Abstract
Objective
To assess dose, characteristics, and coprescribed analgesics in patients newly prescribed pregabalin for neuropathic pain and fibromyalgia in Japan.
Methods
Based on the medical and prescription information present in the Medical Data Vision database, we analyzed the initial and maximum daily doses, prescription period, coprescribed analgesics, and neuropathic pain-related disorders of patients newly prescribed pregabalin between 01 July 2010 and 31 December 2013.
Results
A total of 45,331 patients (mean age 66.8 years, 48.7% men) were newly prescribed pregabalin during this period. The mean initial and maximum daily doses were 97.3 mg and 127.8 mg, respectively, and decreased yearly. The duration of the prescription period was 111.9 (mean) and 53 (median) days, and the frequently coprescribed analgesics included NSAIDs, opioids, and Neurotropin®. About one half of the patients had spinal disorders.
Conclusion
In Japan during the period examined, the number of newly prescribed pregabalin users increased, but the initial and maximum daily doses decreased yearly after pregabalin went on the market. The maximum daily dose in Japan was lower than those reported in the USA and Europe. These differences might be associated with patient age and physical status and with anxiety about possible adverse events.
1. Introduction
Neuropathic pain and fibromyalgia are intractable chronic pains. Neuropathic pain is defined as “pain caused by a lesion or disease of the somatosensory system” and is classified as peripheral or central neuropathic pain according to the site of the lesion or disease [1, 2]. The prevalence of neuropathic pain was estimated at 6.9% to 10% in some countries [3]. Fibromyalgia is a disorder characterized by systemic pain accompanied by neuropsychiatric symptoms such as insomnia and depression and autonomic symptoms such as irritable bowel syndrome, gastroesophageal reflux disease, and over active bladder [4–6]. In Japan, the prevalence was estimated to be 1.7%–2.1% and about 60%–80% of sufferers were women [7, 8].
Pregabalin is a ligand for the α 2 δ subunit of the calcium channel and is used world wide to treat seizure, generalized anxiety disorder, neuropathic pain, and fibromyalgia. Pregabalin is a first- and/or second-line recommendation for neuropathic pain in many guidelines [9–12] and was approved for fibromyalgia in the USA but not in Europe. In Japan, pregabalin was approved for postherpetic neuralgia in April, 2010, and current indications have been expanded to neuropathic pain and first-line recommendation in the guideline for pharmacologic treatment of neuropathic pain [13]. Pregabalin was also approved for fibromyalgia in June, 2012, in Japan.
Pregabalin is approved to be started at 150 mg daily and titrated up to a maintenance daily dose range. In Japan and the USA, this dose range is 300–600 mg for neuropathic pain and 300–450 mg for fibromyalgia, and in Europe, 150–600 mg for neuropathic pain. However, the efficacy of 150 mg daily was inconsistent [14–16]. In the USA or European observation studies, the mean maximum (or average) daily dose was less than 300 mg (lower limit of approved maintenance dose range in the USA and Japan) or many patients were prescribed <300 mg for neuropathic pain [17–22] and fibromyalgia [23–25].
An interim report of postmarketing surveillance for peripheral neuropathic pain in Japan [26] showed that the mean initial and maximum daily doses of pregabalin were less than the approved initial and maintenance doses. However, the patient population of this study was small (2010 patients), and the observation period was short (13 weeks). Thus, in the present study, the real-world pregabalin prescription for neuropathic pain and fibromyalgia in Japan was examined using the Medical Data Vision database, a large medical and prescription database.
2. Methods
2.1. Data Sources
This descriptive study was conducted using the data collected and aggregated by the Medical Data Vision (MDV) Co. Ltd. from the hospitals using a novel medical reimbursement system for hospitalization, the Diagnosis Procedure Combination/Per-Diem Payment System (DPC/PDPS) in Japan [27, 28]. In April 2016, 1667 hospitals had introduced the DPC/PDPS, encompassing a total of about 495,227 beds. These constituted about 20% of all hospitals and 55% of the total hospital beds in Japan [29]. In May 2016, this database contained the anonymized data of 14,390,000 patients from 247 hospitals. These data contain medical and prescription data from both inpatients and outpatients. Prescription data consisted of individual records each containing one set of information comprising drug name, content, prescription date, daily volume or number of drug formulation, and the number of days prescribed. The study protocol was approved by Kyoto University Graduate School and Faculty of Medicine, Ethics Committee (Kyoto, Japan, Application number E2507).
2.2. Patients
We selected patients newly prescribed pregabalin between 01 July 2010 and 31 December 2013. Newly prescribed was defined as a first prescription in the database with no prescribed pregabalin in the previous 90 days. The date of newly prescribed pregabalin was designated as the first prescription date. Patients were excluded when the hospital's data collection had started within 90 days before the first prescription date. Records of prescribed pregabalin “as-needed” were excluded and patients whose first prescription was only “as-needed” were excluded.
2.3. Daily Dose and Prescription Period
The daily dose of pregabalin was calculated from the content and the daily number of capsules. When there were several records on the same prescription date, daily dose and the number of the days prescribed were estimated based on the number of days until the next pregabalin prescription date. The last prescription date was defined as the last date of pregabalin prescription in the database or as the first date with no pregabalin prescription after 30 days plus the number of days of the last prescription. The prescription period was set to be the time between the first prescription date and the end of the time prescribed by the last prescription. When the duration of the prescription period was over 365 days, this duration was set at 365 days.
2.4. Coprescribed Drugs
Coprescriptions were defined as drugs that were coprescribed at the first prescription date of pregabalin, or before this first date but overlapping it and represcribed within 90 days after it. The number of kinds of coprescribed oral drugs was based on substance names. Coprescribed analgesic drugs were categorized as (1) first- and second-line drugs of the Japanese guideline (1-2-line drugs; oral formulation), including tricyclic antidepressants (TCAs; amitriptyline hydrochloride, imipramine hydrochloride, and nortriptyline hydrochloride), gabapentin, extract of cutaneous tissue of rabbit inoculated with vaccinia virus (Neurotropin), duloxetine hydrochloride, and mexiletine hydrochloride; (2) opioids, including fentanyl (transdermal patch), oxycodone (oral formulation), morphine (oral and injection formulations), buprenorphine (transdermal patch and oral mucosa patch), tramadol (oral and injection formulations), and acetaminophen/tramadol combination (oral formula); and (3) nonsteroidal anti-inflammatory drugs (NSAIDs; ATCcode M01A and N02B0, excluding pentazocine and acetaminophen/tramadol combination; oral formulation). Opioids were subcategorized by strength (weak (tramadol and acetaminophen/tramadol combination) and strong (the others)) and route (oral and nonoral).
2.5. Neuropathic Pain-Related Disorders
Neuropathic pain-related disorders were classified as follows: (1) spinal disorders (ICD-10 code: M47, M48, M50, M51, or M53), (2) postherpetic neuropathy (ICD-10 code: B02.2), (3) diabetic neuropathy (ICD-10 code: E10.4, E11.4, or E14.4), (4) cancer-related pain (disease name: cancer pain, or ICD-10 code: C00-C97 in combination with ICD-10 code: R52.1, R52.2, or R52.9), (5) trigeminal neuralgia (ICD-10 code: G50.0), (6) entrapment peripheral neuropathy of the upper limb (disease name: carpal-tunnel syndrome, Gion tunnel syndrome, cubital tunnel syndrome, or thoracic outlet syndrome), (7) other neuropathic pain (ICD-10 code: G62.9, G64, G96.9, or G98, not classified in the above categories), (8) fibromyalgia (disease name: fibromyalgia), and (9) others (not classified in the above categories).
2.6. Analgesic Drugs Prescribed after Pregabalin Discontinuation Period
Patients were analyzed whose pregabalin prescription period duration was less than 365 days. The after pregabalin discontinuation period was defined as the time between the day after the last prescription date and 90 days after the end of the pregabalin prescription period. The prescriptions of the analgesic drugs described above in this period were then summarized. The prescriptions after the pregabalin discontinuation period were compared with those during the pregabalin prescription period and categorized as follows: (1) continued use: when the same drug had been prescribed during the pregabalin prescription period without prescriptions of other drugs of the same category, (2) new use: when a drug or other drugs of the same category had not been prescribed during the pregabalin prescription period, (3) changed/added drugs: the drugs prescribed after the pregabalin discontinuation period were different from or added to other drugs of the same category prescribed during the pregabalin prescription period, (4) changed/additions of the route: the routes of the drugs coprescribed during the pregabalin prescription period were changed or new administration routes for the same drugs were added after the pregabalin discontinuation period, and (5) changed/additions of the strength: different strengths of opioids were prescribed after the pregabalin discontinuation period to change from or add to opioids prescribed during the pregabalin prescription period.
2.7. Statistical Analysis
Numerical data are presented as means ± their standard deviation, or as medians and their 25th and 75th percentiles, and categorical data are presented as numbers and percentages. Statistical comparisons were made using the t-test for numerical data and the chi-square test for categorical data and were conducted using the SAS version 9.4 (SAS Institute Inc.) statistical package.
3. Results
3.1. Newly Prescribed Pregabalin Patients
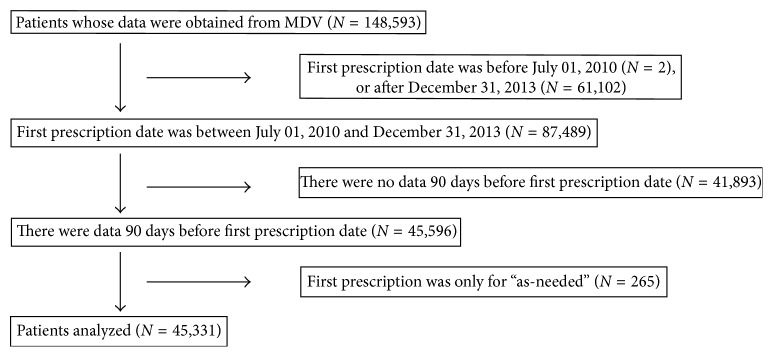
We obtained the data of 148,593 patients from the MDV database who had been prescribed pregabalin; 45,331 of those patients met the necessary criteria, and their data were analyzed further (Figure 1). The number of patients newly prescribed pregabalin increased dramatically in the first year after the launch of pregabalin in the second half of 2010. After that period, the number of these patients increased gradually (Figure 2).
Figure 1.
Patient selection flowchart.
Figure 2.
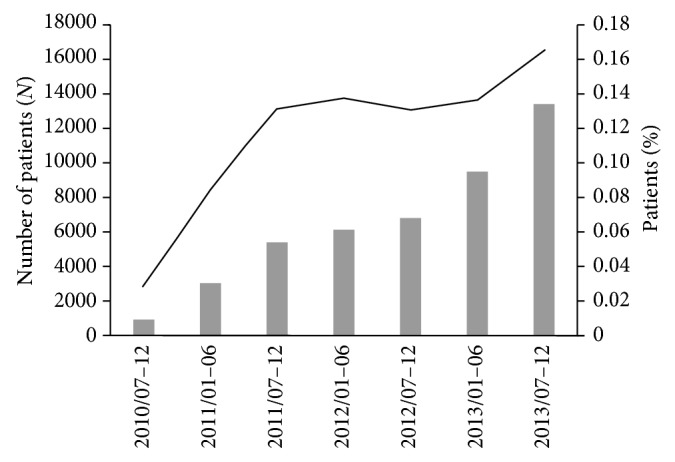
The number of patients newly prescribed pregabalin. Solid columns represent the number of patients newly prescribed pregabalin in each half year. Solid line represents the number of patients newly prescribed pregabalin in each half year divided by the total number of monthly patients in each half year in this database.
3.2. Characteristics of Pregabalin-Prescribed Patients
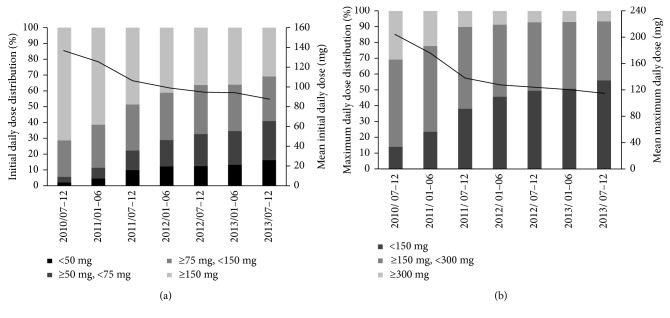
Table 1 shows the characteristics of pregabalin-prescribed patients. At their first prescription date, 37,045 of the patients (81.7%) were prescribed pregabalin in an outpatient setting. The male ratio was 48.7%, and the mean age was 66.8 ± 13.9 years. The male ratio of inpatients was over 50%, and the mean age of inpatients was a little higher than that of outpatients. The mean initial daily dose and maximum daily dose were 97.3 ± 56.7 mg and 127.8 ± 87.0 mg, respectively, but these tended to decrease as time proceeded after the initial launch (Figure 3).
Table 1.
Characteristics of pregabalin-prescribed patients.
| All | Outpatient | Inpatient | P value | |
|---|---|---|---|---|
| N | 45331 | 37045 | 8286 | |
|
| ||||
| Sex (male), N (%) | 22085 (48.7) | 17692 (47.8) | 4393 (53.0) | <0.001 |
|
| ||||
| Age, mean (SD) | 66.8 (13.9) | 66.4 (14.0) | 69.0 (13.3) | <0.001 |
|
| ||||
| Initial daily dose, mg | ||||
| Mean (SD) | 97.3 (56.7) | 95.5 (55.7) | 105.4 (60.5) | <0.001 |
|
| ||||
| N (%) | ||||
| <50 mg | 5737 (12.7) | 4733 (12.8) | 1004 (12.1) | — |
| ≥50 mg, <75 mg | 8691 (19.2) | 7596 (20.5) | 1095 (13.2) | — |
| ≥75 mg, <150 mg | 13139 (29.0) | 10687 (28.8) | 2452 (29.6) | — |
| ≥150 mg | 17764 (39.2) | 14029 (37.9) | 3735 (45.1) | — |
|
| ||||
| Maximum daily dose, mg | ||||
| Mean (SD) | 127.8 (87.0) | 123.6 (82.5) | 146.2 (102.5) | <0.001 |
|
| ||||
| N (%) | ||||
| <150 mg | 21468 (47.4) | 18162 (49.0) | 3306 (39.9) | — |
| ≥150 mg, <300 mg | 19763 (43.6) | 15913 (43.0) | 3850 (46.5) | — |
| ≥300 mg | 4100 (9.0) | 2970 (8.0) | 1130 (13.6) | — |
|
| ||||
| Prescription period, day | ||||
| Mean (SD) | 111.9 (124.2) | 118.5 (126.8) | 82.6 (107.0) | <0.001 |
| Median (25, 75 percentile) | 53 (21, 163) | 56 (21, 182) | 36 (14, 94) | <0.001 |
| Prescription period over 90 days, N (%) | 16762 (37.0) | 14595 (39.4) | 2167 (26.2) | <0.001 |
|
| ||||
| Change in dose, N(%) | 12205 (26.9) | 9584 (25.9) | 2621 (31.6) | <0.001 |
|
| ||||
| Coprescribed oral drugs, mean (SD) | 4.2 (3.7) | 3.9 (3.6) | 5.7 (4.0) | <0.001 |
|
| ||||
| Coprescribed analgesics, N (%) | ||||
| 1-2-Line drugs | 4698 (10.4) | 4017 (10.8) | 681 (8.2) | <0.001 |
| TCAs | 615 (1.4) | 473 (1.3) | 142 (1.7) | — |
| Gabapentin | 160 (0.4) | 120 (0.3) | 40 (0.5) | — |
| Neurotropin | 3656 (8.1) | 3256 (8.8) | 400 (4.8) | — |
| Duloxetine | 258 (0.6) | 178 (0.5) | 80 (1.0) | — |
| Mexiletine | 217 (0.5) | 162 (0.4) | 55 (0.7) | — |
| Opioids | 3843 (8.5) | 2117 (5.7) | 1726 (20.8) | <0.001 |
| Oral opioids | 3071 (6.8) | 1825 (4.9) | 1246 (15.0) | — |
| Nonoral opioids | 790 (1.7) | 275 (0.7) | 515 (6.2) | — |
| Strong opioids | 2219 (4.9) | 954 (2.6) | 1265 (15.3) | — |
| Weak opioids | 1688 (3.7) | 1184 (3.2) | 504 (6.1) | — |
| NSAIDs | 17303 (38.2) | 13657 (36.9) | 3646 (44.0) | <0.001 |
|
| ||||
| Neuropathic pain-related disorders∗, N (%) | ||||
| Spinal disorders | 23502 (51.8) | 21144 (57.1) | 2358 (28.5) | <0.001 |
| Postherpetic neuropathy | 2795 (6.2) | 2435 (6.6) | 360 (4.3) | <0.001 |
| Diabetic neuropathy | 1478 (3.3) | 1244 (3.4) | 234 (2.8) | 0.014 |
| Cancer-related pain | 3933 (8.7) | 2172 (5.9) | 1761 (21.3) | <0.001 |
| Trigeminal neuralgia | 780 (1.7) | 713 (1.9) | 67 (0.8) | <0.001 |
| Entrapment peripheral neuropathy of the upper limb | 1329 (2.9) | 1251 (3.4) | 78 (0.9) | <0.001 |
| Other neuropathic pain disorders | 11564 (25.5) | 9728 (26.3) | 1836 (22.2) | — |
| Fibromyalgia | 153 (0.3) | 127 (0.3) | 26 (0.3) | 0.681 |
| Others | 3178 (7.0) | 1113 (3.0) | 2065 (24.9) | — |
1-2-line drugs: first- and second-line drugs of the Japanese guideline; TCAs: tricyclic antidepressants (amitriptyline hydrochloride, imipramine hydrochloride, and nortriptyline hydrochloride); neurotropin; extract of cutaneous tissue of rabbit inoculated with vaccinia virus. ∗Patients in these categories are not mutually exclusive. —, statistical analyses not performed.
Figure 3.
The initial (a) and maximum (b) daily doses. Solid columns represent the daily dose distribution. Solid line represents the mean daily dose.
The mean and median durations of the prescription period were 111.9 ± 124.2 and 53 days, respectively. The duration of the prescription period was longer for outpatients than that for inpatients. The fraction of patients who changed from the initial daily dose to at least one another dose during the prescription period (change in dose) was only 26.9%, and these changes were higher for inpatients than they were for outpatients. The mean number of coprescribed oral drugs was 4.2 ± 3.7, and the number for inpatients was higher than that for outpatients.
The most frequently coprescribed analgesics for outpatients at the first pregabalin prescription date were NSAIDs (36.9%); the second most frequent was Neurotropin. Opioids were coprescribed for 5.7% of the outpatients, and most of these were oral formulas. For inpatients, the most frequently coprescribed analgesics were NSAIDs, followed by opioids; strong opioids were more frequently coprescribed than weak ones.
About one half of the outpatients had spinal disorders; other disorders, in descending order of frequency, included postherpetic neuropathy, cancer-related pain, and diabetic neuropathy. About 30% of inpatients had spinal disorders and about 20% had cancer-related pain.
To exclude the effects of short-trial pregabalin use on the results, a subgroup analysis of 12-month continuous users was conducted (Supplementary Table S1). Compared with the results of the main analysis, the mean maximum daily dose increased to 153.5 ± 106.2, 16.4% of the patients were prescribed ≥300 mg as the maximum dose, and 51.7% of them underwent a change in dose. Other parameters were comparable to those in the main analysis.
3.3. Pregabalin Prescription Patterns according to Gender and Age
Table 2 shows the pregabalin prescription patterns according to gender and age. The initial and maximum daily doses prescribed for males were higher than those prescribed for females, and were also higher for patients younger than 65 years than for those 65 years or older. On the other hand, there were only small differences in the durations of the prescription period between males and females, while the duration of the prescription period for patients younger than 65 was shorter than that of those 65 years or older. The maximum daily dose tended to increase with increasing first daily doses. The prescription period tended to be shorter for those receiving initial daily doses of more than 75 mg/day.
Table 2.
Prescription patterns according to gender and age.
| Initial daily dose | Maximum daily dose | Prescription period | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | Mean (SD) | Mean (SD) | Median (25, 75 percentile) | ||||||||||
| Age | ||||||||||||||
| <65 | ≥65 | <65a | ≥65 | P value | <65 | ≥65 | P value | <65 | ≥65 | P value | <65 | ≥65 | P value | |
| Sex | ||||||||||||||
| Male | 8578 (50.8) | 13507 (47.5) | 110.6 (57.8) | 98.0 (58.4) | <0.001 | 149.7 (97.2) | 130.4 (88.9) | <0.001 | 104.7 (118.4) | 118.8 (126.5) | <0.001 | 49 (21,144) | 57 (22,184) | <0.001 |
| Female | 8318 (49.2) | 14928 (52.5) | 101.9 (55.2) | 86.6 (53.2) | <0.001 | 132.3 (85.3) | 110.2 (75.7) | <0.001 | 100.5 (118.0) | 116.2 (128.1) | <0.001 | 43 (16,132) | 54 (21,178) | <0.001 |
|
| ||||||||||||||
| Initial daily dose | ||||||||||||||
| <50 mg | 1470 (8.7) | 4267 (15.0) | — | — | — | 55.7 (55.6) | 51.4 (54.7) | 0.001 | 112.4 (124.5) | 130.1 (132.2) | <0.001 | 56 (21,161) | 63 (27,225) | <0.001 |
| ≥50 mg, <75 mg | 2714 (16.1) | 5977 (21.0) | — | — | — | 86.2 (66.7) | 78.8 (54.0) | <0.001 | 113.5 (122.5) | 126.9 (129.1) | <0.001 | 56 (24,164) | 65 (28,205) | <0.001 |
| ≥75 mg, <150 mg | 4886 (28.9) | 8253 (29.0) | — | — | — | 116.2 (71.6) | 109.8 (67.4) | <0.001 | 98.4 (114.7) | 117.3 (127.4) | <0.001 | 44 (20,127) | 56 (21,182) | <0.001 |
| ≥150 mg | 7826 (46.3) | 9938 (34.9) | — | — | — | 191.9 (86.9) | 182.2 (76.4) | <0.001 | 99.7 (117.4) | 106.5 (123.0) | <0.001 | 42 (14,135) | 46 (16,149) | 0.013 |
3.4. Subgroup Analysis of Individual Disorders
The use of pregabalin and coprescribed drugs was analyzed in subgroups of the following individual disorders: spinal disorders, postherpetic neuropathy, diabetic neuropathy, cancer-related pain, trigeminal neuralgia, and entrapment peripheral neuropathy of the upper limb (Table 3). The male ratio was higher for diabetic neuropathy and cancer-related pain. The mean age of patients was greater than 60 years for all disorders. The initial and maximum daily doses of pregabalin tended to be slightly lower in outpatients with spinal disorders, diabetic neuropathy, and entrapment neuropathy of the upper limb than in those with other disorders. The maximum daily dose of pregabalin was higher in inpatients than in outpatients with spinal disorders, postherpetic neuropathy, cancer-related pain, and entrapment neuropathy of the upper limb. Inpatients tended to have shorter durations of the prescription period than did outpatients with spinal disorders, diabetic neuropathy, cancer-related pain, and trigeminal neuralgia.
Table 3.
Subgroup analysis of individual disorders.
| Spinal disorders | P value | Postherpetic neuropathy | P value | Diabetic neuropathy | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Outpatient | Inpatient | Outpatient | Inpatient | Outpatient | Inpatient | ||||
| N | 21144 | 2358 | 2435 | 360 | 1244 | 234 | |||
| Sex (male), N (%) | 10219 (48.3) | 1211 (51.4) | 0.006 | 1142 (46.9) | 168 (46.7) | 0.935 | 745 (59.9) | 146 (62.4) | 0.473 |
| Age, mean (SD) | 67.3 (13.9) | 70.0 (13.1) | <0.001 | 69.1 (13.2) | 70.8 (13.0) | 0.030 | 67.7 (11.7) | 69.1 (11.6) | 0.084 |
| Initial daily dose (mg), mean (SD) | 89.5 (54.1) | 101.4 (56.8) | <0.001 | 108.0 (58.7) | 104.8 (52.6) | 0.321 | 96.9 (54.2) | 99.1 (57.8) | 0.570 |
| Maximum daily dose (mg), mean (SD) | 115.2 (76.4) | 140.0 (92.9) | <0.001 | 146.5 (96.9) | 164.5 (106.2) | 0.002 | 129.9 (85.1) | 139.1 (92.6) | 0.135 |
| Prescription period (day) | |||||||||
| Mean (SD) | 123.6 (128.2) | 95.7 (116.1) | <0.001 | 95.2 (116.5) | 94.0 (112.8) | 0.857 | 160.3 (143.6) | 103.2 (124.1) | <.0.001 |
| Median (25, 75 percentile) | 62 (28, 196) | 43 (15, 122) | <0.001 | 42 (14, 119) | 43.5 (20, 111.5) | 0.600 | 91 (30, 365) | 43 (17, 129) | <0.001 |
| Prescription period over 90 days, N (%) | 8726 (41.3) | 724 (30.7) | <0.001 | 747 (30.7) | 105 (29.2) | 0.562 | 634 (51.0) | 74 (31.6) | <0.001 |
| Coprescribed oral drugs, mean (SD) | 4.0 (3.7) | 5.5 (4.2) | <0.001 | 4.3 (3.7) | 5.4 (4.0) | <0.001 | 7.0 (4.4) | 7.4 (4.6) | 0.232 |
| 1-2-line drugs, N (%) | 2798 (13.2) | 272 (11.5) | 0.021 | 350 (14.4) | 49 (13.6) | 0.670 | 171 (13.7) | 31 (13.2) | 0.839 |
| Opioids, N (%) | 881 (4.2) | 322 (13.7) | <0.001 | 95 (3.9) | 33 (9.2) | <0.001 | 33 (2.7) | 21 (9.0) | <0.001 |
| NSAIDs, N (%) | 8813 (41.7) | 1119 (47.5) | <0.001 | 959 (39.4) | 164 (45.6) | 0.026 | 264 (21.2) | 63 (26.9) | 0.054 |
|
| |||||||||
| Cancer-related pain | P value | Trigeminal neuralgia | P value | Entrapment neuropathy of the upper limb | P value | ||||
| Outpatient | Inpatient | Outpatient | Inpatient | Outpatient | Inpatient | ||||
|
| |||||||||
| N | 2172 | 1761 | 713 | 67 | 1251 | 78 | |||
| Sex (male), N(%) | 1167 (53.7) | 1089 (61.8) | <0.001 | 320 (44.9) | 31 (46.3) | 0.828 | 499 (39.9) | 37 (47.4) | 0.188 |
| Age, mean (SD) | 64.3 (12.2) | 65.0 (12.6) | 0.060 | 65.9 (13.7) | 68.9 (12.8) | 0.079 | 65.4 (14.2) | 68.7 (12.7) | 0.044 |
| Initial daily dose (mg), mean (SD) | 108.3 (54.2) | 107.1 (58.8) | 0.515 | 109.9 (60.2) | 117.4 (69.8) | 0.337 | 84.4 (52.7) | 93.1 (60.7) | 0.161 |
| Maximum daily dose (mg), mean (SD) | 150.0 (96.4) | 165.1 (116.7) | <0.001 | 148.7 (97.3) | 165.5 (93.2) | 0.176 | 107.8 (74.3) | 142.8 (124.8) | <0.001 |
| Prescription period (day) | |||||||||
| Mean (SD) | 118.3 (123.2) | 72.3 (92.4) | <0.001 | 124.0 (135.2) | 76.6 (100.3) | 0.006 | 125.6 (128.4) | 126.9 (137.0) | 0.935 |
| Median (25, 75 percentile) | 63 (28, 177) | 36 (14, 84) | <0.001 | 51 (14, 220) | 39 (16, 88) | 0.371 | 63 (28, 204) | 54 (21, 228) | 0.631 |
| Prescription period over 90 days, N(%) | 888 (40.9) | 418 (23.7) | <0.001 | 287 (40.3) | 15 (22.4) | 0.005 | 520 | (41.6) | 31 (39.7) |
| Coprescribed oral drugs, mean (SD) | 5.1 (3.4) | 5.7 (3.5) | <0.001 | 4.1 (4.0) | 6.4 (4.3) | <0.001 | 3.8 (3.9) | 5.8 (4.2) | <0.001 |
| 1-2-line drugs, N(%) | 93 (4.3) | 84 (4.8) | 0.463 | 82 (11.5) | 7 (10.4) | 0.796 | 201 (16.1) | 11 (14.1) | 0.646 |
| Opioids, N(%) | 1001 (46.1) | 1127 (64.0) | <0.001 | 25 (3.5) | 8 (11.9) | 0.001 | 28 (2.2) | 7 (9.0) | <0.001 |
| NSAIDs, N(%) | 1111 (51.2) | 1035 (58.8) | <0.001 | 161 (22.6) | 33 (49.3) | <0.001 | 363 (29.0) | 34 (43.6) | 0.007 |
1-2-line drugs: first- and second-line drugs of the Japanese guideline.
Only about 5% of patients with cancer-related pain were coprescribed 1-2-line analgesic drugs, while these were prescribed for 10.4% to 16.1% of the patients with other disorders. By contrast, about half of the patients with cancer-related pain were coprescribed opioids, while at most 15% of the patients with other pain disorders received opioids. More inpatients were coprescribed opioids along with the first prescription than were outpatients. Smaller percentages of diabetic neuropathy patients (both inpatients and outpatients) as well as outpatients with trigeminal neuropathy and entrapment neuropathy of the upper limb were coprescribed NSAIDs.
3.5. After the Pregabalin Discontinuation Period
After the pregabalin discontinuation period (Table 4), 10.2% of patients were represcribed pregabalin, and 8.2% and 15.2% of patients were prescribed 1-2-line drugs and opioids, respectively. About 5% of patients continued receiving prescriptions of the same 1-2-line drugs, and 3.4% received new 1-2-line prescriptions, while 4.0% continued previously prescribed opioids, and 7.4% received new opioid prescriptions.
Table 4.
Analgesic drugs used before and after the pregabalin discontinuation.
| Before | After | |
|---|---|---|
| N | 39249 | 39249 |
| Analgesics (N, %) | ||
| Represcribed pregabalin use | — | 4009 (10.2) |
|
| ||
| 1-2-line drugs | 5416 (13.8) | 3213 (8.2) |
| Continued use | — | 1761 (4.5) |
| New use | — | 1348 (3.4) |
| Changed/added drugs | — | 104 (0.3) |
| TCAs | 837 (2.1) | 448 (1.1) |
| Gabapentin | 355 (0.9) | 197 (0.5) |
| Neurotropin | 3775 (9.6) | 2106 (5.4) |
| Duloxetine | 501 (1.3) | 455 (1.2) |
| Mexiletine | 310 (0.8) | 221 (0.6) |
|
| ||
| Opioids | 8171 (20.8) | 5954 (15.2) |
| Continued use | — | 1580 (4.0) |
| New use | — | 2894 (7.4) |
| Changed/additions of drugs/route | — | 1480 (3.8) |
|
| ||
| Oral opioids | 5008 (12.8) | 3350 (8.5) |
| Continued use | — | 1358 (3.5) |
| New use | — | 1649 (4.2) |
| Changed/added drugs | — | 160 (0.4) |
| Changed/additions of route | — | 183 (0.5) |
|
| ||
| Nonoral opioids | 4737 (12.1) | 3368 (8.6) |
| Continued use | — | 702 (1.8) |
| New use | — | 1465 (3.7) |
| Changed/added drugs | — | 527 (1.3) |
| Changed/additions of route | — | 674 (1.7) |
|
| ||
| Weak opioids | 3238 (8.2) | 2377 (6.1) |
| Continued use | — | 710 (1.8) |
| New use | — | 1444 (3.7) |
| Changed/added drugs | — | 76 (0.2) |
| Changed/additions of the strength | — | 147 (0.4) |
|
| ||
| Strong opioids | 5928 (15.1) | 3963 (10.1) |
| Continued use | — | 1010 (2.6) |
| New use | — | 1644 (4.2) |
| Changed/added drugs | — | 1062 (2.7) |
| Changed/additions of the strength | — | 247 (0.6) |
1-2-line drugs:, first- and second-line drugs of the Japanese guideline; TCAs: tricyclic antidepressants (amitriptyline hydrochloride, imipramine hydrochloride, and nortriptyline hydrochloride); neurotropin: extract of cutaneous tissue of rabbit inoculated with vaccinia virus.
When individual pain disorders were analyzed separately (Supplementary Table S2), pregabalin was represcribed for less than 10% of postherpetic neuralgia and cancer-related pain patients. The frequency of prescribed opioids for cancer-related pain (50.2%) was highest in patients with these disorders, and most were continued prescriptions or changes/additions of the drugs/routes (all greater than 10%).
4. Discussion
The data for the 45,331 patients newly prescribed pregabalin included in the MDV database, showed that the number of new users increased considerably from the launch of pregabalin in the second half of 2010 to the second half of 2011, when this increase leveled off. Indications for pregabalin were expanded in 2012 and 2013; however, these expansions were for fibromyalgia and central neuropathic pain and had little affect on the number of pregabalin prescriptions, as there are fewer of these patients than those with the prior indications for peripheral neuropathic pain.
The initial daily dose was lower than the approved initial daily dose in Japan (150 mg), and decreased yearly; 60.8% of patients were prescribed less than the approved dose. The reason for this decrease may be due to the many adverse events observed early in the launch of pregabalin [16]. A notice for elderly patients was released by the Pharmaceuticals and Medical Devices Agency of Japan in July 2012 [30] stating that “In elderly patients, some cases of falls due to dizziness, somnolence, loss of consciousness, etc. leading to fractures have been reported; therefore extra caution should be exercised.” The initial daily doses prescribed for males were higher than those for females and were also higher for patients younger than 65 years than for those 65 years or older. Differences in physical status (weight and height) or excretory function and older age being a risk factor for early onset of adverse events, such as somnolence and dizziness [31], were considered possible reasons for these differences. The duration of the prescription period was shorter in those patients who received more than 75 mg/day as an initial daily dose. The incidence of adverse events in those receiving more than 75 mg/day may have increased, resulting in a shorter duration of the prescription period.
The maximum daily dose was lower than both the approved initial (150 mg) and maintenance (≥300 mg) daily doses in Japan; the proportions of patients prescribed less than approved doses were 47.4% and 91.0%, respectively. In the subgroup analysis of 12-month continuous users, the maximum daily dose (153.5 mg) was comparable to the approved initial daily dose in Japan, and the proportions of patients prescribed less than the approved doses were 38.6% and 83.6%, respectively. Such low doses were also reported in another Japanese study (134.9 mg) [16]. In USA and European studies, the maximum or average daily doses (about 170–280 mg) [18–24] were less than 300 mg and although not many patients were prescribed more than 300 mg (about 20–30%) [18, 19, 21, 23, 25], these numbers were still higher than those in Japan. In randomized control trials, the efficacy of 150 mg/day was inconsistent [14–16]. Especially in those conducted in Japan [32–34], only one study for postherpetic neuralgia had a 150 mg group as the maintenance dose, but significant effects were not shown [32]. The maximum daily dose in Japan was considered much lower, this might be associated with differences in age; the patients in this study (67 years old) were older than those in other studies (45–67 years old). There were also differences in physical status; Japanese people are generally less heavy and shorter than Westerners. Prescribing doctors may also have had anxiety about the reported adverse events; the initial daily dose was being decreased and many patients were prescribed the same daily dose during the prescription period.
The ratio of males, initial daily dose, maximum daily dose, and number of coprescribed opioids were higher in inpatients than in outpatients. The high ratio of males was considered to result from the high ratio of cancer-related pain patients and a low ratio of spinal disorders because there was a higher ratio of cancer-related pain in males and a lower ratio of males with spinal disorders. The higher ratio of coprescribed opioids in inpatients was observed for all disorders, including cancer-related pain. This may be because severer pain patients may more often be inpatients than outpatients.
The 1-2-line drugs were coprescribed for about 15% patients with all disorders except cancer-related pain, and NSAIDs were coprescribed for 20% to 40% of patients. The high frequency of coprescribed NSAIDs may be due to many patients also having nociceptive pain, another component of pain. Approximately half of the patients with cancer-related pain were coprescribed opioids, and one half of the patients were coprescribed NSAIDs. NSAIDs and opioids were recommended for cancer pain, and pregabalin was coprescribed for adjuvant analgesics or relief of neuropathic pain from cancer or cancer treatment and/or the coexistence of these pains.
This study had several strengths. First, a large population of patients who were prescribed pregabalin was analyzed about 10 times that of other drug-use investigations in Japan [15], and the maximum follow-up period was 4 times longer. Second, the database contained the data of patients who visited hospitals regardless of their kind of insurance. There were patients of a variety of ages, including later-stage elderly (75 or older) and a variety of jobs. Third, pregabalin was only approved in Japan for pain disorder, neuropathic pain, and fibromyalgia, and unapproved for other symptoms, such as seizure and generalized anxiety disorder; therefore, the analysis in this study was able to specialize in its use for pain.
There were also some limitations. First, the follow-up of patients was limited because this was a database of hospital-based data. After discontinuation of pregabalin, other analgesics were prescribed for about 30% of patients. When there were no data of analgesic prescriptions, it was not possible to know whether no analgesics had been prescribed or the patients had consulted a different hospital. However, these patients were considered to be unsatisfied with the effectiveness of pregabalin. Second, the reasons for discontinuation were not confirmed because the effects and the most adverse drug reactions of pregabalin, such as dizziness and somnolence, were not collected for this database. Third, there is a possibility of bias in the patient population because many small scale hospitals have not introduced the DPC/PDPS [29]. Moreover, as the DPC/PDPS is a system for hospitalization, this database has no data from hospitals without inpatient facilities and little data from small scale hospitals. Forth, the disorders for which pregabalin was prescribed were not confirmed. In this database, prescribed drugs and the disorders for which they were prescribed were not combined; therefore, for example, the neuropathic pain-related disorders were categorized from those treated in the same month of the first prescription date.
5. Conclusion
In Japan, the number of patients being newly prescribed pregabalin increased over the course of the study period, but the initial and maximum daily doses decreased yearly after pregabalin went on the market. The maximum daily dose in Japan was lower than those reported in the USA and Europe, which may be associated with differences in age and physical status and anxiety about possible adverse events.
Acknowledgments
The authors thank Dr. Sei Fukui, Clinical Professor of Pain Management Clinic, Interdisciplinary Pain Management Center, Shiga University of Medical Science Hospital, for helpful discussions. The authors also thank Dr. Yoshika Onishi, Dr. Isao Nahara, Dr. Chikashi Takeda, and Dr. Hiroshi Yonekura, Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University, for helpful considerations.
Conflicts of Interest
Mikito Hirakata is an employee of Toray Industries, Inc. Koji Kawakami received research funds from Dainippon Sumitomo Pharma, Olympus, Stella Pharma, Medical Platform Co., Novartis Pharmaceutical K.K., Bayer, and Maruho; honorariums from Astellas, Taisho Pharmaceutical, Hikari Pharmaceutical, Eisai, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Company Limited, and Sanofi K.K.; and consulting fees from Olympus, Kyowa Hakko Kirin, Kaken Pharmaceutical, and Otsuka Pharmaceuticals. The other authors have no conflicts of interest.
Authors' Contributions
Mikito Hirakata contributed to conception and design, acquisition of data, analysis and interpretation of data, and drafting the manuscript, Satomi Yoshida contributed to analysis and interpretation of data, and the other authors contributed to conception and design and analysis and interpretation of the data. All authors contributed to critically revising the article for important intellectual content and had final approval of the version to be published.
Supplementary Materials
Table S1: subgroup analysis of characteristics of 12-month continuous pregabalin-prescribed patients. Table S2: subgroup analysis of individual disorders for analgesics drug use before and after the discontinuation of pregabalin.
References
- 1.Loeser J. D., Treede R. D. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137(3):473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Jensen T. S., Baron R., Haanpää M., et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 3.van Hecke O., Austin S. K., Khan R. A., Smith B. H., Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Wallace D. J., Hallegua D. S. Fibromyalgia: the gastrointestinal link. Current Pain and Headache Report. 2004;8(5):364–368. doi: 10.1007/s11916-996-0009-z. [DOI] [PubMed] [Google Scholar]
- 5.Chung J. H., Kim S. A., Choi B. Y., et al. The association between overactive bladder and fibromyalgia syndrome: a community survey. Neurourology and Urodynamics. 2013;32(1):66–69. doi: 10.1002/nau.22277. [DOI] [PubMed] [Google Scholar]
- 6.Queiroz L. P. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Report. 2013;17(8):p. 356. doi: 10.1007/s11916-013-0356-5. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M. Epidemiology of fibromyalgia. Pharma Medica. 2006;24(6):35–39. in Japanese. [Google Scholar]
- 8.Nakamura I., Nishioka K., Usui C., et al. An epidemiologic internet survey of fibromyalgia and chronic pain in Japan. Arthritis Care and Research. 2014;66(7):1093–1101. doi: 10.1002/acr.22277. [DOI] [PubMed] [Google Scholar]
- 9.Attal N., Cruccu G., Baron R., et al. European federation of neurological societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European Journal of Neurology. 2010;17(9):1113–1123. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 10.NICE. Neuropathic Pain—Pharmacological Management: The Pharmacological Management of Neuropathic Pain in Adults in Nonspecialist Settings. 2016. http://www.nice.org.uk/guidance/cg173.
- 11.Finnerup N. B., Attal N., Haroutounian S., et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–173. doi: 10.1016/s1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler D., Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. Journal of Diabetes and its Complications. 2015;29(1):146–156. doi: 10.1016/j.jdiacomp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 13.The Committee for the Guidelines for the Pharmacologic Management of Neuropathic Pain of JSPC. Guidelines for the Pharmacologic Management of Neuropathic Pain. Hyogo, Japan: Publishing Department, Shinko Trading Co. Ltd.; 2011. [Google Scholar]
- 14.Freeman R., Durso-Decruz E., Emir B. Efficacy, safety and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy; findings from seven randomized controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–1454. doi: 10.2337/dc07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore R. A., Straube S., Wiffen P. J., Derry S., McQuay H. J. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews. 2009;3:p. CD007076. doi: 10.1002/14651858.cd007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finnerup N. B., Sindrup S. H., Jensen T. S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Navarro A., Saldaña M. T., Pérez C., Torrades S., Rejas J. Patient-reported outcomes in subjects with neuropathic pain receiving pregabalin: evidence from medical practice in primary care settings. Pain Medicine. 2010;11(5):719–731. doi: 10.1111/j.1526-4637.2010.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun P., Zhao Y., Zhao Z., Bernauer M., Watson P. Dosing pattern comparison between duloxetine and pregabalin among patients with diabetic peripheral neuropathic pain. Pain Practice. 2012;12(8):641–648. doi: 10.1111/j.1533-2500.2012.00537.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P., Becker L., Halpern R., Sweeney M. Real-world treatment of post-herpetic neuralgia with gabapentin or pregabalin. Clinical Drug Investigation. 2013;33(1):35–44. doi: 10.1007/s40261-012-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettermark B., Brandt L., Kieler H., Bodén R. Pregabalin is increasingly prescribed for neuropathic pain, generalised anxiety disorder and epilepsy but many patients discontinue treatment. International Journal of Clinical Practice. 2014;68(1):104–110. doi: 10.1111/ijcp.12182. [DOI] [PubMed] [Google Scholar]
- 21.Happich M., Schneider E., Boess F. G., et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clinical Journal of Pain. 2014;30(10):875–885. doi: 10.1097/ajp.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 22.Asomaning K., Abramsky S., Liu Q., Zhou X., Sobe R. E., Watt S. Pregabalin prescriptions in the United Kingdom: a drug utilisation study of The Health Improvement Network (THIN) primary care database. International Journal of Clinical Practice. 2016;70(5):380–388. doi: 10.1111/ijcp.12791. [DOI] [PubMed] [Google Scholar]
- 23.Gore M., Sadosky A. B., Zlateva G., Clauw D. J. Clinical characteristics, pharmacotherapy and healthcare resource use among patients with fibromyalgia newly prescribed gabapentin or pregabalin. Pain Practice. 2009;9(5):363–374. doi: 10.1111/j.1533-2500.2009.00292.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun P., Zhao Y., Zhao Z., Watson P. Medication dosing patterns associated with duloxetine and pregabalin among patients with fibromyalgia. Current Medical Research and Opinion. 2011;27(9):1793–1801. doi: 10.1185/03007995.2011.605113. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Qian C., Yang M. Treatment patterns associated with ACR-recommended medications in the management of fibromyalgia in the United States. Journal of Managed Care and Specialty Pharmacy. 2016;22(3):263–271. doi: 10.18553/jmcp.2016.22.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa S., Komatsu M., Ohno S., Yamane H., Hayakawa K. Interim report of drug use investigation of pregabalin (Lyrica®) Progress in Medicine. 2013;33(10):2159–2171. in Japanese. [Google Scholar]
- 27.Tanaka S., Seto K., Kawakami K. Pharmacoepidemiology in Japan: medical databases and research achievements. Journal of Pharmaceutical Health Care and Sciences. 2015;1(1):p. 16. doi: 10.1186/s40780-015-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueyama H., Hinotsu S., Tanaka S., et al. Application of a self-controlled case series study to a database study in children. Drug Safety. 2014;37(4):259–268. doi: 10.1007/s40264-014-0148-9. [DOI] [PubMed] [Google Scholar]
- 29. Ministry of Health, Labour and Welfare of Japan, 2016, http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000121094.pdf, in Japanese.
- 30.Pharmaceuticals and Medical Devices Agency. 2017. http://www.pmda.go.jp/files/000153585.pdf.
- 31.Kato H., Miyazaki M., Takeuchi M., et al. A retrospective study to identify risk factors for somnolence and dizziness in patients treated with pregabalin. Journal of Pharmaceutical Health Care and Sciences. 2015;1(1):p. 22. doi: 10.1186/s40780-015-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa S., Suzuki M., Arakawa A., Araki S., Yoshiyama T. Efficacy and tolerability for postherpetic neuralgia: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Journal of the Japan Society of Pain Clinicians. 2010;17(2):141–152. in Japanese. [Google Scholar]
- 33.Satoh J., Yagihashi S., Baba M., et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabetic Medicine. 2011;28(1):109–116. doi: 10.1111/j.1464-5491.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 34.Ohta H., Oka H., Usui C., Ohkura M., Suzuki M., Nishioka K. A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Research and Therapy. 2012;14(5):p. R217. doi: 10.1186/ar4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: subgroup analysis of characteristics of 12-month continuous pregabalin-prescribed patients. Table S2: subgroup analysis of individual disorders for analgesics drug use before and after the discontinuation of pregabalin.