Abstract
In humans, cardiovascular disease (CVD) is the most frequent cause of death worldwide. Myocardial infarction (MI) is a leading cause of heart failure due to myocardial impairment, yet the progression of the resultant dysfunction is often undetected after incidental or induced myocardial infarction. In this study we tracked the progression of left-sided heart failure in 6-mo-old male castrated sheep in which we created 2 models of myocardial infarction, small and large. Myocardial infarction was induced through ligation of a single branch (obtuse marginal [OM] 1) of the left circumflex coronary artery to create small (mild) infarcts and of 2 branches (OM1 and OM2) for large (severe) infarcts. Progression of heart failure was evaluated by assessing scar size, the left ventricular ejection fraction, hematology, cardiac serum biochemical biomarkers, ST elevation, and clinical observation. All parameters were assessed at baseline and at 3 wk and 3 mo after infarction, except that clinical observation of the animals was conducted daily. The different parameters differed in their usefulness: some verified appropriate creation of the model, whereas others enabled assessment of the progression of heart disease. We hypothesize that myocardial scar size, as a function of induced ischemia, coupled with left ventricular ejection fraction are predictive indicators of postinfarction cardiac dysfunction.
Abbreviations: CVD, cardiovascular disease; LVD, left ventricular dysfunction; MI, myocardial infarction; LVEF, left ventricular ejection fraction; OM, obtuse marginal branch of the left circumflex coronary artery
The prevalence of cardiovascular disease (CVD) in humans achieved global significance over the past 2 decades.2,15 In the Asian Pacific region, ischemic heart disease accounted for an estimated 7.3 million deaths in 2008, whereas CVD, in general, is the leading cause of death in the Caribbean region.1,12 In addition, ischemic heart disease is responsible for 5.2% of the worldwide burden due to heart disease.14 Many risk factors including diet, tobacco use, obesity, diabetes mellitus, and hypertension predispose the human population to CVD, but occupational hazards such as ambient pollution by factories coupled with debilitating preexisting conditions such as HIV–AIDS are important contributors also.3,7,11,14,16,17 Pigs and sheep, which have poor coronary collateral circulation, are 2 important animal models used to study ischemic heart disease.4,9,13 Depending on the nature of the study, sheep may be chosen over pigs, which are prone to ventricular arrhythmia and have a tendency to develop irreversible ventricular fibrillation. Sheep are used for diverse cardiac studies including evaluation of valvular prostheses and research involving myocardial ischemia. In addition, the sheep heart is similar to that of humans both in terms of size and the composition of cardiomyocytes.5,6,18 The current study provides valuable insights regarding the use of infarction scar size in conjunction with left ventricular ejection fraction as a useful predictor of advanced left ventricular dysfunction.
Materials and Methods
This study was conducted in an AAALAC-accredited facility at the Icahn School of Medicine at Mt Sinai (New York, New York). All animals were maintained with husbandry practices consistent with the Guide for Care and Use of Laboratory Animals.10 The sheep were assigned to the study of myocardial infarction. All procedures were reviewed and approved by the IACUC at the Icahn School of Medicine at Mt Sinai. The procedures were conducted in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals.10 Castrated male Suffolk-cross sheep (n = 13; age, 6 mo; weight, 35 to 55 kg) were obtained from Barton's West End Farms (Oxford, NJ). The animals were screened at the vendor and were negative for Coxiella burnetti (Q fever), brucellosis, ovine progressive pneumonia, and Pasteurella spp. On arrival to our facility, all sheep received a complete physical exam and a health profile, including CBC and serum biochemistry analysis. The animals were received in good body condition, with a score of at least 3 on a scale of 5 and were free of clinical signs of disease. The sheep were group-housed and were acclimated for 2 to 7 d prior to use. They were fed twice daily with a species-specific diet (Rumilab 5508, LabDiet, Brentwood, MO) and were provided with water at all times. Room environmental parameters were monitored twice daily and maintained on a 12:12-h light:dark cycle, a temperature of 61 to 81 °F (16.1 to 27.2 °C), and a relative humidity of 30% to 70%.
Presurgical evaluation and preparation.
Sheep underwent MRI, ECG, hematology, serum biochemical analysis, and cardiac biomarker measurement at baseline and at 3 wk and 3 mo after infarction for verification of model creation and for tracking the progression of ischemic disease states. Food was withheld for 12 h before surgery; water was available without restriction. Sheep received buprenorphine (0.01mg /kg IM) and cefazolin (25 mg/kg IM) preemptively; ketamine (10 mg/kg IV) and midazolam (0.3 mg /kg IV) were used for induction of anesthesia. Each animal was intubated with a 7.0-mm endotracheal tube and an 18-gauge angiocatheter, which was placed in the cephalic vein of the right forelimb. Intravenous fluids consisting of 0.9% NaCl were administered at rate of 0.5 to 1 mL/kg/h. The left side of the chest was shaved and scrubbed by using chlorhexidine–impregnated sponges, with 70% alcohol as the final scrub. The animal was transported to the surgical suite, placed in right lateral recumbency, and ventilated mechanically. General anesthesia was provided with 2% isoflurane in oxygen (1 to 2 L/min). Equipment was installed to monitor SpO2 transcutaneously; end-tidal volume CO2 concentration, heart rate, respiratory rate, body temperature, and blood pressure were assessed by using a noninvasive blood pressure monitor. Surgical intervention was undertaken through left thoracotomy.
Surgical procedure to create small and large acute transmural myocardial infarctions.
The sheep were of similar health status, weight, and body condition score and were randomly allocated for infarct creation. Control animals remained untreated. At 1 h prior to infarction, central venous infusion of amiodarone (75 mg/kg/h IV) and lidocaine (35 mg/kg/h IV) was initiated to prevent cardiac arrhythmias. A left anterolateral thoracotomy incision was created in the fourth or fifth intercostal space. The left lung was collapsed. The pericardium was opened longitudinally posterior to the phrenic nerve. Next step was to identify the circumflex artery. The 2 primary branches of the coronary left circumflex artery are obtuse marginal (OM) 1 and 2, which supply blood to the lateral and posterior parts respectively, of the left ventricle. To create large infarcts, the proximal portion of both OM1 and OM2 was ligated by using 4-0 polypropylene suture, with close attention to ECG changes. For small infarcts, we ligated OM1 only. Acute ST elevation and discoloration of surrounding tissue confirmed the creation of an infarct. The heart was inspected for residual bleeding. The pericardium was loosely approximated by using 3-0 silk suture. A 28-French angled thoracostomy tube was placed in the left pleural space and connected to low, continuous wall suction through a drainage system (PleurEvac, Teleflex, Morrisville, NC) for 30 min. The thoracotomy incision was repaired routinely, after which suction was discontinued and the tube removed. After extubation, the sheep was transported to a recovery room for routine postoperative care including buprenorphine (0.01mg/kg IM twice daily for 3 d), flunixin meglumine (1 mg/kg IM once daily for 3 d), and cefazolin (25 mg/kg IM twice daily for 7 d).
Electrocardiography.
Six-lead electrocardiography (MAC 1600, Hewlett-Packard, Palo Alto, CA) was performed in all sheep at baseline and at 3 and 12 wk after infarction, with analysis of the waveform components and segment depression or elevation.
MRI analysis of cardiac hemodynamics.
Cardiac MRI (Sigma LX, GE Healthcare, Chalfont St Giles, United Kingdom) acquisition and analysis of cardiac hemodynamics were conducted on each sheep at baseline and 3 and 12 wk after infarction. The same anesthesia regimen was used for MRI and ECG as for surgery. The heart was imaged from apex to base in 10 imaging levels. Contrast-enhanced images were acquired approximately 10 min after bolus injection of gadolinium (0.20 mmol/kg IV). Cardiac cycles were evaluated by using manufacturer-provided software.
Left ventricular cardiac mechanics measures including volumes, dimensions, and ejection fraction were tracked over time by MRI at baseline and at 3 and 12 wk after myocardial infarction; in parallel, scar size was measured by using delayed contrast enhancement imaging. All global and regional hemodynamic measures, including stroke volume, cardiac index, end-diastolic volume index, end-systolic volume index, left ventricular radial contraction velocity, left ventricular fractional wall thickening, preload recruitable stroke work, and infarct size (% of left ventricular wall), were obtained directly from the MRI evaluation. Data were normalized according to body surface area, and analytic models including the modified Simpson rule were implemented to assess functional parameters. Quantitative evaluation of cardiac output was computed by multiplying stroke volume (area underflow curve within one cardiac cycle) by the corresponding heart rate of the animal.
Clinical evaluation.
All 13 sheep were received in good physical health. The animals received additional physical exams prior to sedation for surgery and other procedures at the various time points of the study; only healthy animals were cleared for surgery or the selected procedures. Biochemical tests for serum biochemistry and cardiac biomarkers (troponin, creatinine kinase) were conducted at baseline 3 wk and 3 mo after infarction. Although results were elevated at baseline, cardiac markers normalized by the 3-wk time point and for the remainder of the study. All 13 animals were monitored twice daily until 3 mo postsurgery. Parameters routinely monitored were level of activity; appetite; body temperature; heart rate and quality of beats as determined through auscultation, respiratory rate and integrity of breaths as determined through auscultation, and body condition score (scale of 1 [poor] to 5 [extremely obese]). Body condition scoring included assessment of spinous processes, fat cover, and muscle fullness along the dorsum of the animal; a score of 1 indicated sharp, prominent spinous processes without fat cover or muscle fullness, and scores 2 through 5 indicated decreasing sharpness of spinous processes and increasing fat cover and muscle fullness for grades 2, 3 and 4. Grade 5 represented spinous processes with thick fat cover and full muscles. A trained veterinary technician blinded to the study assessed body condition scores and monitored the animals daily. The animals were weighed at the time of sedation for the selected procedures and were found to conform to expected growth curves. The 3 animals that were used as controls had similar clinical health profiles and body condition scores to those of the experimental groups except for minor cardiac-unrelated findings that were recorded for the experimental groups.
Pathology.
For characterization of the damage and remodeling in the affected hearts, multiple samples from 2 affected hearts (one from the mild group and one from the severe group) were submitted for histopathology. The samples submitted from each heart included myocardium from a grossly unaffected area distant from the infarct, an infarcted area, and the border zone between the infarcted area and adjacent normal tissue. Sections were stained with hematoxylin and eosin, Masson trichrome, and picrosirius red (for collagen) according to standard protocols and were evaluated by an experienced comparative pathologist.
Statistical analysis.
All data are presented as mean ± SEM. The Kolmogorov–Smirnov test for normality was applied to each dataset. Single ANOVA was performed across time (for example, baseline, 3 wk, 12 wk) for each parameter, and then unpaired t tests (significance, P < 0.05) were used to assess differences within a group between any 2 time points or between groups (for example, mild compared with severe) at the same time point.
Results
Clinical features.
Except for minor, cardiac-unrelated clinical findings among some sheep in the experimental groups, all animals were otherwise clinically normal. Each animal's level of activity, body condition scores, and postures were normal. Heart and lung auscultation were murmur-free, with normal sinus rhythm and clear, acoustic and eupneic, respectively. Hematologic parameters were normal for both categories of infarcts at all time points, as were serum biochemical renal and hepatic test profiles, but cardiac enzyme biomarkers were elevated between baseline and 24 h after infarct creation. The presence of ST elevation and increased levels of cardiac enzymes, including troponin 1 and total creatine phosphokinase, within 24 h after infarction confirmed successful creation of the model. Cardiac enzyme concentrations normalized by the 3-wk time point. Scar size was increased at the 3-wk time point in both the small- and large-infarct groups and was further increased at 12 wk only for large infarcts. Left ventricular ejection fraction percentages decreased for each infarct group at the corresponding 3 wk and 12 wk’ time-points.
Mild infarction group.
One sheep developed an elevated temperature of 105.7 °F (40.9 °C) and exhibited reduced appetite at 2 wk after infarction. These conditions resolved after treatment with cefazolin (25 mg/kg IM twice daily for 7 d) and flunixin meglumine (1 mg/kg IM daily for 3 d).
Severe infarction group.
Three animals developed symptoms that were of clinical importance. One sheep had moderate seroma formation at the incision site that resolved within 14 d without additional treatment. Another developed pain with a mild limp in the left forelimb immediately after infarction; the limp and pain persisted through the first 5 d of the postoperative period. We suspected the condition was due to inflammation at the surgery site, and it resolved after treatment with flunixin meglumine (1 mg/kg IM daily for 3 d).
The third animal died within 24 h after infarction. The carcass was submitted for necropsy, and selected tissues were processed for gross and histopathologic evaluation. Pathologic evaluation of specimens from this animal revealed focally extensive, acute myocardial degeneration in the area of the infarct, moderate pulmonary edema and congestion, and subacute to chronic enteritis with intraluminal cestode. All 12 remaining animals were otherwise clinically normal and free of signs of cardiac disease for the remainder of the study. Selected parameters, such as cardiac biomarkers, were elevated at 24 h after model creation but had normalized for the 3- and 12-wk time points (Table 1).
Table 1.
Hematology and clinical chemistry in sheep with mild or severe left ventricular myocardial infarcts
| Baseline |
24 h after infarction |
3 wk after infarction |
12 wk after infarction |
|||||||
| Reference range | Mild | Severe | Mild | Severe | Mild | Severe | Mild | Severe | ||
| Hematology | ||||||||||
| Hgb | 8.5–14.8 g/dL | 12.7 ± 0.4 | 12.3 ± 0.7 | 8.9 ± 1.3 | 9.2 ± 0.7 | 10.2 ± 0.9 | 9.6 ± 0.3 | 10.3 ± 0.4 | 10.6 ± 0.6 | |
| WBC | 3.8–11.5 ×103 cells/μL | 6.3 ± 0.5 | 5.7 ± 0.7 | 8.5 ± 0.3 | 7.4 ± 0.8 | 6.0 ± 0.5 | 4.9 ± 0.9 | 5.2 ± 0.5 | 5.6 ± 0.6 | |
| RBC | 8.5–15.5 ×103 cells/μL | 11.2 ± 0.7 | 10.7 ± 1.3 | 8.6 ± 0.3 | 8.2 ± 0.9 | 9.3 ± 1.9 | 10.3 ± 1.4 | 9.9 ± 0.4 | 9.5 ± 1.0 | |
| Hct | 27%–45% | 36.7 ± 1.6 | 33.9 ± 1.5 | 26.8 ± 1.5 | 28.7 ± 1.1 | 29.6 ± 1.6 | 31.2 ± 1.8 | 32.3 ± 1.9 | 30.8 ± 1.2 | |
| Platelets | 150–750 ×103 cells/μL | 436.3 ± 66.0 | 426.8 ± 57.0 | 322.7 ± 77.0 | 356.8 ± 47.0 | 362.0 ± 52.0 | 432.0 ± 56.0 | 388.7 ± 40.0 | 322.7 ± 87.0 | |
| Neutrophils | 10.5%–55.0% | 30.1 ± 3.2 | 35.5 ± 2.9 | 40.7 ± 3.8 | 43.3 ± 4.2 | 28.9 ± 2.3 | 26.9 ± 3.4 | 29.8 ± 1.9 | 31.8 ± 3.1 | |
| Lymphocytes | 42%–75% | 61.8 ± 4.8 | 65.8 ± 3.2 | 73.8 ± 3.8 | 74.3 ± 2.7 | 63.7 ± 4.7 | 60.6 ± 5.9 | 56.3 ± 3.6 | 52.6 ± 2.9 | |
| Kidney function | ||||||||||
| BUN | 8.5–35 mg/dL | 14.6 ± 2.2 | 13.6 ± 1.9 | 16.5 ± 3.2 | 15.9 ± 3.2 | 14.2 ± 2.9 | 17.9 ± 4.8 | 17.3 ± 3.2 | 16.8 ± 4.0 | |
| Creatinine | 0.4–1.9 mg/dL | 0.75 ± 0.09 | 1.3 ± 0.2 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | |
| Liver function | ||||||||||
| ALT | 20–38 U/L | 26.1 ± 4.4 | 28.3 ± 3.2 | 33.2 ± 3.3 | 35.1 ± 2.8 | 27.9 ± 3.0 | 33.9 ± 3.9 | 25.1 ± 2.9 | 28.4 ± 4.0 | |
| ALP | 70–390 U/L | 165 ± 27 | 155 ± 37 | 288 ± 26 | 302 ± 30 | 187 ± 26 | 245 ± 47 | 193 ± 42 | 266 ± 36 | |
| Total bilirubin | 0.1–0.5 mg/dL | 0.14 ± 0.03 | 0.16 ± 0.04 | 0.43 ± 0.03 | 0.35 ± 0.05 | 0.34 ± 0.08 | 0.20 ± 0.09 | 0.22 ± 0.02 | 0.38 ± 0.03 | |
| Cardiac enzymes | ||||||||||
| Troponin I | < 0.03 ng/mL | 0.01± 0.00 | 0.01 ± 0.00 | 26.7 ± 2.8b | 26.7 ± 2.8c | 0.13 ± 0.00 | 0.09 ± 0.008 | 0.02 ± 0.00 | 0.02 ± 0.01 | |
| Total creatine phosphokinase I | 49–397 U/L | 64 ± 2.1 | 80 ± 3.8 | 2154 ± 344c | 6884 ± 286c | 369 ± 48a | 324 ± 38a | 123 ± 23 | 188 ± 48 | |
P < 0.05 compared with baseline value for this group.
P < 0.01 compared with baseline value for this group.
P < 0.001 compared with baseline value for this group.
Electrocardiography.
ST segment deviation occurred immediately after ligation of the respective circumflex vessels for both the mild (OM1) and severe (OM1 and 2) infarction groups; however ST segment elevation had returned to normal at the 3- and 12-wk time points (Table 2).
Table 2.
Electrocardiographic analysis after mild and severe myocardial infarction in sheep
| 24 h after infarction |
3 wk after infarction |
12 wk after infarction |
|||||
| Baseline | mild | severe | mild | severe | mild | severe | |
| PQ interval (ms) | 121 ± 6.2 | 141 ± 4.8a | 143 ± 7.2a | 133 ± 4.2a | 139 ± 3.4a | 127 ± 3.8a | 129 ± 4.6a |
| QRS complex (ms) | 29.6 ± 2.1 | 33.2 ± 1.5a | 38.6 ± 2.3a | 36.1 ± 2.7a | 38.9 ± 3.9a | 42.3 ± 2.1a | 55.2 ± 3.2a |
| QT interval (ms) | 325 ± 11.2 | 349 ± 10.2 | 366 ± 8.7a | 354 ± 9.8a | 372 ± 11.2a | 355 ± 6.9a | 359 ± 13.2a |
| ST deviation (mV) | –12.4 ± 2.2 | 242 ± 13.5b | 489 ± 15.9b | 71.6 ± 5.6c | 91.1 ± 8.7c | 41 ± 2.5a | 52 ± 4.7a |
P < 0.05 compared with baseline value for this group.
P < 0.01 compared with baseline value for this group.
P < 0.001 compared with baseline value for this group.
MRI.
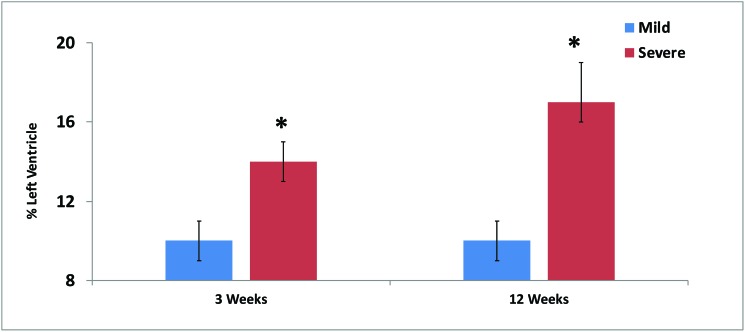
In the small-infarct group, the infarction scar involved 10% ± 1% of the left ventricle at both 3 and 12 wk after infarction, whereas the severe group demonstrated higher percentages at 3 wk 14 ± 1% and worsening to 17 ± 2% at 12 wk, (all P < 0.05; Figure 1). MR images of a typical enlarged ventricle due to severe infarction are shown in Figure 2.
Figure 1.
Infarct size (% of left ventricle) at 3 and 12 wk after ligation of 1 (mild) or 2 (severe) branches of the left circumflex artery in sheep. At both time points, severe infarcts were significantly (*, P < 0.05) larger than mild infarcts.
Figure 2.
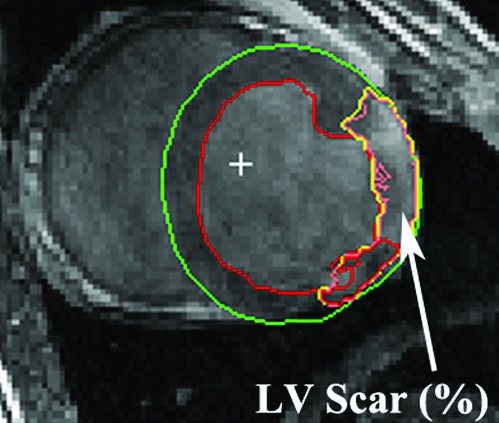
Left ventricular (LV) scar size was measured by using delayed contrast enhancement on MRI. The left ventricle and interventricular septum are outlined in green, the left ventricular lumen is outlined in red, the area between the green and red outlines represents the myocardium, and the infarcted area is outlined in yellow.
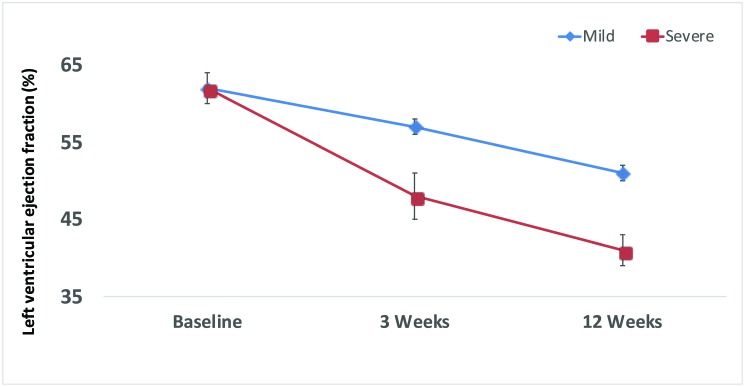
Changes in end-diastolic volume over time (baseline, 3 wk, 12 wk) were modest in the mild-infarction group (94 ± 4, 111 ± 11, 104 ± 4 mL) but were much more accentuated in the severe-infarction group (94 ± 6, 109 ± 5, 127 ± 7 mL; P < 0.05). End-systolic volumes followed a similar trend, with those of 35 ± 2, 48 ± 5, and 51 ± 3 mL in the small-infarct group compared with 36 ± 3, 56 ± 4, and 75 ± 5 mL in the large-infarct group. The group that received severe infarction had a much smaller functional ejection fraction over time compared with the mild group. Both groups had an ejection fraction of 62% ± 2% at baseline; but, at 3 wk, sheep that underwent mild infarction had an ejection fraction of 57% ± 1% compared with 48% ± 5% for the severe-infarction group; at 12 wk, these data were 51% ± 1% for the small-infarct sheep compared with severe 41% ± 2% for the large-infarct animals (P < 0.05, Figure 3).
Figure 3.
Left ventricular ejection fraction over time after mild compared with severe infarction.
Pathology.
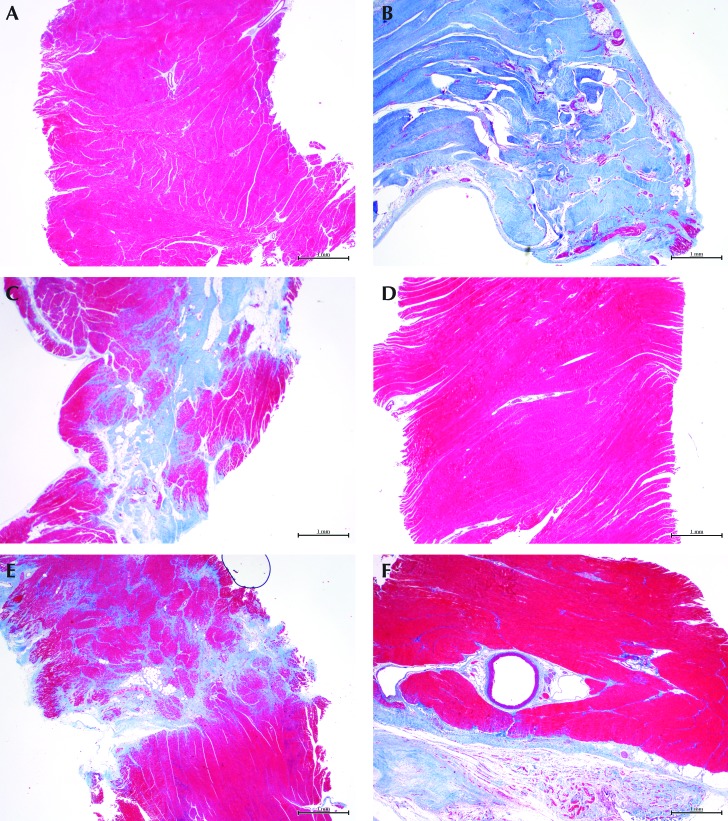
Myocardium in areas distant from the infarct showed no replacement fibrosis in either the mild- or severe-infarction sheep heart (Figure 4 A and D). The infarcted area from the severely affected heart revealed transmural and nearly complete replacement of the myocardium by dense fibrous tissue after staining with Masson trichrome (Figure 4 B). In contrast, the infarcted area from the mildly affected heart had only multifocal deposits of a mixture of dense and loose, poorly organized fibrous tissue (Figure 4 E). The border zone in the severely affected heart had multifocal replacement fibrosis, which was composed of loose bundles of collagen that dissected into adjacent muscle (Figure 4 C). The border zone in the mildly affected heart had a focal, distinctly demarcated area of fibrosis, which was composed of dense and loose collagen, within the myocardium (Figure 4 F).
Figure 4.
Panels A through C represent the severe-infarction group; panels D through F represent animals with mild infarcts. (A and D) Grossly unaffected areas of the myocardium away from the infarct show no replacement fibrosis. (B) The infarcted area from a severely affected heart has extensive and transmural replacement fibrosis, whereas (E) the mildly affected heart demonstrates multifocal distribution in the infarcted area. (C) The border zone from the severely affected heart shows multifocal and poorly demarcated replacement fibrosis, whereas that from (D) the mildly affected group has less replacement of muscle. Masson trichrome stain (red, muscle; blue, collagen); magnification, 20×.
Discussion
Sheep have been commonly used as an animal model for many cardiovascular studies.6,8 In this study, we evaluated the usefulness of scar size in combination with selected parameters to track the progression of left ventricular myocardial disease. Daily routine clinical evaluation of the animals was uninformative in the assessment of the onset and progression of disease. The observed signs of ill health were not directly related to myocardial disease and resultant dysfunction of the left ventricle. Although some parameters, such as cardiac biomarkers (troponin, total creatine phosphokinase) and electrocardiography (ST elevation), were useful indicators of acute ischemic insult, they were of less value for tracking the progression of left ventricular dysfunction. Scar size combined with the left ventricular ejection fraction was the most robust indicator of progressive failure of the myocardium and the resultant dysfunction.
Comparison of scar size and ejection fraction for the 3 and 12-wk time points revealed an inverse relationship for mild infarction (10% and 5%, respectively) and almost direct proportionality for severe infarction (14% and 15%, respectively). Comparative ratios of scar size to left ventricular ejection fraction for the 12-wk point were 1:4.7% for small infarcts and 1:2.2% for large infarcts. These values indicate the level of myocardial remodeling experience for each model and suggest the likelihood of survival of animals in each group. Although we expected both models to show a decline in myocardial function over time, this study provides precise correlation of scar size relative to the left ventricular ejection fraction, thus enabling researchers of ischemic heart disease to make more accurate decisions relative to experimental and humane endpoints in sheep. The data obtained from the severe model provided solid evidence of the functional deterioration of the left ventricle; researchers who use cardioprotective substances such as ACE inhibitors and β-blockers as a therapeutic component of these studies can now make incisive decisions regarding their use. Although these substances are potential confounders of studies of myocardial ischemia, our findings will help to delineate any actual contribution and potential adverse events due to their use. We conclude that the information yielded by this study has considerable prognostic value regarding the identification of the critical limits of left ventricular function after the creation of experimental models of small and large myocardial infarcts in sheep.
Although the large infarct model yielded robust data that verified significant left ventricular dysfunction, the sheep were euthanized for tissue harvest shortly after the 12-wk time point. Our findings define the critical limits of survival; However correlating scar size and ejection fractions with death as an endpoint would be informative.
Acknowledgments
We thank Dr Lupita Leyva-Grado and the staff of the Comparative Pathology Laboratory at Icahn School of Medicine at Mt. Sinai for assistance with histologic preparation of the specimens. We also thank Carlos J Rodriguez, Certified Veterinary Technician for technical support.
References
- 1.Abdulkadri AO, Tulloch-Reid MK, Francis DK, Gordon-Strachan GM, Younger-Coleman NO, Rocke KD, McFarlane SR, Cunningham-Myrie CA, Ferguson TS, Wilks RJ, Anderson SG. 2015. WHO/ISH total risk approach for primary prevention of cardiovascular disease shows greater decrease in costs for women but not the elderly in Jamaica. J Clin Epidemiol 68:994–1001. https://doi.org/10.1016/j.jclinepi.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Barquera S, Pedro-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. 2015. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res 46: 328–338. Doi.org/10.1016/j.arcmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 3.Cioe PA, Crawford SL, Stein MD. 2014. Cardiovascular risk-factor knowledge and risk perception among HIV-infected adults. J Assoc Nurses AIDS Care 25:60–69. https://doi.org/10.1016/j.jana.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon JA, Spinale FG. 2009. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2:262–271. https://doi.org/10.1161/CIRCHEART-FAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fallon AM, Goodchild TT, Cox JL, Matheny RG. 2014. In vivo remodeling potential of a novel bioprosthetic tricuspid valve in an ovine model. J Thorac Cardiovasc Surg 148:333–340.e1. https://doi.org/10.1016/j.jtcvs.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 6.Fathi E, Farahzadi R, Pishgahzadeh E. 2013. Hematological changes in an ovine model of acute myocardial infarction. Journal of Biology and Life Science 4:272–281. https://doi.org/10.5296/jbls.v4i2.3648. [Google Scholar]
- 7.Gallagher LG, Ray RM, Li W, Psaty BM, Gao DL, Thomas DB, Checkoway H. 2012. Occupational exposures and mortality from cardiovascular disease among women textile workers in Shanghai, China. Am J Ind Med 55:991–999. https://doi.org/10.1002/ajim.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerelli S, Van Steenberghe M, Patel M, Van Aerschot I, Boudjemline Y. 2013. Feasibility of creating a novel animal heart model to test transcatheter techniques for a cavocaval connection that mimics a Fontan completion. J Thorac Cardiovasc Surg 146:408–412. https://doi.org/10.1016/j.jtcvs.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 9.Gorman JH, 3rd, Gorman RC, Plappert T, Jackson BM, Hiramatsu Y, St John-Sutton MG, Edmunds LH., Jr 1998. Infarct size and location determine development of mitral regurgitation in the sheep model. J Thorac Cardiovasc Surg 115:615–622. [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 11.Kang MY, Kim HR. 2014. Association between voluntary/involuntary job loss and the development of stroke or cardiovascular disease: a prospective study of middle-aged to older workers in a rapidly developing Asian country. PLoS One 9:e113495 https://doi.org/10.1371/journal.pone.0113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendis S, Puska P, Norroving B, editors. 2011. Global atlas on cardiovascular disease prevention and control: policies, strategies, and interventions. Geneva (Switzerland): World Health Organization. [Google Scholar]
- 13.Moainie SL, Gorman JH, 3rd, Guy TS, Bowen FW, 3rd, Jackson BM, Plappert T, Narula N, St. John-Sutton MG, Narula J, Edmunds LH, Jr, Gorman RC. 2002. An ovine model of postinfarction dilated cardiomayopathy. Ann Thorac Surg 74:753–760. https://doi.org/10.1016/S0003-4975(02)03827-4. [DOI] [PubMed] [Google Scholar]
- 14.Moran AE, Roth GA, Narula J, Mensah GA. 2014. 1990–2010 global cardiovascular disease atlas. J. Glob Heart 9:3–16. https://doi.org/10.1016/j.gheart.2014.03.1220. [DOI] [PubMed] [Google Scholar]
- 15.Nag T, Ghosh A. 2013. Cardiovascular disease risk factors in Asian Indian population: a systematic review. J Cardiovasc Dis Res 4:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neto MG, Zwirtes R, Brites C. 2013. A literature review on cardiovascular risk in human immunodeficiency virus-infected patients: implications for clinical management. Braz J Infect Dis 17:691–700. PubMed https://doi.org/10.1016/j.bjid.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco S, Pistoia F, Degan D, Carolei A. 2015. Conventional vascular risk factors: their role in the association between migraine and cardiovascular diseases. Caphalalgia 35:146 –164. https://doi:10.1177/0333102414559551 [DOI] [PubMed] [Google Scholar]
- 18.Sellers R, Gloster J. 2008. Foot-and-mouth disease: a review of intranasal infection of cattle, sheep and pigs. Vet J 177:159–168. https://doi.org/10.1016/j.tvjl.2007.03.009. [DOI] [PubMed] [Google Scholar]