Abstract
OBJECTIVE
Pyridoxine is converted to its biologically active form pyridoxal-5-phosphate (P5P) by the enzyme pyridox(am)ine 5′-phosphate oxidase and serves as a cofactor in nearly 200 reactions in the central nervous system. Pyridox(am)ine 5′-phosphate oxidase deficiency leads to P5P dependent epilepsy, typically a neonatal- or infantile-onset epileptic encephalopathy treatable with P5P or in some cases, pyridoxine. Following identification of retinopathy in a patient with pyridox(am)ine 5′-phosphate oxidase deficiency that was reversible with P5P therapy, we describe the systemic manifestations of pyridox(am)ine 5′-phosphate oxidase deficiency.
METHODS
A series of six patients with homozygous mutations of PNPO, the gene coding pyridox(am)ine 5′-phosphate oxidase, were evaluated in our center over the course of two years for phenotyping of neurological and systemic manifestations.
RESULTS
Five of six were born prematurely, three had anemia and failure to thrive, and two had elevated alkaline phosphatase. A movement disorder was observed in two children, and a reversible retinopathy was observed in the most severely affected infant. All patients had neonatal-onset epilepsy and were on a continuum of developmental delay to profound encephalopathy. Electroencephalographic features included background slowing and disorganization, absent sleep features, and multifocal and generalized epileptiform discharges. All the affected probands carried a homozygous PNPO mutation (c.674 G>T, c.686 G>A and c.352G>A).
CONCLUSION
In addition to the well-described epileptic encephalopathy, pyridox(am)ine 5′-phosphate oxidase deficiency causes a range of neurological and systemic manifestations. A movement disorder, developmental delay, and encephalopathy, as well as retinopathy, anemia, and failure to thrive add to the broadening clinical spectrum of P5P dependent epilepsy.
Keywords: pyridoxine, epileptic encephalopathy, pyridoxal-5-phosphate, reversible retinopathy
Introduction
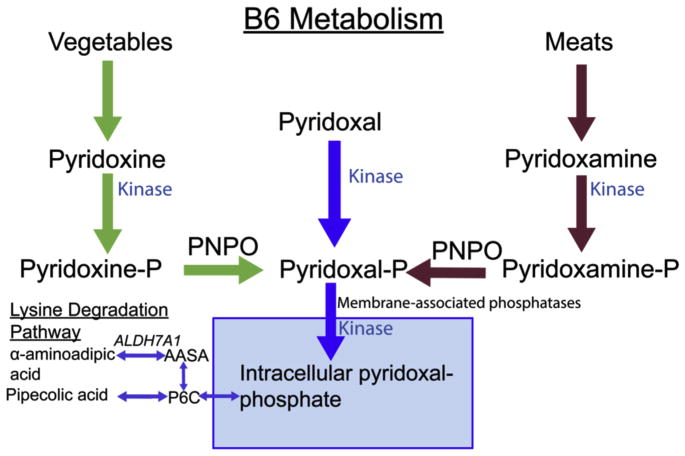
Pyridoxine (vitamin B6) is a cofactor in nearly 200 biochemical reactions of the central nervous system. Pyridoxine is converted to its biologically active form, pyridoxal-5-phosphate (P5P), by the enzyme pyridox(am)ine 5′-phosphate oxidase (PNPO). The PNPO gene (OMIM 6032870) has a cytogenetic location of 17q21.32. PNPO is the rate-limiting step of conversion of vitamin B6 to P5P. P5P-dependent enzymes account for 4% of enzyme-catalyzed reactions,1 including reactions within amino acid and glycogen metabolism and the synthesis of nucleic acids, hemoglobin, sphingomyelin, and multiple neurotransmitters (Fig 1).
FIGURE 1.
B6 metabolism. Pyridoxine and pyridoxamine are derived from vegetables and meat, respectively. These two are ultimately converted into pyridoxal-5-phosphate (P5P) by PNPO. Defects in antiquitin, an enzyme in the lysine degradation pathway, are the cause of pyridoxine-responsive seizures leading to accumulation of the upstream intermediary alpha-aminoadipic semialdehyde (AASA) and sequestration of P5P. P6C, piperideine-6-carboxylic acid; ALDH7A1, antiquitin or aldehyde dehydrogenase 7 family, member A1.
Pyridoxine-dependent epilepsy takes on two primary forms. The first identified cause of pyridoxine-responsive seizures is a defect in antiquitin, a gene encoding the enzyme alpha aminoadipic semialdehyde dehydrogenase in the lysine degradation pathway.2 This defect leads to toxic intermediates and decreases the amount of available P5P, a product sequestrated by the accumulation of a carboxylate that forms as a result of this defect. Treatment with pyridoxine can overcome this deficit. More recently, defects in both the quantity and functional configuration of PNPO have also been identified in children with early epileptic encephalopathies.3–7 Children with PNPO deficiency require treatment with P5P to circumvent the need for conversion of pyridoxine to P5P Mills et al8; however, some may have a partial response to pyridoxine Pearl et al.3–5,9
In addition to an encephalopathy with prominent epilepsy, other neurological manifestations include paroxysmal events or movement disorders.6,10 In addition, systemic manifestations may occur in PNPO deficiency and may serve to distinguish it from antiquitin deficiency.6,11–13 Following identification of a pigmentary retinopathy in our sentinel patient, we sought to elaborate on systemic manifestations of PNPO deficiency.
Methods
Following the identification of our index patient, five additional children with confirmed PNPO deficiency were evaluated for phenotypic characterization. Demographic, clinical, laboratory electroencephalographic (EEG), and imaging data were collected (see Tables 1–3). A chart review was conducted for details regarding initial presentation and management, as well as response to P5P and other medical interventions. The Boston Children’s Hospital Institutional Review Board approved the study.
TABLE 1.
Neurological Manifestations in PNPO Deficiency
| Case | Paroxysmal or Movement Disorder | Regression | CSF P5P (30–80 nmol/L) | Seizures at Presentation | Ongoing Seizures at Discharge |
|---|---|---|---|---|---|
| 1 | Yes | No | <5 | Yes | Yes |
| 2* | Yes | Yes | 16 | Yes | Yes |
| 3* | No | No | 25 | Yes | Yes |
| 4 | No | No | 15 | Yes | Yes |
| 5 | No | No | 52 | Yes | Rare |
| 6† | Yes | No | 23 | Yes | Yes |
| Summary | 3/6 | 1/6 | 5/6 | 6/6 | 5/6 |
Abbreviations:
CSF = Cerebrospinal fluid
MRI = Magnetic resonance imaging
P5P = Pyridoxal-5-phosphate
PVL = Periventricular leukomalacia
SDH = Subdural hemorrhage.
Patients 2 and 3 are sisters.
Patient 6 was first described in Pearl et al.9
TABLE 3.
EEG, MRI, P5P, and Genetic Characteristics
| Case | EEG Background | Epileptiform Discharges | Notable MRI Findings | CSF P5P (30–80 nmol/L) | DNA Sequence Change | Amino Acid Substitution (Protein Change) |
|---|---|---|---|---|---|---|
| 1 | No normal background features, discontinuous | Multifocal and generalized | Venous sinus thrombosis | <5 | Homozygous c.686 G>A | pArg229Gln (R229Q) |
| 2* | No normal background features, increased discontinuity | Frequent, multifocal | Atrophy | 16 | Homozygous c.674G>T | p.Arg225Leu (R225L) |
| 3* | Background preserved over one hemisphere | Frequent, multifocal | Atrophy | 25 | Homozygous c.674G>T | p.Arg225Leu (R225L) |
| 4 | Intact awake and sleep graphoelements and background organization | Multifocal | PVL | 15 | Homozygous c.674G>T | p.Arg225Leu (R225L) |
| 5 | Intact awake and sleep graphoelements and background organization | Rare | Small SDH | 52 | Homozygous c.674G>T | p.Arg225Leu (R225L) |
| 6† | Neonatal EEG with evidence of sleep features and state changes | Multifocal and generalized | Normal | 23 | Homozygous c.352G>A | p.Arg118Gln (G118R) |
Abbreviations:
CSF = Cerebrospinal fluid
MRI = Magnetic resonance imaging
P5P = Pyridoxyl-5-phosphate
PVL = Periventricular leukomalacia
SDH = Subdural hemorrhage.
Patients 2 and 3 are sisters.
Patient 6 was first described in Pearl et al.9
Results
Six children with PNPO deficiency (three males) were collected over a two-year period. One was previously reported Pearl et al.9 Five children were born in the Middle East (one in Qatar and four in United Arab Emirates) and were referred for evaluation. Two patients were siblings. All were born between 32 and 37 weeks’ gestation. Based on the earliest documentation available, normocytic anemia was present in three, constipation in four, and hypothyroidism in one. Liver function tests were abnormal in four, including two with significantly elevated alkaline phosphatase serum levels that were present before P5P administration (Table 1). All had documented homozygous mutations in the PNPO gene, including four with c.674G>T;p.Arg225Leu and one each with c.686 G>A;p.Arg229Gln and c.352G>A;p.Arg118Gln (Table 2). All the three variants are extremely rare and have never been seen in a homozygous state in the publicly available Exome Aggregation Consortium database (exac.broadinstitute.org).
TABLE 2.
Systemic Manifestations in PNPO Deficiency
| Case | Prematurity | Perinatal Distress | Anemia | FTT | Constipation | Thyroid Dysfunction | Alkphos (110–400 U/L) | AST (10–65 U/L) | Ophtho |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 weeks | Yes | Yes | Yes | Yes | Not assessed | 1011 | N/a | Retinopathy |
| 2* | 35 weeks | Yes | Yes | Yes | Yes | Not assessed | WNL | 50 | Disc pallor |
| 3* | 35 weeks | Yes | No | No | Yes | Hypo | WNL | WNL | WNL |
| 4 | 32 weeks | Yes | No | Yes | Yes | No | WNL | WNL | WNL |
| 5 | 35 weeks | Yes | Yes | No | No | No | 2548 | WNL | Not assessed |
| 6† | 37 weeks | No | No | Yes | No | Not assessed | WNL | WNL | Not assessed |
| Summary | 5/6 | 5/6 | 3/6 | 4/6 | 4/6 | 1/6 | 2/6 | 1/6 | 1/6 |
Abbreviations:
Alkphos = Alkaline phosphate
AST = Aspartate aminotransferase
FTT = Failure to thrive
Ophtho = Ophthalmology
PNPO = Pyridox(am)ine 5′-phosphate oxidase
WNL = Within normal limits
Patients 2 and 3 are sisters.
Patient 6 was first described in Pearl et al.9
All children had neonatal-onset epileptic encephalopathy with developmental delay and seizures, including developmental regression in one. EEG background abnormalities were diverse and included lack of organization and state transitioning (two), discontinuity (two), and multi-focal and generalized epileptiform discharges (six). Magnetic resonance imaging findings were nonspecific and most commonly revealed cerebral atrophy. Two of the children had ophthalmologic pathology, one having optic disc pallor and the other the reversible retinopathy described below.
Representative patient
This girl was born in Qatar to consanguineous parents by spontaneous vaginal delivery at 33 weeks’ gestation weighing 1870 grams. The pregnancy was significant for mild gestational diabetes and preterm labor. Her Apgar scores were 5 and 8 (at one and five minutes), and the cord pH was 7.13 with base excess of 6. Seizures began at three hours of life with unilateral eye twitching and clonic movements of one leg. An EEG from day 12 of life was excessively and persistently discontinuous with high-voltage asynchronous bursts of theta and delta activity superimposed upon a low-voltage background with inter-burst intervals greater than 20 seconds.
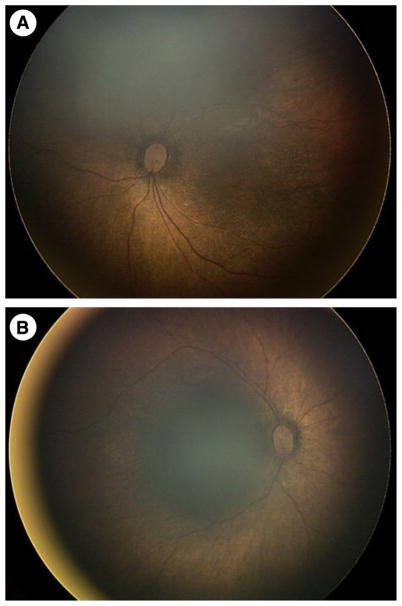
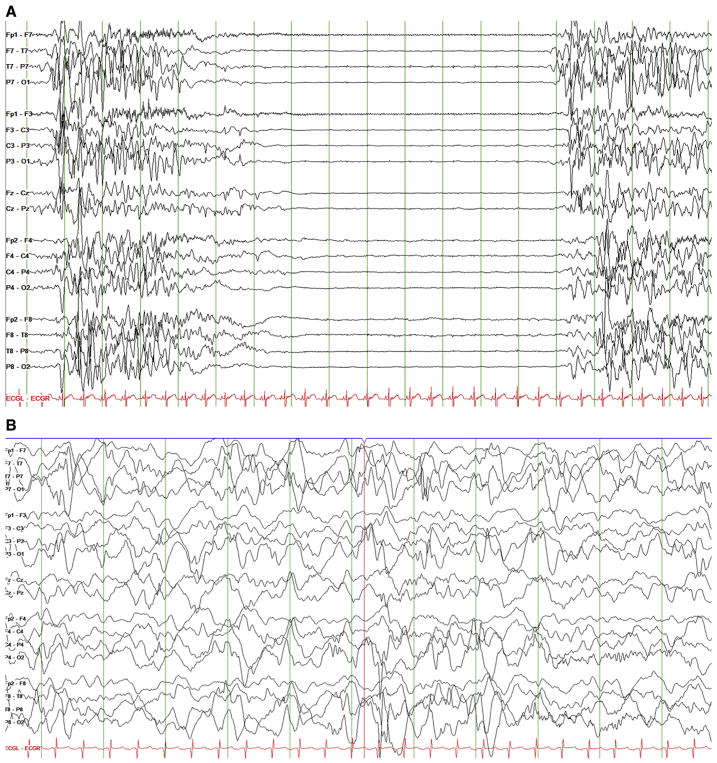
Seizures were refractory to phenobarbital, phenytoin, and midazolam, followed by a trial of intravenous pyridoxine without change. Upon transfer at age eight weeks, evaluation revealed a nondysmorphic infant with mild hypotonia, normocytic anemia with hematocrit of 22.9%, and a pigmentary retinopathy. The retinopathy was described as a bull’s-eye maculopathy with attenuated vessels and salt-and-pepper pigmentary changes (Fig 2). EEG continued to reveal an invariant suppression-burst pattern (Fig 3), ictal discharges were focal from the right posterior quadrant, and there were also electrodecremental responses associated with flexor spasms.
FIGURE 2.
“Salt-and-pepper” retinopathy. Bulls-eye maculopathy of the left (A) and right (B) eyes with attenuated retinal vessels, pale optic nerve heads, and salt-and-pepper or black-and-white pigmentary changes in the retina seen on fundoscopic examination.
FIGURE 3.
Electroencephalography (EEG) before and after pyridoxal-5-phosphate treatment. (A) EEG before P5P treatment with burst-suppression pattern. (B) EEG evolved with more continuity on P5P and vigabatrin by age four months.
P5P and folinic acid reduced clinical spasms but did not initially improve the EEG. Over the subsequent three days, the EEG improved from burst suppression to a more continuous pattern (Fig 3). A phenotype of irritability combined with a mixed movement disorder characterized by erratic eye movements, oromotor dyskinesias, and chorea gradually improved over the first two to four weeks of P5P therapy. The retinopathy improved following four weeks of P5P. The infant was discharged at age four months on P5P, vigabatrin, phenytoin, and clobazam with occasional breakthrough seizures attributed to the severity of her disease as well as difficulties with P5P administration and precipitation of the compound with storage.
Laboratory analysis showed persistent hyperphosphatasia and a low cerebrospinal fluid (CSF) P5P level of less than 5 nmol/L, with normal urine alpha-aminoadipic semi-aldehyde and serum pipecolic acid. Pyridoxamine 5-phosphate oxidase (PNPO) gene testing revealed homozygous, missense mutations: C.686G>A;p.R229Q. Trio testing confirmed heterozygosity for the mutation in each parent.
Discussion
PNPO deficiency causes not only P5P-dependent epilepsy and associated encephalopathy but also a range of neurological and systemic manifestations. Our patients presented with a neonatal epileptic encephalopathy and varying severity of movement disorder, retinopathy, normocytic anemia, and hyperphosphatasia. The systemic symptoms help to distinguish the phenotype from antiquitin deficiency causing pyridoxine-dependent epilepsy, and the pigmentary retinopathy appears reversible with specific treatment.
Our most severely affected child with the lowest P5P level required prolonged treatment with P5P, and improvement in EEG background lagged behind clinical improvement (Fig 3), which has been seen by others.14,15 The delay in EEG treatment response is clinically instructive, as in pyridoxine- or P5P-dependent epilepsies, reduction in clinical seizures should be seen within days of treatment introduction but improvement of the EEG background may take hours to weeks and lack of immediate response should not be considered a treatment failure. Nonepileptic movements including irritability, oromotor movements, and choreawere also prominent in two of our patients and improved with P5P treatment. Movement disorders have been observed previously and offered as a potential way to distinguish the PNPO phenotype from traditional pyridoxine-dependent epileptic encephalopathy.6,10
Our series suggests that there may be a relationship between PNPO deficiency and hyperphosphatasia-related disorders. Subjects 1 and 5 both had dramatically elevated alkaline phosphatase levels, without evidence of jaundice or cholestasis. Hyperphosphatasia and epilepsy can also be seen in Mabry syndrome, a group of genetic disorders in the biosynthesis of an important membrane linking protein called the glycophosphatidylinositol anchor. Mabry syndrome is associated with intellectual disability, epilepsy, and characteristic dysmorphism. In Mabry syndrome, CSF P5P levels may also be decreased, and seizures may be responsive to therapy with pyridoxine. The precise mechanism for reduced CSF P5P levels in Mabry syndrome is not known, but may relate to increased dephosphorylation of P5P in the central nervous system due to increased local phosphatase activity.16
The implications of hyperphosphatasia in our patients are not entirely clear. To cross the blood-brain barrier, circulating P5P must first be dephosphorylated to pyridoxine by alkaline phosphatase. The effects of a failure of this process are exemplified by the autosomal recessive disorder hypophosphatasia, in which there is a deficiency in the production or function of alkaline phosphatase resulting in CSF pyridoxine deficiency.17–19 For this reason, hypophosphatasia has been associated with pyridoxine-dependent seizures.20,21 Based on this presumption, it is possible that the elevated alkaline phosphatase levels in our patients may be a compensatory mechanism to increase pyridoxine transport across the blood-brain barrier. The alkaline phosphatase level decreased in patient 1 following P5P administration from 1011 to 596 U/L, but this correlation did not occur in patient 5, wherein it fluctuated from 100 to 2000s U/L without a relationship to P5P administration. The CSF P5P level in patient 5 was higher (52 nmol/L) and may not have been as responsive to exogenous administration. These results should be interpreted with caution, though, because raised alkaline phosphatase may be a nonspecific finding in premature or systemically ill infants. Nevertheless, in children with early-onset epileptic encephalopathy, attention to routine laboratory values may point to a cause and attention to such disturbances may lead to earlier diagnosis and treatment.
The retinopathy observed in our index patient is not typically associated with P5P-dependent epileptic encephalopathy. Mechanistically, this may resemble gyrate atrophy, which is responsive to supplementation with P5P.11,22,23 Gyrate atrophy is an autosomal recessive retinopathy characterized by sharply demarcated retinal pigment epithelium and choroidal atrophy resembling cerebral gyri and is associated with deficiency of ornithine aminotransferase (OAT), which relies upon P5P as a cofactor.11,22 OAT is the key enzyme that converts arginine and ornithine to the neurotransmitters glutamate and GABA. Gyrate atrophy differs in appearance from salt-and-pepper retinopathy; however, the retinal manifestations may exist along a spectrum as was observed in affected siblings with gyrate atrophy.24 Although the exact mechanism for the retinopathy in OAT deficiency is unclear, we hypothesize that the retinopathy we report in PNPO deficiency may be explained by decreased functionality of OAT due to the absence of its cofactor P5P. Other causes of retinopathy are possible, particularly in the setting of consanguinity; however, given the fact that our patient’s retinopathy improved with P5P treatment over the course of four weeks, a PNPO-related mechanism is likely.
Prematurity, failure to thrive, constipation, and normocytic anemia were common features in our patients and have been observed in PNPO deficiency and in the context of B6-related diseases.4,11,25,26 In conclusion, this report highlights a range of neurological and systemic manifestations in PNPO deficiency including the presence of a retinopathy that appears responsive to P5P therapy. The degree of encephalopathy and the severity of neurological symptoms appears to correlate with the degree of systemic illness.
Footnotes
Financial Disclosures: None.
References
- 1.Mozzarelli A, Bettati S. Exploring the pyridoxal 5′-phosphate-dependent enzymes. Chem Rec. 2006;6:275–287. doi: 10.1002/tcr.20094. [DOI] [PubMed] [Google Scholar]
- 2.Mills PB, Struys E, Jakobs C, et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med. 2006;12:307–309. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 3.Jaeger B, Abeling NG, Salomons GS, et al. Pyridoxine responsive epilepsy caused by a novel homozygous PNPO mutation. Mol Genet Metab Rep. 2016;6:60–63. doi: 10.1016/j.ymgmr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills PB, Camuzeaux SSM, Footitt EJ, et al. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain. 2014;137:1350–1360. doi: 10.1093/brain/awu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plecko B, Paul K, Mills P, et al. Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology. 2014;82:1425–1433. doi: 10.1212/WNL.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt B, Baumgartner M, Mills PB, et al. Seizures and paroxysmal events: symptoms pointing to the diagnosis of pyridoxine-dependent epilepsy and pyridoxine phosphate oxidase deficiency. Dev Med Child Neurol. 2010;52:e133–e142. doi: 10.1111/j.1469-8749.2010.03660.x. [DOI] [PubMed] [Google Scholar]
- 7.Veeravigrom M, Damrongphol P, Ittiwut R, Ittiwut C, Suphapeetiporn K, Shotelersuk V. Pyridoxal 5′-phosphate-responsive epilepsy with novel mutations in the PNPO gene: a case report. Genet Mol Res. 2015;14:14130–14135. doi: 10.4238/2015.October.29.34. [DOI] [PubMed] [Google Scholar]
- 8.Mills PB, Surtees RAH, Champion MP, et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5′-phosphate oxidase. Hum Mol Genet. 2005;14:1077–1086. doi: 10.1093/hmg/ddi120. [DOI] [PubMed] [Google Scholar]
- 9.Pearl PL, Hyland K, Chiles J, McGavin CL, Yu Y, Taylor D. Partial pyridoxine responsiveness in PNPO deficiency. JIMD Rep. 2013;9:139–142. doi: 10.1007/8904_2012_194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware TL, Earl J, Salomons GS, et al. Typical and atypical phenotypes of PNPO deficiency with elevated CSF and plasma pyridoxamine on treatment. Dev Med Child Neurol. 2013;56:498–502. doi: 10.1111/dmcn.12346. [DOI] [PubMed] [Google Scholar]
- 11.Cellini B, Montioli R, Oppici E, Astegno A, Voltattorni CB. The chaperone role of the pyridoxal 5′-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin Biochem. 2014;47:158–165. doi: 10.1016/j.clinbiochem.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Pearl PL. New treatment paradigms in neonatal metabolic epilepsies. J Inherit Metab Dis. 2009;32:204–213. doi: 10.1007/s10545-009-1045-8. [DOI] [PubMed] [Google Scholar]
- 13.Thompson MD, Killoran A, Percy ME, Nezarati M, Cole DEC, Hwang PA. Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder? Pediatr Neurol. 2006;34:303–307. doi: 10.1016/j.pediatrneurol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Gospe SM. Pyridoxine-Dependent Epilepsy. In: Pagon RA, Adam MP, Ardinger HH, editors. GeneReviews. Seattle: 2014. pp. 1–18. [Google Scholar]
- 15.Naasan G, Yabroudi M, Rahi A, Mikati MA. Electroencephalographic changes in pyridoxine-dependant epilepsy: new observations. Epileptic Disord. 2009;11:293–300. doi: 10.1684/epd.2009.0280. [DOI] [PubMed] [Google Scholar]
- 16.Cole DEC, Thompson MD. Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP). Subcellular Biochemistry. Dordrecht, Netherlands: Springer; 2015. Neurogenetic Aspects of Hyperphosphatasia in Mabry syndrome; pp. 343–361. [DOI] [PubMed] [Google Scholar]
- 17.Chodirker BN, Evans JA, Seargeant LE, Cheang MS, Greenberg CR. Hyperphosphatemia in infantile hypophosphatasia: implications for carrier diagnosis and screening. Am J Hum Genet. 1990;46:280–285. [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramaniam S, Bowling F, Carpenter K, et al. Perinatal hypophosphatasia presenting as neonatal epileptic encephalopathy with abnormal neurotransmitter metabolism secondary to reduced cofactor pyridoxal-5′-phosphate availability. J Inherit Metab Dis. 2010;33:25–33. doi: 10.1007/s10545-009-9012-y. [DOI] [PubMed] [Google Scholar]
- 19.de Roo MGA, Abeling NGGM, Majoie CB, et al. Infantile hypophosphatasia without bone deformities presenting with severe pyridoxine-resistant seizures. Mol Genet Metab. 2014;111:404–407. doi: 10.1016/j.ymgme.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Belachew D, Kazmerski T, Libman I, et al. JIMD Reports–Case and Research Reports, 2012/6. JIMD Reports. Berlin, Heidelberg: Springer; 2013. Infantile Hypophosphatasia Secondary to a Novel Compound Heterozygous Mutation Presenting with Pyridoxine-Responsive Seizures; pp. 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Güzel Nur B, Çelmeli G, Manguoglu E, Soyucen E, Bircan İ, Mıh¸ı E. Pyridoxine-responsive seizures in infantile hypophosphatasia and a novel homozygous mutation in ALPL gene. J Clin Res Pediatr Endocrinol. 2016;8:360–364. doi: 10.4274/jcrpe.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton PT. B6-responsive disorders: A model of vitamin dependency. J Inherit Metab Dis. 2006;29:317–326. doi: 10.1007/s10545-005-0243-2. [DOI] [PubMed] [Google Scholar]
- 23.Michaud J, Thompson GN, Brody LC, et al. Pyridoxine-responsive gyrate atrophy of the choroid and retina: clinical and biochemical correlates of the mutation A226V. Am J Hum Genet. 1995;56:616–622. [PMC free article] [PubMed] [Google Scholar]
- 24.Büyüktortop N, Alp MN, Sivri S, Coşkun T, Kural G. Gyrate atrophy of the choroid and retina: a case report. Turk J Pediatr. 2011;53:94–96. [PubMed] [Google Scholar]
- 25.Gospe SM. Current perspectives on pyridoxine-dependent seizures. J Pediatr. 1998;132:919–923. doi: 10.1016/s0022-3476(98)70384-1. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann GF, Schmitt B, Windfuhr M, et al. Pyridoxal 5′-phosphate may be curative in early-onset epileptic encephalopathy. J Inherit Metab Dis. 2006;30:96–99. doi: 10.1007/s10545-006-0508-4. [DOI] [PubMed] [Google Scholar]