Highlights
-
•
We assessed vasomotor symptoms in patients on hormone therapy (ADT) for prostate cancer.
-
•
The commonest symptoms were hot flushes & sweats followed by fatigue & sleep disturbances.
-
•
The short term side-effect profile of ADT for prostate cancer was favourable.
-
•
Younger age and higher BMI predicted for severe toxicity.
Keywords: Prostate cancer, Hormone therapy, Vasomotor symptoms, Predictive factors
Abstract
Background & purpose
The impact of vasomotor symptoms (VMS) occurring in prostate cancer (PC) patients whilst on androgen deprivation therapy (ADT) has not been extensively researched. This longitudinal study sought to assess the VMS and identify any predictive factors.
Material & methods
Data from 250 PC patients on ADT were prospectively evaluated between January 10 and August 13 using a physician-directed questionnaire, to assess the impact of VMS. Parameters including height, weight, body surface area (BSA), body mass index (BMI), duration/type of ADT, co-morbidities and ethnicity were recorded.
Results
Fifty (20%) men reported no toxicity, whilst 171 (68.4%), and 29 (11.6%) reported mild to moderate and severe symptoms, respectively. Drenching sweats and hot flashes were common, and coexisted with sleep disturbances and fatigue. Patients with severe toxicity were younger (73 vs. 77 yrs; p = 0.04), had higher BMI (28 vs. 26; p = 0.02), and higher BSA (1.99 vs. 1.90; p = 0.04), when compared with those experiencing no toxicity. On multivariate analysis, younger age was predictive of sweats and hot flushes, whilst Afro-Caribbean men were twice as likely to experience sweats (OR 2.03, p = 0.05).
Conclusions
The short-term side-effect profile of ADT for prostate cancer was favourable, though debilitating VMS can occur in a significant minority of cases. Younger age and higher BMI predicted for severe toxicity but not the duration of ADT.
Introduction
The hormone responsive nature of prostate cancer lends itself to effective treatment with therapeutic agents that decrease the levels of testosterone (Luteinizing hormone-releasing hormone agonists (LHRHa), such as Goserelin, Buserelin and orchidectomy) or agents that block the androgen receptor (anti-androgens (AA), such as Bicalutamide, Cyproterone Acetate and Enzalutamide). Since, the demonstration of hormone responsive nature of prostate cancer by Huggins and Hodges in 1941 [1], androgen deprivation therapy (ADT) has been the standard of care for prostate cancer [2], [3]. LHRHa, and AA, have become the main form of ADT used [4], [5], replacing orchidectomy.
Long-term ADT (2–3 years or indefinite) plays an important role in the management of both locally advanced and metastatic prostate cancer [6], [7], [8]. In addition short-term (up to 6 months) ADT has been increasingly used to enhance the effect and clinical outcome of radiotherapy in organ-confined disease [9]. The long-term effects of ADT on libido, erectile function, and bone health through the lowering of testosterone and oestrogen levels are widely acknowledged [10]. In addition by increasing fat mass and decreasing lean body mass, ADT can also induce a metabolic syndrome with an increased risk of diabetic and cardiovascular complications [11], [12], [13], [14]. Aggressive risk factor modification for diabetes and heart disease, together with earlier detection and treatment of osteoporosis, and greater therapeutic options for erectile dysfunction have contributed significantly to the tolerability and acceptability of long term ADT treatment [15], [16].
However, little attention has been focussed on assessing short-term vasomotor symptoms (VMS) and associated psychological effects of ADT which can affect quality of life (QoL) in prostate cancer patients and disrupt daily activities during their treatment. This in turn can lead to poor compliance and in particular may influence treatment decisions for localised disease.
The most common VMS include hot flushes and sweats caused by a result of the release of hypothalamic catecholamines, such as norepinephrine in response to the decreased levels of LH and FSH as a result of LHRH agonist administration. These catecholamines disrupt the thermoregulation centre in the upper hypothalamus, resulting in abnormal and poorly regulated peripheral vasodilatation and the occurrence of hot flushes and perspiration [17]. Moreover there is now increasing recognition of psychological sequelae as a consequence of low testosterone levels notably on sleep [18], mood and cognitive decline [19].
Most of the data on VMS and ADT in men is extrapolated from breast cancer patients, and methodology for data collection has focussed mainly on hot flashes and sweats, requiring patients to keep diaries to record daily frequency of their symptoms.
This longitudinal study was conducted on prostate cancer patients attending a local oncology clinic receiving ADT. A physician directed objective questionnaire filled out at the time of consultation asking about common VMS and using a simple scoring system to asses impact of toxicity on daily activities (see Appendix 1) was used to determine the following issues:
-
1.
To assess the prevalence of VMS in men receiving ADT for their prostate cancer.
-
2.
The severity of VMS toxicity in patients undergoing such treatment.
-
3.
To identify whether there are any predictive factors for developing ADT related toxicity.
Methods
This was a prospective study using a physician completed questionnaire on a series of consecutive patients attending the uro-oncology clinic at Imperial College Healthcare NHS Trust, who were actively receiving ADT for their prostate cancer.
The aim of the questionnaire was to allow for a simple and objective assessment of a variety of VMS symptoms that could be completed with negligible impact on consultation time. The questionnaire specifically asked about common VMS symptoms including sweats, hot flashes, sleep disturbance, nervousness, low mood, tiredness, joint pains, headaches palpitations and unsteadiness. In particular the assessment was focussed on the extent to which the symptoms were interfering with their daily life. A scoring system of 1–4 was designated by the Clinician as follows:
-
1.
No toxicity (Absent),
-
2.
Self reported symptoms but not disrupting daily activities (Mild),
-
3.
Bothersome toxicity affecting quality of life but not disabling or severe enough for patients to seek intervention (Moderate).
-
4.
Toxicity disrupting daily activities requiring medical intervention-discontinuation of ADT (Severe).
To aid in reproducibility of data collection for hot flashes and to distinguish from sweats, duration of flashes was used to define toxicity, with an abnormal heat sensation lasting less than 1 minute, between 1 and 5 minutes and greater than 5 minutes used to designate mild, moderate and severe toxicity respectively. The degree to which these flushes were associated with perspiration and the effect on daily activity were then subsequently captured in the data for sweats. Patients were asked specifically to score the worse toxicity they had experienced up until the time of assessment. The duration of ADT was recorded from the start of ADT to the date of assessment or the time to worse documented toxicity (whichever was soonest).
Physical parameters such as height, weight body mass index (BMI), body surface area (BSA), ethnicity and medical co-morbidities, such as presence or absence of diabetes, hypertension and ischemic heart disease were collected (these common conditions or their treatment can be associated with VMS). Treatment related factors were also recorded such as duration and type of ADT. Although repeated assessments were allowed for patients at subsequent visits, only data collected at the time of first assessment during the time period of the study was included. The follow-up data will be analysed and published at a later date. The assessments were carried out at random time points in the patient’s treatment pathway to get an idea of prevalence of such symptoms.
Statistical analysis
The analysis was set to establish the prevalence of VMS in prostate cancer patients receiving ADT at various stages in their treatment pathway, and explore predictive factors for such toxicity. Based on the assumption that the prevalence of VMS is in the order of approximately 30%, and assuming a 90% confidence with a 5% precision, then 227 patients would be needed for an adequate sample size. Thus this study was set to collect data sets on 250 patients.
Continuous variables were summarised using descriptive statistics (median and interquartile range: IQR) and categorical variables using frequencies and percentages. Multivariate logistic regression models were used to explore the effects of independent variables (i.e. age, height, weight, hormone treatment, BMI, treatment time, ethnic, diabetes, ischemic heart disease (IHD) and blood pressure (BP)) on the vasomotor toxicity variables. Each toxicity variable was grouped as absent/slight vs. moderate/severe. The occurrence of moderate/severe toxicity was modelled in the logistic regression model. In addition, independent samples t-test and Chi-square test were applied to detect the differences of baseline data between no toxicity and severe toxicity. Statistical significance was set at P < 0.05 (two-tailed). All statistical analyses were performed using SAS9.4.
Results
Two hundred and fifty consecutive patients were recruited between Jan 2010 and Aug 2013 of which complete data sets with full radiological staging data were available on 241. None refused or declined the assessment of VMS. The patient demographics, clinical stage, co morbidities and ethnicity are shown in Table 1. Most men were treated with LHRH analogue injections, the commonest of which was Goserelin (reflecting local practice prescribing trends). The median duration of ADT was 9 months (IQR: 4–24 months) at the time of assessment.
Table 1.
Patient characteristics.
| Age – median (IQR) | 74 (69–80) | |
| Body Surface Area – median (IQR) | 2.0 (1.85–2.0) | |
| Body Mass index – median (IQR) | 28 (24.4–31) | |
| Stage | T1-T4, N0 M0 | Low risk-6 |
| Intermediate risk-58 | ||
| High risk-103 | ||
| Node positive, M0 | 22 | |
| M1 | 52 | |
| Medical co-morbidity | Diabetes | 64 |
| Hypertension | 128 | |
| Ischemic heart disease | 71 | |
| Ethnicity | Afrocaribbean | 119 |
| Caucasian | 85 | |
| Asian | 40 | |
| Other | 6 | |
| ADT | LHRH agonist | 223 |
| Anti androgen | 7 | |
| CAB# | 20 | |
-Bicalutamide +Goserelin-14, Flutamide+Goserelin-3, Bicalutmaide +Leuprorelin-3.
CAB (Combined Androgen Blockade).
Vasomotor symptoms
The most common moderate to severe symptoms (Grade 3–4) were hot flushes and sweats followed by fatigue and sleep disturbances. The frequency of toxicity for various VMS is summarised in Table 2. As expected the co-existence of sweats and hot flushes was a common occurrence.
Table 2.
A summary of the toxicity profile of ADT VMS.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Sweats | 82 | 91 | 51 | 26 |
| Hot flushes | 84 | 91 | 53 | 22 |
| Sleep disturbances | 186 | 44 | 17 | 3 |
| Nervousness | 231 | 18 | 1 | 0 |
| Low mood | 203 | 40 | 7 | 0 |
| Tiredness/Fatigue | 150 | 79 | 19 | 2 |
| Joint pains | 200 | 41 | 9 | 0 |
| Headache | 238 | 12 | 0 | 0 |
| Palpitations | 241 | 8 | 1 | 0 |
| Unsteadiness | 231 | 17 | 2 | 0 |
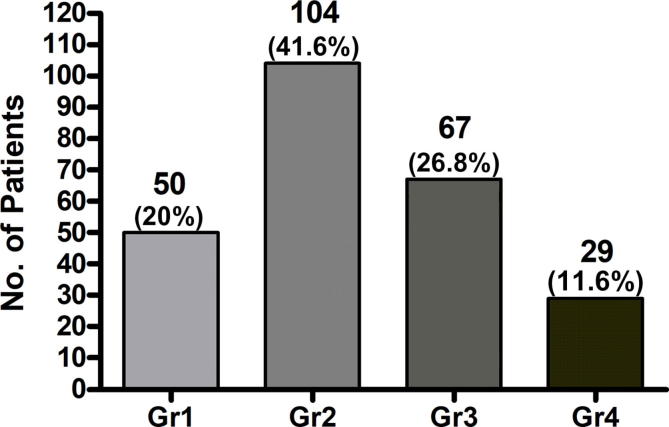
The toxicity according to severity is shown in Fig. 1. In 50 patients (20%) no toxicity was observed, 104 (41.6%) mild, 67 (26.8%) moderate. Twenty nine (11.6%) patients demonstrated severe symptoms such that further medical intervention was required or treatment interrupted. Various interventions, to treat hot flushes include low dose Venlafaxine, Medroxyprogesterone Acetate, Evening Primrose Oil and Black Cohosh [20], [21]. Treatment interruption or discontinuation of ADT occurred in 20% of the cohort with grade 4 toxicity.
Fig. 1.
The toxicity grading and proportion of patients with vasomotor symptoms with androgen deprivation therapy.
On comparison of the cohort experiencing severe VMS to those experiencing no toxicity (Table 3), it was seen that men experiencing significant toxicity were younger (73 vs 77 yrs; p = 0.04), had a higher Body Mass Index (28 vs. 26; p = 0.02) and body surface area (1.99 vs 1.90; p = 0.04.) No significant differences were noted with respect to ethnicity, stage of disease or co-morbidity between the two groups.
Table 3.
Comparing differences between men experiencing no toxicity (Grade 1) with those experiencing significant (Grade 4) vasomotor symptoms (VMS).
| Variables | No VMS-Grade 1 n = 50 (%) | Severe VMS-Grade 4 n = 29 (%) | Statistics (t-test/chi squared)* | p value |
|---|---|---|---|---|
| Age (year)(mean ± SD) | 77.12 ± 7.56 | 73.10 ± 8.84 | 2.14 | 0.04 |
| Height (m)(mean ± SD) | 171.08 ± 7.25 | 172.34 ± 6.66 | -0.77 | 0.44 |
| Weight (Kg)(mean ± SD) | 76.98 ± 12.85 | 83.20 ± 11.05 | -2.18 | 0.03 |
| Body Mass Index (mean ± SD) | 26.18 ± 3.23 | 28.10 ± 3.78 | -2.40 | 0.02 |
| BSA (mean ± SD) | 1.90 ± 0.19 | 1.99 ± 0.15 | -2.14 | 0.04 |
| PSA (mean ± SD) | 316.94 ± 1490.83 | 156.6 ± 368.29 | 0.55 | 0.59 |
| Ethnicity (n, %) | ||||
| Afrocarribean | 23 (47.92) | 18 (66.67) | 2.62 | 0.27 |
| Asian | 9 (18.75) | 4 (14.81) | ||
| Caucasian | 16 (33.33) | 5 (18.52) | ||
| Hormone treatment (n, %) | ||||
| LHRH | 36 (72) | 18 (62.07) | 0.84 | 0.36 |
| Others | 14 (28) | 11 (37.93) | ||
| Treatment time (n, %) | ||||
| <=6 months | 23 (46) | 14 (48.28) | 0.04 | 0.85 |
| >6 months | 27 (54) | 15 (51.72) | ||
| Stage of illness (n, %) | ||||
| N0 & M0 | 30 (66.67) | 16 (59.26) | 0.40 | 0.53 |
| N1 and/or M1 | 15 (33.33) | 11 (40.74) | ||
| Diabetes (n, %) | ||||
| Yes | 11 (23.40) | 10 (35.71) | 1.32 | 0.25 |
| No | 36 (76.60) | 18 (64.29) | ||
| IHD (n, %) | ||||
| Yes | 18 (38.30) | 7 (25) | 1.40 | 0.24 |
| No | 29 (61.70) | 21 (75) | ||
| BP (n, %) | ||||
| Yes | 23 (48.94) | 18 (64.29) | 1.67 | 0.20 |
| No | 24 (51.06) | 10 (35.71) |
SD = standard deviation-t-test numerical variable, chi squared – categorical data.
Chi-square test for categorical variables and t-test for continuous variables.
Predictive factors
Table 4 shows the results of logistic regression analysis in identifying any predictive factors or associations between various parameters and the four most common grade 4 VMS encountered (sweats, flushes, sleep disturbance and tiredness).
Table 4.
Logistic regression analysis to identify predictive factors for common vasomotor symptoms identified.
| Independent variables | Sweating* |
Hot flushes* |
Sleep disturbance* |
Tiredness* |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age (year) | 0.95 (0.91–0.99) | 0.01 | 0.96 (0.92–1.0) | 0.03 | 1.06 (0.99–1.13) | 0.09 | 1.02 (0.96–1.08) | 0.56 |
| Height (m) | 1.11 (0.91–1.35) | 0.30 | 1.08 (0.9–1.31) | 0.4 | 1.04 (0.82–1.32) | 0.75 | 1.33 (0.93–1.88) | 0.11 |
| Weight (kg) | 0.91 (0.75–1.11) | 0.36 | 0.93 (0.77–1.12) | 0.45 | 1.01 (0.81–1.26) | 0.91 | 0.79 (0.56–1.11) | 0.17 |
| Hormones treatment (LHRH vs. others) | 0.89 (0.48–1.66) | 0.71 | 0.91 (0.48–1.7) | 0.76 | 0.54 (0.2–1.42) | 0.21 | 1.55 (0.53–4.56) | 0.42 |
| BMI | 1.37 (0.77–2.45) | 0.29 | 1.29 (0.73–2.27) | 0.37 | 1.03 (0.53--2.01) | 0.94 | 2.15 (0.76–6.10) | 0.15 |
| Treatment time (<6 m vs. >6 m) | 0.9 (0.48–1.69) | 0.74 | 0.85 (0.45–1.61) | 0.62 | 0.82 (0.29–2.32) | 0.71 | 0.86 (0.31–2.43) | 0.78 |
| Ethnic (Afrocarribean vs. Caucasian) | 2.03 (1.0–4.1) | 0.05 | 1.77 (0.88–3.58) | 0.11 | 1.41 (0.46–4.31) | 0.55 | 0.71 (0.24–2.13) | 0.55 |
| Ethnic (Asian vs. Caucasian) | 0.80 (0.29–2.23) | 0.67 | 0.65 (0.23–1.87) | 0.43 | 0.79 (0.14–4.54) | 0.80 | 0.73 (0.13–4.10) | 0.73 |
| DM (Yes/No) | 0.67 (0.31–1.43) | 0.30 | 0.70 (0.33–1.51) | 0.37 | 0.85 (0.28–2.61) | 0.78 | 0.92 (0.27–3.18) | 0.90 |
| IHD (Yes/No) | 0.57 (0.28–1.19) | 0.13 | 0.40 (0.18–0.85) | 0.02 | 1.19 (0.41–3.45) | 0.75 | 0.63 (0.20–2.03) | 0.44 |
| BP (Yes/No) | 1.00 (0.53–1.89) | 1.00 | 1.03 (0.54–1.96) | 0.92 | 2.83 (0.92–8.71) | 0.07 | 0.97 (0.35–2.70) | 0.95 |
The dependent variables are grouped as absent/slight vs. moderate/severe. OR – Odds ratio, CI – confidence interval.
The data suggest that younger age predicts a greater risk of sweats and hot flushes, together with a non-significant trend to greater sleep disturbance in patients receiving ADT. Sweats occurred twice as often in Afrocaribbean men compared to caucasian individuals (Odds ratio: OR 2.03, 95% CI 1.00–4.10, p = 0.05). Duration of hormonal treatment did not predict any of the 4 most disruptive VMS. In men with IHD there was a significant reduction in the occurrence of hot flushes (OR 0.4 95% CI 0.18–0.85, P = 0.02).
Discussion
This, to our knowledge is the largest reported cross-sectional study to consider the effect of ADT on a variety of VMS in terms of how they impact on quality of life in patients with prostate cancer as well as considering any predictive factors including medical co-morbidity (Table 5).
Table 5.
Summary of published trials on the incidence of VMS in men treated with ADT for prostate cancer.
| Author | N | Mechanism of androgen deprivation | Incidence of VMS |
|---|---|---|---|
| Schow et al. [22] | 43 | Neoadjuvant hormonal therapy prior to prostatectomy | 20% no hot flushes 69% hot flushes but resolved after termination of treatment 11% persisted after three months |
| Karling et al. [23] | 77 | Orchidectomy or LHRH |
68% during treatment, 48% persisted after 5 years |
| Tunn et al. [24] | 178 | LHRH (Leuprorelin) 3 m or 6 m |
Hot flushes-43% vs 34%(6monthly depot) Sweats 10% vs 6% (6 monthly depot0 |
| Charig et al. [25] | 75 | Orchidectomy | 76% hot flushes, 30% warranted treatment, commenced 1–12 (mean 2.7 months) post op, lasting on average for 30 months |
| Challapalli et al. (This study) | 250 | LHRHa, AA, CAB | 68.4%, and 11.6% reported mild to moderate and severe symptoms, respectively |
The results of this study show short term VMS, i.e. namely sweats and hot flushes associated with ADT in men treated for prostate cancer is well tolerated. Up to 20% of men experienced no toxicity despite ADT treatment for greater than 6 months, whilst 40% experience symptoms disrupting their daily activities. Less than 11% experienced symptoms sufficiently severe that they either required some form of therapeutic intervention or were unable to continue treatment. The severity of VMS bore no relation to duration or type of ADT (albeit the majority of patients in this study were treated with LHRH analogues) though severe toxicity tended to occur in those who were younger and had a larger Body Mass Index. In addition although these were random single assessments, there did not seem to be a correlation between severity of VMS and duration of ADT.
The reported incidence of VMS in patients with prostate cancer is limited and focuses predominantly on the incidence of hot flushes and their relation to duration of ADT, rather than on the severity of symptoms that affect quality of life. Nevertheless our data seems to be consistent with other reported studies (Table 5) [22], [23], [24], [25]. Schow et al. looked at the prevalence of hot flushes in prostate cancer patients receiving neoadjuvant hormonal therapy by using a simple questionnaire [22]. Of the 43 patients studied full data sets were available on 35. No hot flushes were noted in 20%. Sixty-nine percent of patients experienced hot flushes during treatment but resolved after termination of treatment; and in 11% the flushes continued for at least 3 months after cessation of hormonal therapy. No predictive factors were identified but the prevalence of hot flushes were noted in those who continued with ADT for more than 4 months.
In a similar study using a self reported questionnaire, Karling et al., evaluated hot flushes in 77 men undergoing surgical or medical castration, for their prostate cancer [23]. With full data available on 63 patients 68% had flushes, of which more than 30% persisted 8 years after castration majority persisted over time. Tunn et al. evaluated the safety and clinical efficacy of a 6-month depot formulation of Leuprorelin acetate in patients with prostate cancer in Europe [24]. Hot flushes and sweating were the commonest VMS, with an incidence of 43 and 10% respectively in patients treated over a 12 month period for the three monthly depot compared to 34 and 6% with the 6 monthly preparation.
In terms of predictive factors our results are in part agreement with the prospective study by Gonzalez assessing hot flushes using a hot daily flash interference index age on 60 prostate cancer patients undergoing ADT compared with 83 age and education matched men treated with prostatectomy and 86 similarly matched men with no cancer [26]. Patients who at baseline were younger and with a lower body mass index had a greater increase in hot flushes over time, in addition to those with certain genetic polymorphisms [26].
There is a disparity in relation to hot flushes and body mass index between this study and that of Gonzalez et al. [26]. The greater peripheral conversion of androgens to oestrogen in adipose tissue [27], which can have protective effects on thermoregulation through modulation of the serotonin receptor suggest that those who are overweight should experience less flushes and is at odds to what was observed in this study. However, there may be several other explanations for a high BMI and hot flushes including a greater degree of insulation with adiposity which increases core body temperature [28], [29] and recognition that other hormones produced by adipose tissue such as IL-8 may also be influencing thermoregulation [30]. This may in part account for the BMI not being predictive of hot flushes or sweating in our logistic regression analysis, although heavier patients were more likely to have grade 4 toxicity than experience no toxicity
This study has noteworthy limitations that need to be acknowledged when interpreting the results. Although there were no discernable differences between the incidence of VMS according to type of ADT, the majority of patients in this study were taking LHRH analogue injections only (greater than 90%-Goserelin). It is planned to extend this questionnaire to prostate patients attending an academic oncology centre where there is likely to be a greater cohort of patients on LHRH antagonists, anti-androgens, combined androgen blockade and oestrogen. The use of oestrogens in particular warrant specific scrutiny given that Di-ethyl Stilboestrol has been shown to be associated with a decreased incidence of hot flushes, though at the expense of increased cardiovascular toxicity [31]. The MRC PRO9 (PATCH study) investigating the use of transdermal oestrogen patches which should mitigate against cardiovascular toxicity [32], has already shown early promise by demonstrating a reduction in hot flushes and improved quality of life at 6 months with oestrogen patches compared to LHRH analogues [33]. More robust information will follow now that oestrogen patches will form one of the randomisation arms of the STAMPEDE study.
Our study did not show a relationship between the duration of ADT and prevalence of side effects. The median duration of ADT in those that experienced significant toxicity at the time of interview was similar at 7.0 (IQR4-17) months compared to 7.03 (IQR 2.2–22) months on those who experienced no toxicity. It is difficult to comment on whether there was a lingering effect on discontinuation of ADT, as only patients that were still on ADT were interviewed. In addition whilst it is not possible to tell directly whether the incidence of 20% experiencing no toxicity would be similar for patients undergoing short course androgen deprivation (less than 6 months) with radiotherapy, this can be reliably assumed to be roughly of similar magnitude given the interquartile range of the cohort. Although patients were asked to score their worst toxicity to the date of the actual assessment and the time to occurrence from the start of ADT was recorded, the scoring of symptoms at a single time point has to be interpreted with caution, as there will be a degree of subjectivity with regards to patient recollection of events. Of the patients who described severe VMS, all were offered some form of intervention and serial assessments were performed subsequently. This data is currently being analysed.
Our data suggests that sleep disturbance is a common occurrence, with up to 25% of patients experiencing some disruption to their sleep, and 40% citing fatigue as causing some upset to their daily activities. Whilst this is broadly in keeping with other studies on sleep disturbance in patients on ADT for prostate cancer [34], the questionnaire was not specifically detailed to effectively discriminate between cancer related fatigue (with normal amount of night time sleep), and those that have altered sleep patterns as a result of ADT. Nevertheless it confirms that sleep related issues are an important component which clearly needs more detailed exploration. Further studies using actigraphy to provide a more objective assessment are underway.
Although the study proved useful in identifying patients with grade 4 toxicity, given that data collection was at only one time point, it was unable to evaluate the benefit of any therapeutic interventions suggested. The treatments varied and included Evening Primrose Oil capsules, Medroxyprogesterone Acetate, low dose Venlafaxine and Tamoxifen. Although this was not the main objective of the study, nevertheless follow-up data has been collected at three and six monthly intervals and is currently being analysed. It is hoped that the questionnaire used in this study may prove a useful tool in the designing of a randomised trial to evaluate the effectiveness of various therapeutic options.
The logistic regression analysis has revealed some interesting results in terms of predictive factors for various VMS. The observance of increased sweats in Afro-Carribean men mirrors that of menopausal African-American women, and which remain poorly understood [35], [36]. This group particularly warrants further investigation as they tend to have more aggressive disease presenting at a younger age, and are more likely to require longer term ADT. Likewise the observation between the protective effect of ischaemic heart disease and hot flushes allows for interesting speculation especially for future drug treatments. This study did not collect a detailed drug history on patients, but there is some data to suggest that sympatholytics such as beta-blockers may reduce post menopausal symptoms [37]. In addition nitric oxide mediated cutaneous vasodilation may well play an important part in hot flushes which may be defective or reduced in patients with coronary atherosclerosis/ischemic heart disease [38].
Conclusion
This study confirms that ADT in prostate cancer is generally well tolerated, with up to 20% of patients describing no short-term VMS or psychological sequeale. The main side effects are sweats hot flushes, fatigue and sleep disturbances which can be disabling in upto 11%, and in this study there was no obvious relation to the duration of ADT. Such toxicity tended to affect patients who were younger and had a higher BMI. Younger age, afro-carribean ethnicity were predictive of sweats, whilst patients with ischemic heart disease seemed to have a lower risk of suffering with hot flushes.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the support of the following: S. Ali, M. Asif, H. Bhola-Stewart, K. Biswas, J. Broomes, B. Chikkamuniyappa, R. Lindemane, S. Magwaro, F. Miah, S. Mondal, L. Morland, S. Morris, F. Power, K. Querns, S. Rahman, A. Rice, W. Saleem, A. Servidad, R. Wathes, L. Yazmin. The sponsors had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Role of funding source
This works was supported by a charitable donation from BAPS charities UK, Neasden, London, NW10 8HD, UK.
Appendix 1
The hormone assessment sheet.

References
- 1.Huggins C., Hodges C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 2.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.McLeod D.G. Hormonal therapy: historical perspective to future directions. Urology. 2003;61:3–7. doi: 10.1016/s0090-4295(02)02393-2. [DOI] [PubMed] [Google Scholar]
- 5.Seidenfeld J., Samson D.J., Hasselblad V., Aronson N., Albertsen P.C., Bennett C.L. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132:566–577. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 6.Mason M.D., Parulekar W.R., Sydes M.R., Brundage M., Kirkbride P., Gospodarowicz M. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33:2143–2150. doi: 10.1200/JCO.2014.57.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolla M., Van Tienhoven G., Warde P., Dubois J.B., Mirimanoff R.O., Storme G. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 8.Hanks G.E., Pajak T.F., Porter A., Grignon D., Brereton H., Venkatesan V. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92–02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Dearnaley D.P., Jovic G., Syndikus I., Khoo V., Cowan R.A., Graham J.D. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi H., Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111:543–548. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 11.Basaria S., Lieb J., 2nd, Tang A.M., DeWeese T., Carducci M., Eisenberger M. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol. 2002;56:779–786. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 12.Braga-Basaria M., Dobs A.S., Muller D.C., Carducci M.A., John M., Egan J. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 13.Hakimian P., Blute M., Jr., Kashanian J., Chan S., Silver D., Shabsigh R. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int. 2008;102:1509–1514. doi: 10.1111/j.1464-410X.2008.07933.x. [DOI] [PubMed] [Google Scholar]
- 14.Rezaei M.M., Ghoreifi A., Kerigh B.F. Metabolic syndrome in patients with prostate cancer undergoing intermittent androgen-deprivation therapy. Can Urol Assoc J. 2016;10:E300–E305. doi: 10.5489/cuaj.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmadi H., Daneshmand S. Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes. Patient Relat Outcome Meas. 2014;5:63–70. doi: 10.2147/PROM.S52788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvao D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 17.Kouriefs C., Georgiou M., Ravi R. Hot flushes and prostate cancer: pathogenesis and treatment. BJU Int. 2002;89:379–383. doi: 10.1046/j.1464-4096.2001.01761.x. [DOI] [PubMed] [Google Scholar]
- 18.Savard J., Hervouet S., Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology. 2013;22:1381–1388. doi: 10.1002/pon.3150. [DOI] [PubMed] [Google Scholar]
- 19.Donovan K.A., Walker L.M., Wassersug R.J., Thompson L.M., Robinson J.W. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121:4286–4299. doi: 10.1002/cncr.29672. [DOI] [PubMed] [Google Scholar]
- 20.Irani J., Salomon L., Oba R., Bouchard P., Mottet N. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial. Lancet Oncol. 2010;11:147–154. doi: 10.1016/S1470-2045(09)70338-9. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan M., Mahon S. Hot flash management: update of the evidence for patients with cancer. Clin J Oncol Nurs. 2014;18(Suppl):59–67. doi: 10.1188/14.CJON.S3.59-67. [DOI] [PubMed] [Google Scholar]
- 22.Schow D.A., Renfer L.G., Rozanski T.A., Thompson I.M. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. doi: 10.1097/00007611-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Karling P., Hammar M., Varenhorst E. Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J Urol. 1994;152:1170–1173. doi: 10.1016/s0022-5347(17)32530-2. [DOI] [PubMed] [Google Scholar]
- 24.Tunn U.W., Wiedey K. Safety and clinical efficacy of a new 6-month depot formulation of leuprorelin acetate in patients with prostate cancer in Europe. Prostate Cancer Prostatic Dis. 2009;12:83–87. doi: 10.1038/pcan.2008.52. [DOI] [PubMed] [Google Scholar]
- 25.Charig C.R., Rundle J.S. Flushing. Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. doi: 10.1016/0090-4295(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez B.D., Jim H.S., Donovan K.A., Small B.J., Sutton S.K., Park J. Course and moderators of hot flash interference during androgen deprivation therapy for prostate cancer: a matched comparison. J Urol. 2015;194:690–695. doi: 10.1016/j.juro.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson E.R. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 28.Gallicchio L., Visvanathan K., Miller S.R., Babus J., Lewis L.M., Zacur H. Body mass, estrogen levels, and hot flashes in midlife women. Am J Obstet Gynecol. 2005;193:1353–1360. doi: 10.1016/j.ajog.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Glickman-Weiss E.L., Nelson A.G., Hearon C.M., Prisby R., Caine N. Thermal and metabolic responses of women with high fat versus low fat body composition during exposure to 5 and 27 degrees C for 120 min. Aviat Space Environ Med. 1999;70:284–288. [PubMed] [Google Scholar]
- 30.Yasui T., Uemura H., Tomita J., Miyatani Y., Yamada M., Kuwahara A. Association of interleukin-8 with hot flashes in premenopausal, perimenopausal, and postmenopausal women and bilateral oophorectomized women. J Clin Endocrinol Metab. 2006;91:4805–4808. doi: 10.1210/jc.2006-1100. [DOI] [PubMed] [Google Scholar]
- 31.Smith J.A., Jr. A prospective comparison of treatments for symptomatic hot flushes following endocrine therapy for carcinoma of the prostate. J Urol. 1994;152:132–134. doi: 10.1016/s0022-5347(17)32835-5. [DOI] [PubMed] [Google Scholar]
- 32.Langley R.E., Cafferty F.H., Alhasso A.A., Rosen S.D., Sundaram S.K., Freeman S.C. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09) Lancet Oncol. 2013;14:306–316. doi: 10.1016/S1470-2045(13)70025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert D.C., Duong T., Kynaston H.G., Alhasso A.A., Cafferty F.H., Rosen S.D. Quality-of-life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer. BJU Int. 2017;119:667–675. doi: 10.1111/bju.13687. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Piñeiro L., Antolín A.R., Romero M.E.J., Ramos J.B.G., Bellido D.L., Gracia P.R. Prevalence and severity of fatigue in castration resistant prostate cancer in Spain: VITAL study. Ann Oncol. 2016;27 [Google Scholar]
- 35.Gold E.B., Colvin A., Avis N., Bromberger J., Greendale G.A., Powell L. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpkins J.W., Brown K., Bae S., Ratka A. Role of ethnicity in the expression of features of hot flashes. Maturitas. 2009;63:341–346. doi: 10.1016/j.maturitas.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kujala S.M., Poyhonen-Alho M., Kaaja R.J. Effects of sympatholytic therapy on postmenopausal symptoms in hypertensive postmenopausal women. Climacteric. 2014;17:356–362. doi: 10.3109/13697137.2013.842226. [DOI] [PubMed] [Google Scholar]
- 38.Dusting G.J., Fennessy P., Yin Z.L., Gurevich V. Nitric oxide in atherosclerosis: vascular protector or villain? Clin Exp Pharmacol Physiol Suppl. 1998;25:S34–41. doi: 10.1111/j.1440-1681.1998.tb02298.x. [DOI] [PubMed] [Google Scholar]