Abstract
A 1-y-old spayed female ferret (Mustela putorius furo) was referred for additional diagnostic evaluation after physical examination by the referring veterinarian revealed a cranial abdominal mass. The ferret had a 2-wk history of inappetence, weight loss, and lethargy. On presentation, the ferret was thin, and an approximately 3-cm mass was palpable in the cranial abdomen. No other abnormalities were noted. Abdominal ultrasonography confirmed the presence of a soft-tissue structure, with a moderate blood supply and mesenteric lymphadenopathy. Fine-needle aspirates of the mass were nondiagnostic. Exploratory laparotomy revealed multiple nodules and thickened tissues throughout the mesentery, a thickened and nodular pancreas, and a small amount of free abdominal fluid. Histopathology of mesenteric, lymphatic, and pancreatic biopsies revealed suppurative pancreatitis and necrotizing and pyogranulomatous mesenteric steatitis. Positive immunohistochemistry for feline coronavirus confirmed a diagnosis of ferret systemic coronavirus disease (FSCD). The ferret was treated medically with oral prednisolone, improved dramatically, and was still doing well 22 mo after diagnosis. Although FSCD has been reported extensively, this case is noteworthy for the presence of suppurative pancreatitis and the positive long-term outcome after corticosteroid therapy.
Abbreviations: FCV, ferret coronavirus; FSCD, ferret systemic coronaviral disease
Case Report
A 1-y-old spayed female ferret (Mustela putorius furo) was referred for a 2-wk history of inappetence, weight loss, and lethargy. The referring veterinarian had palpated a firm mass in the abdomen, and radiographs showed a small, circular opacity in the middle to upper right abdominal quadrant. No significant previous medical history was noted. The ferret had previously received one distemper vaccine from a pet store and was housed with 4 other ferrets. None of the other ferrets had any similar clinical signs.
Physical examination revealed a small, firm mass (diameter, approximately 3 cm) on the right side of the abdominal cavity. The ferret was of small body size and thin (body condition of 1 [maximum, 5]), weighing only 458 g. The rest of the physical examination was unremarkable. A blood sample was obtained from the cranial vena cava and submitted for a complete blood count (CBC) and plasma biochemistry profile. The biochemical profile revealed a mild hypoproteinemia (43 g/L; published reference interval, 51 to 75 g/L), mild hypochloremia (99 mmol/L; 121 to 124 mmol/L), mild hyponatremia (137 mmol/L; 142 to 156 mmol/L), mild hypocalcemia (1.8 mmol/L; 1.85 to 2.42 mmol/L), mildly increased ALT (325 U/L; 54 to 280 U/L), and increased creatine kinase (407 U/L; 74 to 294 U/L).5,14 The CBC count was unremarkable.
The ferret was sedated by using midazolam (0.2 mg/kg IM, Sandoz Standard, Boucherville, Quebec, Canada) and butorphanol (0.2 mg/kg IM, Sandoz Standard). Abdominal ultrasonography revealed a poorly demarcated, soft-tissue mass (diameter, 1 to 2 cm) with a moderate blood supply adjacent to the mesenteric lymph nodes. An ultrasound-guided fine needle aspirate was obtained, and smears were submitted for cytologic examination. Cytology was consistent with a reactive lymph node and normal cuboidal to columnar intestinal or related epithelial tissue, but a specific diagnosis could not be made from the cytologic aspirates. An exploratory laparotomy was recommended to remove the mass and obtain a diagnosis. Exploratory laparotomy was scheduled for the following week, and the ferret was sent home on sucralfate (20 mg/kg PO twice daily, Aptalis Pharma Canada, Mont Saint-Hilaire, Quebec, Canada) for gastric protection after the visit and prior to surgery.
Physical examination results prior to surgery were unchanged from the previous visit. The ferret was sedated by using midazolam (0.4 mg/kg IM) and hydromorphone (0.1 mg/kg IM, Sandoz Standard). Anesthesia was induced by using ketamine (10 mg/kg IV, Pfizer, Saint-Laurent, Quebec, Canada) and midazolam (0.5 mg/kg IV). The ferret was intubated with a 3-mm cuffed endotracheal tube and maintained on isoflurane (1% to 1.5%) in 100% oxygen. Intravenous fluid support (Plasmalyte-A, 10 mL/kg/h, Baxter, Mississauga, Ontario, Canada) was continued throughout anesthesia. A single, additional dose of hydromorphone (0.02 mg/kg IV) was administered intraoperatively for analgesia. Anesthesia was monitored by using capnography, electrocardiography, pulse oximetry, and indirect blood pressure measurements with a Doppler unit and a sphygmomanometer. Because the ferret was assessed as being hypotensive, constant-rate intravenous infusion of dopamine (Hospira Healthcare, Montreal, Quebec, Canada) was initiated at 10 μg/kg/min for the first hour and then reduced to 5 μg/kg/min for the remainder of the procedure. Dopamine was discontinued during recovery. Cefazolin (22 mg/kg IV, Hospira Healthcare) was administered approximately 20 min prior to surgery and was repeated 90 min later.
With the patient in dorsal recumbency, a ventral midline laparotomy was performed by using a routine approach, and the abdominal cavity was explored. The right and left lobes of the pancreas were severely hyperemic and irregular in texture, and multiple small, white, firm nodules were present in the surrounding mesenteric fat and were interpreted to be necrotic fat nodules. There were multiple adhesions and nodular lesions extending to the root of the mesentery, which complicated visualization of the adjacent mesenteric lymph node and mesenteric artery. Therefore, complete dissection and removal of the affected area was not feasible. A small amount of free abdominal fluid was noted, and a swab of the fluid was submitted for bacterial culture and sensitivity. Biopsies from the pancreas, thickened and nodular mesentery, mesenteric lymph node, and liver were submitted for histopathology. No foreign body was present in the stomach or intestines, and the rest of the abdominal organs appeared normal. The abdomen was copiously lavaged with warm sterile saline, and the body wall, subcutaneous tissue, and skin were closed in a routine manner. The ferret recovered from anesthesia without complication.
The ferret was hospitalized for 2 d postoperatively, during which time it began to eat and drink normally. During hospitalization, the ferret continued to receive sucralfate (20 mg/kg PO every 12 h), famotidine (0.5 mg/kg IV daily, Merck, Kirkland, Quebec, Canada), tramadol (2 mg/kg PO every 12 h, Sandoz, Boucherville, Quebec, Canada), and meloxicam (0.05 mg/kg PO every 12 h, Boehringer Ingelheim Vetmedica, Burlington, Ontario, Canada). While awaiting histopathology results, the ferret was discharged from the hospital, and the owners were instructed to continue treatment with tramadol, meloxicam, and sucralfate at the described dosages for 5 additional days. In addition, they were instructed to provide a high-protein, low-fat diet for the operated ferret and to keep it separated from the other ferrets.
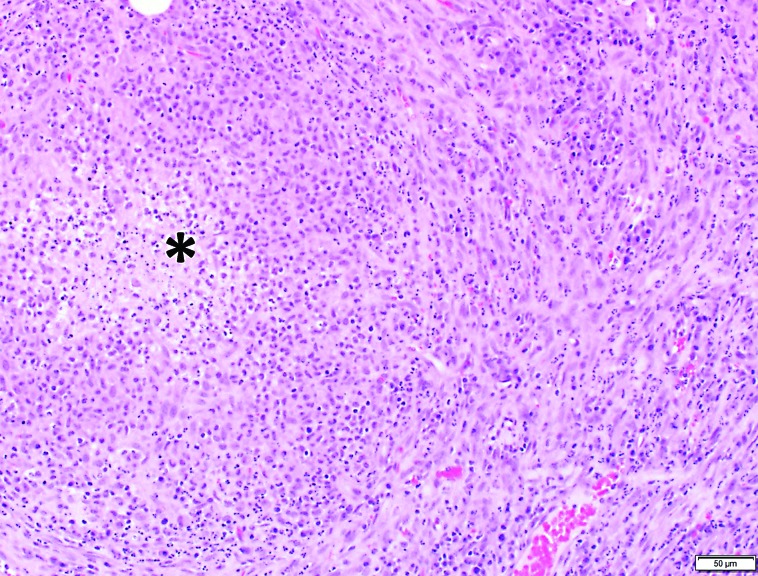
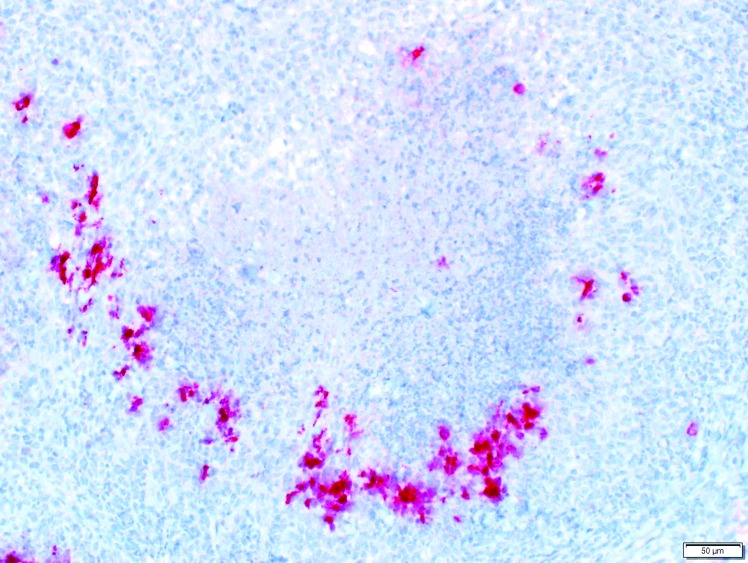
The swab of abdominal fluid yielded no aerobic bacterial growth after 72 h of culture. Microscopic examination of the biopsy samples revealed abnormalities consisting of multifocal necrotizing and pyogranulomatous steatitis of the peripancreatic adipose tissue and acute suppurative pancreatitis accompanied by peripancreatic fibrosis (Figure 1). Immunohistochemistry using FIPV3-70 monoclonal antibody against coronavirus antigen (clone FIPV3-70, Custom Monoclonals International, Sacramento, CA) was performed on samples of peripancreatic adipose and pancreas, and numerous macrophages in the pyogranulomatous inflammation surrounding the regions of fat necrosis stained intensely (Figure 2). A diagnosis of systemic infection with ferret coronavirus (FCV) infection was made in light of the positive immunohistochemical staining, combined with the typical gross and histopathologic lesions.
Figure 1.
Photomicrograph of peripancreatic adipose tissue. A core of necrotic debris and degenerate neutrophils (asterisk) is surrounded by plump, activated macrophages and a thin capsule of fibrous connective tissue mixed with neutrophils. Small amounts of granulation tissue are present. Hematoxylin and eosin stain.
Figure 2.
Photomicrograph of peripancreatic adipose tissue stained with FIPV3-70 monoclonal antibody. Macrophages at the periphery of the pyogranuloma show intense cytoplasmic immunostaining for FCV.
After diagnosis, the ferret was started on prednisolone (1 mg/kg PO daily, Rafter 8 Products, Calgary, Alberta, Canada). Follow-up examination at 1 mo after surgery revealed no change in the size of the mass in the abdomen. According to the owners, the ferret was eating and drinking well, was much more active, and had gained weight (body condition score, 2.5; weight, 490 g). The remainder of the physical examination was unremarkable. CBC analysis revealed thrombocytopenia (111 × 109/L; published reference interval, 250 × 109/L to 600 × 109/L).14 No petechial hemorrhages were noted on physical examination, but the owners were instructed to monitor the ferret for bleeding. The ferret was followed for 22 mo, during which time it remained on prednisolone therapy and ate, drank, and did well overall, although it did not gain body weight.
Discussion
This case documents the occurrence of suppurative pancreatitis and necrotizing mesenteric steatitis in a young ferret concurrent and likely due to FCV infection. Notable features of the case include the antemortem diagnosis by biopsy and histopathology of pancreatitis, a rare occurrence overall in ferrets, and more significantly, long-term survival after confirmation of the diagnosis of infection with FCV.
Ferret systemic coronaviral disease (FSCD) has been diagnosed in ferrets since 2004 and is characterized by pyogranulomatous inflammation of numerous organs.2,4,7,9 FSCD typically is seen in young ferrets and is most commonly a rapidly fatal disease.2,7,9 The disease has been likened to the dry form of feline infectious peritonitis.4,7,9,19 Feline infectious peritonitis is thought to result from a mutated form of feline enteric coronavirus, which occurs randomly in some cats. The exact mechanism underlying this mutation is still unknown. Although molecular evidence supports a similar mechanism for ferret coronaviruses, a clear association between viral recombination or mutation and increased pathogenicity leading to systemic coronaviral disease has not been demonstrated conclusively.10,13 FCV shares more than 96% of the distal one-third of its genome sequence with ferret enteric coronavirus, indicating that FCV is more closely related to feline enteric coronavirus than to other group 1 coronaviruses.19 Another study showed that ferret coronaviruses were closely related to mink coronaviruses and formed a separate clade of mustelid α-coronaviruses.10
FCV infection occurs primarily in ferrets younger than 1 y. The ferret we described here was young and displayed the commonly reported clinical signs of FSCD, including lethargy, anorexia, inability to gain weight, and palpable intraabdominal masses.9 In addition, diarrhea has been reported frequently and neurologic signs occasionally, but neither was seen in this ferret.8,9 These clinical signs are not specific for FSCD, with differential diagnoses including gastrointestinal foreign body and neoplasia, particularly lymphoma. Less-prevalent lesions documented in cases of FSCD include glomerulonephritis and ocular manifestations.6,11
Abdominal ultrasonography, possibly combined with ultrasound-guided fine needle aspiration, may be useful to identify the cause of an abdominal mass. The findings of the abdominal ultrasonography of the ferret we presented here were consistent with those of FSCD (for example, peritonitis, abdominal soft-tissue masses, abdominal lymphadenopathy).3 However, ultrasound-guided fine needle aspirates of the mesenteric lymph nodes and mass were nondiagnostic. In light of the history, clinical signs, and findings from ultrasonography, abdominal exploratory laparotomy was performed, and severe, granulomatous and necrotizing steatitis and acute suppurative pancreatitis were diagnosed through histologic examination of biopsies. FSCD was confirmed according to positive immunohistochemical staining using the monoclonal antibody FIPV3-70, which crossreacts with ferret coronavirus antigens.2,12,19
Pancreatitis is an unusual finding in ferrets but is often identified with FSCD. In a case series of 23 ferrets with FSCD, 3 had concomitant pyogranulomatous lesions in the pancreas.7 Another case series of 5 ferrets with FSCD included one animal with pancreatic pyogranulomatous and necrotic lesions.2 Pancreatitis has occasionally been reported after pancreatic surgery to remove insulinomas.15 Despite the severe mesenteric necrotizing and pyogranulomatous lesions, the CBC analysis was unremarkable in the presented ferret. In previously reported cases, inflammatory leukograms or anemia were present in some cases but overall were inconsistently present.2,7,11,16 Likewise, the results of the plasma biochemistry profile showed no suggestion of pancreatitis, but the diagnostic accuracy of measured plasma pancreatic enzymes is unknown in ferrets. In domestic carnivores, especially cats, increased pancreatic enzyme activities (that is, serum lipase and amylase) are not considered useful for the diagnosis of pancreatitis.1 Other descriptions of ferrets with FCV infection, and pancreatic lesions did not report elevated pancreatic enzymes.2,7 More sensitive tests such as pancreatic lipase immunoreactivity and serum trypsin-like immunoreactivity are highly species-specific immunoassays and are not commercially available for use in ferrets.
The primary treatment goal in FSCD is to control or decrease the inflammation that is typical of the disease. Therapeutic protocols are based on those used in cats with feline infectious peritonitis.9 The ferret we described was treated with prednisolone (1 mg/kg PO once daily) and, for gastric protection, was maintained on sucralfate (20 mg/kg PO twice daily). A low-fat feline diet was recommended, given the evidence of pancreatic disease. Prednisolone therapy generally is recommended only for the initial treatment period to decrease inflammation; however, because of the lack of immunomodulators and antiviral medications, prednisolone therapy was continued for 22 mo in the reported ferret. Ferrets tend to be relatively corticoid-resistant and seldom exhibit side effects after extended prednisolone therapy.17 Therefore, long-term prednisolone use may be beneficial in controlling the systemic inflammation seen with FSCD. Survival after the diagnosis of FSCD typically is low and only a few months in most ferrets.9 However, prolonged survival times of 1 to 2 y have been reported for FSCD in nonpeer-reviewed sources.18
Although regular follow-up visits for assessment of the ferret's physical condition and repletion of bloodwork were recommended, the owners complied with only one such visit, at 1 mo after surgery. At that point, the ferret was eating and drinking normally, showed a normal activity level, and had gained weight. Multiple phone conversations and emails with the owners for 22 mo postoperatively revealed that the ferret was still eating, drinking, and active at home. The ferret was still on prednisolone treatment at 22 mo postoperatively.
This case illustrates the successful management of a ferret diagnosed with FSCD and associated acute, suppurative pancreatitis for 22 mo by using prednisolone, sucralfate, and a low-fat, high-protein diet. FSCD should be part of the differential diagnosis for pancreatitis and runting in ferrets when these conditions occur concurrently with other, more commonly reported clinical signs and lesions associated with this disease.
References
- 1.Allison RW. 2012. Laboratory evaluation of the pancreas and glucose metabolism. p 425–440. In: Thrall M, Weiser G, Allison R, Campbell T, editors. Veterinary hematology and clinical biochemistry. 2nd ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 2.Autieri CR, Miller CL, Scott KE, Kilgore A, Papscoe VA, Garner MM, Haupt JL, Bakthavatchalu V, Muthupalani S, Fox JG. 2015. Systemic coronaviral disease in 5 ferrets. Comp Med 65:508–516. [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez E, Novellas R, Moya A, Espada Y, Martorell J. 2011. Abdominal radiographic and ultrasonographic findings in ferrets (Mustela putorius furo) with systemic coronavirus infection. Vet Rec 169:231–231. https://doi.org/10.1136/vr.d4705. [DOI] [PubMed] [Google Scholar]
- 4.Doria-Torra G, Vidaña B, Ramis A, Amarilla SP, Martínez J. 2016. Coronavirus infection in ferrets: antigen distribution and inflammatory response. Vet Pathol 53:1180–1186. https://doi.org/10.1177/0300985816634809. [DOI] [PubMed] [Google Scholar]
- 5.Fox J. 2014. Normal clinical and biological parameters. p 157–186. In: Fox J, Marini R, editors. Biology and Disease of the Ferret. 3rd ed Ames( IA): Wiley–Blackwell. [Google Scholar]
- 6.Fujii Y, Tochitani T, Kouchi M, Matsumoto I, Yamada T, Funabashi H. 2015. Glomerulonephritis in a ferret with feline coronavirus infection. J Vet Diagn Invest 27:637–640. https://doi.org/10.1177/1040638715599570. [DOI] [PubMed] [Google Scholar]
- 7.Garner MM, Ramsell K, Morera N, Juan-Sallés C, Jiménez J, Ardiaca M, Montesinos A, Teifke JP, Löhr CV, Evermann JF, Baszler TV, Nordhausen RW, Wise AG, Maes RK, Kiupel M. 2008. Clinicopathologic features of a systemic coronavirus-associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius). Vet Pathol 45:236–246. https://doi.org/10.1354/vp.45-2-236. [DOI] [PubMed] [Google Scholar]
- 8.Gnirs K, Quinton JF, Dally C, Nicolier A, Ruel Y. 2015. Cerebral pyogranuloma associated with systemic coronavirus infection in a ferret. J Small Anim Pract 57:36–39. https://doi.org/10.1111/jsap.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiupel M, Perpinan D. 2014. Viral diseases of ferrets. p 439–518. In: Fox J, Marini R, editors. Biology and disease of the ferret. 3rd ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 10.Lamers MM, Smits SL, Hundie GB, Provacia LB, Koopmans M, Osterhaus ADME, Haagmans BL, Raj VS. 2016. Naturally occurring recombination in ferret coronaviruses revealed by complete genome characterization. J Gen Virol 97:2180–2186. https://doi.org/10.1099/jgv.0.000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindemann DM, Eshar D, Schumacher LL, Almes KM, Rankin AJ. 2015. Pyogranulomatous panophthalmitis with systemic coronavirus disease in a domestic ferret (Mustela putorius furo). Vet Ophthalmol 19:167–171. https://doi.org/10.1111/vop.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez J, Reinacher M, Perpiñán D, Ramis A. 2007. Identification of group 1 coronavirus antigen in multisystemic granulomatous lesions in ferrets (Mustela putorius furo). J Comp Pathol 138:54–58. https://doi.org/10.1016/j.jcpa.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minami S, Kuroda Y, Terada Y, Yonemitsu K, Nguyen D Van, Kuwata R, Shimoda H, Takano A, Maeda K. 2016, Detection of novel ferret coronaviruses and evidence of recombination among ferret coronaviruses. Virus Genes 52:858–862. https://doi.org/10.1007/s11262-016-1365-3 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrisey JK. 2013. Ferrets. p 564–594. In: Carpenter J, Marion C, editors. Exotic animal formulary, 4th ed. St Louis (MO): Elsevier. [Google Scholar]
- 15.Perpinan D, Johnson-Delaney CA. 2017. Disorders of the digestive system and liver. p 159–190. In: Johnson-Delaney C, editor. Ferret medicine and surgery. Boca Raton (FL): CRC Press. [Google Scholar]
- 16.Perpiñán D, López C. 2008. Clinical aspects of systemic granulomatous inflammatory syndrome in ferrets (Mustela putorius furo). Vet Rec 162:180–183. https://doi.org/10.1136/vr.162.6.180. [DOI] [PubMed] [Google Scholar]
- 17.Petritz OA, Chen S, 2018. Therapeutic contraindications in exotic pets. Vet Clin North Am Exot Anim Pract 21: 327–340. https://doi.org/10.1016/j.cvex.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Swisher S, Lennox AM. 2017. Disorders of the haemic, immunological and lymphatic systems. p 237–258. In: Johnson-Delaney C, editor. Ferret medicine and surgery. Boca Raton (FL): CRC Press. [Google Scholar]
- 19.Wise AG, Kiupel M, Garner MM, Clark AK, Maes RK. 2010. Comparative sequence analysis of the distal one-third of the genomes of a systemic and an enteric ferret coronavirus. Virus Res 149:42–50. https://doi.org/10.1016/j.virusres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]