Abstract
Background/Aims
Middle East respiratory syndrome coronavirus (MERS-CoV) and Marburg virus (MARV) are among the World Health Organization's top 8 emerging pathogens. Both zoonoses share nonspecific early symptoms, a high lethality rate, and a reduced number of specific treatment options. Therefore, we evaluated extracorporeal virus and glycoprotein (GP) elimination by lectin affinity plasmapheresis (LAP).
Methods
For both MERS-CoV (pseudovirus) as well as MARV (GPs), 4 LAP devices (Mini Hemopurifiers, Aethlon Medical, San Diego, CA, USA) and 4 negative controls were tested. Samples were collected every 30 min and analyzed for reduction in virus infectivity by a flow cytometry-based infectivity assay (MERS-CoV) and in soluble GP content (MARV) by an immunoassay.
Results
The experiments show a time-dependent clearance of MERS-CoV of up to 80% within 3 h (pseudovirus). Up to 70% of MARV-soluble GPs were eliminated at the same time. Substantial saturation of the binding resins was detected within the first treatment hour.
Conclusion
MERS-CoV (pseudovirus) and MARV soluble GPs are eliminated by LAP in vitro. Considering the high lethality and missing established treatment options, LAP should be evaluated in vivo. Especially early initiation, continuous therapy, and timed cartridge exchanges could be of importance.
Keywords: Middle East respiratory syndrome coronavirus, Marburg virus, Extracorporeal purification, Lectin affinity plasmapheresis
Introduction

According to Bill Gates' address at the Munich Security Conference 2017, the next 10–15 years could witness a global pandemic taking more than 30 million victims in less than a year [1]. He therefore called for an accelerated development of new vaccines, therapeutics, and diagnostics for emerging pathogens. Among the World Health Organization's top 8 emerging pathogens are Middle East respiratory syndrome coronavirus (MERS-CoV) as well as Marburg virus (MARV) [2]. Besides a zoonotic transmission chain, their common features are nonspecific early symptoms, a high lethality rate, and a reduced number of effective treatment options [3, 4].
Middle East Respiratory Syndrome Coronavirus
MERS-CoV is an enveloped betacorona virus and the infectious agent of MERS, a severe acute respiratory distress syndrome, often accompanied by acute kidney injury (AKI) [5]. In the past, more than half of adult symptomatic MERS patients had to be admitted to intensive care units (ICU) because of respiratory failure as well as AKI (KDIGO 3): mechanical ventilation was necessary in 70–85% and renal replacement therapy (RRT) in 50–70% of ICU cases [6, 7]. Importantly, persisting bloodstream detection of MERS-CoV is seen in lethal cases, while the virus is cleared from blood in survivors [5, 8, 9, 10]. As of October 2017, the case fatality rate in Saudi Arabia is 41% [11].
Marburg Virus
MARV is, like Ebola virus (EBOV), an enveloped member of the Filovirus family and is the causal agent of Marburg Virus Disease (MVD) [12], a hemorrhagic fever with a case fatality rate of up to 90% [13]. Important characteristics are systemic viral replication, immunosuppression, and an abnormal inflammatory response finally leading to multiorgan failure [14]. This is initially facilitated by bloodstream dissemination to dendritic cells, monocytes, and macrophages. Early replication also takes place in the liver, before MARV spreads to the spleen, kidney, and gonads [13]. During the 2014 EBOV epidemic, AKI (KDIGO 3) was the strongest predictor for mortality besides viral load [15]. In addition, soluble filovirus glycoproteins (GP) appear to be involved in important pathological mechanisms [16]. Soluble filovirus GP can act as decoys for neutralization of antibodies [17] and can induce a shift toward non-neutralizing antibody formation (“antigenic subversion”) [18]. Also, EBOV shed GP have been shown to induce a massive release of cytokines by binding macrophages and dendritic cells and to increase vascular permeability [16]. This is mediated by toll-like receptor 4, which was also targeted by MARV GP [19].
Due to lack of established specific antiviral therapeutics or vaccines, the treatment for both MVD and MERS is still symptomatic [3, 4]. Therefore, we sought to evaluate lectin affinity plasmapheresis (LAP) for the reduction of virus and GP load from fluids.
Methods and Material
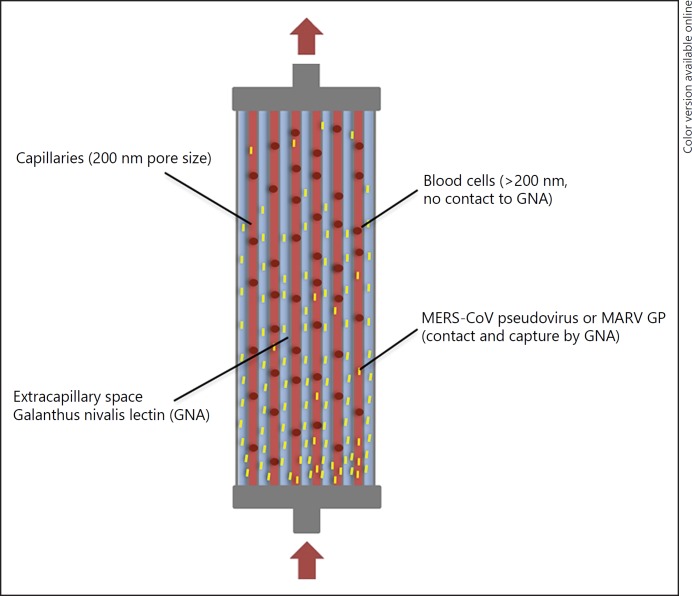
LAP combines plasma separation with virus capture by immobilized affinity agents in the extracapillary spaces of a plasma filter (Fig. 1 and US Patent 20120037564 A1). The method is based on affinity chromatography where the affinity agent defines the binding partner. It has been shown that Galanthus nivalis agglutinin from Galanthus nivalis (the common snowdrop) has a high affinity to mannose-rich GP [20]. These GP are present on enveloped viruses and can also be shed from virus-infected cells [16]. The plasmafilter's 200 nm pores allow plasma, viruses, and viral GP to travel alongside the filter's pressure difference (“starling flow”) in the extracapillary space without activating or harming blood cells. A high pressure at the blood inlet and low pressure at the blood outlet (created by the hollow fiber bundle resistance) allow the filtrate to exit the fibers in the proximal third of the device and binding of viruses and GP to the affinity matrix in the extracapillary space. Later the cleared plasma rejoins the cellular blood components next to the blood outlet. As plasma never leaves the device, no loss of plasma takes place. There was no indication in the human clinical data presented to the German medical device regulatory agency (special approval of LAP treatment for an Ebola patient in 2014) that beneficial biomolecules are being absorbed with a clinically significant level [21]. In addition, it was reported that only about 0.08% of total human plasma proteins are absorbed by LAP devices (primarily albumin, to a smaller amount IgG) [22].
Fig. 1.
LAP device. Galanthus nivalis (common snowdrop) lectin is placed in the extracapillary space of a standard plasma filter with 200 nm pore size. Enveloped viruses are affinity-captured by lectins. The purified plasma returns to the patient. LAP, lectin affinity plasmapheresis; MERS-CoV, middle east respiratory syndrome coronavirus; GNA, galanthus nivalis agglutinin; MARV GP, marburg virus glycoprotein.
Lentivirus particles were pseudotyped with MERS-CoV S-protein derived from a 3 plasmid pseudovirus system (MERS-CoV GP plasmid and lentiviral GagPol and enhanced green fluorescent protein transfer vectors). The MERS-EMC/2012 S-plasmid was provided by Prof. Christian Drosten (Institute of Virology, Charité, Berlin, Germany). MERS-CoV pseudovirus concentrations of 500,000/mL totaling 2.5 million viruses in complete medium (n = 3, DMEM with 10% fetal calf serum, penicillin/streptomycin and glutamine, Gibco Life Technologies, Darmstadt, Germany) as well as human donor serum (n = 1, #H3667, Sigma-Aldrich, Taufkirchen, Germany) were used for testing. LAP devices (Mini Hemopurifiers) were provided by Aethlon Medical Inc. (San Diego, CA, United States). Furthermore, negative controls (MicroKros mPES 0.2 µm cartridges, Spectrum Labs Inc., Breda, The Netherlands) were tested for the clearance of MERS-CoV pseudovirus as well as MARV GP (each n = 4).
MARV-Musoke GP was expressed from a plasmid obtained through BEI Resources (NIAID, NIH, Bethesda, Maryland, United States, vector pCAGGS containing Marburgvirus, Musoke GP with N-Terminal HA Tag, #NR-49353), expressed in HEK/293T cells and purified by immunoprecipitation using the HA Tag (IP Kit-HA Tag Immunomagnetic Beads, #TB100028-1, Sino Biological, Beijing, China). Afterwards, the GP were size controlled by Coomassie staining and quantified by immunoassay (MARV-GP antibody ELISA kit, Alpha Diagnostic Intl. Inc., San Antonio, TX, United States). Prior to the experiment, MARV GP was diluted to 1,500 ng/mL.
The extracorporeal circuit was made by MicroPerpex tubing (GE Healthcare, Freiburg, Germany) in an LKB 2232 MicroPerpex peristaltic pump (LKB, Bromma, Sweden). The columns were equilibrated with 5 mL of sterile phosphate-buffered saline before it came into contact with the samples and the pump was set to a rate of 1 mL/min. Samples were taken every 30 min. For in vivo usage, we refer to the detailed description of our successful LAP treatment of an Ebola patient in 2014 [21]. As there were no animal or human studies in the MERS-CoV/MARV-GP experiment, an approval of the Ethics Committee was not required. For evaluation of MERS-CoV clearance, each sample was used for the infection of epithelial-like tumorigenic cells (HUH7, obtained through ATCC, Manassas, VA, United States). The positive controls were done by infection of cells with virus supernatants prior to establishing the LAP circuit. Expression of the green fluorescent protein marker protein (as surrogate for the number of infected cells) was measured after 4 days by a flow cytometric infectivity assay (LSR Fortessa, Becton-Dickinson, Heidelberg, Germany). For MARV GP, the samples' GP concentration was measured by the immunoassay
Results
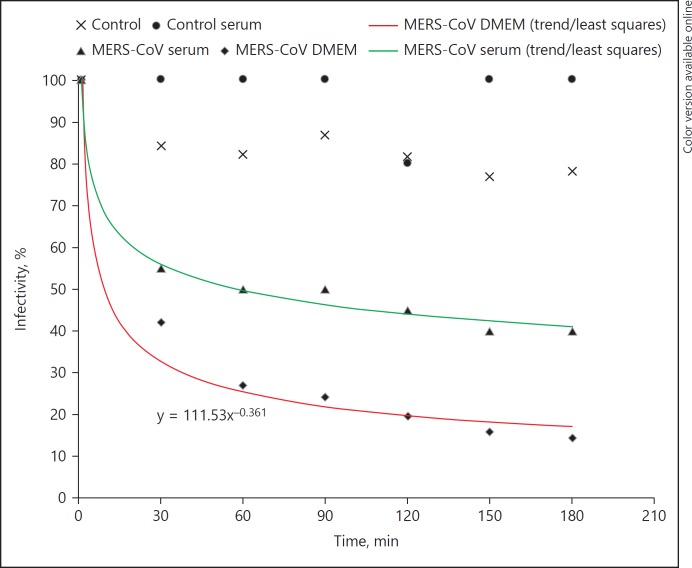
MERS-CoV pseudovirus was passed through LAP devices. Samples were taken every 30 min and used in a cytometry-based infectivity assay. The data from this study are summarized in Figure 2 and Table 1. The experiments show a time-dependent MERS-CoV pseudovirus reduction of infectivity from 100 to 20–40%, which in other terms is a clearance of 60–80% within 3 h. Most of the virus and GP was cleared within the first treatment hour. Negative controls show 0–20% reduction in infectivity, which can be attributed to nonspecific virus binding. A representative flow cytometry dot plot is shown in Figure 3.
Fig. 2.
MERS-CoV decrease in infectivity vs. time during LAP. The lower graph denotes the mean of 3 MERS-CoV pseudovirus experiments in complete medium. The upper graph shows a MERS-CoV pseudovirus serum experiment. Measurements were done by a flow cytometric infectivity assay. Controls (n = 4) were done with LAP devices without GNA. LAP, lectin affinity plasmapheresis; MERS-CoV, middle east respiratory syndrome coronavirus; GNA, galanthus nivalis agglutinin
Table 1.
MERS-CoV pseudovirus clearance by LAP (data for Fig. 2): % infectivity vs. time from 4 experiments. LAP I–III show MERS-CoV pseudovirus in complete medium, LAP IV (S) was virus in human donor serum
| Time, min | Infectivity, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| LAP I | Control I | LAP II | Control II | LAP III | Control III | LAP IV (S) | Control IV (S) | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 30 | 53.7 | 94.7 | 24 | 75 | 52.6 | 87 | 55.3 | 100 |
| 60 | 35 | 100 | 10.7 | 72 | 41.2 | 77 | 50.8 | 100 |
| 90 | 36.2 | 98.5 | 15.3 | 76 | 28.2 | 91 | 50.9 | 100 |
| 120 | 29.5 | 95.5 | 11.3 | 78 | 24.3 | 77 | 45.1 | 80 |
| 150 | 24.1 | 82.6 | 5.1 | 73 | 23.7 | 76 | 40.5 | 100 |
| 180 | 25 | 79.5 | 6.5 | 77 | 17.5 | 77 | 40.6 | 100 |
Fig. 3.
Representative flow cytometric dot plot: MERS-CoV pseudovirus infections in HUH7 cells. LAP 0 vs. 180 min. There was a marked reduction of infected cells after LAP treatment (GFP positive cells). Measurement was by a cytometry-based infectivity assay using MERS-CoV pseudovirus expressing GFP. LAP, lectin affinity plasmapheresis; MERS-CoV, middle east respiratory syndrome coronavirus; GFP, green fluorescent protein.
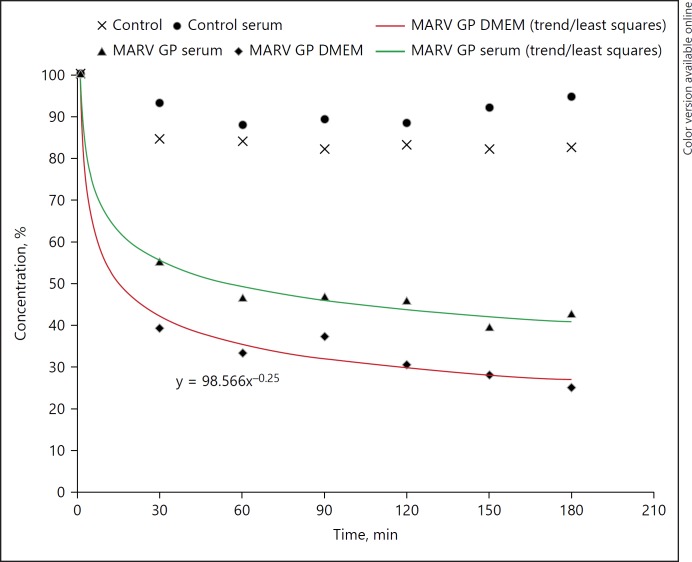
MARV GP at initially 1,500 ng/mL was circulated through LAP devices. Samples were taken every 30 min and MARV GP concentration quantified by an immunoassay. The data from these experiments are summarized in Figure 4 and Table 2. The results show a 50–70% reduction within 3 h. The negative controls show less than 20% reduction in MARV-GP concentration.
Fig. 4.
MARV-GP clearance by LAP: concentration vs. time (measurements by immunoassay). The lower graph denotes the mean of 3 MARV GP experiments in complete medium, the upper one shows a MARV GP serum experiment. The controls were done with LAP devices without GNA. LAP, lectin affinity plasmapheresis; MERS-CoV, middle east respiratory syndrome coronavirus; GNA, galanthus nivalis agglutinin; MARV GP, marburg virus glycoprotein.
Table 2.
MARV GP clearance by LAP (data for Fig. 4): GP concentration (%) vs. time from 4 experiments. LAP I–III show MARV GP in complete medium, LAP IV (S) was MARV GP in human donor serum. Controls (n = 4): LAP devices without GNA
| Time, min | Concentration, % |
|||||||
|---|---|---|---|---|---|---|---|---|
| LAP I | Control I | LAP II | Control II | LAP III | Control III | LAP IV (S) | Control IV (S) | |
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 30 | 37.5 | 79.9 | 39 | 83.5 | 42.3 | 89.8 | 55 | 93 |
| 60 | 27.3 | 82.4 | 34.6 | 81.1 | 38.6 | 89.1 | 46.5 | 87.8 |
| 90 | 34.2 | 78.1 | 39.4 | 86 | 38.2 | 83.2 | 46.9 | 89 |
| 120 | 28.5 | 81.2 | 30.4 | 83.1 | 32.5 | 86.4 | 45.8 | 88.1 |
| 150 | 20.8 | 76.8 | 24.6 | 85 | 39.3 | 86.1 | 39.6 | 91.8 |
| 180 | 17.8 | 78.5 | 23.3 | 83.8 | 34.6 | 86.5 | 42.6 | 94.4 |
Discussion
We investigated the use of LAP for clearance of MERS-CoV pseudotyped viruses as well as MARV GP and found evidence for a significant reduction in all experiments. Further reduction could likely be achieved by scaling up the Galanthus nivalis agglutinin amount in LAP devices or by setting up a timed device exchange interval. According to our measurements, a suitable time point would be after the near saturation interval, that is, after about 60 min. Considering our data from the first LAP treatment done for a patient affected by EBOV – more than 253 million EBOV copies, in addition to soluble viral GP, were eliminated safely [21, 23, 24] – LAP is feasible in vivo and already has a rationale especially during early infection [25]. These data have been preceeded by the successful treatment of viral hepatitis in dialysis patients with a combination therapy made of LAP and antiviral medications [26]. The virus elimination by LAP is fast and can also clear viral immunosuppressive molecules, that is, free viral proteins and fragments [27].
However, the key question is if there could be a relevance of plasma virus load reduction for patients with MERS-CoV or MARV infection.
Currently, no specific treatments for MERS or MVD are available [3, 4]. Standard of care is ICU support in a high containment facility (mandatory for MARV, preferential for MERS), especially calculated replacement of volume and electrolyte loss as well as RRT and/or respiratory support (respectively extracorporeal membrane oxygenation) and treatment of co-infections. Currently, many efforts for effective vaccines are made (MERS-CoV see e.g., [28], MARV, EBOV see e.g., [29]) and inter alia promising virus-like particles evaluated for immunization [30]. Also, existing medications like interferon-α2b, teicoplanin, or eritoran are considered for usage, convalescent plasma is given, and promising antiviral therapies are developed [19, 31, 32, 33]. Still, none of them has qualified for wide scale use up to now.
Pathophysiologically, MERS-CoV inhibits the host's early antiviral response by suppressing interferon signaling and provoking cytokine dysregulation [34, 35]. AKI is common after MERS-CoV infection [6, 7, 36, 37]. While kidney function appears to be normal in the early course, AKI may start between day 10 and 16 [36, 37] and is a mark of those patients with severe disease: up to 70% of ICU patients are dependent on RRT [6, 7]. This is in accordance with MERS-CoV producing almost thousand-fold more infective progeny in renal epithelial cells than in bronchial epithelial cells [38]. Taken together, renal involvement implies a preceding bloodstream-based dissemination. Yet, MERS-CoV blood kinetics are poorly understood [39]. Nevertheless, MERS-CoV RNA has been shown in an ICU patient in whole blood [8] with increasing virus load, respectively, decreasing cycle threshold values during the ICU stay (cycle threshold 35 to 29–30) and persisting presence in blood as well as in urine: from days 13 to 30 when the patient passed away because of refractory acute respiratory distress syndrome, AKI (KDIGO 3), and an impaired type I interferon response [5]. Another study classified MERS patients (n = 14) according to disease severity and mortality and found sustained viremia in plasma of all fatal (n = 5, average of 32,000 copies/mL) and severe cases (n = 4). In contrast, less severe cases had stronger antibody responses, less lymphopenia, and either no viremia or they cleared plasma until day 18 after symptom onset [9]. Also, it was reported (n = 21) that patients without traceable blood MERS-CoV on admission had a survival rate of about 90% compared to 40% in blood viral RNA-positive patients [10].
Marburg fever has a case fatality rate of up to 90%, but the pathogenesis remains poorly understood [14]. Important characteristics are systemic viral replication, immunosuppression, and an abnormal inflammatory response finally leading to multiorgan failure. However, it must be noted that visible pathological changes caused by MARV in single organs are not severe enough to explain the disease's fatal outcome [40]. Importantly, this outcome in filovirus infections is correlated with plasma virus load: fatal cases show a marked higher virus load compared to survivors [41, 42, 43, 44, 45]. An important feature of filovirus infections appears to be the deployment of soluble GP as decoys to subvert and escape the immune response [17]. Also, EBOV shed GP have been reported to be responsible for an extensive cytokine release and increase in vascular permeability [16]. Therefore, elimination of soluble GP could reduce inflammation and free up the available antibodies to fight remaining viruses.
LAP can be easily included in the existing RRT circuits, as most severe MERS cases are dependent on RRT [6, 7] and KDIGO stage 3 AKI is one of the strongest predictors for death in filovirus disease [15]. For Ebola treatment, we did incorporate the LAP device in the arterial line upstream of the dialyzer. Anticoagulation of the extracorporeal circuit was achieved using regional citrate anticoagulation [21]. Because of early virus and GP blood stream dissemination, an early initiation of LAP should be evaluated for mitigation or even suppression of systemic effects. In the event of coinfections or the need of cytokine respectively specific toxin elimination (e.g., CytoSorbent's CytoSorb®), the clearance of many enveloped viruses by LAP (Aethlon's Hemopurifier®) could be complemented by ongoing efforts of the exciting Dialysis-Like Therapeutics project [46], like adding specific bacterial and fungal elimination (e.g., Exthera's Seraph® Microbind®).
Conclusion
MERS-CoV (pseudovirus) and MARV-soluble GP are eliminated by LAP in vitro. Considering the high lethality and missing established treatment options, LAP should be evaluated in vivo. Especially early initiation, continuous therapy, and timed cartridge exchanges could be of importance.
Disclosure Statement
Support for this project was received in part from Aethlon Medical Inc., San Diego, CA, USA.
References
- 1.Gates B. Speech by Bill Gates at the 53rd Munich Security Conference. 2017 https://www.securityconference.de/aktivitaeten/munich-security-conference/msc-2017/reden/speech-by-bill-gates/ (accessed September 26, 2017) [Google Scholar]
- 2.Sweileh WM. Global research trends of World Health Organization's top eight emerging pathogens. Global Health. 2017;13:233. doi: 10.1186/s12992-017-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntosh K, Perlman S, Coronaviruses Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett's Infectious Disease Essentials. Philadelphia, Elsevier. 2017:p 215. [Google Scholar]
- 4.Geisbert TW, Marburg and Ebola hemorrhagic fevers Bennet JE, Dolin R, Blaser MJ, editors. Mandell, Douglas and Bennett's Infectious Disease Essentials. Philadelphia, Elsevier. 2017:p 227. [Google Scholar]
- 5.Poissy J, Goffard A, Parmentier-Decrucq E, Favory R, Kauv M, Kipnis E, Mathieu D, van der Werf S, Guery B. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J Clin Virol. 2014;61:275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senga M, Arabi YM, Fowler RA. Clinical spectrum of the Middle East respiratory syndrome coronavirus (MERS-CoV) J Infect Public Health. 2016;10:191–194. doi: 10.1016/j.jiph.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, Shalhoub S, et al. Critically Ill patients with the Middle East Respiratory Syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 8.Guery B, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, Vuotto F, Goffard A, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Park SJ, Cho SY, Cha RH, Jee HG, Kim G, et al. Viral RNA in Blood as Indicator of severe outcome in Middle East Respiratory Syndrome Coronavirus infection. Emerg Infect Dis. 2016;22:1813–1816. doi: 10.3201/eid2210.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ISID 2017 PRO/AH/EDR> MERS-CoV (64), published on October 12, 2017, Archive Number 20171012.5375933 http://www.promedmail.org/post/5375933 (accessed October 16, 2017) [Google Scholar]
- 12.Siegert R, Shu HL, Slenczka W, Peters D, Müller G. On the cause of a previously unknown human infection transmitted from monkeys. Dtsch Med Wochenschr. 1967;92:2341–2343. doi: 10.1055/s-0028-1106144. [DOI] [PubMed] [Google Scholar]
- 13.Feldmann H, Sanchez A, Geisbert TW, Filoviridae: Ebola -and Marburgvirus Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, Lippincott Williams & Wilkins. 2013:pp 923–956. [Google Scholar]
- 14.Mehedi M, Groseth A, Feldmann H, Ebihara H. Clinical aspects of Marburg hemorrhagic fever. Future Virol. 2011;6:1091–1106. doi: 10.2217/fvl.11.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt L, Gupta-Wright A, Simms V, Tamba F, Knott V, Tamba K, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15:1292–1299. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 16.Escudero-Pérez B, Volchkova VA, Dolnik O, Lawrence P, Volchkov VE. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog. 2014;10:e1004509. doi: 10.1371/journal.ppat.1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1–9. doi: 10.1016/j.jaut.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Mohan GS, Li W, Ye L, Compans RW, Yang C. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathog. 2012;8:e1003065. doi: 10.1371/journal.ppat.1003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younan P, Ramanathan P, Graber J, Gusovsky F, Bukreyev A. The toll-like receptor 4 antagonist eritoran protects mice from lethal filovirus challenge. MBio. 2017;8:pii:e00226-17. doi: 10.1128/mBio.00226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmood N, Hay AJ. An ELISA utilizing immobilised snowdrop lectin GNA for the detection of envelope glycoproteins of HIV and SIV. J Immunol Methods. 1992;151:9–13. doi: 10.1016/0022-1759(92)90101-x. [DOI] [PubMed] [Google Scholar]
- 21.Büttner S, Koch B, Dolnik O, Eickmann M, Freiwald T, Rudolf S, et al. Extracorporeal virus elimination for the treatment of severe Ebola virus disease – first experience with lectin affinity plasmapheresis. Blood Purif. 2014;38:286–291. doi: 10.1159/000375229. [DOI] [PubMed] [Google Scholar]
- 22.Tullis RH, Ichim T, Affinity hemodialysis and plasmapheresis . Dialysis. History, Development and Promise. In: Ing TS, Rahman MA, Kjellstrand CM, editors. Singapore: World Scientific; 2012. p. p 841. [Google Scholar]
- 23.Koch B, Büttner S, Geiger H. Schneeglöckchen zur Senkung der Viruslast bei lebensbedrohlichen Viruserkrankungen. Dtsch Med Wochenschr. 2016;141:1868–1871. doi: 10.1055/s-0042-118682. [DOI] [PubMed] [Google Scholar]
- 24.Koch B, Büttner S, Dolnik O, Eickmann M, Freiwald T, Rudolf S, et al. FP472glycoprotein and virus elimination by lectin affinity plasmapheresis. Nephrol Dial Transplant. 2015;30:iii229. [Google Scholar]
- 25.Ronco C. Ebola virus disease and blood purification techniques. Blood Purif. 2014;38:273–275. doi: 10.1159/000375257. [DOI] [PubMed] [Google Scholar]
- 26.Tullis RH, Duffin RP, Handley HH, Sodhi P, Menon J, Joyce JA, et al. Reduction of hepatitis C virus using lectin affinity plasmapheresis in dialysis patients. Blood Purif. 2009;27:64–69. doi: 10.1159/000167011. [DOI] [PubMed] [Google Scholar]
- 27.Tullis RH, Duffin RP, Ichim TE, Joyce JA, Levin NW. Modeling hepatitis C virus therapies combining drugs and lectin affinity plasmapheresis. Blood Purif. 2010;29:210–215. doi: 10.1159/000245649. [DOI] [PubMed] [Google Scholar]
- 28.Volz A, Kupke A, Song F, Jany S, Fux R, Shams-Eldin H, et al. Protective efficacy of recombinant modified vaccinia virus ankara delivering Middle East Respiratory Syndrome Coronavirus spike glycoprotein. J Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds P, Marzi A. Ebola and Marburg virus vaccines. Virus Genes. 2017;53:501–515. doi: 10.1007/s11262-017-1455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196:S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 31.Rougeron V, Feldmann H, Grard G, Becker S, Leroy EM. Ebola and Marburg haemorrhagic fever. J Clin Virol. 2015;64:111–119. doi: 10.1016/j.jcv.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabaan AA, Alahmed SH, Bazzi AM, Alhani HM. A review of candidate therapies for Middle East respiratory syndrome from a molecular perspective. J Med Microbiol. 2017;66:1261–1274. doi: 10.1099/jmm.0.000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou N, Pan T, Zhang J, Li Q, Zhang X, Bai C, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J Biol Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Ye F, Zhu N, Wang W, Deng Y, Zhao Z, et al. Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets. Sci Rep. 2015;5:17554. doi: 10.1038/srep17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel Coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 37.Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, Guggemos W, Kallies R, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13:745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckerle I, Müller MA, Kallies S, Gotthardt DN, Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al Johani S, Hajeer AH. MERS-CoV diagnosis: an update. J Infect Public Health. 2016;9:216–219. doi: 10.1016/j.jiph.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falzarano D, Feldmann H. Marburg virus; in: Mahy BWJ, van Regenmortel MHV (eds): Desk Encyclopedia of Human and Medical Virology. Oxford, Academic Press. 2010:pp 434–441. [Google Scholar]
- 41.Ajelli M, Merler S. Transmission potential and design of adequate control measures for Marburg hemorrhagic fever. PLoS One 2012 Dec 10. 7:e50948. doi: 10.1371/journal.pone.0050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewers EC, Pratt WD, Twenhafel NA, Shamblin J, Donnelly G, Esham H, et al. Natural history of aerosol exposure with Marburg virus in rhesus macaques. Viruses. 2016;8:87. doi: 10.3390/v8040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, et al. Biomarker correlates of survival in pediatric patients with Ebola virus disease. Emerg Infect Dis. 2014;20:1683–1690. doi: 10.3201/eid2010.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, et al. Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210:558–566. doi: 10.1093/infdis/jiu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knust B, Schafer IJ, Wamala J, Nyakarahuka L, Okot C, Shoemaker T, et al. Multidistrict outbreak of Marburg virus disease-Uganda, 2012. J Infect Dis. 2015;212((suppl 2)):S119–S128. doi: 10.1093/infdis/jiv351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DARPA 2017 Dialysis-Like Therapeutics (DLT) https://www.darpa.mil/program/dialysis-like-therapeutics* (accessed October 11, 2017)