Abstract
Target spot, a recently observed citrus disease that is caused by Pseudofabraea citricarpa, can cause substantial economic losses in citrus production. In this study, a 797 bp marker specific to Ps. citricarpa was identified via random amplified polymorphic DNA (RAPD) technique. The primer pair Pc-SFP/Pc-SRP, which was designed from RAPD amplicons, was utilized as a sequence-characterized amplified region (SCAR) marker. This marker identified Ps. citricarpa with a single and distinct band of 389 bp but did not amplify DNA from other tested fungal species. The PCR assay was highly sensitive to the target DNA at picogram levels and could reliably amplify Ps. citricarpa sequences with the Pc-SFP/Pc-SRP primer pair. The SCAR marker that was identified in the present study can facilitate rapid decision-making and precise disease forecasting and management.
1. Introduction
Target spot, a new leaf-spotting disease of citrus first described in China, has caused considerable economic losses in local citrus production [1]. The target spot pathogen was identified as Cryptosporiopsis citricarpa based on Koch's postulates and morphological and molecular phylogenetic characteristics [1] and then reclassified to the monotypic genus Pseudofabraea [2]. This fungal pathogen could infect both Satsuma mandarin (Citrus unshiu) and kumquat (Fortunella margarita) in orchards [1]. Unlike diseases that usually occur on the young leaves of citrus during warm and humid seasons, target spot occurs during late winter and early spring and causes severe leaf spotting or even defoliation (Figure 1). However, target spot is difficult to diagnose accurately based solely on experience and subjective judgment. Once the disease becomes epidemic, fungicide application was difficult to control effectively. Therefore, monitoring the disease in the citrus orchards plays a key role in effective control of target spot.
Figure 1.

Symptoms of citrus target spot caused by Pseudofabraea citricarpa.
Citrus infected by Ps. citricarpa does not show any symptoms at early stages of invasion, which is difficult to determine the primary infection potential, and early molecular detection of this pathogen. In recent decades, molecular methods, particularly nucleic acid-based methods, have been applied to identify and detect plant pathogens; these methods can overcome uncertain diagnosis or pathogen taxonomy and enable the rapid and accurate detection and quantification of pathogens [3, 4]. Sequence-characterized amplified region (SCAR), a kind of reliable PCR-based molecular marker, has been developed to detect plant pathogens, such as Magnaporthe grisea [5], Puccinia striiformis [6], and Fusarium oxysporum [7]. The use of the SCAR markers simplifies identification and promotes the development of prevention strategies that are superior to traditional methods.
In the current study, we developed a useful SCAR marker via the simple random amplified polymorphic DNA (RAPD) technique [8, 9] and establish a sensitive and simple PCR-based method for the rapid molecular identification and differentiation of Ps. citricarpa from other fungal pathogens of citrus.
2. Materials and Methods
2.1. Fungal Pathogens
Ps. citricarpa strains were isolated from citrus leaves or shoots with disease symptoms. The diseased plant materials were obtained from local orchards. Five fungal pathogens of citrus leaves were collected from Citrus Research Institute, Southwest University. The pathogens included Alternaria alternata, Colletotrichum gloeosporioides, Diaporthe citri, Botrytis cinerea, and Phyllosticta citricarpa. Three fungal pathogens of citrus fruit were collected from the College of Food Science, Southwest University. The pathogens included Oospora citri-aurantii, Penicillium italicum, and Pe. digitatum. Except for Ps. citricarpa, which was cultured at 20°C, all tested strains were cultured at 25°C on potato dextrose agar media until the mycelium covered approximately three-quarters of the plates.
2.2. DNA Isolation
Approximately 1 g of fresh fungal mycelium and approximately 0.3 g of field-infected citrus tissues were snap-frozen in liquid nitrogen, and ground to a fine powder with a mortar and pestle. Genomic DNA was extracted via the CTAB method [10]. DNA samples were dissolved in 0.1x TE buffer, quantified, and adjusted to a final concentration of 100 ng/μL for PCR amplification.
2.3. RAPD Analysis
RAPD amplification was conducted with 15 μL of reaction mixture with 40 random primers (Table S1). Each reaction tube contained 100 ng of DNA, 1 U of rTaq DNA polymerase (Takara Co., China), 100 μmol/L of each dNTP, 1.5 μL of 10x Taq DNA polymerase buffer with 1.5 mmol/L MgCl2, and 1.0 μL of random primer (10 mmol/L). PCR amplification was performed in a DNA thermocycler (Bio-Rad S1000™) with the following conditions: 94°C for 5 min, 35 cycles at 94°C for 30 s, 36°C for 30 s, and 72°C for 90 s with a final extension at 72°C for 10 min. The amplified PCR products were resolved on 1.5% agarose gels, followed by GoldView staining and visualization under UV light.
2.4. Amplicon Cloning and Sequencing
The amplicon, which was specific to Ps. citricarpa but absent in the other eight species, was identified and purified with a gel extraction mini kit (Tiangen Biotech Co., China). The purified DNA products were cloned into a pGEM-T Easy vector (Promega Co., USA) and introduced into the competent cells of Escherichia coli strain DH5α in accordance with manufacturer's instructions. Subsequently, the positive clones were sequenced by Shanghai Biotech Co.
2.5. Primer Design and Establishment of Detection System
Based on the sequenced RAPD amplicons, the specific SCAR primers (Table 1) Pc-SFP (specific forward primer) and Pc-SRP (specific reverse primer) were designed using Primer Premier 6 software (Premier Biosoft International, USA). A 20 μL reaction system was developed to simplify the detection system. The system contained 10 μL of Premix Taq Version 2.0 plus dye (Takara Co., China), 1.0 μL of forward primer (10 mmol/L), 1.0 μL of reverse primer (10 mmol/L), and 100 ng of genomic DNA. Amplifications were conducted in a DNA thermocycler (Bio-Rad S1000) with the following conditions: 94°C for 5 min, 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s with a final extension at 72°C for 10 min.
Table 1.
Pseudofabraea citricarpa-specific SCAR primers designed from sequenced RAPD amplicons.
| RAPD primer | SCAR marker | Number of base pairs (bp) | Nucleotide sequence | G + C content (%) | Annealing temperature |
|---|---|---|---|---|---|
| CS38 | Pc-SFP | 20 | 5′-GCTGATTGAGTGCCCATAGA-3′ | 50 | 55°C |
| Pc-SRP | 22 | 5′-ACTCCAACCAACGAGATGATAG-3′ | 45 |
2.6. Specificity and Sensitivity of the SCAR Marker
All DNA samples, including those from six foliar pathogens and three postharvest pathogens of citrus, were amplified via PCR with the Pc-SPF and Pc-SPR primers (Table 1) to verify the specificity of the SCAR marker. To test detection sensitivity, 50 ng/μL to 5 fg/μL serial dilutions of the DNA of Ps. citricarpa strain were used as the DNA templates for PCR amplification under the above thermocycling conditions.
2.7. Validating SCAR Marker in Citrus Tissues Collected from Orchards
To confirm the effectiveness of the primer pairs Pc-SPF and Pc-SPR for detecting Ps. citricarpa in the field, the primers were used to amplify DNA samples from symptomatic and asymptomatic citrus tissues that were collected diseased orchards. DNA was extracted from leaves and shoots in accordance with the method described above. Ps. citricarpa DNA was used as positive control, and the DNA of healthy citrus leaves obtained from greenhouse were used as negative control. PCR amplification was performed with the primers Pc-SPF and Pc-SPR under the above conditions.
3. Results
3.1. Screening and Sequencing of RAPD Markers for Ps. citricarpa
Of the 40 screened RAPD primers, CS38 (5′-TGCTGACGAC-3′) consistently amplified a single intense band of over 750 bp from Ps. citricarpa. This band was absent in the eight other pathogens (Figure 2). This differential band was selected to develop a species-specific SCAR marker and subsequently was cloned and sequenced. The sequencing result showed that the length of the specific amplicon was 797 bp with 50% G + C content (A = 188, T = 212, C = 176, and G = 221) (Figure 3). BLAST result revealed that no significant similar sequence had been found at different levels.
Figure 2.
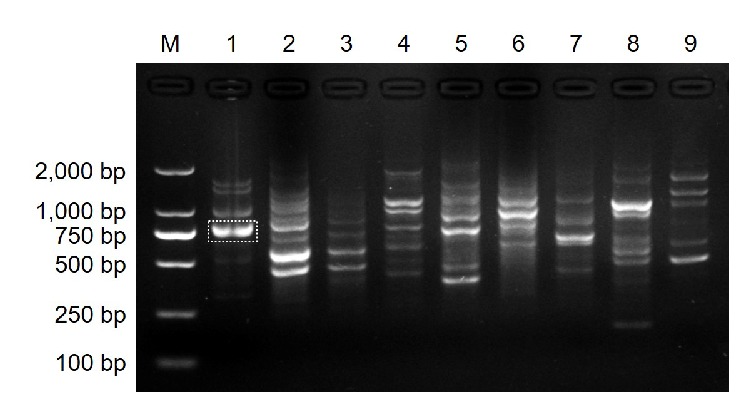
Random amplified polymorphic DNA (RAPD) profiles of Pseudofabraea citricarpa and other citrus fungal pathogens obtained with random primer CS38. M, DNA ladder 2000; lane 1, Ps. citricarpa; lane 2, Alternaria alternata; lane 3, Colletotrichum gloeosporioides; lane 4, Diaporthe citri; lane 5, Botrytis cinerea; lane 6, Oospora citri-aurantii; lane 7, Phyllosticta citricarpa; lane 8, Penicillium italicum; lane 9, Pe. digitatum. The dotted box represents the location of the Ps. citricarpa-specific band.
Figure 3.

Specific DNA sequence of Pseudofabraea citricarpa obtained with the RAPD primer CS38. The gray region indicates the sequence that was amplified by the primer pair Pc-SFP/Pc-SRP (the sequence of the primer pairs were in bold). The first 10 nucleotides of the obtained sequence completely matched the corresponding RAPD primer CS38.
3.2. Specific SCAR Marker Design and Amplification
The primer pair Pc-SFP/Pc-SRP (Table 1) was designed using Primer Premier 6.0 software (Premier Biosoft International) based on the sequence of the specific amplicon. When Pc-SFP and Pc-SRP were used to amplify genomic DNA from the nine selected pathogens, a single and distinct band of 389 bp was only observed in Ps. citricarpa (Figure 4). Sequencing analysis showed the amplicon was the expected Ps. citricarpa fragment, indicating that the designed SCAR marker is specific for the citrus target spot pathogen.
Figure 4.
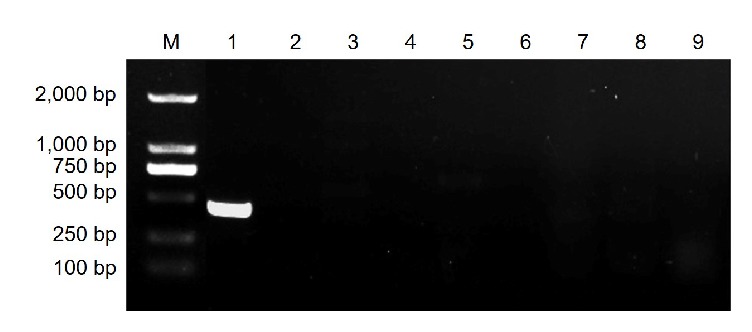
The specificity of PCR product for the detection of Pseudofabraea citricarpa using the primer pair Pc-SFP/Pc-SRP. M, DNA ladder 2000; lane 1, Ps. citricarpa; lane 2, Alternaria alternata; lane 3, Colletotrichum gloeosporioides; lane 4, Diaporthe citri; lane 5, Botrytis cinerea; lane 6, Oospora citri-aurantii; lane 7, Phyllosticta citricarpa; lane 8, Penicillium italicum; lane 9, Pe. digitatum.
3.3. Sensitivity Test of the Detection System
To test the sensitivity of the specific marker for detecting Ps. citricarpa, serial dilutions of Ps. citricarpa DNA were used as templates in the PCR assay with Pc-SFP and Pc-SRP primers. The results revealed that the SCAR marker could detect Ps. citricarpa DNA at levels as low as 50 pg/μL (Figure 5).
Figure 5.
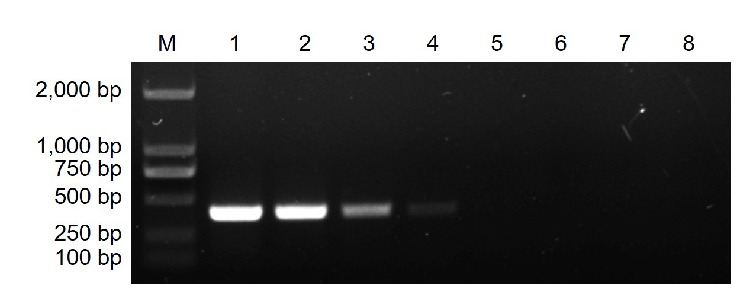
The PCR sensitivity of the primer pair Pc-SFP/Pc-SRP with a serial dilution of Pseudofabraea citricarpa DNA. M, DNA ladder 2000; lane 1, 50 ng/μL; lane 2, 5 ng/μL; lane 3, 500 pg/μL; lane 4, 50 pg/μL; lane 5, 5 pg/μL; lane 6, 500 fg/μL; lane 7, 50 fg/μL; lane 8, 5 fg/μL.
3.4. Detection of Ps. citricarpa in Orchards
To test the reliability of the Ps. citricarpa-specific SCAR marker Pc-SFP and Pc-SRP, citrus leaves and shoots without any visible symptoms were collected from diseased orchards and were used for the verification test. The expected 389 bp bands were obtained from portions of the selected samples (Figure 6). No PCR product was amplified in the negative control (uninfected citrus leaves). The results validated the reliability of the designed SCAR marker.
Figure 6.
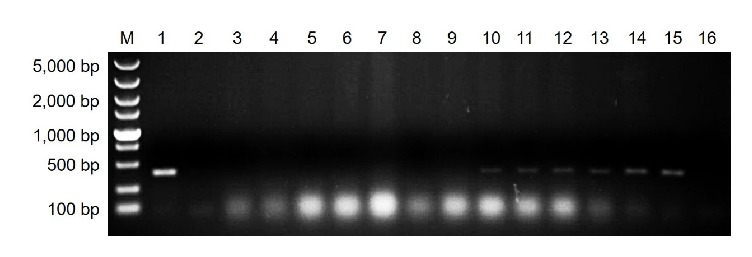
PCR amplification using DNA extracted from citrus samples that were collected from orchards with target spot. M, DNA ladder 5000; lane 1, positive control (Ps. citricarpa DNA); lanes 2–15, citrus leaves or shoots without any visible symptoms; lane 16, negative control (uninfected citrus leaf DNA).
4. Discussion
Given that knowledge on the infection cycle and disease epidemics of citrus target spot is limited, the disease has been mistaken as a brown spot or anthracnose for prevention and control for a long time, which caused poor control effects [11]. The sensitivity tests showed that the SCAR marker could detect as low as 50 pg/μL of Ps. citricarpa DNA extracted from mycelia and from citrus leaves or shoots collected diseased orchards, but not from healthy leaves (Figure 5). These results indicated that the proposed amplification system could help illustrate the oversummering mechanism and occurrence characteristics of citrus target spot, which will be useful for the effective forecasting and management of this disease.
RAPD analysis reveals a high degree of polymorphism even without the DNA sequence information of the species; moreover, RAPD is easy to perform [12]. Given the advantages of low workload, rapidity, and high efficiency compared with traditional identification methods, RAPD-based SCAR markers are extensively used for the in planta detection of several plant pathogens [5, 13, 14]. The SCAR marker developed in this study can also facilitate rapid decision-making and precise early season disease management to reduce the risk of Ps. citricarpa epidemics.
Acknowledgments
This study was funded by the Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0316), the Chongqing Postdoctoral Science Foundation (Xm2016124), the Fundamental Research Funds for the Central Universities (XDJK2016A020), and the China Scholarship Council (201706995068). The authors thank Professor Kaifang Zeng, Southwest University, for providing them with the fungal pathogens of citrus fruits used in this study.
Contributor Information
Yuheng Yang, Email: yyh023@swu.edu.cn.
Changyong Zhou, Email: zhoucy@cric.cn.
Conflicts of Interest
The authors declared no conflicts of interest.
Supplementary Materials
Table S1: sequence of random amplified polymorphic DNA (RAPD) PCR primers used in this study.
References
- 1.Zhu L., Wang X., Huang F., et al. A destructive new disease of citrus in China caused by Cryptosporiopsis citricarpa sp. nov. Plant Disease. 2012;96(6):804–812. doi: 10.1094/PDIS-09-11-0775. [DOI] [PubMed] [Google Scholar]
- 2.Chen C., Verkley G. J. M., Sun G., Groenewald J. Z., Crous P. W. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biology. 2016;120(11):1291–1322. doi: 10.1016/j.funbio.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Martinelli F., Scalenghe R., Davino S., et al. Advanced methods of plant disease detection. A review. Agronomy for Sustainable Development. 2015;35(1):1–25. doi: 10.1007/s13593-014-0246-1. [DOI] [Google Scholar]
- 4.López M. M., Llop P., Olmos A., Marco-Noales E., Cambra M., Bertolini E. Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Current Issues in Molecular Biology. 2009;11:13–46. [PubMed] [Google Scholar]
- 5.Jesumaharaja L. G., Manikandan R., Raguchander T. SCAR marker specific to detect Magnaporthe grisea infecting finger millets (Eleusine coracana) Journal of Applied Microbiology. 2016;121(3):778–786. doi: 10.1111/jam.13209. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J., Wang X. J., Chen C. Q., Huang L. L., Kang Z. S. A PCR-based assay for detection of Puccinia striiformis f. sp. tritici in wheat. Plant Disease. 2007;91(12):1669–1674. doi: 10.1094/PDIS-91-12-1669. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y.-H., Su C.-C., Chao C.-P., et al. A molecular diagnosis method using real-time PCR for quantification and detection of Fusarium oxysporum f. sp. cubense race 4. European Journal of Plant Pathology. 2013;135(2):395–405. doi: 10.1007/s10658-012-0096-0. [DOI] [Google Scholar]
- 8.McDonald B. A. The population genetics of fungi: Tools and techniques. Journal of Phytopathology. 1997;87(4):448–453. doi: 10.1094/PHYTO.1997.87.4.448. [DOI] [PubMed] [Google Scholar]
- 9.Williams J. G. K., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research. 1990;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velegraki A., Kambouris M., Kostourou A., Chalevelakis G., Legakis N. J. Rapid extraction of fungal DNA from clinical samples for PCR amplification. Medical Mycology. 1999;37(1):69–73. doi: 10.1046/j.1365-280X.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Tian W., Wei G. Feature and prevention of citrus target spot. Fruit Grower's Friend. 2015;no. 5:p. 36. [Google Scholar]
- 12.Dnyaneshwar W., Preeti C., Kalpana J., Bhushan P. Development and application of RAPD-SCAR marker for identification of Phyllanthus emblica Linn. Biological & Pharmaceutical Bulletin. 2006;29(11):2313–2316. doi: 10.1248/bpb.29.2313. [DOI] [PubMed] [Google Scholar]
- 13.Nithya K., Bukhari K. A. I. M., Valluvaparidasan V., Paranidharan V., Velazhahan R. Molecular detection of Colletotrichum falcatum causing red rot disease of sugarcane (Saccharum officinarum) using a SCAR marker. Annals of Applied Biology. 2012;160(2):168–173. doi: 10.1111/j.1744-7348.2011.00529.x. [DOI] [Google Scholar]
- 14.Ladhalakshmi D., Vijayasamundeeswari A., Paranidharan V., Samiyappan R., Velazhahan R. Molecular identification of isolates of Peronosclerospora sorghi from maize using PCR-based SCAR marker. World Journal of Microbiology and Biotechnology. 2009;25(12):2129–2135. doi: 10.1007/s11274-009-0117-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: sequence of random amplified polymorphic DNA (RAPD) PCR primers used in this study.


