Abstract
We performed a systematic review and meta-analysis to determine the risk of immune-related pancreatitis associated with the treatment by immune checkpoint inhibitors (ICIs) for solid tumors. Eligible studies were selected from multiple databases including phase II/III randomized controlled trials (RCTs) with ICIs in solid tumor patients. The data were analyzed with Stata version 12.0 software. After excluding ineligible studies, a total of 15 clinical trials were considered eligible for the meta-analysis, which included 9099 patients. Compared with chemotherapy or placebo, the risk ratio (RR) for all-grade lipase elevation after CTLA-4 inhibitor treatment was 1.05 (95% confidence interval (CI): 1.01–2.24, p = 0.047). However, the risk for pancreatitis after ICI treatment in any subgroup was not significantly higher than that after control therapy. In addition, compared with ipilimumab/nivolumab alone, the RR for all-grade and high-grade lipase elevation under combination treatment of nivolumab and ipilimumab was 6.43 (95% CI: 1.43–28.99, p = 0.015) and 6.44 (95% CI: 1.39–29.79, p = 0.017), respectively, and the RR for all-grade amylase elevation under combination treatment was 6.08 (95% CI: 1.51–24.44, p = 0.011). Our meta-analysis has demonstrated that both CTLA-4 inhibitors alone and combination treatment of nivolumab and ipilimumab could increase the risk of amylase or lipase elevation, but not significantly increase the risk of pancreatitis when compared with controls.
1. Introduction
Up to now, cancers have been treated with surgery, chemotherapy, radiotherapy, and targeted molecular therapy including EGFR-TKI (epidermal growth factor receptor-tyrosine kinase inhibitor) [1]. Recently, immunotherapies involving immune checkpoint inhibitors (ICIs) include cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death protein 1 and ligand 1 (PD-1 and PD-L1) monoclonal antibodies. ICIs have emerged as a new effective treatment of advanced solid tumors such as advanced melanoma (MM), nonsmall cell lung cancer (NSCLC), and urothelial carcinoma [2–4]. In 2011, ipilimumab, the first ICI, has received US Food and Drug Administration (FDA) approval for the use in advanced melanoma [3, 4]. Since 2011, there have been additional five ICIs approved for the treatment of various solid tumors [2]. Unfortunately, when used both alone and combined, ICIs have generated new kinds of toxicity profiles, specifically referred to as immune-related adverse events (irAEs), which included but were not limited to thyroid dysfunction, colitis, pneumonitis, dermatitis, and hepatitis [5]. A less commonly seen irAE was immune-related pancreatitis [6–16]. The diagnosis of acute pancreatitis (AP) could be supported by increases of serum amylase and lipase. Values of serum amylase or lipase in excess of three times the upper limit of normal were characteristic of AP [17]. This study is conducted to evaluate the risk of immune-associated pancreatitis in cancer patients treated with ICIs. To our knowledge, this study is the first meta-analysis to report on the risk of immune-related pancreatitis under ICI treatment. We believe this meta-analysis study will improve awareness of the incidence and characteristics of immune-related pancreatitis, which may lead to more appropriate utilization of immune checkpoint inhibitors in clinical practice.
2. Methods
Our systematic review and meta-analysis was conducted according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions [18] and the PRISMA Statement [19].
2.1. Strategy of Literature Searching
Random controlled trials (RCTs) exploring CTLA-4, PD-1, and PD-L1 antibodies in solid tumors were searched on the following databases: Embase, PubMed, and ClinicalTrials.gov. RCTs conducted between January 1990 and July 2017 were included. The medical subject heading (MeSH) terms included for searching the relevant studies contained one term that refers to cancer (neoplasm, carcinoma, cancer, or tumor), one term indicating the ICIs (anti-CTLA-4, anti-PD-1, ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, or avelumab), and one term related to randomized controlled trials, connected by “and” (Supplementary Table 1).
2.2. Inclusion and Exclusion Criteria
Studies with the following information were included in our meta-analysis: (1) phase II/III RCTs with primary endpoints such as overall survival (OS), progression-free survival (PFS), or objective response rate (ORR); (2) histologically confirmed solid cancer such as lung cancer and melanoma (MM); (3) containing the information of ICIs and pancreatitis or amylase or lipase; and (4) sharing some similarity in experimental method across different studies.
However, studies were excluded if they were (1) reviews, duplicate reports, letters, unfinished studies, or conference reports; (2) papers in languages other than English; (3) studies where pancreatitis could not be confirmed due to insufficient data; (4) studies conducted with cell lines, animal models, or on nonsolid cancers; (5) studies whose experimental method was substantially different from other selected RCTs; and (6) RCTs in phase I.
2.3. Data Extraction
Two reviewers (Qiang Su and Yan-li Hou) independently searched all the relevant studies and read the titles, abstracts, and full texts of the identified studies. Cases of disagreement were resolved through discussion with the third reviewer (Chen-guang Zhang). The following information was extracted from the selected studies: year of publication, authors' family names, methods of trails, number of ICI treatment type, number of control treatment, number of pancreatitis, amylase, and lipase of all grades (grades 1–5) and high grade (grades 3–5).
2.4. Data Analysis
In our meta-analysis, risk of bias analysis was performed using Review Manager 5.3 software (Cochrane Collaboration 2014, Nordic Cochrane Center, Copenhagen, Denmark). Two independent reviewers (Qiang Su and Yan-li Hou) assessed the quality of the included studies according to the Cochrane risk of bias tool. Specifically, the following seven domains were assessed: selection bias (including both random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other biases. The Stata version 12.0 statistical software (Stata Corporation, College Station, Texas, USA) was used for meta-analysis. Risk ratio (RR) was used to estimate pancreatitis of grades 1–5 and grades 3–5. RR > 1.0 indicates higher risk or higher incidence of pancreatitis, amylase, or lipase in patients treated with ICIs. In addition, the I 2 statistics was used to assess the heterogeneity among the RCTs. I 2 values of <30%, 30%–59%, 60%–75%, and >75% were classified as low, moderate, substantial, and considerable heterogeneity, respectively [20]. We used the random effects model (REM) [21] to calculate pooled RR and 95% confidence interval (CI). Sources of heterogeneity were explored using subgroup analyses (different ICIs). The Begg's and Egger's tests were used to analyze the publication bias across RCTs. All p values were 2 tailed, and a probability level < 0.05 was considered statistically significant.
2.5. Quality Assessment
Our analysis was performed by pair-wise comparisons of the ICI arms and the control arms. Among the studies (see Table 1), there were five three-arm trials [11, 13, 16, 17, 22]. During the statistical process, one arm in each three-arm RCT maybe was entered twice in our database. The number of patients in this arm, which was used twice, was divided by two to avoid an increased influence of the arm on the overall result. In addition, we paid attention to the heterogeneity among the RCTs by using subgroup analysis. REM was employed for our meta-analysis to test and verify the statistical results.
Table 1.
Characteristics of the eligible RCTs.
| Study (year) | Study type | Histology | Endpoint | Treatment arm | Patient (no.) | Pancreatitis | AMY | Lipase | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (G1–5) | (G3–5) | (1–5) | (3–5) | (1–5) | (3–5) | ||||||
| Ribas et al., 2013 [6] | RCT III | MM | OS | Tremelimumab at 15 mg/kg 90 ds | 325 | 3 | 3 | NA | NA | NA | NA |
| Chemotherapy control | 319 | 0 | 0 | NA | NA | NA | NA | ||||
|
| |||||||||||
| Know, 2014 | RCT III | prostate Ca | OS | Ipilimumab 10 mg/kg q3w | 393 | NA | NA | 2 | 2 | 3 | 1 |
| Placebo | 396 | NA | NA | 1 | 0 | 3 | 2 | ||||
|
| |||||||||||
| Eggermont et al., 2015 [7] | RCT III | MM | PFS | Ipilimumab 10 mg/kg q3w | 471 | NA | 1 | NA | NA | 43 | 1 |
| Placebo | 474 | NA | 0 | NA | NA | 30 | 0 | ||||
|
| |||||||||||
| Maio et al., 2017 [8] | RCT IIb | Mesothelioma | OS | Tremelimumab 10 mg/kg q4w | 380 | 2 | 1 | NA | 0 | 18 | 11 |
| Placebo | 189 | 0 | 0 | NA | 1 | 4 | 3 | ||||
|
| |||||||||||
| Brahmer et al., 2015 [24] | RCT III | NSCLC | OS | Nivolumab 3 mg/kg q2w | 131 | NA | NA | NA | NA | 1 | 1 |
| Chemotherapy control | 129 | NA | NA | NA | NA | 0 | 0 | ||||
|
| |||||||||||
| Robert et al., 2011 [4] | RCT III | MM | OS | Nivolumab 3 mg/kg q2w | 206 | NA | NA | NA | 1 | NA | NA |
| Chemotherapy control | 205 | NA | NA | NA | 0 | NA | NA | ||||
|
| |||||||||||
| Weber et al., 2015 [9] | RCT III | MM | ORR | Nivolumab 3 mg/kg q2w | 268 | NA | 2 | NA | NA | NA | 3 |
| Chemotherapy control | 102 | NA | 0 | NA | NA | NA | 1 | ||||
|
| |||||||||||
| Herbst et al., 2016 [10] | RCT III | NSCLC | OS | Pembrolizumab 2 mg/kg q2w | 339 | 3 | 2 | NA | NA | NA | NA |
| Pembrolizumab 10 mg/kg q2w | 343 | 0 | 0 | NA | NA | NA | NA | ||||
| Chemotherapy control | 309 | 0 | 0 | NA | NA | NA | NA | ||||
|
| |||||||||||
| Reck et al., 2016 [11] | RCTIII | NSCLC | PFS | Pembrolizumab 200 mg q3w | 154 | NA | 1 | NA | NA | NA | NA |
| Chemotherapy control | 150 | NA | 0 | NA | NA | NA | NA | ||||
|
| |||||||||||
| Ribas et al., 2015 [12] | RCT II | MM | ORR | Pembrolizumab 2 mg/kg q2w | 178 | 1 | 1 | NA | NA | NA | NA |
| Pembrolizumab 10 mg/kg q2w | 179 | 3 | 1 | NA | NA | NA | NA | ||||
| Chemotherapy control | 171 | 1 | 1 | NA | NA | NA | NA | ||||
|
| |||||||||||
| Rittmeyer et al., 2017 [13] | RCT II | NSCLC | OS | Atezolizumab 1200 mg q3w | 609 | NA | 0 | NA | NA | NA | NA |
| Chemotherapy control | 578 | NA | 1 | NA | NA | NA | NA | ||||
|
| |||||||||||
| Hodi et al., 2016 [14] | RCT II | MM | ORR | Ipilimumab 3 mg/kg q3w +nivolumab 1 mg/kg q3w |
94 | 2 | 2 | 11 | 2 | 17 | 2 |
| Ipilimumab 3 mg/kg q3w | 46 | 0 | 0 | 0 | 0 | 2 | 0 | ||||
|
| |||||||||||
| Antonia et al., 2016 [22] | RCT II | SCLC | ORR | Ipilimumab 3 mg/kg q3w +nivolumab 1 mg/kg q3w |
61 | NA | NA | 4 | 1 | 7 | 5 |
| Ipilimumab 1 mg/kg q3w +nivolumab 3 mg/kg q3w |
54 | NA | NA | 2 | 0 | 0 | 0 | ||||
| Nivolumab 3 mg/kg q2w | 98 | NA | NA | 1 | 1 | 0 | 0 | ||||
|
| |||||||||||
| Larkin et al., 2015 [15] | RCT III | MM | OS/PFS | Ipilimumab 3 mg/kg q3w +nivolumab 1 mg/kg q3w |
313 | NA | 2 | NA | NA | NA | 2 |
| Nivolumab 3 mg/kg q3w | 313 | NA | 1 | NA | NA | NA | 0 | ||||
| Ipilimumab 3 mg/kg q3w | 311 | NA | 1 | NA | NA | NA | 0 | ||||
|
| |||||||||||
| Robert et al., 2015 [16] | RCT III | MM | OS | Pembrolizumab 10 mg/kg q2w | 278 | NA | 0 | NA | NA | NA | NA |
| Pembrolizumab 10 mg/kg q3w | 277 | NA | 1 | NA | NA | NA | NA | ||||
| Ipilimumab 3 mg/kg q3w | 256 | NA | 0 | NA | NA | NA | NA | ||||
MM: melanoma; NSCLC: nonsmall cell lung cancer; DTIC: Dacarbazine; gp100: gp100 vaccine; DOX: docetaxel; OS: overall survival; ORR: objective response rate; NA: not available.
3. Results
3.1. Selection of Studies
We initially identified 3877 studies from our search of the databases mentioned above. Among those 3877 studies, 15 RCTs met our inclusion criteria (Supplementary Figure 1). All 15 trials compared the effectiveness of ICI therapies with control treatments in solid tumors, representing data from a total of 9099 patients (Table 1). Among the 15 studies, four studies [7–9, 23] involved CTLA-4 antibodies (ipilimumab: 2 cohorts, n = 864; tremelimumab: 2 cohorts, n = 705), seven [10–13, 17, 24, 25] involved PD-1 antibodies (nivolumab: 3 cohorts, n = 605; pembrolizumab: 4 cohorts, n = 1748), one [14] involved PD-L1 (atezolizumab: n = 609), and the other three [15, 16, 22] studies involved combination treatment of nivolumab and ipilimumab (3 cohorts; n = 522). Eight trials [7, 8, 10, 13, 15–17] involved patients with malignant melanoma (MM), four [11, 12, 14, 24] involved patients with nonsmall cell lung cancer (NSCLC), and the other three [9, 22, 23] involved patients with mesothelioma, prostate cancer, or small cell lung cancer (SCLC).
Cochrane risk of bias tool was used to measure the quality of the included studies. The results are shown in Supplementary Figure 2. All of the included studies described the detail of random sequence generation and blinding of outcome assessment. However, some of them described incomplete outcome data and allocation concealment. Some studies failed to mention blinding of participants and personnel and selective reporting. Other indices of bias lacked specific description in the included clinical studies.
3.2. Analysis of Pancreatitis Risk: Comparison between ICI Treatments and Controls
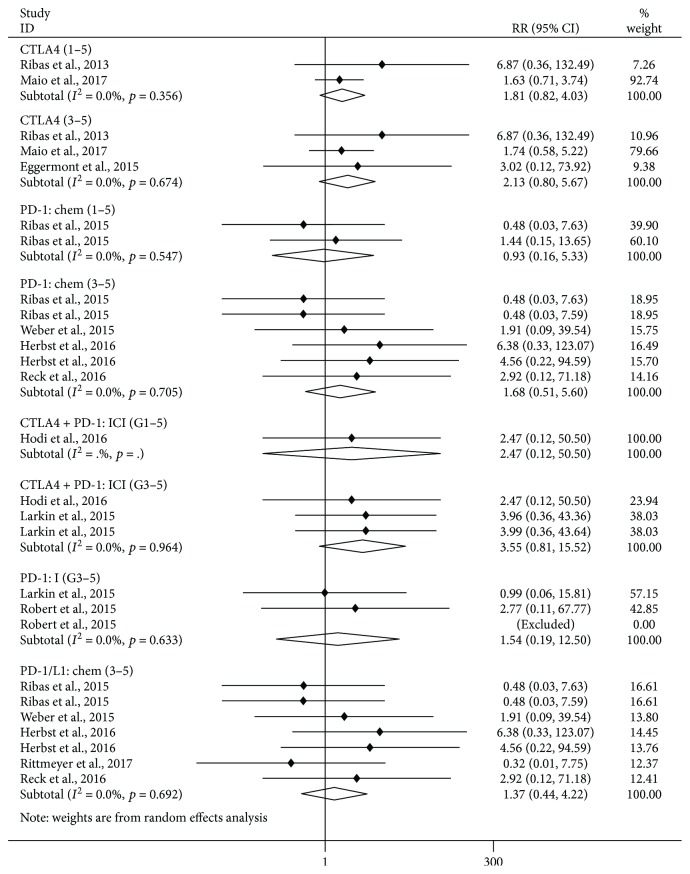
As shown in Figure 1, compared with control treatments, there was no significant increase of risk of grade 1–5 pancreatitis in the CTLA-4 inhibitor subgroup (versus chemotherapy/placebo, RR 1.81, 95% CI: 0.82–4.03, p = 0.143) and in the PD-1 inhibitor subgroup (versus chemotherapy, RR = 0.93, 95% CI: 0.16–5.33, p = 0.937). Furthermore, we observed no significant increase in the risk of grade 3–5 pancreatitis in the CTLA-4 inhibitor subgroup (versus chemotherapy/placebo, RR = 2.13, 95% CI: 0.80–5.67, p = 0.130), in the PD-1 inhibitor subgroup (versus chemotherapy, RR = 1.68, 95% CI: 0.51–5.60, p = 0.395; versus ipilimumab, RR = 1.54, 95% CI: 0.19–12.50, p = 0.685), in the combination treatment of nivolumab and ipilimumab subgroup (versus ipilimumab, RR = 3.55, 95% CI: 0.81–15.52, p = 0.093), and in the PD-1/PD-L1 inhibitor subgroup (versus chemotherapy, RR 1.37, 95% CI: 0.44–4.22, p = 0.584).
Figure 1.
Forest plot analysis of pancreatitis in patients treated with PD-1/CTLA-4 antibodies compared with control therapy PD-1: chem: PD-1 inhibitor versus chemotherapy; PD-1: I: PD-1 inhibitor versus ipilimumab; PD-1/L1: chem: PD-1/L1 inhibitor versus chemotherapy; CTLA-4: CTLA-4 inhibitor versus chemotherapy/placebo; CTLA-4 + PD-1: ICI: nivolumab + ipilimumab subgroup versus nivolumab/ipilimumab; G1–5: grade1–5; G3–5: grade3–5.
3.3. Analysis of the Risk of Amylase Elevation: Comparison between ICI Treatments and Controls
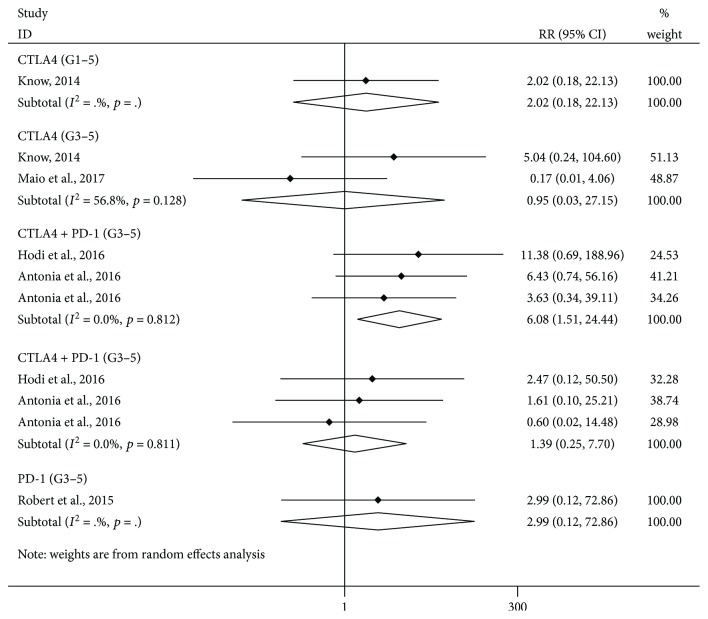
As shown in Figure 2, compared with control treatment, there was a significant increase of risk of grade 1–5 amylase elevation in the combination treatment of nivolumab and ipilimumab subgroup (versus ipilimumab or nivolumab alone, RR = 6.08, 95% CI: 1.51–24.44, p = 0.011). Furthermore, we observed no significant increase in the risk of grade 3–5 amylase elevation in the CTLA-4 inhibitor subgroup (versus chemotherapy/placebo, RR = 0.95, 95% CI: 0.03–27.15, p = 0.977) and in the combination treatment of nivolumab and ipilimumab subgroup (versus ipilimumab/nivolumab, RR = 1.39, 95% CI: 0.25–7.70, p = 0.708).
Figure 2.
Forest plot analysis of amylase in patients treated with PD-1/CTLA-4 antibodies compared with control therapy CTLA-4: CTLA-4 inhibitor versus chemotherapy/placebo; PD-1: PD-1 inhibitor versus chemotherapy; CTLA-4 + PD-1: nivolumab + ipilimumab subgroup versus nivolumab/ipilimumab; G1–5: grade1–5; G3–5: grade3–5.
3.4. Analysis of the Risk of Lipase Elevation: Comparison between ICI Treatments and Controls
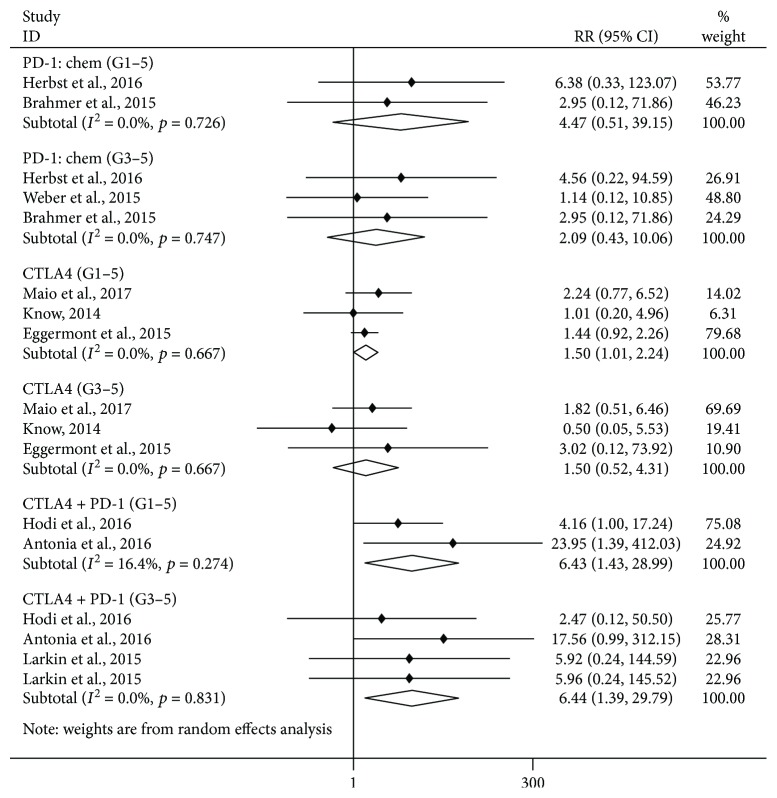
As shown in Figure 3, we observed a significant increase in the risk of grade 1–5 lipase elevation in the CTLA-4 inhibitor subgroup (versus chemotherapy/placebo, RR = 1.50, 95% CI: 1.01–2.24, p = 0.047) and in the combination treatment of nivolumab and ipilimumab subgroup (versus ipilimumab/nivolumab, RR = 6.43, 95% CI: 1.43–28.99, p = 0.015). There was no significant increase in the risk of grade 1–5 lipase elevation in the PD-1 inhibitor subgroup (versus chemotherapy, RR = 4.47, 95% CI: 0.51–39.15, p = 0.176). Furthermore, we observed a significant increase in the risk of grade 3–5 lipase elevation in the combination treatment of nivolumab and ipilimumab subgroup (versus ipilimumab/nivolumab, RR = 6.44, 95% CI: 1.39–29.79, p = 0.017), but not in the CTLA-4 inhibitor subgroup (versus chemotherapy/placebo, RR = 1.50, 95% CI: 0.52–4.31, p = 0.451), or in the PD-1 inhibitor subgroup (versus chemotherapy, RR = 2.09, 95% CI: 0.43–10.06, p = 0.359).
Figure 3.
Forest plot analysis of lipase in patients treated with PD-1/CTLA-4 antibodies compared with control therapy. PD-1: chem: PD-1 inhibitor versus chemotherapy; CTLA-4: CTLA-4 inhibitor versus chemotherapy/placebo; CTLA-4 + PD1: nivolumab + ipilimumab subgroup versus nivolumab/ipilimumab; G1–5: grade1–5; G3–5: grade3–5.
3.4.1. Heterogeneity of All the Subgroups
Heterogeneity in meta-analysis refers to the variation in study outcomes between studies. In nearly all the subgroups, we found the low overall heterogeneity of grade 1–5/3–5 pancreatitis, amylase, and lipase elevation incidence which displayed I 2 values of 0.0%, but not in this subgroup (CTLA-4 inhibitor subgroup versus chemotherapy/placebo: I 2 = 56.8%, p = 0.128).
3.5. Analysis of Publication Bias
Egger's test and Begg's test, conducted in Stata 12.0 software, were utilized to evaluate the publication bias between different RCTs. As presented in Supplementary Table 2 and Supplementary Figure 3, all p values were >0.05 after both tests. Therefore, there was no significant publication bias in this meta-analysis.
4. Discussion
ICI-induced pancreatitis remains a complicated irAE. The incidence of immune-related pancreatitis caused by ICIs is rare (CTLA4: 0.9–3%, PD-1: 0.5–1.6%, CTLA4 + PD-1: 1.2–2.1%) [6–16]. Since ICI-induced pancreatitis can be considered an immune-related pancreatitis, it is reasonable to propose that its diagnosis can be based on the diagnostic criteria of autoimmune pancreatitis (AIP) [26, 27]; the diagnosis of autoimmune pancreatitis is based on results from these five factors: imaging, serology, histology, extrapancreatic involvement, and perhaps steroid responsiveness. Although the mechanism of immune-induced pancreatitis caused by ICIs is largely unknown, it is postulated that ICIs could unavoidably disturb the balance of autologous tolerance, which could result in some immune-related side effects.
Our meta-analysis shows that compared with chemotherapy or placebo, using CTLA-4 inhibitors as single treatment significantly increases (RR = 1.50) the risk of all-grade lipase elevation among solid cancer patients. The risk of pancreatitis was not significantly elevated under CTLA-4 and PD-1 inhibitors alone or in combination. Compared with ipilimumab or nivolumab alone, combination treatment with nivolumab and ipilimumab might increase the risk of all-grade lipase elevation (RR = 6.43) and amylase elevation (RR = 1.39), as well as the risk of high-grade lipase elevation (RR = 6.44) among solid cancer patients.
We found that the CTLA-4 inhibitor increased the risk of lipase elevation (all-grade); however, neither CTLA-4 nor PD-1 inhibitor increased the risk of pancreatitis. Such a discrepancy between some elevated serologic test results and clinically significant pancreatitis further supported our proposal to use the diagnostic criteria of AIP to confirm ICI-induced pancreatitis. According to the mentioned criteria of ICI-induced pancreatitis, we noted that amylase/lipase elevation was only one out of the five key aspects for diagnosis; images including contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) seem more important clinically to discern immune-related pancreatitis [26]. Consistently, because of the rare incidence of ICI-induced pancreatitis [6–16, 28, 29], some studies propose that routine monitoring of amylase/lipase in asymptomatic individuals is not recommended. Also, the mechanism of elevated amylase/lipase in solid cancer patients treated with ICIs in the absence of clinically significant pancreatitis remains unclear. Given that combined ipilimumab and nivolumab results in a more frequent elevation of amylase and lipase suggests that the elevation of amylase and lipase is likely immune related.
Undoubtedly, the meta-analysis itself based upon published data had some limitations [30]. One limitation of this meta-analysis was the lack of individual patient data, the use of which would have provided more detail about immune-related pancreatitis with ICIs. Secondly, because of the paucity of the study number on immune-related pancreatitis, the sensitivity analysis was not employed in this meta-analysis. Thirdly, imaging data with CT or MRI to contain radiographic evidence of pancreatitis was absent. However, we have made efforts on the overall quality assessment that may make our results more steady and credible: (1) two independent reviewers searched all the relevant trails with well-defined inclusion criteria. They assessed the appropriate studies for meta-analysis evaluated by using PICO chart and assessed the risk of bias for the included RCTs according to the Cochrane Handbook. (2) Two independent reviewers verified data in our meta-analysis that was obtained from pair-wise comparisons. (3) The REM was statistically employed in this meta-analysis.
With the increasing morbidity of cancer and the lengthening of overall survival, although the incidence is very low, the cases of ICI-induced pancreatitis are estimated to grow [31, 32]. These should be useful in order for the clinicians to comprehend the incidence and risk of ICI-induced pancreatitis. Further study on the molecular mechanisms underlying ICI-induced pancreatitis could help us to prevent or relieve this adverse event during ICI treatment [33].
5. Conclusion
In summary, this meta-analysis study has demonstrated that CTLA-4 inhibitor therapy may result in a higher risk of all-grade lipase elevation when compared to chemotherapy. However, neither CTLA-4 nor PD-1 inhibitor when given alone or in combination increased the risk of immune-induced pancreatitis when compared to controls. Compared with nivolumab or ipilimumab, the combination of nivolumab and ipilimumab could increase the risk of all-grade and high-grade lipase elevation, as well as the risk of all-grade amylase elevation.
Acknowledgments
The working group wishes to thank Professor Zuhua Gao (Department of Pathology, Research Institute of McGill University Health Center, Montreal, Quebec, Canada) and Gordon (YTL) (College Jean-de-Brebeuf, Montreal, Quebec, Canada) for their assistance in the preparation and review of this manuscript. This work is funded through the Foundation Clinical Research of Capital Medical University (no. 15JL33) and the Foundation of Beijing Friendship Hospital (yyqdkt 2014-12).
Contributor Information
Yan-li Hou, Email: hyl_0730@126.com.
Yu-xia Yao, Email: 13717601652@163.com.
Bang-wei Cao, Email: oncology@ccmu.edu.cn.
Conflicts of Interest
The authors declare no conflicts of interest in preparing this article.
Authors' Contributions
Qiang Su and Yan-li Hou had entire access to all the data included in the study and takes duty for the completeness of the data and the accuracy of our analysis. Chen-guang Zhang and Yu-xia Yao helped in designing of the study; Qiang Su and Yan-li Hou contributed to the statistical analysis and the revision of this manuscript; Xiao-chen Zhang, Qiang Su, and Bang-wei Cao approved the final manuscript.
Supplementary Materials
Supplementary file (including Supplementary Tables 1-2 and Supplementary Figures 1–3).
References
- 1.Rossi A., Sacco P. C., Santabarbara G., et al. Developments in pharmacotherapy for treating metastatic non-small cell lung cancer. Expert Opinion on Pharmacotherapy. 2017;18(2):151–163. doi: 10.1080/14656566.2017.1280460. [DOI] [PubMed] [Google Scholar]
- 2.Fesus V. Recent advances of immunooncology in the treatment of solid tumours and haematological malignancies: the immune checkpoint inhibitors. Magyar Onkologia. 2017;61(2):116–125. [PubMed] [Google Scholar]
- 3.Hodi F. S., O'Day S. J., McDermott D. F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C., Thomas L., Bondarenko I., et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England Journal of Medicine. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D. B., Sullivan R. J., Menzies A. M. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123(11):1904–1911. doi: 10.1002/cncr.30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribas A., Kefford R., Marshall M. A., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. Journal of Clinical Oncology. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggermont A. M., Chiarion-Sileni V., Grob J. J. Correction to Lancet Oncol 2015; 16: 522–30. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. The Lancet Oncology. 2015;16(6, article e262) doi: 10.1016/S1470-2045(15)70271-8. [DOI] [PubMed] [Google Scholar]
- 8.Maio M., Scherpereel A., Calabrò L., et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. The Lancet Oncology. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 9.Weber J. S., D'Angelo S. P., Minor D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The Lancet Oncology. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 10.Herbst R. S., Baas P., Kim D. W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Reck M., Rodríguez-Abreu D., Robinson A. G., et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung Cancer. The New England Journal of Medicine. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Ribas A., Puzanov I., Dummer R., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The Lancet Oncology. 2015;16(8):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi F. S., Chesney J., Pavlick A. C., et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. The Lancet Oncology. 2016;17(11):1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J., Hodi F. S., Wolchok J. D. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. The New England Journal of Medicine. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 16.Robert C., Schachter J., Long G. V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. The New England Journal of Medicine. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 17.Banks P. A., Bollen T. L., Dervenis C., et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 18.JPT H., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Version 5.1.0., http://handbook-5-1.cochrane.org/ [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials. 2015;45(Part A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonia S. J., López-Martin J. A., Bendell J., et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet Oncology. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 23.Kwon E. D., Drake C. G., Scher H. I., et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J., Reckamp K. L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. The New England Journal of Medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C., Long G. V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England Journal of Medicine. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 26.Shimosegawa T., Chari S. T., Frulloni L., et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 27.Sureka B., Rastogi A. Autoimmune pancreatitis. Polish Journal of Radiology. 2017;82:233–239. doi: 10.12659/PJR.900899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V., Chaudhary N., Garg M., Floudas C. S., Soni P., Chandra A. B. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Frontiers in Pharmacology. 2017;8:p. 49. doi: 10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sznol M., Postow M. A., Davies M. J., et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treatment Reviews. 2017;58:70–76. doi: 10.1016/j.ctrv.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Lyman G. H., Kuderer N. M. The strengths and limitations of meta-analyses based on aggregate data. BMC Medical Research Methodology. 2005;5(1) doi: 10.1186/1471-2288-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller K. D., Siegel R. L., Lin C. C., et al. Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 32.van der Vlist M., Kuball J., Radstake T. R. D., Meyaard L. Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nature Reviews Rheumatology. 2016;12(10):593–604. doi: 10.1038/nrrheum.2016.131. [DOI] [PubMed] [Google Scholar]
- 33.Friedman C. F., Proverbs-Singh T. A., Postow M. A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncology. 2016;2(10):1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file (including Supplementary Tables 1-2 and Supplementary Figures 1–3).