Abstract
Labyrinthitis is inflammation of the membranous and bony labyrinth of the inner ear. Typical portals of entry include hematogenous spread from the cochlear vasculature, passage of otitis media pathogens through the round window, and most commonly, meningogenic spread from the subarachnoid space. The sequela of chronic inner ear inflammation is labyrinthitis ossificans, in which inner ear structures are replaced by fibrous and osseous tissues. Labyrinthitis in humans has been reported concurrently with infection due to various viruses (for example, varicella–zoster, measles, mumps) and bacteria (for example, Treponema pallidum, Streptococcus pneumoniae) and may be associated with vertebrobasilar ischemia and meningitis. Profound sensorineural hearing loss is a common, serious complication of this disease. Here, we report a case of labyrinthitis ossificans in a cynomolgus macaque (Macaca fascicularis) with a potential infectious etiology. Historically, this animal had an indwelling femoral intravenous catheter for more than 4 y. He presented with a right-sided head tilt and incoordination of 2 mo duration. The macaque was treated with NSAID and antibiotics, which corrected the incoordination but not the head tilt. MRI revealed right-sided labyrinthitis, and euthanasia was elected due to clinical signs that were refractory to treatment. Gross pathology was unremarkable, but histopathology revealed chronic labyrinthitis ossificans with local fibroplasia and vestibuloauditory neuritis. We describe here the clinical features, imaging, and histologic lesions of labyrinthitis in a macaque.
Case Report
An 18-y-old cynomolgus macaque that had been group-housed presented with a right-sided head tilt and incoordination in January 2016, when it was singly housed for observation. This animal was managed in accordance with federal, state, and institutional guidelines, at an AAALAC-accredited facility. Treatment with ceftiofur and meloxicam resolved the incoordination, but the macaque continued to present with head tilt. MRI (Magnetom Skyra 3-T Scanner, Siemens Medical Solutions, Malvern, PA) of the brain and temporal bones conducted in March 2016 revealed abnormal hypointense signal on a T2-weighted sequence and abnormal enhancement on a postcontrast T1-weighted sequence in the right inner ear structures, presumed to be right-sided labyrinthitis (Figures 1 and 2). The macaque had positive antibody titers to herpes B virus and measles (due to previous vaccination) in April 2016. The animal previously had an indwelling femoral catheter with a subcutaneous port for more than 4 y and was part of an IACUC-approved experimental study that required cognitive tasks. Blood reports from January through April 2016 demonstrated moderately elevated liver enzymes (ALP, 496 U/L [reference, 89 to 166 U/L]; ALT, 213 U/L [reference, 23 to 61 U/L]) and low platelet numbers (230,000/μL [reference, 300 to 512/μL]). Euthanasia (sodium pentobarbital overdose) was elected in light of the treatment-refractory clinical signs and poor long-term prognosis.
Figure 1.
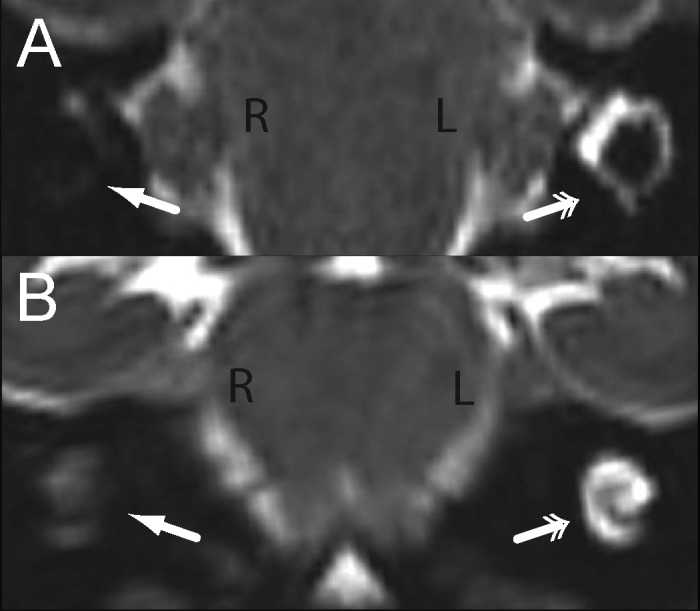
Labyrinthitis ossificans, cynomolgus macaque, MRI. High-resolution, 0.5-mm isotropic T2-weighted (A) axial and (B) coronal images in radiologic convention demonstrate abnormal hypointensity of the (A) vestibule and horizontal semicircular canal and (B) cochlea of the right ear (single arrows) compared with the normal hyperintense appearance of these structures in the left ear (double arrows).
Figure 2.
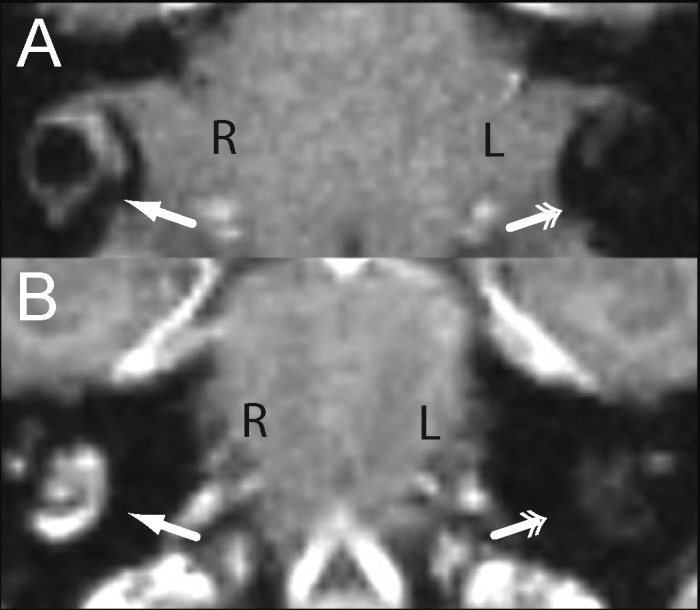
Labyrinthitis ossificans, cynomolgus macaque, MRI. High-resolution, 0.5-mm isotropic postcontrast T1-weighted (A) axial and (B) coronal images in radiologic convention demonstrate abnormal enhancement of the vestibule and horizontal semicircular canal and cochlea of the right ear (single arrows) compared with the normal nonenhancing appearance of these structures in the left ear (double arrows).
At necropsy, the right cranial and middle lung lobes were tightly attached to the thoracic wall by fibrous adhesions. Both caudal lung lobes were attached to the diaphragm, and there were fibrous adhesions between the left lung lobes. The vertebral body of the eighth thoracic vertebrae was thickened. The liver had several fluid-filled cysts (diameter, 1 to 3 mm) randomly scattered throughout the parenchyma. Gross examination of 4 serial sections of both petrous bones after fixation and decalcification was normal.
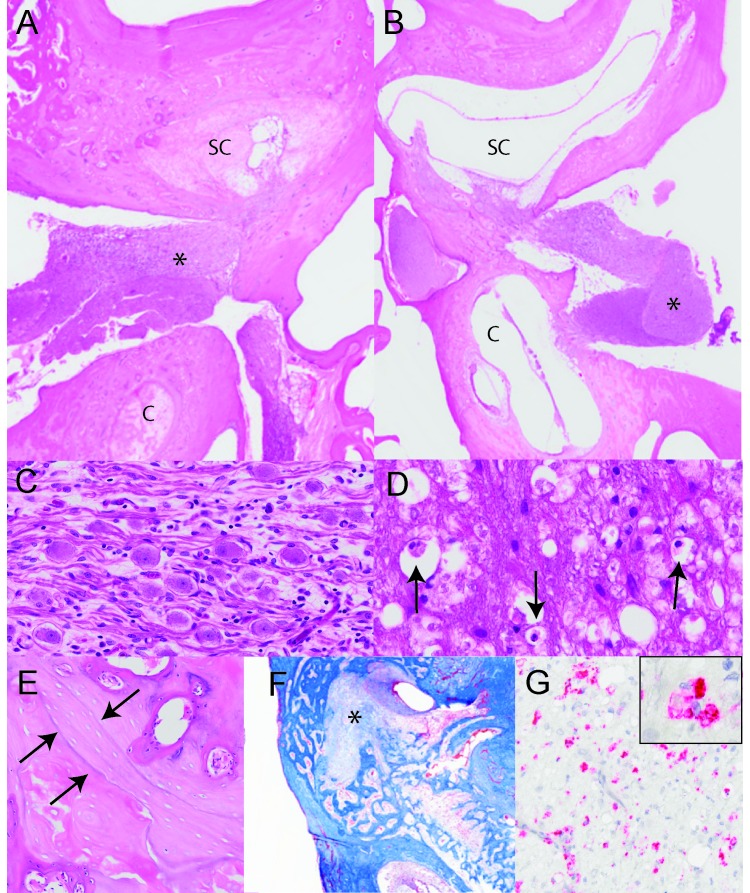
Histopathology of the right petrous bone revealed the cochlea (scala tympani, scala media, scala vestibule, and organ of Corti) and vestibule (semicircular canals, utricle, and saccule) were distorted by fibroplasia and fibrosis, bony metaplasia, and few histiocytes. (Figure 3 A). In contrast, the contralateral inner ear appeared histologically normal (Figure 3 B). Fibrous tissue also extended into the vestibulocochlear nerve of the affected inner ear (Figure 3 C). Occasional axonal sheaths within the nerve were vacuolated and contained shrunken axons or macrophages (Figure 3 D, arrows). The surrounding bone tissue had an irregular surface, basophilic reversal lines, and multiple trabeculae that extended into the effaced cochlea (Figure 3 E). Masson trichrome staining confirmed the presence of mature fibrous connective tissue (Figure 3 F). The affected structures were negative for CD3 (dilution, 1:300 dilution; A0452, clone F7.2.38, Dako, Carpinteria, CA), CD20 (1:1500; M0755, clone L26, Dako), and myeloperoxidase (1:200; NCL-L-MYELO, clone 5D11, Leica Biosystems, Buffalo Grove, IL). However, a cluster of neutrophils was present in the cochlea of a single hematoxylin- and eosin-stained section (not shown). Within the affected area, the cochlea was replaced by loose connective tissue and a few HAM56-positive macrophages (1:50; 279M-16, clone HAM56, Sigma, St Louis, MO), which indicated chronic inflammation (Figure 3 G). All immunohistochemistry was performed on an automated slide stainer (Leica Bond, Leica Biosystems) by using Vector Red chromogen (Vector Laboratories, Burlingame, CA). Images were captured by using a VS120 imaging system (Olympus, Tokyo, Japan).
Figure 3.
Labyrinthitis ossificans, cynomolgus macaque. (A) Subgross image of the structures of the inner ear. The semicircular canals (SC) and cochlea (c) of the right side are infiltrated by fibrous connective tissue and surrounded by remodeled bone. The vestibulocochlear nerve (*) is vacuolated. Hematoxylin and eosin stain; magnification, 0.75×. (B) The semicircular canals (SC) and cochlea (c) of the left inner ear are clear, with appropriate placement of both the boney and membranous components. Hematoxylin and eosin stain; magnification, 0.75×. (C) A higher magnification of the affected vestibulocochlear nerve ganglion shows infiltration of lymphocytes, macrophages and fibroblasts. Hematoxylin and eosin stain; magnification, 10×. (D) Axonal sheaths are often vacuolated and contain shrunken axons or macrophages (arrows). Hematoxylin and eosin stain; magnification 40×. (E) Bone surrounding the cochlea is irregular and forms basophilic lines, which indicates remodeling. Hematoxylin and eosin stain; magnification, 8×. (F) Masson trichrome staining confirms the mature fibrous connective tissue invasion of the semicircular canals (*); magnification, 0.75×. (G) Fibrous connective tissue within the cochlea was immunopositive for HAM56; magnification, 40×. Inset is an enlarged section of the fibrous connective tissue, showing cellular detail.
Liver histology comprised a mild neutrophilic and lymphocytic hepatitis, which might have contributed to the elevated liver enzymes. The histology of the bone marrow, meninges, and vestibular nucleus was within normal limits. Fibrous adhesions involved the lung lobes, but no histologic evidence of an active inflammatory process involving the lungs or pleura was present. The number of platelets in the peripheral blood was slightly below the reference interval but not sufficient to cause clinical thrombocytopenia.
Discussion
The inner ear has cochlear and semicircular canals that are composed of a membranous labyrinth within a bony labyrinth. Labyrinthitis is inflammation of the inner ear and can involve the membranous or bony labyrinth or both structures. It is a rare disease of the inner ear that can result from infectious, toxic, or traumatic causes. In fact, many labyrinthitis cases in humans have been associated with several viruses, whereas others have been attributed to previous upper respiratory infections. Viral causes of labyrinthitis include herpes simplex virus, cytomegalovirus, mumps virus, measles virus, and varicella–zoster virus.4,13,15 Bacterial otitis infections in adult humans are rare in the postantibiotic era, but suppurative meningitis and otitis can lead to labyrinthitis after infection with Streptococcus pneumoniae, Treponema pallidum, or Bartonella henselae.9,10,18 Regardless of the underlying cause, the main clinical signs and symptoms in humans include hearing loss and vertigo or head tilt.16 In addition, labyrinthitis has been reported in a variety of veterinary species. A litter of Doberman pinschers developed congenital labyrinthitis after suspected viral infection, and a flock of turkey poults developed labyrinthitis after infection with Salmonella enterica arizonae.6,14 Furthermore, Cryptococcus neoformans has been implicated in the formation of labyrinthitis in cats.12 Moreover, labyrinthitis has been reported in mice and guinea pigs, 2 species frequently used to model inner ear inflammation.1,5
Labyrinthitis ossificans is the sequela of labyrinthitis and comprises progressive fibrosis and ossification of the granulation tissue within the membranous labyrinth. The cochlear fibrosis and ossification usually starts at the base of the cochlea, near the round window.19 In experimental Streptococcus pneumoniae-induced meningitis in gerbils, fibrosis can be observed as early as 2 wk after infection, and ossification occurs within 2 mo.11 In another study using Mongolian gerbils, intrathecal injection of Streptococcus pneumoniae induced osteoid deposition and mineralization as early as 3 d.17 Other factors that may lead to the progression of labyrinthitis ossificans include trauma, neoplasia, and allergies.19 Depending on the inciting cause, the severity of the fibrosis and ossification differ: S. pneumoniae causes particularly pronounced ossification after suppurative labyrinthitis. The incidence of labyrinthitis in humans has increased recently with cochlear implants.2,8
Why the macaque in this case developed unilateral disease is unclear. Although he was positive for herpes B virus, an agent associated with immunosuppression,7 he was able to mount an immune response, suggesting that he was not immunosuppressed. The macaque had a long-term indwelling catheter, which may have served as an inciting nidus of bacterial infection. In the current case, a venous catheter extended from the left femoral vein to the heart, which was removed several months prior to necropsy and was not available for culture at that time. However, no evidence of inflammation involving the procedure site or heart or of thrombus was observed. Several cases of unilateral labyrinthitis with suspected viral etiology have been reported, including one of a 5-y-old girl who developed unilateral labyrinthitis after a viral upper respiratory tract infection.3 Therefore, our macaque might have developed this condition due to viral infection or secondary bacterial infection due to immunosuppression. However, no overt signs of systemic disease or infection were present at the time of necropsy. In addition, although mild hepatitis was present on histologic examination, the meninges, vestibular nucleus, and bone marrow were all within normal limits.
In summary, labyrinthitis should be considered in macaques that present for ataxia or head tilt, especially when the animals have been previously exposed to radiation or had an indwelling catheter or another source of chronic immune system stimulation. This case report describes the clinical and radiologic findings, histology, and immune cell response associated with chronic inflammation of the inner ear.
Acknowledgments
We thank the Comparative Pathology Laboratory for their histotechnical support, which is funded by the National Center for Advancing Translational Sciences (NCATS), NIH, through grant award numbers UL1TR001420 and R37 DA010584 (to MAN) and supported the import and maintenance of this animal. Partial funding also was provided by the Wake Forest School of Medicine ARP and its NIH-sponsored T32 fellowship grant (T32OD010957).
References
- 1.Barkdull GC, Hondarrague Y, Meyer T, Harris JP, Keithley EM. 2007. AM-111 reduces hearing loss in a guinea pig model of acute labyrinthitis. Laryngoscope 117:2174–2182. [DOI] [PubMed] [Google Scholar]
- 2.Benatti A, Castiglione A, Trevisi P, Bovo R, Rosignoli M, Manara R, Martini A. 2013. Endocochlear inflammation in cochlear implant users: case report and literature review. Int J Pediatr Otorhinolaryngol 77:885–893. [DOI] [PubMed] [Google Scholar]
- 3.Cohen BE, Durstenfeld A, Roehm PC. 2014. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis LE, Johnsson LG, Kornfeld M. 1981. Cytomegalovirus labyrinthitis in an infant: morphological, virological, and immunofluorescent studies. J Neuropathol Exp Neurol 40:9–19. [PubMed] [Google Scholar]
- 5.Esaki S, Goshima F, Kimura H, Ikeda S, Katsumi S, Kabaya K, Watanabe N, Hashiba M, Nishiyama Y, Murakami S. 2011. Auditory and vestibular defects induced by experimental labyrinthitis following herpes simplex virus in mice. Acta Otolaryngol 131:684–691. [DOI] [PubMed] [Google Scholar]
- 6.Forbes S, Cook JR., Jr 1991. Congenital peripheral vestibular disease attributed to lymphocytic labyrinthitis in 2 related litters of Doberman pinscher pups. J Am Vet Med Assoc 198:447–449. [PubMed] [Google Scholar]
- 7.Huff JL, Barry PA. 2003. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg Infect Dis 9:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itayem DA, Sladen D, Driscoll CL, Neff BA, Beatty CW, Carlson ML. 2017. Cochlear implant associated labyrinthitis: a previously unrecognized phenomenon with a distinct clinical and electrophysiological impedance pattern. Otol Neurotol 38:e445–e450. [DOI] [PubMed] [Google Scholar]
- 9.Kantas I, Katotomichelakis M, Vafiadis M, Kaloutsa ZV, Papadakis CE. 2009. Serous labyrinthitis as a manifestation of cat scratch disease: a case report. J Med Case Rep 3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr AG, Smyth GD, Landau HD. 1970. Congenital syphilitic labyrinthitis. Arch Otolaryngol 91:474–478. [DOI] [PubMed] [Google Scholar]
- 11.Nabili V, Brodie HA, Neverov NI, Tinling SP. 1999. Chronology of labyrinthitis ossificans induced by streptococcus pneumoniae meningitis. Laryngoscope 109:931–935. [DOI] [PubMed] [Google Scholar]
- 12.Paulin J, Morshed M, Armien AG. 2012. Otitis interna induced by Cryptococcus neoformans var. grubii in a cat. Vet Pathol 50:260–263. [DOI] [PubMed] [Google Scholar]
- 13.Rubin S, Eckhaus M, Rennick LJ, Bamford CG, Duprex WP. 2015. Molecular biology, pathogenesis, and pathology of mumps virus. J Pathol 235:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivaprasad HL, Cortes P, Crespo R. 2006. Otitis interna (labyrinthitis) associated with Salmonella enterica arizonae in turkey poults. Avian Dis 50:135–138. [DOI] [PubMed] [Google Scholar]
- 15.Stokroos RJ, Albers FW, Schirm J. 1998. The etiology of idiopathic sudden sensorineural hearing loss. Experimental herpes simplex virus infection of the inner ear. Am J Otol 19:447–452. [PubMed] [Google Scholar]
- 16.Thompson TL, Amedee R. 2009. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J 9:20–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Tinling SP, Nabili V, Brodie HA. 2005. Fine structure histopathology of labyrinthitis ossificans in the gerbil model. Ann Otol Rhinol Laryngol 114:161–166. [DOI] [PubMed] [Google Scholar]
- 18.Vyas S, Bhatia V, Panda NK, Singh P, Khandelwal N. 2016. Labyrinthitis ossificans after meningitis: Superiority of high-resolution magnetic resonance imaging in demonstration of disease extent compared to high-resolution computed tomography. J Neurosci Rural Pract 7:327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu HX, Joglekar SS, Paparella MM. 2009. Labyrinthitis ossificans. Otol Neurotol 30:579–580. [DOI] [PubMed] [Google Scholar]