of remote serum creatinine (0.2 mg/dL increase in the 3D-CRT group, p = 0.02). On the other hand, this study did not reveal any significant improvement in overall survival. Another dosimetric analysis published in 2013 compared the treatment plans of 15 patients between 3D-CRT, IMRT with 5 treatment beams, and IMRT with 7 treatment beams [47]. In this study, IMRT had a significant advantage over several dosimetric parameters (conformation index, homogeneity index) and a significantly lower dose received by the spinal cord (p = 0.009). However, the doses received by the liver and kidneys were similar. In 2014, Stiekema et al. compared dosimetric and clinical data from 87 patients, 31 treated with 2D radiation therapy with 2 anteroposterior beams, 25 with 3D-RTC, and 31 with IMRT [48]. In all three groups, the dose received by the left kidney exceeded the maximum tolerated dose, while the right kidney was spared. However, IMRT decreased the dose received by the left kidney, which resulted in the statistically significant preservation of renal function.
In a prospective study, adjuvant CRT (IMRT 45 Gy and concurrent 5FU) was compared with adjuvant LV5FU2 in 68 patients who underwent surgery with D1 or D2 lymphadenectomy for locally advanced gastric adenocarcinoma (T3, T4 and / or N+) [49]. There was a significant benefit in the CRT group for overall survival at 1, 2 and 3 years (86%, 73% and 68%, respectively, in the CRT group versus 68%, 50%, and 44% in the chemotherapy group). Grade 3 and 4 toxicity rates were similar in both groups.
In a study published in 2009 and updated in 2013, a benefit in overall survival with IMRT has been also reported (median survival: 43 months versus 18 months with 3D-CRT, p = 0.06) [50], [51]. However, the two groups were not homogeneous because most of the patients in the 3D-CRT group received concurrent 5FU and the patients in the IMRT group received concomitant XELOX.
In another large-scale trial, after surgery with extensive D2 nodal dissection, 380 patients were randomized between postoperative CRT with IMRT (45 Gy and concurrent LV5FU2) and adjuvant chemotherapy (LV5FU2). This trial failed to show a significant benefit on overall survival with CRT, but found an improvement in progression-free survival in the CRT arm (50 months versus 36 months, HR = 1.35, CI 95%=1.03–1.78; p = 0.029) [52]. Grade 3–4 gastrointestinal toxicity rate was significantly higher in the CRT group.
In a phase II trial published in 2015, a cohort of 110 patients treated with 3D-CRT or IMRT at a 50.4 Gy dose with concurrent FOLFOX was included. The authors reported a good tolerance with 90% of patients receiving the entire treatment (compared to 64% in the MacDonald princeps study in 2001 with 2D treatment) [18].
The definition of planning treatment volume (PTV) must consider the movements of the organs related to the respiratory cycle. In a study including 22 postoperative patients, a mean abdominal movement of 11.1 mm in the cranio-caudal direction, 1.9 mm in the lateral direction and 5.5 mm in the anteroposterior direction was reported with conventional treatment in free breathing versus respectively, and 3.7, 1.6 and 2.8 mm with breath hold treatment, respectively. A margin of 1 cm seems to be the minimum acceptable. A study evaluating the relevance of respiratory motion management showed that IMRT with the breath-hold technique could decrease organ movement and allow a dose escalation to 54 Gy [53].
A comparison of treatment plans by IMRT, SA-VMAT and DA-VMAT (Single Arc or Double Arc Volumetric Modulated Arc Therapy) has also recently been published [54]. The results suggested that treatment with DA-VMAT improved the coverage of the target volume compared to a 5-beam or 7-beam IMRT and SA-VMAT. However, DA-VMAT did not reduce the dose received by the liver compared to IMRT with 5 beams.
The benefit of protontherapy in the treatment of resected gastric cancer has been discussed in a dosimetric study. A 6-beam IMRT treatment plan was compared to a 2 to 3 beam protontherapy plan in 13 patients [55]. IMRT treatment plans generated a better homogeneity index (median: 0.04 ± 0.01 in IMRT versus 0.07 ± 0.01 with protontherapy, p = 0.03), but the protontherapy plan allowed fewer low and intermediate doses to be delivered to the surrounding tissue. Four patients received protontherapy and CT imaging performed during treatment showed good target volume coverage. Protontherapy could thus provide robust treatment plans in this indication.
Proposal
Treatment with IMRT reduces the dose received by the organs at risk and optimizes the distribution of the dose to the target volume. This irradiation technique could allow a dose escalation to at least 50.4 Gy. When available, patients should be treated with a breath hold technique in order to reduce respiratory motion. If not, an ITV based on a 4D CT scan should be added to the CTV.
Concurrent chemotherapy
Adjuvant setting
There is currently no consensus regarding the best concurrent chemotherapy to combine with radiation therapy for the adjuvant treatment of gastric cancer. In the SWOG/INT0116 trial, patients received concurrent FUFOL regimen during the first four days and last three days of radiation therapy [6], [56]. Due to a significant rate of haematological and digestive grade 3–4 toxicities (54% and 33%, respectively), a modified LV5FU2 protocol was then proposed in a prospective study published in 2005 [57]. In this study, 4% haematological toxicities and 4% grade 3–4 digestive toxicity were observed, with treatment discontinuation for only one patient (4%) [57]. These results warrant an improved tolerance of this administration scheme.
In the Korean phase III ARTIST trial, concurrent capecitabine was proposed [12]. Despite the lack of superiority of CRT in terms of survival in this trial, tolerance was acceptable with 52% of grade 3–4 neutropenia. Gastrointestinal tolerance was better compared with FUFOL. In another prospective trial including 30 patients with complete resection, the same dose of capecitabine was used [58]. The authors reported a similar tolerance, with 48% and 3% of grade 3–4 neutropenia or diarrhea, respectively. The dosage of capecitabine in these two trials seems optimal. This scheme is already used in common practice for rectal and pancreatic cancer [59], [60].
A recent prospective phase II study evaluated the efficacy and tolerance of concurrent FOLFOX combined with 50.4 Gy 3D-CRT or IMRT [18], [61]. Authors reported grade 3–4 nausea/vomiting, anorexia, or neutropenia toxicities in 15%, 12%, and 9% patients, respectively. IMRT with concomitant XELOX was also assessed in a retrospective study including 24 patients [50], [51]. Late toxicity, especially renal and digestive, appeared to be lower with XELOX compared to the MacDonald-treated cohort [50], [51]. Concerning the use of taxanes, there are currently two published trials. In the phase II RTOG 0114, all the patients received radiation therapy and were randomized between a concurrent combination of 5FU and paclitaxel versus a combination of cisplatin and paclitaxel [62]. There were 43% and 54% grade 3 and grade 4 toxicities in the 5FU-paclitaxel arm, respectively, and 51% and 22% in the cisplatin-paclitaxel arm, respectively, mostly haematological. In a second trial, patients received a 45 Gy radiation therapy with concurrent platinum and docetaxel [63]. A modification of the protocol with replacement of cisplatin with carboplatin after the inclusion of the first 45 patients was required because nausea and vomiting rates were too high.
Locally-advanced inoperable tumors
Data from the National Cancer Data Base from 2000 to 2009 demonstrated that approximately 20–30% stage I-III gastric cancer patients did not receive surgical resection [64], [65]. There is relatively little data regarding CRT for inoperable gastric cancer patients.
A phase II trial assessing radiation therapy with concurrent weekly docetaxel plus pre- and post-radiation chemotherapy with modified DCF regimen (mDCF) in inoperable gastric cancer patients showed promising efficacy (36% and 83% of complete or objective response rate, respectively), with acceptable toxicities (31% and 14% of ≥grade 3 nausea and neutropenia, respectively) [65].
Proposal
Currently, there are alternatives to the FUFOL regimen used by MacDonald, mainly represented by capecitabine monotherapy or the XELOX combination. However, none of these protocols has been directly compared to this standard in a randomized trial so far.
Organs at risk: dose constraints
In its 2007 edition, which is currently being updated, the Radiation Therapy Procedures Guide proposed a maximum dose of 45 Gy in one point of the spinal cord, a maximum of 30 Gy in 50% of the liver volume or 26 Gy in 100% of the liver, and a maximum of 20 Gy delivered in a total volume equivalent to one kidney. These recommendations are only valid for patients with normal liver and kidney functions. The maximal dose in the whole heart was 35 Gy, limiting to the maximum the volume of heart receiving 40 Gy or more [24].
A randomized controlled multicentric trial proposed a V15Gy received by the kidney ≤40%, V30Gy in the liver ≤60%, and a dose <40 Gy at a point in the spinal cord [52]. Another trial proposed a V20 Gy <20% and a total dose <10 Gy for the kidneys, a V30Gy <60% for the liver, a spinal cord dose <45 Gy [49]. A retrospective study recommended a V20Gy <75% of the volume of a kidney and a maximal dose to the spinal cord of 50 Gy. Considering IMRT planning, the maximum dose was 12 Gy to the kidneys (considering both in a single volume), 24 Gy to the whole liver, and 40 Gy to the spinal cord. In a phase II trial, the dose constraints were V18Gy <66% of one kidney or V25Gy <30% of each kidney, V30Gy <30% of the liver, and a maximum dose of 40 Gy on the spinal cord [18]. The QUANTEC criteria defined the upper-abdominal dose constraints as: (i) mean dose <30–32 Gy to the liver, and (ii) <15–18 Gy to the kidneys to have less than 5% risk of severe complications [66]. The small bowel dose limitation was V15 <120 cc to have less than 10% severe complications [66].
Finally, the nephrotoxicity of adjuvant radiation therapy has been assessed in a prospective study including 44 patients. Irradiation at a dose greater than 20 Gy on more than 20% of one kidney resulted in renal failure in 11% of patients six months after the completion of radiation therapy and in 52% after one year [67].
Proposal
The organs at risk for which dose should be controlled during adjuvant radiation therapy of gastric cancer are the kidneys (mostly left kidney), the liver, the spinal cord, and the heart. An assessment of the dose received by the small intestine seems also mandatory, especially as new techniques such as IMRT allow a dose escalation. It seems important to note that in the 10-year follow-up update of patients in the INT 0116 trial, a larger number of second cancers was reported in the postoperative CRT group compared to the control arm (21 versus 8), but it remained non significant (p = 0.21) [7].
Perspectives
Both perioperative chemotherapy and postoperative CRT have a significant survival advantage over surgery alone for the treatment of patients with resectable gastric cancer. In a recent retrospective analysis including approximately 5000 patients with adenocarcinoma of the stomach or gastro-esophageal junction of stages II or III (National Cancer Data Base) the authors showed a survival benefit from perioperative chemotherapy versus postoperative chemoradiotherapy [68]. On the other hand, in a randomized trial between adjuvant chemotherapy alone (5FU-LV, 5 cycles) or CRT (a cycle of 5FU-LV, then CRT 45 Gy with 2 cycles 5FU-LV followed by 2 5FU-LV cycles) including 90 patients, CRT significantly improved 5-year locoregional recurrence-free survival and progression-free survival in locally advanced stage III tumors (93% vs 67%, p = 0.014 and 74% vs 55%, p = 0.056) [69]. Eighty-seven percent of patients in the CRT arm received the complete planned treatment.
In addition, in a subgroup analysis of the ARTIST trial, there was a benefit of CRT in the group of node-positive patients with intestinal (non-diffuse) adenocarcinoma. Moreover, in another subgroup analysis from the ARTIST trial, adjuvant CRT improved significantly locoregional control, especially in patients with lymph node metastases [13]. Thus, the ARTIST II randomized clinical trial has been initiated to confirm these findings and is currently going on in Korea (NCT01761461). In this trial, the chemotherapy used is S1 combined with oxaliplatin and the dose of radiation therapy is 45 Gy.
In Europe, CRITICS was an international, multicenter, randomized, phase III study including patients with resectable gastric adenocarcinoma localized to the stomach or the gastroesophageal junction. All patients received preoperative chemotherapy (ECF or ECX). Following surgery, patients were randomly assigned to receive either chemotherapy (393 patients) or CRT (395 patients). Radiation therapy was administered at 45 Gy in 25 fractions with concurrent cisplatin and capecitabine. The first results were presented at ASCO meeting in 2016 [70]. Five-year OS was similar for patients across the two arms: 40.8% for chemotherapy and 40.9% for CRT. However, only 47% and 52% of patients completed postoperative chemotherapy and CRT, respectively. Patients did not receive postoperative treatment for several reasons, including personal preference, progressive disease, and toxicity in the preoperative setting, enhancing the interest for a neoadjuvant approach.
On this purpose, an international phase III neoadjuvant trial is also on going (TOPGEAR NCT01924819), whose primary objective is to investigate whether the addition of CRT to chemotherapy is superior to chemotherapy alone in the neoadjuvant setting by improving pathological complete response rates in patients undergoing adequate surgery (D1+ dissection) for resectable gastric cancer. The interim results have been published after the inclusion of 120 patients [71]. The compliance to neoadjuvant treatment was very good (93% in the ECF arm versus 98% in the CRT arm) with 90% and 85% of patients proceeding to surgery in the ECF arm and in the CRT arm, respectively. The rates of grade 3–4 gastrointestinal toxicity were 32% and 30% in the ECF arm and in the chemoradiation arm, respectively while grade 3–4 hematological toxicity rates were 50% and 52%, respectively. The rate of surgical complications was similar between the two groups. In this trial, a strong QA programme is included with an online pretreatment validation of the target volume definition. The neoadjuvant approach seems feasible and is very promising.
CRITICS II (NCT02931890) will randomize patients between neoadjuvant chemotherapy followed by surgery vs. neoadjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery vs. neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. Also, combined approach with novel molecules such as check point inhibitors are ongoing: the PROCEED trial (NCT03064490) investigate wether addition of pembrolizumab improves the efficacy of neoadjuvant chemoradiotherapy (45 Gy in 25 fractions with concurrent, weekly carboplatin and paclitaxel). Finally, the FLOT4 trial showed that FLOT is superior over the ECF regime and is therefore the new benchmark for trials of rtchx in gastric cancer [72].
Conclusions
Since the publication of the results of INT0116 phase II trial by MacDonald et al. in 2001, evidence have been made that gastric cancer patients benefit from radiation therapy in combination with chemotherapy. Debates persist between the superiority of perioperative chemotherapy or adjuvant chemoradiotherapy. These regimens have not been compared in a randomized clinical trial. Among patients with lymph node-positive disease, more aggressive therapy before or after surgery is under consideration. We have reviewed the current state of knowledge, as well as modern techniques for prescribing chemoradiotherapy. Results from ongoing prospective trials on preoperative or adjuvant chemoradiotherapy are mandatory. Moreover, gastric cancer has genotypic subtypes. The cancer genome should drive future adjuvant trials of targeted therapies.
Conflict of interest
None.
Financial disclosure
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.02.005.
Appendix A. Supplementary data
References
- 1.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman D., Burley V.J. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20(4):633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Moertel C.G., Childs D.S., O’Fallon J.R., Holbrook M.A., Schutt A.J., Reitemeier R.J. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2(11):1249–1254. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 4.Dent D.M., Werner I.D., Novis B., Cheverton P., Brice P. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44(2):385–391. doi: 10.1002/1097-0142(197908)44:2<385::aid-cncr2820440203>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Allum W.H., Hallissey M.T., Ward L.C., Hockey M.S. A controlled, prospective, randomised trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: interim report. British Stomach Cancer Group. Br J Cancer. 1989;60(5):739–744. doi: 10.1038/bjc.1989.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdonald J.S., Smalley S.R., Benedetti J., Hundahl S.A., Estes N.C., Stemmermann G.N. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.Smalley S.R., Benedetti J.K., Haller D.G., Hundahl S.A., Estes N.C., Ajani J.A. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.Baniak N., Senger J.-L., Ahmed S., Kanthan S.C., Kanthan R. Gastric biomarkers: a global review. World J Surg Oncol. 2016;14(1) doi: 10.1186/s12957-016-0969-3. Available from: http://wjso.biomedcentral.com/articles/10.1186/s12957-016-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H.-H., Lin W., Tsai K.-W. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16 doi: 10.1017/erm.2013.16. Available from: http://www.journals.cambridge.org/abstract_S1462399413000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Z., Jiang W., Wang L. Biomarkers for gastric cancer: Progression in early diagnosis and prognosis (Review) Oncol Lett. 2015;9(4):1502–1508. doi: 10.3892/ol.2015.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Lim D.H., Kim S., Park S.H., Park J.O., Park Y.S. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 13.Yu J.I., Lim D.H., Ahn Y.C., Lee J., Kang W.K., Park S.H. Effects of adjuvant radiotherapy on completely resected gastric cancer: A radiation oncologist’s view of the ARTIST randomized phase III trial. Radiother Oncol. 2015;117(1):171–177. doi: 10.1016/j.radonc.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Park S.H., Sohn T.S., Lee J., Lim D.H., Hong M.E., Kim K.-M. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.3930. Available from: http://jco.ascopubs.org/cgi/doi/10.1200/JCO.2014.58.3930. [DOI] [PubMed] [Google Scholar]
- 15.Chung H.T., Lee B., Park E., Lu J.J., Xia P. Can all centers plan intensity-modulated radiotherapy (IMRT) effectively? An external audit of dosimetric comparisons between three-dimensional conformal radiotherapy and IMRT for adjuvant chemoradiation for gastric cancer. Int J Radiat Oncol. 2008;71(4):1167–1174. doi: 10.1016/j.ijrobp.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Murthy K., Shukeili K., Kumar S., Davis C., Chandran R., Namrata S. Evaluation of dose coverage to target volume and normal tissue sparing in the adjuvant radiotherapy of gastric cancers: 3D-CRT compared with dynamic IMRT. Biomed Imaging Interv J. 2010;6(3) doi: 10.2349/biij.6.3.e29. Available from: http://www.biij.org/2010/3/e29/e29.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G.-F.F., Bair R.J., Bair E., Liauw S.L., Koshy M. Clinical outcomes for gastric cancer following adjuvant chemoradiation utilizing intensity modulated versus three-dimensional conformal radiotherapy. de Mello RA, editor. PLoS One. 2014;9(1):e82642. doi: 10.1371/journal.pone.0082642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Shen Y, Zhu H, Zhao Y, Li Z, Qiu M, et al. A phase II trial of concurrent 3D-CRT/IMRT and oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) in gastric cancer patients with R0 gastrectomy and D2 lymph node dissection. Gastric Cancer [Internet]. 2015 Jan 22 [cited 2015 Mar 14]; Available from: http://link.springer.com/10.1007/s10120-015-0461-8. [DOI] [PubMed]
- 19.Zanoni A., Verlato G., Giacopuzzi S., Weindelmayer J., Casella F., Pasini F. Neoadjuvant concurrent chemoradiotherapy for locally advanced esophageal cancer in a single high-volume center. Ann Surg Oncol. 2013;20(6):1993–1999. doi: 10.1245/s10434-012-2822-4. [DOI] [PubMed] [Google Scholar]
- 20.Hu J.-B., Sun X.-N., Gu B.-X., Wang Q., Hu W.-X. Effect of intensity modulated radiotherapy combined with S-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat. 2014;37(1–2):11–16. doi: 10.1159/000358164. [DOI] [PubMed] [Google Scholar]
- 21.Wang X. Phase I study of postoperative radiotherapy combined with capecitabine for gastric cancer. World J Gastroenterol. 2014;20(4):1067. doi: 10.3748/wjg.v20.i4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyfer V., Geva R., Michelson M., Inbar M., Shacham-Shmueli E., Corn B.W. The impact of overall radiotherapy treatment time and delay in initiation of radiotherapy on local control and distant metastases in gastric cancer. Radiat Oncol. 2014;9(1):81. doi: 10.1186/1748-717X-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonenkamp J.J., Hermans J., Sasako M., van de Velde C.J., Welvaart K., Songun I. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340(12):908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 24.Ortholan C., Estivalet S., Barillot I., Costa A., Gérard J.-P. [Guide for external beam radiotherapy. Procedures 2007]. Cancer Radiother. 2007;11(6–7):329–330. doi: 10.1016/j.canrad.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ahn H.S., Kim S.H., Kodera Y., Yang H.-K. Gastric cancer staging with radiologic imaging modalities and UICC staging system. Dig Surg. 2013;30(2):142–149. doi: 10.1159/000350881. [DOI] [PubMed] [Google Scholar]
- 26.Wu C.-X. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol. 2014;20(16):4574. doi: 10.3748/wjg.v20.i16.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park M.J., Lee W.J., Lim H.K., Park K.W., Choi J.Y., Kim B.-T. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009;34(4):441–447. doi: 10.1007/s00261-008-9424-4. [DOI] [PubMed] [Google Scholar]
- 28.Grabinska K., Pelak M., Wydmanski J., Tukiendorf A., d’Amico A. Prognostic value and clinical correlations of 18-fluorodeoxyglucose metabolism quantifiers in gastric cancer. World J Gastroenterol. 2015;21(19):5901–5909. doi: 10.3748/wjg.v21.i19.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajj C., Goodman K.A. Role of radiotherapy and newer techniques in the treatment of GI cancers. J Clin Oncol. 2015;33(16):1737–1744. doi: 10.1200/JCO.2014.59.9787. [DOI] [PubMed] [Google Scholar]
- 30.Dębiec K, Wydmański J, Gorczewska I, Leszczyńska P, Gorczewski K, Leszczynski W, et al. 18-Fluorodeoxy-Glucose Positron Emission Tomography- Computed Tomography (18-FDG-PET/CT) for Gross Tumor Volume (GTV) Delineation in Gastric Cancer Radiotherapy. Asian Pac J Cancer Prev [Internet]. 2017 Nov [cited 2018 Feb 21];(11). Available from: http://journal.waocp.org/article_51914.html. [DOI] [PMC free article] [PubMed]
- 31.Chi A, Nguyen NP. 4D PET/CT as a Strategy to Reduce Respiratory Motion Artifacts in FDG-PET/CT. Front Oncol [Internet]. 2014 Aug 4 [cited 2018 Feb 21];4. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2014.00205/abstract. [DOI] [PMC free article] [PubMed]
- 32.Schmidt M.A., Payne G.S. Radiotherapy planning using MRI. Phys Med Biol. 2015;60(22):R323–R361. doi: 10.1088/0031-9155/60/22/R323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caudry M., Ratoanina J.L., Escarmant P., Maire J.P. Les volumes–cibles de la radiothérapie des adénocarcinomes gastriques. Cancer/Radiothérapie. 2001;5(5):523–533. doi: 10.1016/s1278-3218(01)00106-8. [DOI] [PubMed] [Google Scholar]
- 34.Smalley S.R., Gunderson L., Tepper J., Martenson J.A., Minsky B., Willett C. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52(2):283–293. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 35.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14(2):101–12. [DOI] [PubMed]
- 36.Hartgrink H.H., van de Velde C.J.H., Putter H., Bonenkamp J.J., Klein Kranenbarg E., Songun I. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22(11):2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Mocellin S., McCulloch P., Kazi H., Gama-Rodrigues J.J., Yuan Y., Nitti D. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2015;8:CD001964. doi: 10.1002/14651858.CD001964.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dikken J.L., Jansen E.P.M., Cats A., Bakker B., Hartgrink H.H., Kranenbarg E.M.-K. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28(14):2430–2436. doi: 10.1200/JCO.2009.26.9654. [DOI] [PubMed] [Google Scholar]
- 39.Hennequin C., Quero L., Mineur L. Cancer de l’estomac : doses et volumes-cibles. Cancer/Radiothérapie. 2008;12(6–7):659–662. doi: 10.1016/j.canrad.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Créhange G., Huguet F., Quero L., N’Guyen T.V., Mirabel X., Lacornerie T. Radiothérapie des cancers de l’œsophage, du cardia et de l’estomac. Cancer/Radiothérapie. 2016;20:S161–S168. doi: 10.1016/j.canrad.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Jansen E.P.M., Nijkamp J., Gubanski M., Lind P.A.R.M., Verheij M. Interobserver variation of clinical target volume delineation in gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77(4):1166–1170. doi: 10.1016/j.ijrobp.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Goodman K.A., Regine W.F., Dawson L.A., Ben-Josef E., Haustermans K., Bosch W.R. Radiation therapy oncology group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol. 2012;83(3):901–908. doi: 10.1016/j.ijrobp.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leong T., Willis D., Joon D.L., Condron S., Hui A., Ngan S.Y.K. 3D conformal radiotherapy for gastric cancer–results of a comparative planning study. Radiother Oncol. 2005;74(3):301–306. doi: 10.1016/j.radonc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Ringash J., Perkins G., Brierley J., Lockwood G., Islam M., Catton P. IMRT for adjuvant radiation in gastric cancer: A preferred plan? Int J Radiat Oncol. 2005;63(3):732–738. doi: 10.1016/j.ijrobp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Trip A.K., Nijkamp J., van Tinteren H., Cats A., Boot H., Jansen E.P.M. IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiother Oncol. 2014;112(2):289–294. doi: 10.1016/j.radonc.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Minn A.Y., Hsu A., La T., Kunz P., Fisher G.A., Ford J.M. Comparison of intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer. 2010;116(16):3943–3952. doi: 10.1002/cncr.25246. [DOI] [PubMed] [Google Scholar]
- 47.Ma H., Han J., Zhang T., Ke Y. Comparison of dosiology between three dimensional conformal and intensity-modulated radiotherapies (5 and 7 fields) in gastric cancer post-surgery. J Huazhong Univ Sci Technolog Med Sci. 2013;33(5):759–764. doi: 10.1007/s11596-013-1193-9. [DOI] [PubMed] [Google Scholar]
- 48.Stiekema J., Trip A.K., Jansen E.P.M., Aarts M.J., Boot H., Cats A. Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an r1 resection? Results from a Dutch cohort study. Ann Surg Oncol. 2015;22(2):581–588. doi: 10.1245/s10434-014-4032-8. [DOI] [PubMed] [Google Scholar]
- 49.Yu C., Yu R., Zhu W., Song Y., Li T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol. 2012;138(2):255–259. doi: 10.1007/s00432-011-1085-y. [DOI] [PubMed] [Google Scholar]
- 50.Boda-Heggemann J., Hofheinz R.-D., Weiss C., Mennemeyer P., Mai S.K., Hermes P. Combined adjuvant radiochemotherapy with IMRT/XELOX improves outcome with low renal toxicity in gastric cancer. Int J Radiat Oncol. 2009;75(4):1187–1195. doi: 10.1016/j.ijrobp.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 51.Boda-Heggemann J., Weiss C., Schneider V., Hofheinz R.-D., Haneder S., Michaely H. Adjuvant IMRT/XELOX radiochemotherapy improves long-term overall- and disease-free survival in advanced gastric cancer. Strahlenther Onkol. 2013;189(5):417–423. doi: 10.1007/s00066-013-0309-2. [DOI] [PubMed] [Google Scholar]
- 52.Zhu W., Xua D., Pu J., Zong C., Li T., Tao G. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104(3):361–366. doi: 10.1016/j.radonc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Hu W., Ye J., Wang J., Xu Q., Zhang Z. Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: a dosimetric study. Radiat Oncol. 2012;7:98. doi: 10.1186/1748-717X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z, Zeng J, Wang Z, Zhu H, Wei Y. Dosimetric comparison of intensity modulated and volumetric arc radiation therapy for gastric cancer. Oncol Lett [Internet]. 2014 Jul 18 [cited 2015 Mar 14]; Available from: http://www.spandidos-publications.com/10.3892/ol.2014.2363. [DOI] [PMC free article] [PubMed]
- 55.Dionisi F., Avery S., Lukens J.N., Ding X., Kralik J., Kirk M. Proton therapy in adjuvant treatment of gastric cancer: planning comparison with advanced x-ray therapy and feasibility report. Acta Oncol. 2014;53(10):1312–1320. doi: 10.3109/0284186X.2014.912351. [DOI] [PubMed] [Google Scholar]
- 56.Machover D., Goldschmidt E., Chollet P., Metzger G., Zittoun J., Marquet J. Treatment of advanced colorectal and gastric adenocarcinomas with 5-fluorouracil and high-dose folinic acid. J Clin Oncol. 1986;4(5):685–696. doi: 10.1200/JCO.1986.4.5.685. [DOI] [PubMed] [Google Scholar]
- 57.Dahan L., Atlan D., Bouché O., Mitry E., Ries P., Artru P. Postoperative chemoradiotherapy after surgical resection of gastric adenocarcinoma: can LV5FU2 reduce the toxic effects of the MacDonald regimen? A report on 23 patients. Gastroenterol Clin Biol. 2005;29(1):11–15. doi: 10.1016/s0399-8320(05)80688-8. [DOI] [PubMed] [Google Scholar]
- 58.Kwon H.-C., Kim M.C., Kim K.H., Jang J.S., Oh S.Y., Kim S.-H. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol. 2010;6(4):278–285. doi: 10.1111/j.1743-7563.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 59.Gérard J.-P., Azria D., Gourgou-Bourgade S., Martel-Laffay I., Hennequin C., Etienne P.-L. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee S., Hurt C.N., Bridgewater J., Falk S., Cummins S., Wasan H. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14(4):317–326. doi: 10.1016/S1470-2045(13)70021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., Wang Y., Qiu M., Li Q., Li Z., He B. Postoperative chemoradiotherapy in gastric cancer: a phase I study of radiotherapy with dose escalation of oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX regimen) Med Oncol. 2011;28(Suppl. 1):S274–S279. doi: 10.1007/s12032-010-9741-7. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz G.K., Winter K., Minsky B.D., Crane C., Thomson P.J., Anne P. Randomized phase II trial evaluating two paclitaxel and cisplatin-containing chemoradiation regimens as adjuvant therapy in resected gastric cancer (RTOG-0114) J Clin Oncol. 2009;27(12):1956–1962. doi: 10.1200/JCO.2008.20.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bamias A., Karina M., Papakostas P., Kostopoulos I., Bobos M., Vourli G. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol. 2010;65(6):1009–1021. doi: 10.1007/s00280-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 64.Raigani S., Hardacre J.M., Kim J., Ammori J.B. Trends in the surgical treatment of gastric adenocarcinoma. Ann Surg Oncol. 2014;21(2):569–574. doi: 10.1245/s10434-013-3314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Zhao G., Xu Y., He X., Li X., Chen H. Multicenter phase 2 study of peri-irradiation chemotherapy plus intensity modulated radiation therapy with concurrent weekly docetaxel for inoperable or medically unresectable nonmetastatic gastric cancer. Int J Radiat Oncol. 2017;98(5):1096–1105. doi: 10.1016/j.ijrobp.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 66.Marks L.B., Yorke E.D., Jackson A., Ten Haken R.K., Constine L.S., Eisbruch A. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol. 2010;76(3):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jansen E.P.M., Saunders M.P., Boot H., Oppedijk V., Dubbelman R., Porritt B. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67(3):781–785. doi: 10.1016/j.ijrobp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Fitzgerald TL, Efird JT, Bellamy N, Russo SM, Jindal C, Mosquera C, et al. Perioperative chemotherapy versus postoperative chemoradiotherapy in patients with resectable gastric/gastroesophageal junction adenocarcinomas: A survival analysis of 5058 patients: PECT Versus POCRT. Cancer [Internet]. 2017 Apr [cited 2017 Jun 14]; Available from: http://doi.wiley.com/10.1002/cncr.30692. [DOI] [PubMed]
- 69.Kim T.H., Park S.R., Ryu K.W., Kim Y.-W., Bae J.-M., Lee J.H. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys. 2012;84(5):e585–e592. doi: 10.1016/j.ijrobp.2012.07.2378. [DOI] [PubMed] [Google Scholar]
- 70.Verheij M, Jansen EPM, Cats A, van Grieken NCT, Aaronson NK, Boot H, et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. J Clin Oncol 34, no 15_suppl (May 2016) 4000-4000 [Internet]. Available from: 10.1200/JCO.2016.34.15_suppl.4000.
- 71.Leong T., Smithers B.M., Haustermans K., Michael M., Gebski V., Miller D. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24(8):2252–2258. doi: 10.1245/s10434-017-5830-6. [DOI] [PubMed] [Google Scholar]
- 72.Al-Batran S-E, Homann N, Schmalenberg H, Kopp H-G, Haag GM, Luley KB. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. Journal of Clinical Oncology 35, no 15_suppl (May 2017) 4004–4004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.