Abstract
Sinapic acid is a natural phenolic acid found in fruits, vegetables, and cereals, exerting numerous pharmacological effects. The aim of the study was to investigate the influence of sinapic acid on biochemical parameters related to glucose and lipid metabolism, as well as markers of antioxidant abilities and parameters of oxidative damage in the blood serum in estrogen-deficient rats. The study was performed on 3-month-old female Wistar rats, divided into 5 groups, including sham-operated control rats, ovariectomized control rats, and ovariectomized rats administered orally with estradiol (0.2 mg/kg) or sinapic acid (5 and 25 mg/kg) for 28 days. The levels of estradiol, progesterone, interleukin 18, insulin, glucose, fructosamine, lipids, and enzymatic and nonenzymatic antioxidants (superoxide dismutase, catalase, and glutathione); total antioxidant capacity; and oxidative damage parameters (thiobarbituric acid-reactive substances, protein carbonyl groups, and advanced oxidation protein products) were determined in the serum. Estradiol counteracted the carbohydrate and cholesterol metabolism disorders induced by estrogen deficiency. Sinapic acid increased the serum estradiol concentration; decreased insulin resistance and the triglyceride and total cholesterol concentrations; and favorably affected the parameters of antioxidant abilities (reduced glutathione, superoxide dismutase) and oxidative damage (advanced oxidation protein products).
1. Introduction
Estrogens affect the body's metabolic homeostasis by regulating many signaling pathways. Estrogens participate, among others, in the control of energy homeostasis and glucose metabolism. By acting in the hypothalamus, they control food intake, energy expenditure, and the deposition of white adipose tissue. They play an important role in the regulation of insulin secretion in the pancreas and in the response to insulin in the skeletal muscle, liver, and adipose tissue [1–3]. After menopause, estrogen deficiency contributes to the development of obesity, dyslipidemia, hypertension, and insulin resistance and, consequently, it increases the risk of cardiovascular diseases and type 2 diabetes [1, 4, 5]. The current state of knowledge on the estrogen effects on metabolism has been recently comprehensively reviewed by Mauvais-Jarvis et al. [1, 4], Sharma et al. [6], and Coyoy et al. [7].
Oxidative stress is considered to be involved in development of disorders resulting from estrogen deficiency, such as: hot flushes, cardiovascular diseases, and osteoporosis [8–10]. Numerous phenolic compounds of plant origin, which are present in food, belong to the factors that modify development of oxidative stress [11, 12]. Phenolic compounds include, among others, flavonoids and phenolic acids. The antioxidant activity of flavonoids (mainly soy isoflavones) has been the subject of studies (e.g., [13–16]) in estrogen-deficient rats. Although the antioxidant activity of phenolic acids is also well documented [17–19], there is still insufficient data on the effect of phenolic acids on oxidative stress in conditions of estrogen deficiency. Soy isoflavones are phytoestrogens, and their effect on oxidative stress parameters may result from their effects on estrogen receptors. Phenolic acids have rather not been demonstrated to have affinity for estrogen receptors [20]; however, our earlier studies indicated that some hydroxycinnamic phenolic acids (especially caffeic acid) may increase the serum estradiol levels in estrogen-deficient rats [21].
Sinapic acid is a phenolic acid, found, among others, in fruits (e.g., strawberries or lemons), grains (oat), and vegetables (especially from the Brassicaceae family, like tronchuda cabbage, broccoli, and turnip) as well as in some medicinal plants and species (for instance borage, sage, mace, or rosemary) [22–24]. Sinapic acid, a hydroxycinnamic acid derivative, has antioxidant activity. There are reports on its numerous peripheral activities (anti-inflammatory [25], hypoglycemic [26, 27], cardioprotective [28, 29], hepatoprotective [30], and nephroprotective [31]) and central activities (neuroprotective [32–34], anticonvulsant [34], and anxiolytic [35]).
Taking into account that phenolic acids may influence both the oxidative stress parameters and estrogen levels, the aim of the study was to investigate the effects of administration of sinapic acid on the serum biochemical parameters related to the development of metabolic disorders resulting from estrogen deficiency in rats. So far, there are no data on the effects of sinapic acid on parameters related to lipid and glucose metabolism in conditions of estrogen deficiency.
2. Materials and Methods
2.1. Animals and Drugs
The experiment was conducted on mature, 3-month-old female Wistar rats, with the approval of the Local Ethics Commission in Katowice (permission numbers 38/2015, 148/2015, and 66/2016). The animals were provided by the Center of Experimental Medicine, Medical University of Silesia, Katowice, Poland. Rats had unlimited access to drinking water and standard laboratory feed (Labofeed B; Wytwórnia Pasz “Morawski”, Kcynia, Poland). The following substances and drugs were used: sinapic acid (Sigma-Aldrich, St. Louis, MO, USA), estradiol hemihydrate (Estrofem, Novo Nordisk A/S, Bagsvard, Denmark), ketamine (Ketamina 10%, Biowet Puławy, Puławy, Poland), xylazine (Xylapan, Vetoquinol Biowet, Gorzów Wlkp., Poland).
2.2. Experimental Design
During the 13-day adaptation period, the animals were divided into the following groups (n = 10): SHAM—sham-operated control rats; OVX—ovariectomized control rats; ESTR—ovariectomized rats receiving estradiol (0.2 mg/kg p.o. daily); SA5—ovariectomized rats receiving sinapic acid (5 mg/kg p.o. daily); SA25—ovariectomized rats receiving sinapic acid (25 mg/kg p.o. daily). The rats of the ESTR group served as a positive control group. Bilateral ovariectomy (in rats of the OVX, ESTR, SA5, and SA25 groups) and sham surgery (in rats of the SHAM group) were performed 7 days before the start of the estradiol or sinapic acid administration. The animals were anesthetized with the mixture of ketamine and xylazine (87.5 and 12.5 mg/kg i.p., resp.). Sinapic acid and estradiol were administered orally by an intragastric tube once a day for 4 weeks as water suspension prepared with the addition of Tween 20 q.s. (maximum 1 μl of Tween 20 per 1 ml of water). The doses were within the range of doses administered in previous experimental studies of sinapic acid [27, 28, 30, 31, 33] and estradiol [36] in rats. The SHAM and OVX control rats received water with the same amount of Tween 20, in the same volume of 2 ml/kg p.o. All animals were weighed twice a week. The final measurements of the body mass were made before the last administration of sinapic acid, estradiol, or vehicle.
The day after the last administration of sinapic acid or estradiol, the rats were sacrificed by cardiac exsanguination under ketamine/xylazine anesthesia. The animals were fasted overnight prior to euthanasia. The serum was obtained from the clotted blood by centrifugation and frozen until the biochemical measurements were performed. The uterus, thymus, liver, and right kidney were isolated from the sacrificed rats and weighed. All spectrophotometric measurements were carried out using the Tecan Infinite M200 PRO plate reader with Magellan 2.0 software.
The timeline of the experiment is shown in Figure 1.
Figure 1.
Timeline of the experiment.
2.3. Determination of Sex Hormones
The serum concentrations of estradiol and progesterone were determined by ELISA, using DiaMetra (Segrate-Milano, Italy) kits according to instructions provided by the manufacturer.
2.4. Determination of Parameters Related to Glucose Homeostasis
The serum concentrations of glucose and fructosamine were determined spectrophotometrically, using Pointe Scientific (Canton, MI, USA) kits, while insulin concentration was determined by ELISA, using a BioVendor (Brno, Czech Republic) kit, according to the manufacturer's instructions. The HOMA-IR index (homeostasis model assessment for insulin resistance) was calculated using formula (1) [37, 38]:
| (1) |
2.5. Determination of Lipid Concentrations
The serum concentrations of triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were determined spectrophotometrically using Pointe Scientific (Canton, MI, USA) kits, according to instructions provided by the manufacturer.
2.6. Determination of Nonenzymatic and Enzymatic Antioxidants
The serum reduced glutathione (GSH), and oxidized glutathione (GSSG) levels, total antioxidant capacity (TAC), superoxide dismutase (SOD) activity, and catalase (CAT) activity were determined using Cayman Chemical (Ann Arbor, MI, USA) kits.
The activities of SOD and CAT were converted to mg of protein. The serum protein level was determined by the biuret method with the use of a Pointe Scientific (Canton, MI, USA) kit. The measurements were performed according to the instructions provided by the manufacturers.
2.7. Determination of Oxidation Damage Parameters
The serum content of TBARS (thiobarbituric acid-reactive substances) was determined using the method of Ohkawa et al. [39]. The method is based on the reaction between lipid peroxidation products and thiobarbituric acid. The intensity of the resulting color was determined spectrophotometrically at the wavelength of 535 nm. To establish a standard curve, 1,1,3,3-tetraethoxypropane (Sigma-Aldrich, St. Louis, MO, USA) was used.
Spectrophotometric determination of advanced oxidation protein products (AOPP) was carried out on the basis of the protocol described by Witko-Sarsat et al. [40]. The calibration curve was made using chloramine T (Sigma-Aldrich, St. Louis, MO, USA), and the absorbance was measured at the wavelength of 340 nm. The concentration of AOPP was presented in μmol of chloramine T equivalents/l.
The concentration of protein carbonyl groups (PCG) was measured spectrophotometrically using a commercially available kit (Cell Biolabs, San Diego, CA, USA), according to the instructions provided by the manufacturer.
2.8. Determination of Interleukin 18
The serum concentration of interleukin 18 (IL-18) was determined by ELISA with the use of a Cloud-Clone (Houston, TX, USA) kit, following the instructions provided by the manufacturer.
2.9. Determination of Biochemical Markers of Liver and Kidney Function
The activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as the concentrations of uric acid and urea, were determined spectrophotometrically with the use of kits produced by BioSystems (Costa Brava, Barcelona, Spain) in the serum. The concentration of creatinine was determined using a Pointe Scientific (Canton, MI, USA) kit. The measurements were made according to the instructions provided by manufacturers.
2.10. Statistical Analysis
The results are presented as the arithmetic mean ± SEM. To assess the statistical significance of the results, one-way ANOVA followed by Fisher's LSD post hoc test was used. The results were considered statistically significant with p ≤ 0.05.
3. Results
3.1. Effect of Estradiol and Sinapic Acid on the Body Mass and Organ Mass
The body mass and body mass gain after 4 weeks of observation increased statistically significantly in the ovariectomized control rats (OVX) compared to the sham-operated control rats (SHAM). In the OVX control rats, the mass of the uterus decreased significantly, while the mass of the thymus increased. The mass of the liver and kidney did not change significantly in comparison with the mass of these organs in the SHAM control rats. Administration of estradiol at a dose of 0.2 mg/kg caused a decrease in the body mass gain, an increase in the uterine mass, and a reduction in the thymus mass, while it did not affect the liver and kidney mass, as compared to the OVX control rats. Sinapic acid administration at both doses did not lead to any changes in the body mass, body mass gain, and mass of all examined organs (Table 1).
Table 1.
Effect of estradiol and sinapic acid on the body mass gain and mass of selected organs in ovariectomized rats.
| Parameter/group | SHAM | OVX | ESTR | SA5 | SA25 |
|---|---|---|---|---|---|
| Body mass at the start of drug administration (g) | 212.2 ± 3.0 | 222.0 ± 4.3 | 222.5 ± 3.0 | 225.0 ± 4.8 | 224.4 ± 4.9 |
| Body mass after 4 weeks of drug administration (g) | 235.3 ± 3.5 | 271.8 ± 5.0∗∗∗ | 257.7 ± 4.3∗∗ | 272.1 ± 6.5∗∗∗ | 271.0 ± 6.4∗∗∗ |
| Body mass gain after 4 weeks (g) | 23.1 ± 1.9 | 49.8 ± 1.8∗∗∗ | 35.2 ± 3.2∗∗### | 47.1 ± 2.4∗∗∗ | 46.6 ± 2.7∗∗∗ |
| Uterus mass (g) | 0.434 ± 0.036 | 0.082 ± 0.005∗∗∗ | 0.181 ± 0.010∗∗∗### | 0.068 ± 0.005∗∗∗ | 0.071 ± 0.002∗∗∗ |
| Thymus mass (g) | 0.377 ± 0.022 | 0.596 ± 0.044∗∗∗ | 0.500 ± 0.023∗# | 0.625 ± 0.037∗∗∗ | 0.613 ± 0.038∗∗∗ |
| Kidney mass (g) | 0.758 ± 0.020 | 0.789 ± 0.016 | 0.785 ± 0.029 | 0.760 ± 0.014 | 0.768 ± 0.013 |
| Liver mass (g) | 5.906 ± 0.149 | 6.382 ± 0.156 | 6.518 ± 0.132 | 6.365 ± 0.315 | 6.330 ± 0.175 |
Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results.∗p ≤ 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001: significantly different from the SHAM control rats. #p ≤ 0.05, ###p < 0.001: significantly different from the OVX control rats. No statistically significant differences in results for the body mass at the start of drug administration, as well as kidney and liver mass, were demonstrated in ANOVA.
3.2. Effect of Estradiol and Sinapic Acid on the Concentration of Sex Hormones in the Serum
In the OVX control rats, a statistically significant reduction in the estradiol and progesterone concentrations was observed, compared to the SHAM control rats. Administration of estradiol did not trigger any changes in the levels of both hormones tested. Sinapic acid administration at both used doses resulted in a statistically significant increase in the serum estradiol concentration in comparison with the OVX control rats. Sinapic acid did not affect the serum progesterone concentration (Figure 2).
Figure 2.
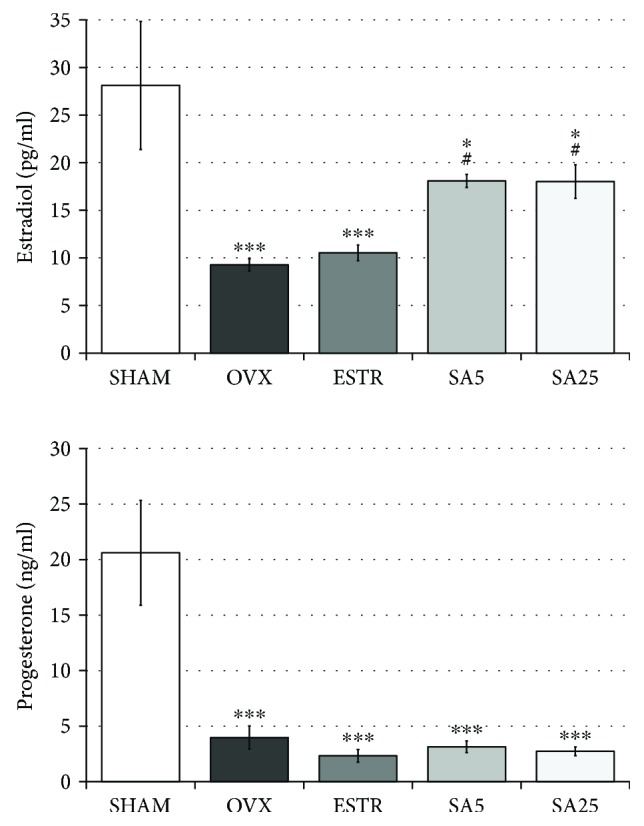
Effect of estradiol and sinapic acid on the serum concentration of estradiol and progesterone in ovariectomized rats. Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to ovariectomized rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results. ∗p ≤ 0.05, ∗∗∗p < 0.001: significantly different from the SHAM control rats. #p ≤ 0.05: significantly different from the OVX control rats.
3.3. Effect of Estradiol and Sinapic Acid on the Glucose Homeostasis Parameters in the Serum
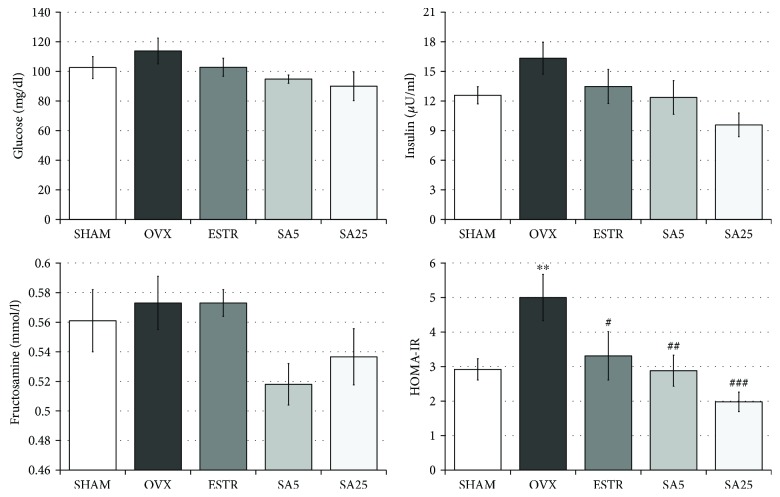
In the OVX control rats, no statistically significant changes in the glucose, insulin, and fructosamine concentrations were found; however, the HOMA-IR index significantly increased compared to that of the SHAM control rats. Estradiol and sinapic acid at both doses did not significantly affect the glucose, insulin, and fructosamine concentrations, but they led to a reduction in the HOMA-IR index, compared to that of the OVX control rats. Sinapic acid at a dose of 5 mg/kg showed a tendency to lower fructosamine concentration, while the higher dose (25 mg/kg) revealed a tendency to lower the insulin concentration in the serum of OVX rats (Figure 3).
Figure 3.
Effect of estradiol and sinapic acid on the serum parameters related to glucose homeostasis in ovariectomized rats. HOMA-IR: homeostasis model assessment of insulin resistance. Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to ovariectomized rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results. ∗∗p < 0.01: significantly different from the SHAM control rats. #p ≤ 0.05, ##p < 0.01, and ###p < 0.001: significantly different from the OVX control rats. No statistically significant differences in results for glucose, insulin, and fructosamine were demonstrated in ANOVA.
3.4. Effect of Estradiol and Sinapic Acid on the Concentration of Lipids in the Serum
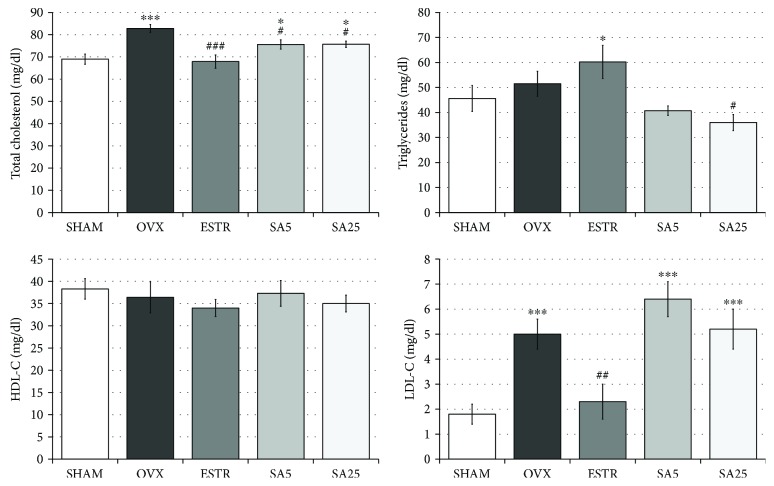
In the OVX control rats, a statistically significant increase in the total cholesterol and LDL-C concentrations was found, while no changes in the triglyceride and HDL-C concentrations were observed in comparison to those in the SHAM control rats. Administration of estradiol caused decreases in the total cholesterol and LDL-C concentrations as compared to the OVX control rats, without any significant effect on the triglyceride and HDL-C concentrations. Sinapic acid at both doses lowered the total cholesterol concentration and, when used at a dose of 25 mg/kg, it decreased the triglyceride concentration. No effect of sinapic acid on the HDL-C and LDL-C concentrations was observed (Figure 4).
Figure 4.
Effect of estradiol and sinapic acid on the serum lipid levels in ovariectomized rats: total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to ovariectomized rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results. ∗p ≤ 0.05, ∗∗∗p < 0.001: significantly different from the SHAM control rats. #p ≤ 0.05, ##p < 0.01, and ###p < 0.001: significantly different from the OVX control rats. No statistically significant differences in results for HDL-C were demonstrated in ANOVA.
3.5. Effect of Estradiol and Sinapic Acid on the Concentration of Nonenzymatic and Enzymatic Antioxidants in the Serum
In the group of the OVX control rats, a significant decrease in the GSH concentration, tendency to decrease the GSH/GSSG ratio, and nonsignificant decrease in TAC were found as compared to the SHAM control rats. There was no effect on the GSSG concentration. The SOD activity was significantly increased; an increase in the CAT activity was not significant. Administration of estradiol resulted in the increased GSH concentration and decreased SOD activity. Administration of sinapic acid at the lower dose resulted in a tendency to increase the GSH concentration, and its administration at the higher dose led to a statistically significant increase in this parameter in comparison to the OVX control rats. Only the higher sinapic acid dose reduced the activity of SOD. Other parameters did not change significantly after administration of estradiol or sinapic acid (Table 2).
Table 2.
Effect of estradiol and sinapic acid on the serum concentrations of nonenzymatic antioxidants and the serum activity of antioxidative enzymes.
| Parameter/group | SHAM | OVX | ESTR | SA5 | SA25 |
|---|---|---|---|---|---|
| GSH (nmol/ml) | 1.197 ± 0.032 | 1.090 ± 0.024∗ | 1.187 ± 0.041# | 1.183 ± 0.033 | 1.239 ± 0.026## |
| GSSG (nmol/ml) | 0.305 ± 0.013 | 0.357 ± 0.030 | 0.317 ± 0.027 | 0.377 ± 0.024 | 0.324 ± 0.029 |
| GSH/GSSG | 3.994 ± 0.245 | 3.161 ± 0.266 | 3.892 ± 0.356 | 3.231 ± 0.297 | 4.060 ± 0.532 |
| TAC (μmol/ml) | 1.27 ± 0.18 | 0.89 ± 0.06 | 1.07 ± 0.13 | 1.18 ± 0.21 | 0.94 ± 0.04 |
| SOD (U/mg of protein) | 5.22 ± 0.19 | 6.20 ± 0.34∗∗ | 5.35 ± 0.15## | 5.87 ± 0.14∗ | 5.57 ± 0.16# |
| CAT (nmol/min/mg of protein) | 0.52 ± 0.08 | 0.74 ± 0.19 | 0.60 ± 0.08 | 0.70 ± 0.13 | 0.63 ± 0.10 |
Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to rats once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats; GSH: reduced glutathione; GSSG: oxidized glutathione; TAC: total antioxidant capacity; SOD: superoxide dismutase; CAT: catalase. Results are presented as the mean ± SEM. The level of TAC is presented in Trolox equivalents. One unit of SOD was the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results. ∗p ≤ 0.05, ∗∗p < 0.01: significantly different from the SHAM control rats. # p ≤ 0.05, ##p < 0.01: significantly different from the OVX control rats. No statistically significant differences in results for GSSG, GSH/GSSG ratio, TAC, and CAT were demonstrated in ANOVA.
3.6. Effect of Estradiol and Sinapic Acid on the Concentration of Oxidative Damage Indicators in the Serum
Estrogen deficiency in the OVX control rats resulted in insignificant increases in the concentrations of TBARS, AOPP, and PCG compared to those in the SHAM control rats. Administration of estradiol did not significantly affect the level of the oxidative damage parameters investigated, although there was a tendency to decrease the PCG concentration in comparison with the OVX control rats. Sinapic acid at both doses significantly lowered the AOPP concentration in the OVX rats but did not cause any significant changes in the concentration of PCG. Sinapic acid at both doses showed a tendency to lower the concentration of TBARS in the OVX rats (Figure 5).
Figure 5.
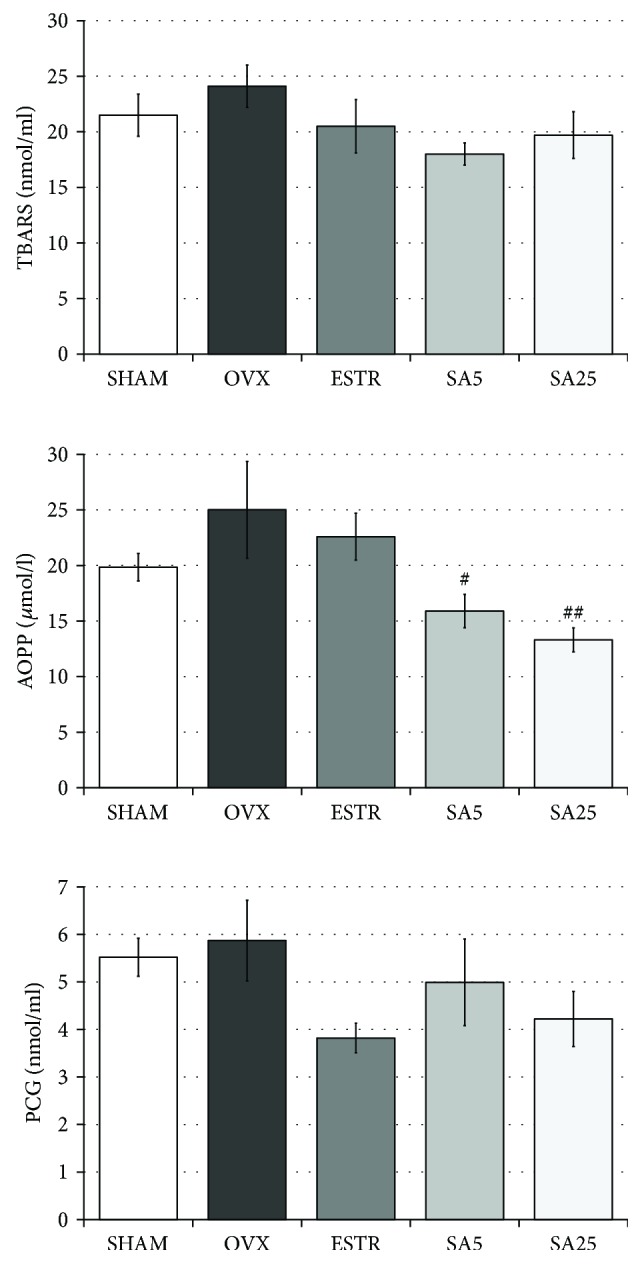
Effect of estradiol and sinapic acid on the serum concentrations of oxidative damage markers: thiobarbituric acid-reactive substances (TBARS), advanced oxidation protein products (AOPP), and protein carbonyl groups (PCG) in ovariectomized rats. Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to ovariectomized rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. The concentration of AOPP is presented in chloramine T equivalents. One-way ANOVA followed by Fisher's LSD test was used for evaluation of statistical significance of the results. #p ≤ 0.05, ##p < 0.01: significantly different from the OVX control rats. No statistically significant differences in results for TBARS and PCG were demonstrated in ANOVA.
3.7. Effect of Estradiol and Sinapic Acid on the IL-18 Concentration in the Serum
In the OVX control rats, a tendency to decrease the IL-18 concentration, compared to the SHAM control rats, was observed. Administration of estradiol and sinapic acid at both doses resulted in a statistically insignificant increase in the IL-18 concentration in comparison with the OVX control rats (Figure 6).
Figure 6.
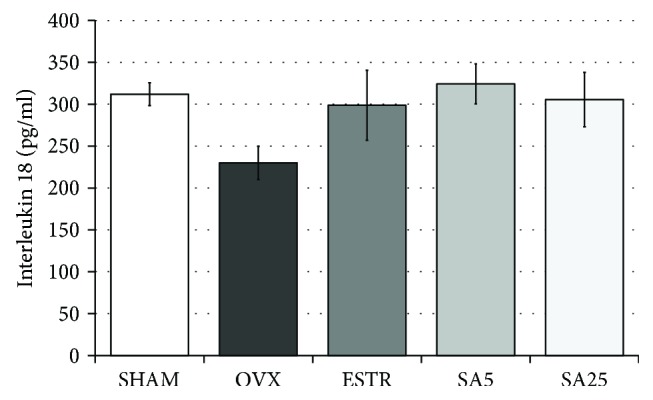
Effect of estradiol and sinapic acid on the serum concentration of interleukin 18 (IL-18) in ovariectomized rats. Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA was used for evaluation of statistical significance of the results. No statistically significant differences were demonstrated in ANOVA.
3.8. Effect of Estradiol and Sinapic Acid on the Serum Biochemical Markers of Liver and Kidney Function
Estrogen deficiency did not alter the AST or ALT activity as well as the concentration of uric acid, urea, and creatinine in comparison with that of the SHAM control rats. Administration of estradiol or sinapic acid at both doses did not affect these parameters, when compared to the OVX control rats (Table 3).
Table 3.
Effect of estradiol and sinapic acid on the serum biochemical markers of liver and kidney function in ovariectomized rats.
| Parameter/group | SHAM | OVX | ESTR | SA5 | SA25 |
|---|---|---|---|---|---|
| AST (U/l) | 39.22 ± 3.38 | 34.72 ± 2.78 | 35.83 ± 3.62 | 38.14 ± 4.08 | 40.35 ± 3.73 |
| ALT (U/l) | 23.68 ± 2.19 | 23.52 ± 1.38 | 22.78 ± 1.83 | 23.69 ± 2.08 | 28.38 ± 3.61 |
| Uric acid (mg/dl) | 1.13 ± 0.07 | 1.18 ± 0.17 | 1.10 ± 0.10 | 0.86 ± 0.06 | 1.00 ± 0.06 |
| Urea (mg/dl) | 25.72 ± 4.74 | 35.36 ± 4.19 | 38.45 ± 5.61 | 32.46 ± 3.63 | 37.49 ± 2.46 |
| Creatinine (mg/dl) | 0.449 ± 0.022 | 0.476 ± 0.019 | 0.431 ± 0.035 | 0.472 ± 0.031 | 0.415 ± 0.053 |
Sinapic acid at doses of 5 mg/kg (SA5) and 25 mg/kg (SA25) or estradiol ((ESTR) 0.2 mg/kg) was administered orally to rats, once daily for 28 days. SHAM: sham-operated control rats; OVX: ovariectomized control rats. Results are presented as the mean ± SEM. One-way ANOVA was used for evaluation of statistical significance of the results. No statistically significant differences in results for AST, ALT, uric acid, urea, and creatinine were demonstrated in ANOVA.
4. Discussion
In the present study, attempting to investigate the effect of sinapic acid on biochemical parameters related to glucose and lipid metabolism as well as oxidative stress in the blood serum in conditions of estrogen deficiency, the rats were administered two doses of sinapic acid: 5 and 25 mg/kg p.o. The content of sinapic acid in food products is reported to be very diverse. For instance, it varies from 0.26 μg/g to 450.3 μg/g in fruits, from 0.5 μg/g to 180.1 μg/g in vegetables, and from 0.07 to 56.05 μg/g in cereals [22]. Assuming a daily intake of 150–250 g of vegetables or fruits rich in sinapic acid by an adult human weighing 70 kg and taking into consideration much faster metabolism in rats [41], it can be assumed that the dose of 5 mg/kg corresponds to maximum human doses achievable by a high dietary intake. The higher dose used in this study (25 mg/kg) was more effective in lowering glucose than the 5 mg/kg dose in rats with experimental type 1 diabetes [27].
In the present study, 5 weeks after performing the bilateral ovariectomy, decreases in the serum levels of sex hormones (estradiol and progesterone) and characteristic changes in estrogen-dependent organ mass (a decreased uterine mass and increased thymus mass), as well as an increased body mass gain, were demonstrated, indicating that the rats were estrogen deficient. Typical disorders of carbohydrate metabolism (insulin resistance) and lipid homeostasis (increases in the total cholesterol and LDL-C concentrations) were observed, consistent with literature data [21, 42, 43].
Although 24 h after the last estradiol administration its serum level did not differ from that of the control rats (due to the fact that the free estradiol half-life in rat circulation is only 2 hours [44]), the estrogenic effect was manifested by an increase in the uterus mass and a decrease in the thymus mass. Administration of estradiol at a dose 0.2 mg/kg p.o. for 4 weeks had a counteracting effect on all the abovementioned disturbances of carbohydrate and lipid metabolism resulting from estrogen deficiency, consistent with the literature data [45, 46].
After administration of sinapic acid at both doses, there was an increase in the serum estradiol concentration, without effect on the progesterone concentration. The increase in the estradiol concentration is consistent with the results of our previous study [21], in which we demonstrated that another phenolic acid (caffeic acid; 10 mg/kg p.o. daily for 28 days) increased the estradiol level in the serum of estrogen-deficient rats. This suggests the possibility of enhancing the estradiol synthesis in extraovarian tissues (muscle, adipose tissue) by different phenolic acids. Both caffeic acid and sinapic acid are derivatives of hydroxycinnamic acid: 3,4-dihydroxycinnamic acid (caffeic acid) and 3,5-dimethoxy-4-hydroxycinnamic acid (sinapic acid). Interestingly, administration of sinapic acid did not lead to any estrogen-specific changes in the estrogen-dependent organ mass (such as the increased uterine mass or decreased thymus mass). The uterine mass in ovariectomized rats receiving sinapic acid even showed a strong tendency to decrease, which in turn may indicate antiestrogenic activity in the uterus.
In our study, sinapic acid reduced insulin resistance determined by the calculated HOMA-IR index, which is widely used to assess insulin sensitivity in humans and experimental animals [8, 36, 37]. Reduction of this parameter both by estradiol and sinapic acid may indicate that sinapic acid acts via estrogen pathways. The beneficial effect of sinapic acid on glucose homeostasis was previously demonstrated in rat models of type 1 diabetes induced by streptozotocin and type 2 diabetes induced by a fructose-rich diet [27]. In type 1 diabetes, sinapic acid reduced the glucose concentration in plasma and increased GLUT4 gene expression in skeletal muscle [27]. In type 2 diabetes, sinapic acid increased the sensitivity of cells to insulin [27].
In this study, administration of sinapic acid at both doses reduced the total cholesterol concentration and, at the higher dose, it also lowered the triglyceride concentration in ovariectomized rats. The results concerning the lowering of the level of total cholesterol are consistent with our previous study on other phenolic acids (caffeic, p-coumaric, and chlorogenic acids) [21]. Also the beneficial effect of sinapic acid on the lipid profile has been demonstrated in the isoproterenol-induced myocardial infarction model [28] and L-NAME-induced hypertension [29]. In those models, sinapic acid reduced the activity of HMG-CoA reductase (the rate-controlling enzyme in the cholesterol synthesis pathway) in the liver [28, 29] and serum [29]. However, in the present study, no effect of sinapic acid on the LDL-C serum concentration was observed, as opposed to estradiol.
In order to broaden the knowledge about mechanisms of action of sinapic acid on the development of metabolic disorders induced by estrogen deficiency, the studies on oxidative stress parameters in the serum were carried out. It is suggested that estradiol is protective against oxidative damage prior to menopause [9]. Numerous in vivo studies performed in the rat model of menopause induced by bilateral ovariectomy have shown that, under estrogen deficiency, the oxidative-antioxidative balance is disturbed, which manifests in the changes in antioxidative parameters, both enzymatic and nonenzymatic, as well as in oxidative damage parameters in various organs [42, 47–54]. However, the number of reports on the measurement of those parameters in the serum of ovariectomized experimental animals is less numerous [42, 54–58].
The most commonly studied enzyme markers of antioxidative abilities are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). These enzymes form an integrated, very sensitive, and specific antioxidant system [59]. SOD participates in the neutralization of superoxide anion radicals, while CAT and GPx break down the hydrogen peroxide formed as a result of SOD activity. In our study, in the OVX control rats, there were increases in the SOD and CAT activities, consistent with the results of previous studies [55]. However, it should be pointed out that there are also reports indicating the reduction of activity of these enzymes in the serum of ovariectomized rats [56]. GSH (reduced glutathione), an important nonenzymatic antioxidant, is a major nonproteinaceous thiol, which protects proteins against oxidation [60]. In our study, the concentration of GSH in the serum of estrogen-deficient rats decreased, which is consistent with previous observations [55, 57]. Moreover, the serum level of TAC, statistically insignificantly decreased. Similar results in ovariectomized rats have been reported earlier [58].
The oxidative damage parameters of lipids (TBARS) and protein (PCG, AOPP) in the serum were also evaluated. Under the influence of estrogen deficiency, in animal models, the concentration of TBARS usually increases [42, 43, 54], although there are also publications indicating the lack of changes in TBARS [55]. The concentration of PCG also increases in estrogen deficiency [42]; to our knowledge, there are no reports describing the effect of estrogen deficiency on the AOPP concentration in laboratory animals. In our experiment, 5 weeks after ovariectomy, the concentrations of all oxidative damage indicators examined (TBARS, PCG, and AOPP) insignificantly increased.
In the present study, the effects of sinapic acid and estradiol on some antioxidant parameters were similar. Under the influence of sinapic acid and estradiol, the concentration of GSH in the serum increased, and, in case of estradiol and the higher dose of sinapic acid, the activity of SOD decreased. Those particular activities of sinapic acid may partly result from an increase in the serum estradiol levels.
On the other hand, sinapic acid, unlike estradiol, improved one of the oxidative damage parameters—AOPP. Apart from being markers of oxidative damage, AOPP are recognized as markers of inflammation [40, 61]. AOPP bind to RAGE receptor, which is the receptor for advanced glycation products (AGEs). Stimulation of this receptor results in production of proinflammatory cytokines and adhesion molecules, which exacerbates inflammation and activates NADPH oxidase, which in turn induces ROS formation and intensification of oxidative stress [62]. The decrease of the AOPP concentration in the serum of ovariectomized rats under the influence of sinapic acid indicates the potential of sinapic acid to reduce oxidative stress and exert anti-inflammatory activity. Another manifestation of antioxidative properties of sinapic acid is a tendency to decrease the TBARS concentration in the serum of ovariectomized rats. The antioxidant activity of sinapic acid may be due to the fact that the hydroxycinnamic acid derivatives neutralize free radicals by liberating a hydrogen atom followed by the formation of a phenoxy radical. The resulting radical is stabilized by the conjugated system of the arene and the alkenyl carboxylate side chain [23, 24].
In addition to the oxidative stress parameters, the concentration of a proinflammatory cytokine IL-18 was examined. IL-18 was included in the research because its concentration was reported to be estradiol dependent [63]. However, the role of IL-18 in metabolic syndrome, diabetes, or obesity is not clear [64–68]. In our study, in the ovariectomized rats, which were characterized by increased body mass and insulin resistance, a reduced concentration of IL-18 was demonstrated and administration of estradiol caused an insignificant increase in the IL-18 concentration, while administration of sinapic acid (5 mg/kg) resulted in a strong tendency to increase the level of IL-18 in relation to the ovariectomized controls. Similarly, Russell et al. [63] reported a slight increase in IL-18 levels in estradiol-treated ovariectomized rats fed a standard diet. However, an estradiol-induced decrease in IL-18 concentration was demonstrated in ovariectomized rats fed an isoflavone-free diet [63]. The present study was performed in rats fed a standard laboratory diet containing soy. Taking into consideration the abovementioned results of Russell et al. [63], it is possible that the effects of sinapic acid on the IL-18 concentration and other parameters observed in the present study also may depend on the diet. Such a possibility should be taken into account because, in case of caffeic acid, the estradiol increasing effect depended on the diet [21, 36]. Although administration of caffeic acid (10 mg/kg p.o.) in ovariectomized rats fed standard laboratory diet resulted in an increase in the serum estradiol concentration [21], the administration of caffeic acid (5 and 50 mg/kg p.o.) did not increase the estradiol concentration in rats fed a soy-free diet (and with low content of phenolic acids) [36]. Results of the present study suggest that more data on the impact of food phytoestrogens on the activity of other diet components or medicines are needed.
5. Conclusion
In conclusion, administration of sinapic acid to ovariectomized rats reduced the concentration of total cholesterol, triglyceride, and HOMA-IR index, and also normalized some serum parameters of antioxidative abilities and oxidative damage, which were disordered by estrogen deficiency. The effects of sinapic acid could partly be due to an increase in the serum estradiol concentration. However, differential effects of sinapic acid and estradiol (e.g., on the body mass gain and uterine mass, the serum concentrations of triglycerides, LDL-C, and AOPP) indicate that sinapic acid may also act according to mechanisms other than estrogenic.
Acknowledgments
The study was financed by Medical University of Silesia, Katowice, Poland.
Data Availability
Data will be made available upon request.
Conflicts of Interest
The authors state that there is no conflict of interest.
References
- 1.Mauvais-Jarvis F., Clegg D. J., Hevener A. L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ropero A., Alonso-Magdalena P., Quesada I., Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73(9-10):874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 3.López M., Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in Endocrinology & Metabolism. 2015;26(8):411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F., Manson J. E., Stevenson J. C., Fonseca V. A. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocrine Reviews. 2017;38(3):173–188. doi: 10.1210/er.2016-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creatsas G., Christodoulakos G., Lambrinoudaki I. Cardiovascular disease: screening and management of the a-symptomatic high-risk post-menopausal woman. Maturitas. 2005;52(Supplement 1):32–37. doi: 10.1016/j.maturitas.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Sharma G., Mauvais-Jarvis F., Prossnitz E. R. Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. The Journal of Steroid Biochemistry and Molecular Biology. 2018;176:31–37. doi: 10.1016/j.jsbmb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyoy A., Guerra-Araiza C., Camacho-Arroyo I. Metabolism regulation by estrogens and their receptors in the central nervous system before and after menopause. Hormone and Metabolic Research. 2016;48(8):489–496. doi: 10.1055/s-0042-110320. [DOI] [PubMed] [Google Scholar]
- 8.Cagnacci A., Cannoletta M., Palma F., Bellafronte M., Romani C., Palmieri B. Relation between oxidative stress and climacteric symptoms in early postmenopausal women. Climacteric. 2015;18(4):631–636. doi: 10.3109/13697137.2014.999659. [DOI] [PubMed] [Google Scholar]
- 9.Cervellati C., Bergamini C. M. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clinical Chemistry and Laboratory Medicine. 2016;54(5):739–753. doi: 10.1515/cclm-2015-0807. [DOI] [PubMed] [Google Scholar]
- 10.Manolagas S. C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocrine Reviews. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths K., Aggarwal B., Singh R., Buttar H., Wilson D., de Meester F. Food antioxidants and their anti-inflammatory properties: a potential role in cardiovascular diseases and cancer prevention. Diseases. 2016;4(4):p. 28. doi: 10.3390/diseases4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapa A., Carroll N. Dietary modulation of oxidative stress in Alzheimer’s disease. International Journal of Molecular Sciences. 2017;18(7):p. 1583. doi: 10.3390/ijms18071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sankar P., Zachariah B., Vickneshwaran V., Jacob S. E., Sridhar M. G. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: the molecular mechanisms. Experimental Gerontology. 2015;63:67–75. doi: 10.1016/j.exger.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y., Li S., Zhang P., et al. Soy isoflavone protects myocardial ischemia/reperfusion injury through increasing endothelial nitric oxide synthase and decreasing oxidative stress in ovariectomized rats. Oxidative Medicine and Cellular Longevity. 2016;2016:14. doi: 10.1155/2016/5057405.5057405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y., Sullivan J. C., Schreihofer D. A. Dietary genistein and equol (4′, 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010;299(3):R871–R877. doi: 10.1152/ajpregu.00031.2010. [DOI] [PubMed] [Google Scholar]
- 16.Evsen M. S., Ozler A., Gocmez C., et al. Effects of estrogen, estrogen/progesteron combination and genistein treatments on oxidant/antioxidant status in the brain of ovariectomized rats. European Review for Medical and Pharmacological Sciences. 2013;17(14):1869–1873. [PubMed] [Google Scholar]
- 17.Heleno S. A., Martins A., Queiroz M. J. R. P., Ferreira I. C. F. R. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chemistry. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 18.Khan F. A., Maalik A., Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. Journal of Food and Drug Analysis. 2016;24(4):695–702. doi: 10.1016/j.jfda.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang N., Kitts D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2016;8(1):p. 16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampa M., Alexaki V. I., Notas G., et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Research. 2004;6(2):R63–R74. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zych M., Folwarczna J., Trzeciak H. I. Natural phenolic acids may increase serum estradiol level in ovariectomized rats. Acta Biochimica Polonica. 2009;56(3):503–507. [PubMed] [Google Scholar]
- 22.Nićiforović N., Abramovič H. Sinapic acid and its derivatives: natural sources and bioactivity. Comprehensive Reviews in Food Science and Food Safety. 2014;13(1):34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 23.Chen C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/3571614.3571614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hameed H., Aydin S., Başaran N. Sinapic acid: is it safe for humans? FABAD Journal of Pharmaceutical Sciences. 2016;41(1):p. 39. [Google Scholar]
- 25.Yun K.-J., Koh D.-J., Kim S.-H., et al. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. Journal of Agricultural and Food Chemistry. 2008;56(21):10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 26.Kanchana G., Shyni W. J., Rajadurai M., Periasamy R. Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. Global Journal of Pharmacology. 2011;5(1):33–39. [Google Scholar]
- 27.Cherng Y. G., Tsai C. C., Chung H. H., Lai Y. W., Kuo S. C., Cheng J. T. Antihyperglycemic action of sinapic acid in diabetic rats. Journal of Agricultural and Food Chemistry. 2013;61(49):12053–12059. doi: 10.1021/jf403092b. [DOI] [PubMed] [Google Scholar]
- 28.Roy S. J., Mainzen Prince P. S. Protective effects of sinapic acid on cardiac hypertrophy, dyslipidaemia and altered electrocardiogram in isoproterenol-induced myocardial infarcted rats. European Journal of Pharmacology. 2013;699(1–3):213–218. doi: 10.1016/j.ejphar.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Silambarasan T., Manivannan J., Raja B., Chatterjee S. Prevention of cardiac dysfunction, kidney fibrosis and lipid metabolic alterations in L-NAME hypertensive rats by sinapic acid—role of HMG-CoA reductase. European Journal of Pharmacology. 2016;777:113–123. doi: 10.1016/j.ejphar.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Shin D. S., Kim K. W., Chung H. Y., Yoon S., Moon J. O. Effect of sinapic acid against dimethylnitrosamine-induced hepatic fibrosis in rats. Archives of Pharmacal Research. 2013;36(5):608–618. doi: 10.1007/s12272-013-0033-6. [DOI] [PubMed] [Google Scholar]
- 31.Ansari M. A., Raish M., Ahmad A., et al. Sinapic acid ameliorate cadmium-induced nephrotoxicity: in vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-κB downregulation. Environmental Toxicology and Pharmacology. 2017;51:100–107. doi: 10.1016/j.etap.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Lee H. E., Kim D. H., Park S. J., et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease. Pharmacology Biochemistry and Behavior. 2012;103(2):260–266. doi: 10.1016/j.pbb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Zare K., Eidi A., Roghani M., Rohani A. H. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metabolic Brain Disease. 2015;30(1):205–213. doi: 10.1007/s11011-014-9604-6. [DOI] [PubMed] [Google Scholar]
- 34.Kim D. H., Yoon B. H., Jung W. Y., et al. Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology. 2010;59(1-2):20–30. doi: 10.1016/j.neuropharm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Yoon B. H., Jung J. W., Lee J. J., et al. Anxiolytic-like effects of sinapic acid in mice. Life Sciences. 2007;81(3):234–240. doi: 10.1016/j.lfs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Folwarczna J., Pytlik M., Zych M., et al. Effects of caffeic and chlorogenic acids on the rat skeletal system. European Review for Medical and Pharmacological Sciences. 2015;19(4):682–693. [PubMed] [Google Scholar]
- 37.Akarte A. S., Srinivasan B. P., Gandhi S. A novel long acting DPP-IV inhibitor PKF-275-055 stimulates β-cell proliferation resulting in improved glucose homeostasis in diabetic rats. Biochemical Pharmacology. 2012;83(2):241–252. doi: 10.1016/j.bcp.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Ali M. A., El-Abhar H. S., Kamel M. A., Attia A. S. Antidiabetic effect of galantamine: novel effect for a known centrally acting drug. PLoS One. 2015;10(8, article e0134648) doi: 10.1371/journal.pone.0134648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Witko-Sarsat V., Friedlander M., Capeillère-Blandin C., et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International. 1996;49(5):1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 41.Shin J.-W., Seol I.-C., Son C.-G. Interpretation of animal dose and human equivalent dose for drug development. The Journal of Korean Oriental Medicine. 2010;31(3):1–7. [Google Scholar]
- 42.Morrone M. d. S., Schnorr C. E., Behr G. A., et al. Curcumin supplementation decreases intestinal adiposity accumulation, serum cholesterol alterations, and oxidative stress in ovariectomized rats. Oxidative Medicine and Cellular Longevity. 2016;2016:12. doi: 10.1155/2016/5719291.5719291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sivasinprasasn S., Sa-Nguanmoo P., Pratchayasakul W., Kumfu S., Chattipakorn S. C., Chattipakorn N. Obese-insulin resistance accelerates and aggravates cardiometabolic disorders and cardiac mitochondrial dysfunction in estrogen-deprived female rats. Age. 2015;37(2):p. 28. doi: 10.1007/s11357-015-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theodorsson A., Hilke S., Rugarn O., Linghammar D., Theodorsson E. Serum concentrations of 17β‐estradiol in ovariectomized rats during two times six weeks crossover treatment by daily injections in comparison with slow‐release pellets. Scandinavian Journal of Clinical and Laboratory Investigation. 2005;65(8):699–706. doi: 10.1080/00365510500375206. [DOI] [PubMed] [Google Scholar]
- 45.Lampert C., Arcego D. M., Laureano D. P., et al. Effect of chronic administration of tamoxifen and/or estradiol on feeding behavior, palatable food and metabolic parameters in ovariectomized rats. Physiology & Behavior. 2013;119:17–24. doi: 10.1016/j.physbeh.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Abbas A. M., Elsamanoudy A. Z. Effects of 17β-estradiol and antioxidant administration on oxidative stress and insulin resistance in ovariectomized rats. Canadian Journal of Physiology and Pharmacology. 2011;89(7):497–504. doi: 10.1139/y11-053. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed H. H., Estefan S. F., Mohamd E. M., Farrag A. E.-R. H., Salah R. S. Does melatonin ameliorate neurological changes associated with Alzheimer’s disease in ovariectomized rat model? Indian Journal of Clinical Biochemistry. 2013;28(4):381–389. doi: 10.1007/s12291-012-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno F. N., Campos-Shimada L. B., Costa S. C. d., et al. Vitex agnus-castus L. (Verbenaceae) improves the liver lipid metabolism and redox state of ovariectomized rats. Evidence-Based Complementary and Alternative Medicine. 2015;2015:14. doi: 10.1155/2015/212378.212378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul Désiré D. D., Yolande Sandrine M. N., Danielle Claude B., et al. In vivo estrogenic-like activities of Gouania longipetala Hemsl. (Rhamnaceae) bark extracts in a post-menopause-like model of ovariectomized Wistar rats. Journal of Ethnopharmacology. 2015;168:122–128. doi: 10.1016/j.jep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 50.Hermoso D. A. M., Shimada L. B. C., Gilglioni E. H., et al. Melatonin protects female rats against steatosis and liver oxidative stress induced by oestrogen deficiency. Life Sciences. 2016;157:178–186. doi: 10.1016/j.lfs.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 51.Freire Machi J., da Silva Dias D., Freitas S., et al. Impact of aging on cardiac function in a female rat model of menopause: role of autonomic control, inflammation, and oxidative stress. Clinical Interventions in Aging. 2016;11:341–350. doi: 10.2147/CIA.S88441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Z., Wang Y., Zhu X., Ni X., Lu J. Exercise increases cystathionine-γ-lyase expression and decreases the status of oxidative stress in myocardium of ovariectomized rats. International Heart Journal. 2016;57(1):96–103. doi: 10.1536/ihj.15-099. [DOI] [PubMed] [Google Scholar]
- 53.Steagall R. J., Yao F., Shaikh S. R., Abdel-Rahman A. A. Estrogen receptor α activation enhances its cell surface localization and improves myocardial redox status in ovariectomized rats. Life Sciences. 2017;182:41–49. doi: 10.1016/j.lfs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Ma R., Guo Y., et al. Antioxidant effect of Fructus Ligustri Lucidi aqueous extract in ovariectomized rats is mediated through Nox4-ROS-NF-κB pathway. Frontiers in Pharmacology. 2017;8:p. 266. doi: 10.3389/fphar.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Behr G. A., Schnorr C. E., Moreira J. C. F. Increased blood oxidative stress in experimental menopause rat model: the effects of vitamin A low-dose supplementation upon antioxidant status in bilateral ovariectomized rats. Fundamental & Clinical Pharmacology. 2012;26(2):235–249. doi: 10.1111/j.1472-8206.2010.00923.x. [DOI] [PubMed] [Google Scholar]
- 56.Yoo J. H., Liu Y., Kim H. S. Hawthorn fruit extract elevates expression of Nrf2/HO-1 and improves lipid profiles in ovariectomized rats. Nutrients. 2016;8(5):p. 283. doi: 10.3390/nu8050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munoz-Castaneda J., Muntane J., Munoz M., Bujalance I., Montilla P., Tunez I. Estradiol and catecholestrogens protect against adriamycin-induced oxidative stress in erythrocytes of ovariectomized rats. Toxicology Letters. 2006;160(3):196–203. doi: 10.1016/j.toxlet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Tahmasbi S., Heidarpour M., Jafari A. M., Mehrjerdi H. K. Effects of pomegranate seed oil on oxidative stress parameters and lipid profiles in ovariectomized rats. Iranian Journal of Veterinary Surgery. 2013;8(2):p. 19. [Google Scholar]
- 59.Skólmowska M., Kmieć M. Antioxidant enzymosomes – properties and application. Postępy Higieny i Medycyny Doświadczalnej. 2011;65:640–644. doi: 10.5604/17322693.962163. [DOI] [PubMed] [Google Scholar]
- 60.Dickinson D. A., Forman H. J. Glutathione in defense and signaling: lessons from a small thiol. Annals of the New York Academy of Sciences. 2002;973(1):488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 61.Piwowar A. Perspectives on the pharmacotherapy of diseases extending with advanced oxidation protein products participation. Postępy Higieny i Medycyny Doświadczalnej. 2014;68:1264–1275. doi: 10.5604/17322693.1127949. [DOI] [PubMed] [Google Scholar]
- 62.Piwowar A. Advanced oxidation protein products. Part I. Mechanism of the formation, characteristics and property. Polski Merkuriusz Lekarski. 2010;28(164):166–169. [PubMed] [Google Scholar]
- 63.Russell A. L., Grimes J. M., Cruthirds D. F., et al. Dietary isoflavone-dependent and estradiol replacement effects on body weight in the ovariectomized (OVX) rat. Hormone and Metabolic Research. 2017;49(6):457–465. doi: 10.1055/s-0043-108250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunological Reviews. 2018;281(1):138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zilverschoon G. R. C., Tack C. J., Joosten L. A. B., Kullberg B. J., van der Meer J. W. M., Netea M. G. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. International Journal of Obesity. 2008;32(9):1407–1414. doi: 10.1038/ijo.2008.109. [DOI] [PubMed] [Google Scholar]
- 66.Zaharieva E., Kamenov Z., Velikova T., Tsakova A., El-Darawish Y., Okamura H. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocrine Connections. 2018;7(1):179–185. doi: 10.1530/EC-17-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Netea M. G., Joosten L. A. B., Lewis E., et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature Medicine. 2006;12(6):650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 68.Murphy A. J., Kraakman M. J., Kammoun H. L., et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metabolism. 2016;23(1):155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request.