 A metal-free C–B bond forming reaction of aryl halides under batch and continuous-flow conditions is described.
A metal-free C–B bond forming reaction of aryl halides under batch and continuous-flow conditions is described.
Abstract
A rapid, chemoselective and metal-free C–B bond-forming reaction of aryl iodides and bromides in aqueous solution at low temperatures was discovered. This reaction is amenable to batch and continuous-flow conditions and shows exceptional functional group tolerance and broad substrate scope regarding both the aryl halide and the borylating reagent. Initial mechanistic experiments indicated a photolytically generated aryl radical as the key intermediate.
Introduction
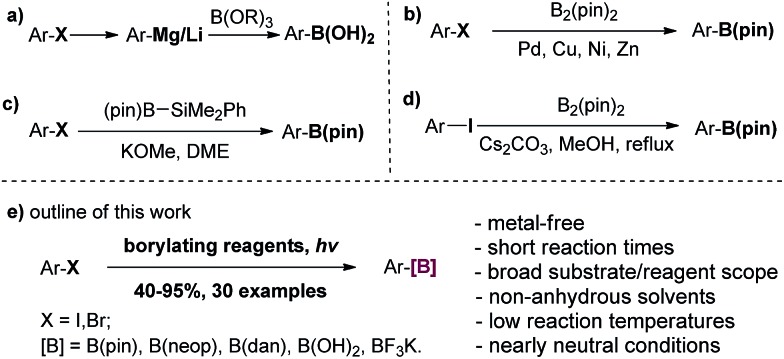
Arylboronic acids and esters have found broad applications in chemical, medicinal and materials sciences. In synthetic organic chemistry, in particular, they are versatile synthons for the formation of carbon–carbon or carbon–heteroatom bonds.1 Conventional methods for generating arylboron compounds involve reactions of arylmetallic intermediates with trialkyl borates, followed by transesterification or hydrolysis. These reactions suffer some major drawbacks such as limited functional group tolerance as well as the necessity of rigorous anhydrous conditions (Scheme 1a).2 In recent decades, transition metal-catalyzed borylation reactions using palladium, nickel, copper and zinc have emerged as highly useful methods for the conversion of C–X bonds to C–B bonds (Scheme 1b).3 More recently, direct C–H borylation methods based on transition-metal catalysts have also been developed.4 In order to reduce the costs and the amount of heavy metal residue in the final products, several transition-metal-free methods for C–B bond formation have been developed. Ito and coworkers discovered an alkali alkoxide-mediated borylation of aryl halides with a silylborane as the unique borylating reagent (Scheme 1c).5 Zhang and coworkers reported that aryl iodides could be borylated with 4.0 equivalents of bis(pinacolato)diboron in refluxing methanol using 2.0 equivalents of Ce2CO3 as the promoter. The reaction time ranged from several hours to days and the yields were generally moderate (Scheme 1d).6 Fernándes and Muñiz transformed diaryliodonium acetates to arylboronates under mild conditions.7 Using aryl amines as the starting material, Wang developed a mild and efficient Sandmeyer-type borylation process.8a–c Borylation of aryl diazonium salts8d–f and aryl triazenes8g has also been reported. In addition, innovative methods for direct C–H borylation under transition metal-free conditions have been reported,9 although the substrates were limited to either electron rich arenes or heterocycles, and air and moisture sensitive reagents were needed. Consequently, a practical, metal-free method that is rapid and effective, works under mild conditions with various readily available borylating reagents, shows high functional group tolerance and avoids strong acids, bases and hazardous reagents is still highly desirable. Herein, we wish to report our discovery and development of a new borylation reaction of aryl halides using light as a clean reagent (Scheme 1e).10
Scheme 1. Summary of borylation reactions of aryl halides and outline of this work.
Results and discussion
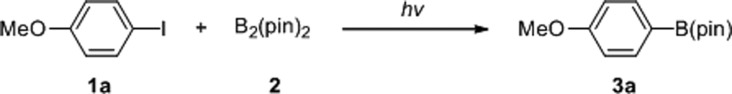
Initially, a solution of 4-iodoanisole (1a) and bis(pinacolato)-diboron (2) in acetonitrile was placed in a quartz test tube and irradiated with a 300 W high pressure mercury lamp (maximum at 365 nm) for 4 hours. Encouragingly, the desired aryl-B(pin) product 3a was formed in 29% yield based on 1H NMR analysis of the crude product (Table 1, entry 1). Other polar solvents such as trifluoroethanol and methanol did not improve the reaction (entries 2 and 3). Adding water and acetone as co-solvents was beneficial in both cases and increased the yield to 46% (entries 4 and 5). Screening of various organic and inorganic additives revealed that an organic base, N,N,N′,N′-tetramethyldiaminomethane (TMDAM), could further improve the yield to 58% (entry 9). By comparison, other bases led to inferior results (entries 6–8). Interestingly, a greater amount of TMDAM led to a significantly lower yield (entry 10). Using two equivalents of B2(pin)2 could improve the yield to 72% (entry 11). Further optimization by changing the reaction concentration of 1a resulted in a higher yield (c = 0.1 M, 81% yield) (entry 12 vs. 11 and 13).
Table 1. Reaction optimization under batch and continuous-flow conditions.
| ||||
| Entry | 2 (eq.) | Solvent | Additive (mol%) | Yield c [%] |
| Batch conditions a | ||||
| 1 | 1.0 | MeCN | None | 29 |
| 2 | 1.0 | TFE | None | 26 |
| 3 | 1.0 | MeOH | None | 15 |
| 4 | 1.0 | MeCN/H2O | None | 42 |
| 5 | 1.0 | MeCN/H2O/acetone | None | 46 |
| 6 | 1.0 | MeCN/H2O/acetone | Cs2CO3 (100) | 16 |
| 7 | 1.0 | MeCN/H2O/acetone | KOtBu (100) | 12 |
| 8 | 1.0 | MeCN/H2O/acetone | TMEDA (50) | 52 |
| 9 | 1.0 | MeCN/H2O/acetone | TMDAM (50) | 58 |
| 10 | 1.0 | MeCN/H2O/acetone | TMDAM (100) | 39 |
| 11 | 2.0 | MeCN/H2O/acetone | TMDAM (50) | 72 |
| 12 d | 2.0 | MeCN/H 2 O/acetone | TMDAM (50) | 81 |
| 13 e | 2.0 | MeCN/H2O/acetone | TMDAM (50) | 55 |
| Flow conditions b | ||||
| 14 | 2.0 | MeCN/H2O/acetone | TMDAM (50) | 87 |
| 15 | 1.5 | MeCN/H 2 O/acetone | TMDAM (50) | 88 |
aBatch conditions: 1a (0.1–0.2 mmol, c = 0.05 M/0.1 M), 2 (0.1–0.4 mmol), RT, 4 h.
bFlow conditions: 1a (c = 0.1 M), –5 °C, residence time 15 min.
cDetermined by 1H NMR with 1,3,5-trimethoxybenzene as an internal standard.
d c = 0.1 M.
e c = 0.2 M; TMEDA: N,N,N,N-tetramethylethylenediamine; TMDAM: N,N,N′,N′-tetramethyldiaminomethane.
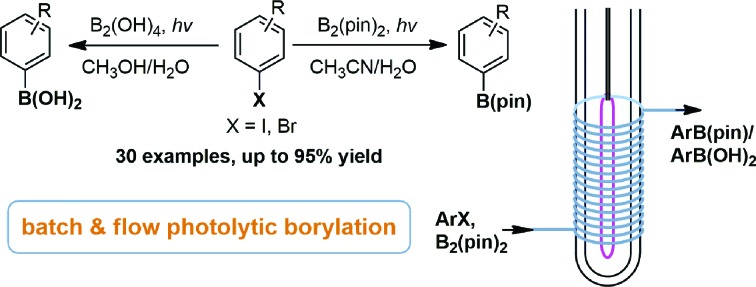
During the study, we observed gradual decomposition of B2(pin)2. We felt that continuous-flow photolytic conditions might help in reducing the amount of B2(pin)2 by competitively accelerating the desired reaction. In comparison with a typical batch photoreactor, microchannel photochemical reactors have significant benefits for reaction efficiency, yield, reproducibility, material throughput and scale-up.11–13 Based on the method developed by Booker-Milburn11a and our own experience in flow chemistry,14 we designed and assembled a continuous-flow photochemical reactor. Thus, transparent fluorinated ethylene propylene (FEP) tubing (reaction volume 780 μL) was coiled around a jacketed quartz immersion well in which the mercury lamp was situated. The reaction temperature was regulated by a cooling liquid circulating pump (see ESI†). A stock solution containing all reactants and reagents was introduced into the tubing using a syringe pump. To our delight, running the reaction under the same conditions as entry 12 but in continuous-flow mode gave 3a in excellent yield (87%, entry 14) with a residence time of only 15 minutes. Indeed, the amount of B2(pin)2 could be reduced to 1.5 equivalents without affecting the reaction efficiency (88% yield, entry 15).
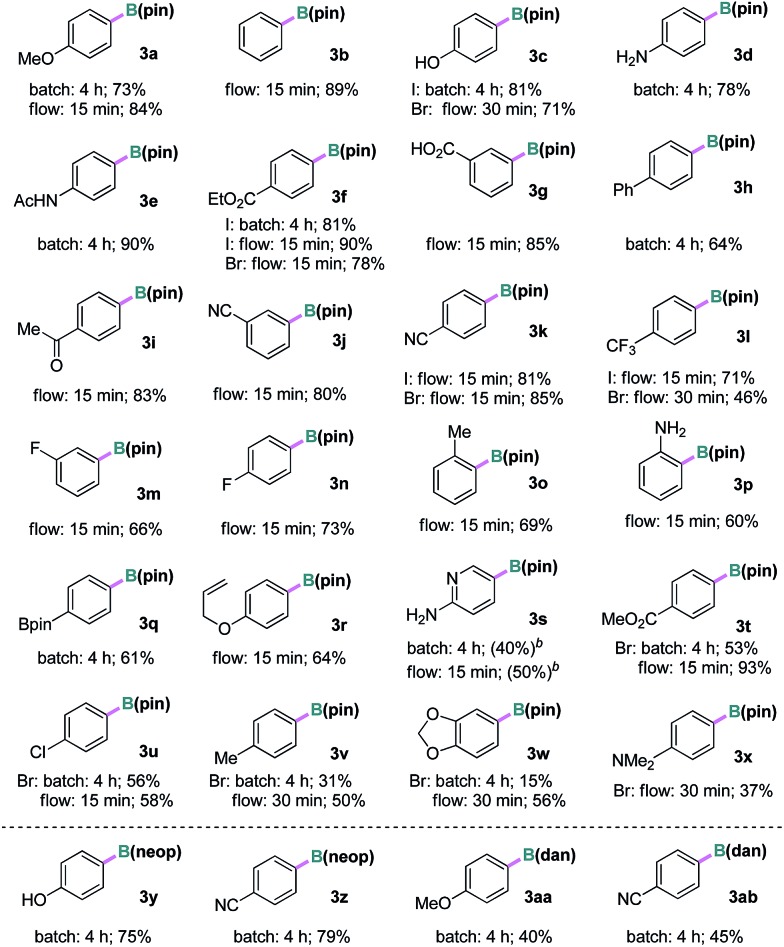
With the optimized conditions in hand, we examined the substrate scope of the current borylation reaction under batch and/or continuous-flow conditions, as summarized in Table 2. Iodoarenes with various electron-donating, -neutral and -withdrawing groups at the para-, meta-, or ortho-positions, including hydroxyl, amino, amide, ester, acid, ketone, cyano, fluorine, boronate and trifluoromethyl groups, were all efficiently converted to the corresponding aryl pinacol boronates in good to excellent yields (3a–3r). Groups potentially reactive under UV light such as aryl ketone (for 3i) and biaryl (for 3h) were compatible. A substrate containing an allyl ether group was also viable (3r), which is interesting considering that the reaction might involve a reactive carbon-based radical and the double bond could be attacked. In addition, the borylation of 2-amino-5-iodopyridine was possible, and a moderate yield of the corresponding boronate 3s was observed by 1H NMR spectroscopic analysis. Attempts to purify 3s were unsuccessful due to its decomposition on silica gel. Furthermore, when aryl bromides were subjected to the same reaction conditions, the desired products were produced in comparable or slightly lower yields than the iodides (3c, 3f, 3k, 3l and 3t–3x). Finally, different borylating reagents were utilized under otherwise identical conditions. Reactions using bis(neopentanediolato)diboron B2(neop)2 successfully afforded the desired products in good yields (3y and 3z). Interestingly, when an unsymmetrical diboron (pin)B–B(dan) was employed, selective introduction of the B(dan) moiety was realized (3aa and 3ab) and no aryl pinacol boronate was observed.15 To demonstrate the stability and usefulness of this reaction in larger scale preparation, the borylation reactions of iodobenzene and 4-iodophenol were carried out at gram scale (10.0 mmol) employing a commercial automated flow chemistry system (reactor volume 7.8 mL, see ESI†). Without any further optimization, the reactions produced the desired arylboronate products in excellent isolated yields (3b 90% and 3c 93%) and the productivity corresponded to ∼3 mmol h–1.
Table 2. Substrate scope of the photolytic borylation a .
|
|
aBatch conditions: 1a (0.2 mmol, c = 0.1 M), 2 (0.4 mmol, 2.0 eq.), TMDAM (0.5 eq.), RT, 4 h; flow conditions: 1a (c = 0.1 M), 2 (1.5 eq.), TMDAM (0.5 eq.), –5 °C, residence time 15–30 min.
bDetermined by 1H NMR with 1,3,5-trimethoxybenzene as an internal standard; TMDAM: N,N,N′,N′-tetramethyldiaminomethane.
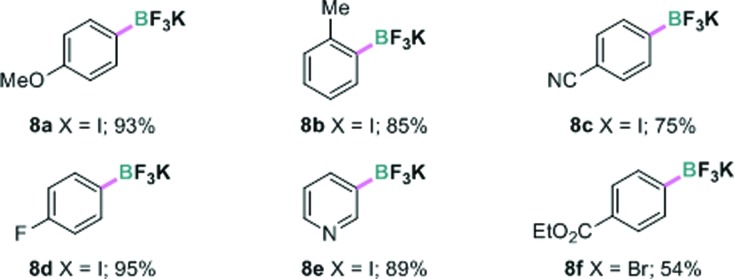
Encouraged by the above results, we further investigated the possibility of using a more atom economical borylating reagent, bis-boronic acid (BBA, 6). Largely because its polar protic properties may not be amenable to most known borylation methods, this reagent has only recently been successfully used in palladium or nickel-catalyzed Miyaura borylation by Molander and coworkers.16 In the present borylation, pleasingly, we were able to convert 4-iodoanisole 1a to the corresponding boronic acid 7a under continuous-flow conditions in quantitative yield based on 1H NMR analysis (residence time 10 minutes). The key variation from the previous conditions was using aqueous methanol (MeOH : H2O = 4 : 1 v/v) as the solvent. Due to the inconvenience of isolating the pure arylboronic acid, aqueous KHF2 was added and the resulting potassium aryltrifluoroborate 8a was obtained in 93% yield. Other aryl and heteroaryl iodides and a bromide were also transformed to the boronates in good to excellent yields in this manner (Table 3).
Table 3. Continuous-flow photolytic borylation with B2(OH)4.
|
|
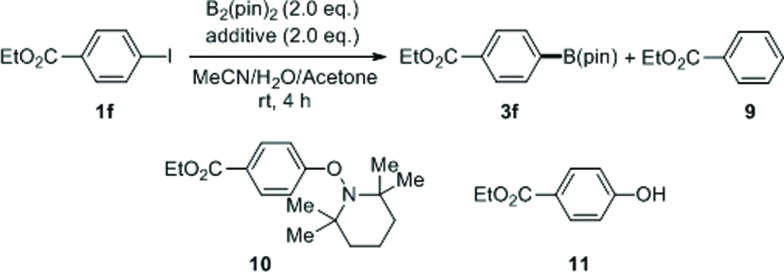
To gain insight into the reaction mechanism, and particularly to probe the role of additives and light, we conducted a series of control experiments (Table 4). When the batch reaction of 1f with B2(pin)2 was run under the standard conditions, deiodination product 9 was formed in 7% yield in addition to the borylation product 3f (entry 1). In the absence of both TMDAM and light (entry 2), no conversion was observed. However, the reaction with 0.5 equivalents of TMDAM in the dark led to a small amount of 3f (entry 3); higher reaction temperatures and prolonged reaction time had little influence on the outcome. A hydrogen atom donor, Bu3SnH, increased the conversion but led to 9 as the major product (entry 4). Furthermore, the reaction with Bu3SnH under UV irradiation afforded 9 in high yield (entry 5). Similarly, using 9,10-dihydroanthracene instead of Bu3SnH, 9 (26%) and concomitant anthracene (11%) were observed (entry 6). Finally, when TEMPO was added as a radical scavenger, the conversion was low and four products including 3f (15%), 9 (11%), the aryl-TEMPO adduct 10 (14%) and ethyl 4-hydroxybenzoate 11 (26%) were formed (entry 7).
Table 4. Control experiments for preliminary mechanistic studies a .
| ||||||
| Entry | Light | TMDAM | Additive | Conversion [%] | Yield of 3f [%] | Yield of 9 [%] |
| 1 | + | + | – | 100 | 81 | 7 |
| 2 | – | – | – | 0 | 0 | 0 |
| 3 | – | + | – | 13 | 13 | 0 |
| 4 | – | + | Bu3SnH | 46 | 17 | 26 |
| 5 | + | + | Bu3SnH | 100 | 18 | 80 |
| 6 | + | + | DHA | 68 | 42 | 26 b |
| 7 | + | + | TEMPO | 69 | 15 | 11 |
aReactions were run in batch and yields were determined by 1H NMR spectroscopic analysis with 1,3,5-trimethoxybenzene as an internal standard.
b11% of anthracene was formed. TMDAM: N,N,N′,N′-tetramethyldiaminomethane; DHA: 9,10-dihydroanthracene; TEMPO: (2,2,6,6-tetramethylpiperidin-1-yl)oxyl.
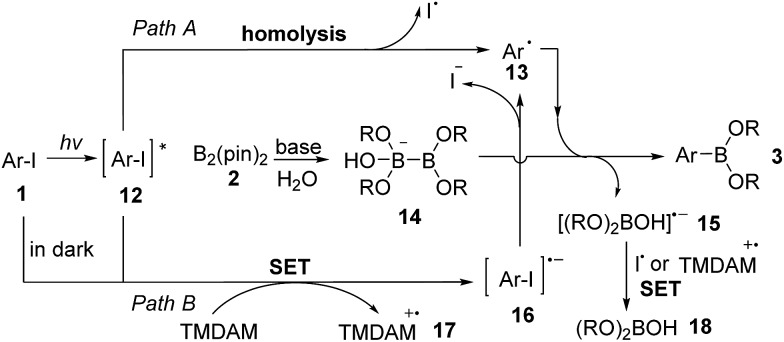
Based on the experimental results and related reports on photolytic reactions of aryl iodides,17 we propose two pathways both involving an aryl radical intermediate as the possible reaction mechanism (Scheme 2). The excited state 12 is generated by UV irradiation of aryl iodide 1. In path A, 12 undergoes homolytic C–I bond cleavage to form aryl radical 13 and an iodine atom. Under aqueous conditions, TMDAM activates a water molecule, combining with B2(pin)2 (2) to form a sp3–sp2 diboron species 14.7,8f,18 Aryl radical 13 then reacts with 14 to produce arylboronate 3 and a boryl radical anion 15.1915 can also be viewed as an anionic base-stabilized boryl radical.20 Alternatively, in path B, the excited state 12 or the starting aryl iodide 1 (when in darkness, although with low efficiency) is reduced by TMDAM via a single electron transfer (SET) process to form radical anion 16 and TMDAM-derived radical cation 17. 16 then undergoes C–I bond cleavage to generate aryl radical 13 and iodide anion. Finally, 15 is oxidized by the iodine atom from path A or TMDAM-derived radical cation 17 from path B to form borate 18 as a byproduct.
Scheme 2. Proposed reaction mechanism.
Conclusions
In summary, we have discovered a novel and efficient photolytic borylation reaction of aryl halides using diboron reagents. This metal-free reaction features very mild conditions, short reaction times, generally high yields and broad functional group tolerance. Considering the reaction conditions, borylating reagent types and possible reaction mechanism, this work represents an important complementary approach to the existing C–B bond formation methods. Further studies on the mechanism and synthetic applications of this reaction are ongoing.
Supplementary Material
Acknowledgments
This work was financially supported by the NSFC (No. 21472146), the Department of Science and Technology of Shaanxi Province (No. 2015KJXX-02) and the Ministry of Science and Technology of the People's Republic of China (No. 2014CB548200). We thank Prof. Que (Xi'an Jiaotong University) and Dr Duncan Guthrie (Vapourtec) for their generous sharing of the batch and flow photochemistry equipment.
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures and characterization of new compounds are provided. See DOI: 10.1039/c5sc04521e
References
- For reviews on arylboronic acid derivatives, see: ; (a) Miyaura N., Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]; (b) Hall D. G., in Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, ed. D. G. Hall, Wiley-VCH, Weinheim, 2011, pp. 1–134. [Google Scholar]; (c) Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; (d) Xu L., Zhang S., Li P. Chem. Soc. Rev. 2015;44:8848. doi: 10.1039/c5cs00338e. [DOI] [PubMed] [Google Scholar]
- (a) Brown H. C., Cole T. E. Organometallics. 1983;2:1316. [Google Scholar]; (b) Brown H. C., Srebnik M., Cole T. E. Organometallics. 1986;5:2300. [Google Scholar]; (c) Baron O., Knochel P. Angew. Chem., Int. Ed. 2005;44:3133. doi: 10.1002/anie.200462928. [DOI] [PubMed] [Google Scholar]; (d) Pintaric C., Olivero S., Gimbert Y., Chavant P. Y., Duñach E. J. Am. Chem. Soc. 2010;132:11825. doi: 10.1021/ja1052973. [DOI] [PubMed] [Google Scholar]
- (a) Ishiyama T., Murata M., Miyaura N. J. Org. Chem. 1995;60:7508. [Google Scholar]; (b) Zhu W., Ma D. Org. Lett. 2006;8:261. doi: 10.1021/ol052633u. [DOI] [PubMed] [Google Scholar]; (c) Billingsley K. L., Barder T. E., Buchwald S. L. Angew. Chem., Int. Ed. 2007;46:5359. doi: 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]; (d) So C. M., Lau C. P., Kwong F. Y. Angew. Chem., Int. Ed. 2008;47:8059. doi: 10.1002/anie.200803193. [DOI] [PubMed] [Google Scholar]; (e) Kleeberg C., Dang L., Lin Z., Marder T. B. Angew. Chem., Int. Ed. 2009;48:5350. doi: 10.1002/anie.200901879. [DOI] [PubMed] [Google Scholar]; (f) Wilson D. A., Wilson C. J., Moldoveanu C., Resmerita A. M., Corcoran P., Hoang L. M., Rosen B. M., Percec V. J. Am. Chem. Soc. 2010;132:1800. doi: 10.1021/ja910808x. [DOI] [PubMed] [Google Scholar]; (g) Nagashima Y., Takita R., Yoshida K., Hirano K., Uchiyama M. J. Am. Chem. Soc. 2013;135:18730. doi: 10.1021/ja409748m. [DOI] [PubMed] [Google Scholar]; (h) Zarate C., Manzano R., Martin R. J. Am. Chem. Soc. 2015;137:6754. doi: 10.1021/jacs.5b03955. [DOI] [PubMed] [Google Scholar]; (i) Bose S. K., Marder T. B. Org. Lett. 2014;16:4562. doi: 10.1021/ol502120q. [DOI] [PubMed] [Google Scholar]; (j) Chow W. K., Yuen O. Y., Choy P. Y., So C. M., Lau C. P., Wong W. T., Kwong F. Y. RSC Adv. 2013;3:12518. [Google Scholar]; (k) Niwa T., Ochiai H., Watanabe Y., Hosoya T. J. Am. Chem. Soc. 2015;137:14313. doi: 10.1021/jacs.5b10119. [DOI] [PubMed] [Google Scholar]; (l) Bose S. K., Deiβenberger A., Eichhorn A., Steel P. G., Lin Z., Marder T. B. Angew. Chem., Int. Ed. 2015;54:11843. doi: 10.1002/anie.201505603. [DOI] [PubMed] [Google Scholar]
- For selected references on catalytic C–H borylation, see: ; (a) Mkhalid I. A. I., Barnard J. H., Marder T. B., Murphy J. M., Hartwig J. F. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]; (b) Iverson C. N., Smith III M. R. J. Am. Chem. Soc. 1999;121:7696. [Google Scholar]; (c) Chen H., Schlecht S., Semple T. C., Hartwig J. F. Science. 2000;287:1995. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]; (d) Ishiyama T., Takagi J., Ishida K., Miyaura N., Anastasi N. R., Hartwig J. F. J. Am. Chem. Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; (e) Kawamorita S., Ohmiya H., Hara K., Fukuoka A., Sawamura M. J. Am. Chem. Soc. 2009;131:5058. doi: 10.1021/ja9008419. [DOI] [PubMed] [Google Scholar]; (f) Dai H. X., Yu J. Q. J. Am. Chem. Soc. 2012;134:134. doi: 10.1021/ja2097095. [DOI] [PubMed] [Google Scholar]; (g) Mazzacano T. J., Mankad N. P. J. Am. Chem. Soc. 2013;135:17258. doi: 10.1021/ja408861p. [DOI] [PubMed] [Google Scholar]; (h) Xu L., Ding S., Li P. Angew. Chem., Int. Ed. 2014;53:1822. doi: 10.1002/anie.201309546. [DOI] [PubMed] [Google Scholar]; (i) Obligacion J. V., Semproni S. P., Chirik P. J. J. Am. Chem. Soc. 2014;136:4133. doi: 10.1021/ja500712z. [DOI] [PubMed] [Google Scholar]; (j) Zhang L.-S., Chen G., Wang X., Guo Q.-Y., Zhang X.-S., Pan F., Chen K., Shi Z.-J. Angew. Chem., Int. Ed. 2014;53:3899. doi: 10.1002/anie.201310000. [DOI] [PubMed] [Google Scholar]; (k) Wang G., Xu L., Li P. J. Am. Chem. Soc. 2015;137:8058. doi: 10.1021/jacs.5b05252. [DOI] [PubMed] [Google Scholar]; (l) Shimada S., Batsanov A. S., Howard J. A. K., Marder T. B. Angew. Chem., Int. Ed. 2001;40:2168. doi: 10.1002/1521-3773(20010601)40:11<2168::AID-ANIE2168>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]; (m) Tajuddin H., Harrisson P., Bitterlich B., Collings J. C., Sim N., Batsanov A. S., Cheung M. S., Kawamorita S., Maxwell A. C., Shukla L., Morris J., Lin Z., Marder T. B., Steel P. G. Chem. Sci. 2012;3:3505. [Google Scholar]
- (a) Yamamoto E., Izumi K., Horita Y., Ito H. J. Am. Chem. Soc. 2012;134:19997. doi: 10.1021/ja309578k. [DOI] [PubMed] [Google Scholar]; (b) Uematsu R., Yamamoto E., Maeda S., Ito H., Taketsugu T. J. Am. Chem. Soc. 2015;137:4090. doi: 10.1021/ja507675f. [DOI] [PubMed] [Google Scholar]; (c) Yamamoto E., Ukigai S., Ito H. Chem. Sci. 2015;6:2943. doi: 10.1039/c5sc00384a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wu H.-H., Zhang J. Eur. J. Org. Chem. 2013:6263. [Google Scholar]
- Miralles N., Romero R. M., Fernándes E., Muñiz K. Chem. Commun. 2015;51:14068. doi: 10.1039/c5cc04944j. [DOI] [PubMed] [Google Scholar]
- (a) Mo F., Jiang Y., Qiu D., Zhang Y., Wang J. Angew. Chem., Int. Ed. 2010;49:1846. doi: 10.1002/anie.200905824. [DOI] [PubMed] [Google Scholar]; (b) Erb W., Hellal A., Albini M., Rouden J., Blanchet J. Chem.–Eur. J. 2014;20:6608. doi: 10.1002/chem.201402487. [DOI] [PubMed] [Google Scholar]; (c) Qiu D., Jin L., Zheng Z., Meng H., Mo F., Wang X., Zhang Y., Wang J. J. Org. Chem. 2013;78:1923. doi: 10.1021/jo3018878. [DOI] [PubMed] [Google Scholar]; (d) Yu J., Zhang L., Yan G. Adv. Synth. Catal. 2012;354:2625. doi: 10.1002/adsc.201100831. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Dewhurst R. D., Neeve E. C., Braunschweig H., Marder T. B. Chem. Commun. 2015;51:9594. doi: 10.1039/c5cc02316e. [DOI] [PubMed] [Google Scholar]; (f) Pietch S., Neeve E. C., Apperley D. C., Bertermann R., Mo F., Qiu D., Cheung M. S., Dang L., Wang J., Radius U., Lin Z., Kleeberg C., Marder T. B. Chem.–Eur. J. 2015;21:7082. doi: 10.1002/chem.201500235. [DOI] [PubMed] [Google Scholar]; (g) Zhu C., Yamane M. Org. Lett. 2012;14:4560. doi: 10.1021/ol302024m. [DOI] [PubMed] [Google Scholar]
- (a) Prokofjevs A., Kamf J. W., Vedejs E. Angew. Chem., Int. Ed. 2011;50:2098. doi: 10.1002/anie.201005663. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Niu L., Yang H., Wang R., Fu H. Org. Lett. 2012;14:2618. doi: 10.1021/ol300950r. [DOI] [PubMed] [Google Scholar]; (c) Bagutski V., Grosso A. D., Carrillo J. A., Cade I. A., Helm M. D., Lawson J. R., Singleton P. J., Solomon S. A., Marcelli T., Ingleson M. J. J. Am. Chem. Soc. 2013;135:474. doi: 10.1021/ja3100963. [DOI] [PubMed] [Google Scholar]; (d) Légaré M. A., Courtemanche M. A., Rochette É., Fontaine F.-G. Science. 2015;349:513. doi: 10.1126/science.aab3591. [DOI] [PubMed] [Google Scholar]; (e) Bose S. K., Marder T. B. Science. 2015;349:473. doi: 10.1126/science.aac9244. [DOI] [PubMed] [Google Scholar]
- . For selected recent examples, see: ; (a) Synthetic Organic Chemistry, ed. A. G. Griesbeck and J. Mattay, Marcel Dekker, New York, 2005. [Google Scholar]; (b) Creutz S. E., Lotito K. J., Fu G. C., Peters J. C. Science. 2012;338:647. doi: 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]; (c) Liu W., Li L., Li C. J. Nat. Commun. 2015;6:6526. doi: 10.1038/ncomms7526. [DOI] [PubMed] [Google Scholar]; (d) Maskill K. G., Knowles J. P., Elliott L. D., Alder R. W., Booker-Milburn K. I. Angew. Chem., Int. Ed. 2013;52:1499. doi: 10.1002/anie.201208892. [DOI] [PubMed] [Google Scholar]
- For selected references on continuous-flow photochemical reactions, see: ; (a) Hook B. D. A., Dohle W., Hirst P. R., Pickworth M., Berry M. B., Booker-Milburn K. I. J. Org. Chem. 2005;70:7558. doi: 10.1021/jo050705p. [DOI] [PubMed] [Google Scholar]; (b) Vaske Y. S. M., Mahoney M. E., Konopelski J. P., Rogow D. L., McDonald W. J. J. Am. Chem. Soc. 2010;132:11379. doi: 10.1021/ja1050023. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lévesque F., Seeberger P. H. Angew. Chem., Int. Ed. 2012;51:1706. doi: 10.1002/anie.201107446. [DOI] [PubMed] [Google Scholar]; (d) Harrowven D. C., Mohamed M., Gonçalves T. P., Whitby R. J., Bolien D., Sneddon H. F. Angew. Chem., Int. Ed. 2012;51:4405. doi: 10.1002/anie.201200281. [DOI] [PubMed] [Google Scholar]; (e) Andrews R. S., Becker J. J., Gagne M. R. Angew. Chem., Int. Ed. 2012;51:4140. doi: 10.1002/anie.201200593. [DOI] [PubMed] [Google Scholar]; (f) Tucker J. W., Zhang Y., Jamison T. F., Stephenson C. R. J. Angew. Chem., Int. Ed. 2012;51:4144. doi: 10.1002/anie.201200961. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Maskill K. G., Knowles J. P., Elliott L. D., Alder R. W., Milburn K. I. B. Angew. Chem., Int. Ed. 2013;52:1499. doi: 10.1002/anie.201208892. [DOI] [PubMed] [Google Scholar]; (h) Zhang Y., Blackman M. L., Leduc A. B., Jamison T. F. Angew. Chem., Int. Ed. 2013;52:4251. doi: 10.1002/anie.201300504. [DOI] [PubMed] [Google Scholar]; (i) Wang X., Cuny G. D., Noël T. Angew. Chem., Int. Ed. 2013;52:7860. doi: 10.1002/anie.201303483. [DOI] [PubMed] [Google Scholar]
- For early pioneering work on flow photochemistry, see: ; (a) Lu H., Schmidt M. A., Jensen K. F. Lab Chip. 2001;1:22. doi: 10.1039/b104037p. [DOI] [PubMed] [Google Scholar]; (b) Ueno K., Kitagawa F., Kitamura N. Lab Chip. 2002;2:231. doi: 10.1039/b207991g. [DOI] [PubMed] [Google Scholar]
- For selected recent reviews on flow photochemistry, see: ; (a) Oelgemöller M., Shvydkiv O. Molecules. 2011;16:7522. doi: 10.3390/molecules16097522. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Knowles J. P., Elliott L. D., Booker-Milburn K. I. Beilstein J. Org. Chem. 2012;8:2025. doi: 10.3762/bjoc.8.229. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Elliott L. D., Knowles J. P., Koovits P. J., Maskill K. G., Ralph M. J., Lejeune G., Edwards L. J., Robinson R. I., Clemens I. R., Cox B., Pascoe D. D., Koch G., Eberle M., Berry M. B., Booker-Milburn K. I. Chem.–Eur. J. 2014;20:15226. doi: 10.1002/chem.201404347. [DOI] [PubMed] [Google Scholar]
- (a) Li P., Buchwald S. L. Angew. Chem., Int. Ed. 2011;50:6396. doi: 10.1002/anie.201102401. [DOI] [PubMed] [Google Scholar]; (b) Li P., Moore J. S., Jensen K. F. ChemCatChem. 2013;5:1729. [Google Scholar]
- Xu L., Li P. Chem. Commun. 2015;51:5656. doi: 10.1039/c5cc00231a. [DOI] [PubMed] [Google Scholar]
- (a) Molander G. A., Trice S. L. J., Dreher S. D. J. Am. Chem. Soc. 2010;132:17701. doi: 10.1021/ja1089759. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander G. A., Trice S. L. J., Kennedy S. M., Dreher S. D., Tudge M. T. J. Am. Chem. Soc. 2012;134:11667. doi: 10.1021/ja303181m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander G. A., Cavalcanti L. N., Garcia-Garcia C. J. Org. Chem. 2013;78:6427. doi: 10.1021/jo401104y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Sage A. G., Oliver T. A. A., Murdock D., Crow M. B., Ritchie G. A. D., Harvey J. N., Ashfold M. N. R. Phys. Chem. Chem. Phys. 2011;13:8075. doi: 10.1039/c0cp02390f. [DOI] [PubMed] [Google Scholar]; (b) Budén M., Guastavino J. F., Rossi R. A. Org. Lett. 2013;15:1174. doi: 10.1021/ol3034687. [DOI] [PubMed] [Google Scholar]; (c) Kan J., Huang S., Zhao H., Lin J., Su W. Sci. China: Chem. 2015;58:1329. [Google Scholar]; (d) Discekici E. H., Treat N. J., Poelma S. O., Mattson K. M., Hudson Z. M., Luo Y., Hawker C. J., Alaniz J. R. Chem. Commun. 2015;51:11705. doi: 10.1039/c5cc04677g. [DOI] [PubMed] [Google Scholar]
- (a) Gao M., Thorpe S. B., Santos W. L. Org. Lett. 2009;11:3478. doi: 10.1021/ol901359n. [DOI] [PubMed] [Google Scholar]; (b) Bonet A., Gulyás H., Fernández E. Angew. Chem., Int. Ed. 2010;49:5130. doi: 10.1002/anie.201001198. [DOI] [PubMed] [Google Scholar]; (c) Bonet A., Pubill-Ulldemolins C., Bo C., Gulyás H., Fernández E. Angew. Chem., Int. Ed. 2011;50:7158. doi: 10.1002/anie.201101941. [DOI] [PubMed] [Google Scholar]; (d) Wu H., Garcia J. M., Haeffner F., Radomkit S., Zhugralin A. R., Hoveyda A. H. J. Am. Chem. Soc. 2015;137:10585. doi: 10.1021/jacs.5b06745. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Thorpe S. B., Calderone J. A., Santos W. L., Org. Lett., 2012, 14 , 1918 , and references therein . [DOI] [PubMed] [Google Scholar]
- (a) Power P. P. Chem. Rev. 2003;103:789. doi: 10.1021/cr020406p. [DOI] [PubMed] [Google Scholar]; (b) Braunschweig H., Dyakonov V., Jimenez J. O. C., Kraft K., Krummenacher I., Radacki K., Sperlich A., Wahler J., Angew. Chem., Int. Ed., 2012, 51 , 2977 , and references therein . [DOI] [PubMed] [Google Scholar]
- (a) Ueng S.-H., Brahmi M. M., Derat E., Fensterbank L., Lacôte E., Malacria M., Curran D. P. J. Am. Chem. Soc. 2008;130:10082. doi: 10.1021/ja804150k. [DOI] [PubMed] [Google Scholar]; (b) Lu D., Wu C., Li P. Chem.–Eur. J. 2014;20:1630. doi: 10.1002/chem.201303705. [DOI] [PubMed] [Google Scholar]; (c) Rablen P. R., Hartwig J. F. J. Am. Chem. Soc. 1996;118:4648. [Google Scholar]; (d) Baban J. A., Roberts B. P. J. Chem. Soc., Chem. Commun. 1983:1224. [Google Scholar]; (e) Lu D., Wu C., Li P. Org. Lett. 2014;16:1486. doi: 10.1021/ol500296a. [DOI] [PubMed] [Google Scholar]; (f) Lalevée J., Blanchard N., Tehfe M.-A., Chany A.-C., Fouassier J.-P. Chem.–Eur. J. 2010;16:12920. doi: 10.1002/chem.201001440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.