ABSTRACT
Vitamin A in human milk is critical for meeting infant requirements and building liver stores needed after weaning. A number of studies have measured milk retinol, but only a subset have corrected for fat, which serves as the retinol carrier in breast milk. The purpose of the present work was to review and analyze studies in which human-milk retinol concentrations were reported in relation to milk fat and to compare these results with unadjusted breast-milk retinol concentrations in terms of time trends over the course of lactation, influences of maternal nutritional and constitutional factors, and effects of maternal vitamin A supplementation. A systematic approach was used to search the available literature by using the US National Library of Medicine's MEDLINE/PubMed bibliographic search engine. Observational and intervention studies were included if the research was original and the retinol-to-fat ratio (retinol:fat) in human milk was measured at ≥1 time point during the first 12 mo of lactation. Retinol:fat and retinol were highest in colostrum, declined rapidly in early lactation, and achieved statistical stability by 2 and 4 wk lactation, respectively. In mature milk, retinol concentration was positively correlated with milk fat (r = 0.61, P = 0.008). Breast-milk retinol:fat and retinol were positively associated with maternal vitamin A intake but were associated with plasma retinol only when dietary intake was inadequate. Postpartum supplementation with high-dose vitamin A (200,000–400,000 IU) resulted in significantly higher breast-milk retinol:fat for 3 mo and retinol for 6 mo (P < 0.05). In populations, the 2 indexes show similar trends and associations with maternal factors. Future studies should monitor how the uptake of retinol into the mammary gland affects maternal vitamin reserves, particularly in women who are at risk of vitamin A deficiency.
Keywords: human milk, lactation, vitamin A, deficiency, supplementation, developmental consequences
Introduction
Adequate vitamin A status is critical for normal gene expression, reproduction, growth and physical development, erythropoiesis, immune function, and vision (1). Infants are born with meager retinol reserves (∼5 μmol), even if their mothers were well nourished during pregnancy (2). During the first 6 mo of life, exclusively breastfed infants of mothers with adequate intake and stores accumulate ∼310 μmol vitamin A. Thus, human-milk vitamin A is critical both for meeting the requirements of the infants and for the accumulation of liver stores needed after weaning (2). In cases in which vitamin A in weaning foods is low, milk retinol can be the most important predictor of vitamin A status in infants through the second year of life, when human milk can still supply almost two-thirds of the recommended intake (3). Breastfeeding from vitamin A–depleted mothers predisposes infants to the consequences of deficiency, including xerophthalmia, anemia and impaired iron metabolism, growth retardation, increased infectious morbidity, depressed immune response, diarrheal disease, respiratory tract infections, and increased risk of mortality (4).
In contrast to blood, which contains 95% of vitamin A in the retinol-binding protein (RBP) retinol form, human-milk vitamin A is present almost exclusively as retinyl esters, mainly retinyl palmitate, in the lipid fraction of the milk (2, 5). Although some vitamin A in milk is derived from serum retinol, which is esterified in the mammary gland, newly absorbed dietary retinol converted to retinyl esters passes directly into milk via chylomicrons, bypassing regulation by the liver (2, 6, 7). In regions where vitamin A intake is high, maternal dietary retinol can saturate milk concentrations (8). In contrast, low milk vitamin A concentration suggests insufficient maternal reserves in addition to inadequate intake (9).
Because vitamin A in human milk is associated with the milk-fat globule, retinol concentration measured in a sample of milk is highly dependent on the fat content of the sample. There are wide intraindividual variations in milk fat, with an estimated CV of 47% over 24 h in mature human milk (10). Milk fat in a sample is related to the storage capacity of each breast and the fullness of the breast at the point of sampling, with peak fat occurring 30 min after a feeding (10, 11). Controlling for milk fat has been suggested to be an efficient way to standardize vitamin A measurements across studies the use different breast-milk collection methodologies (2). However, human-milk retinol is corrected for fat in only a fraction of the literature. The purpose of the present work is to systematically review and analyze studies in which human-milk retinol concentrations were reported in the context of milk fat and to compare these results with data on direct human-milk retinol concentrations in terms of time trends over the course of lactation, influences of maternal nutrition and constitutional factors, and effects of maternal vitamin A supplementation.
Methods
A systematic approach was used to search the available literature from 1 January 1966 through 31 May 2017 by using the US National Library of Medicine's MEDLINE/PubMed bibliographic search engine. Multiple PubMed searches were conducted by using various combinations of MeSH (Medical Subject Heading) and title/abstract keywords. MeSH keywords included “Milk, Human,” “Lactation,” “Breast Feeding,” and “Colostrum” as well as “vitamin A,” “beta-carotene,” and “carotenoids.” Title/abstract keywords included “lactation,” “breastfeeding,” “breast feeding,” “breast-feeding,” “breastmilk,” breast milk,” “breast-milk,” “human milk,” “colostrum,” “vitamin A,” “retinol,” “retinyl,” “pro-vitamin A,” “beta-carotene,” and “carotenoids.” Filters limited search results to human studies published in English, French, German, Portuguese, Spanish, Hebrew, Danish, Norwegian, or Swedish. Two individuals independently screened unique article titles and abstracts to identify a list of relevant studies for full-text review. When opinions differed, a third individual reviewed the abstracts to resolve the discrepancy by two-thirds majority. Review and original research articles were examined for references to other relevant studies not identified by the initial search. Studies were included if the research was original and the retinol-to-fat ratio (retinol:fat; micromoles per gram) in human milk was measured at one or more time points during the first 12 mo of lactation. Exclusion criteria were animal studies and studies failing to express human-milk retinol in relation to fat at any time point within the first year of lactation. By using this strategy, 24 original research studies were identified and included in the systematic review and meta-analysis (Figure 1). Risk of bias was assessed at the individual study level through review of methods, data analysis, and reporting (Table 1). The principal summary measures were human-milk retinol:fat (micromoles per gram) and retinol concentration (micromoles per gram) at various stages of lactation and in intervention and control groups.
FIGURE 1.
Flow diagram.
TABLE 1.
Description of studies included in the systematic review and meta-analysis1
| Study, year (ref) | Country | Adequate population VA status | n | Time | Study design and intervention | Percentage taking supplements containing VA | Retinol,2 μmol/L | Retinol:fat,2 μmol/g fat | Fat, g/L | Methods3 | Comments | Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ayah et al., 2007 (12) | Kenya | N/A | 564 | 4, 14, and 26 wk | RCT; 400,000 IU (n = 282) or placebo (n = 282) within 24 h of delivery | None | Intervention vs. control, 4 wk: 0.67 vs. 0.60 (P = 0.04); 14 wk: 0.52 vs. 0.44 (P = 0.001); 26 wk: 0.50 vs. 0.44 (P = 0.04) | Intervention vs. control; 4 wk: 0.025 vs. 0.019 (P = 0.02); 14 wk: 0.020 vs. 0.019 (NS); 26 wk: 0.020 vs. 0.019 (NS) | 4 wk: 34.6 vs. 37.3 (NS);16 wk: 31.5 vs. 29.8 (NS); 26 wk: 31.3 vs. 30.1 (NS) | Manual expression of 2–5 mL foremilk ≥1 h after last feeding; samples saponified with KOH in water, extracted with hexane, retinol measured by HPLC | Supplementation had a modest effect on HM retinol but failed to increase serum retinol or infant stores | HIV status of mothers unknown despite high population prevalence |
| Bahl et al., 2002 (13) | Ghana, India, Peru | N/A | 1875 | 2, 6, and 9 mo | RCT; 60 mg (200,000 IU) retinol palmitate 18–42 d postpartum (n = 938) vs. placebo (n = 937) | Not reported | N/A | Intervention vs. control; 2 mo: 0.050 ± 0.025 vs. 0.043 ± 0.022 (P < 0.05); 4 mo: 0.043 ± 0.022 vs. 0.041 ± 0.026 (NS); 9 mo: 0.044 ± 0.022 vs. 0.045 ± 0.028 (NS) | N/A | 10 mL HM collected by pump between 0900 and 1200 without regard to time of last feeding; samples saponified with KOH in ethanol, extracted with hexane, retinol measured by HPLC | Improvement in HM retinol at 2 mo but not at 6 or 9 mo; effect greatest in India, followed Peru and Ghana | VA status of mothers unknown, but baseline HM retinol:fat lowest and percentage of infant deficiency at 6 wk highest in India |
| Bezerra et al., 2010 (14) | Brazil | Yes | 143 | Postpartum, 4 wk | RCT; S1: 200,000 IU retinyl palmitate (n = 55); S2: 2 doses of 200,000 IU 24 h apart (n = 58); control (n = 33) | None | Delivery (presupplementation): 3.22 ± 1.81 (S1), 3.21 ± 1.87 (S2), 3.31 ± 1.40 (control) (NS); 4 wk: 1.78 ± 1.00 (S1), 1.93 ± 1.10 (S2), 1.28 ± 0.61 (control) (P < 0.05 between control and supplementation groups) | Delivery (presupplementation): 0.171 ± 0.133, 0.172 ± 0.144, 0.176 ± 0.134 (NS); 4 wk: 0.054 ± 0.029 (S1), 0.060 ± 0.031 (S2), 0.044 ± 0.023 (control) (P < 0.05 between S2 and control) | N/A | 1–2 mL colostrum collected by pump, collection at 4 wk not described; samples saponified with KOH in ethanol, extracted with hexane, retinol measured by HPLC; analytical method of Giuliano et al. (15) | Satisfactory population VA status per colostrum retinol content, but 31.5% deemed deficient by HM retinol <1.05 μmol/L | Lower percentage of maternal deficiency based on HM retinol <1.05 μmol/L in S2 group than in S1 and control groups; capsules contained vitamin E |
| Canfield et al., 1997 (16) | United States | Yes | 12 | Mixed | Pre-post design; single dose: 1) 60 mg (n = 6) or 2) 210 mg (n = 6) β-carotene | Not reported | Group 1 at baseline: 1.1 ± 0.3; group 2 at baseline: 1.7 ± 0.3 (group 1 significantly lower than group 2; P value not stated) | Group 1 at baseline: 0.020 ± 0.006; group 2 at baseline: 0.026 ± 0.004 (NS) | 59 ± 17 | Full expression of one breast by using electric pump, 2 samples collected midafternoon 2–3 h after last feeding; samples saponified with KOH in ethanol, extracted with hexane, retinol measured by HPLC | HM retinol measured in only 3 women in each group; no increase observed in response to β-carotene supplementation | Small n; results for 2 women with longitudinal samples presented only graphically |
| Canfield et al., 1998 (17) | United States | Yes | 5 | Average, 279 d | Pre-post design; 30 mg β-carotene for 28 d | Not stated | Baseline: 2.24 ± 0.007 (n = 3) | Baseline: 0.034 ± 0.003 (n = 5) | N/A | Full expression of one breast by using electric pump, 2 samples collected midafternoon 2–3 h after last feeding; samples saponified with KOH in ethanol, extracted with hexane, retinol measured by HPLC | HM retinol measured over course of study in only 2 women; no increase observed in response to β-carotene supplementation | Small n; results for 2 women with longitudinal samples presented graphically |
| Canfield et al., 2001 (18) | Honduras | No | 86 | Mixed | RCT; 90-mg β-carotene as RPO (n = 32), supplements (n = 36), or placebo control (n = 18) in 6 equal doses over 10 d | — | Baseline: 1.63 ± 1.01 (RPO), 1.28 ± 0.59 (supplement), 1.95 ± 0.87 (placebo) (NS); + 10 d: 1.10 ± 0.041 (RPO), 1.28 ± 1.37 (supplement), 1.09 ± 0.63 (placebo control) (NS) | Baseline: 0.04 ± 0.01 (RPO), 0.05 ± 0.04 (supplement), 0.06 ± 0.04 (placebo) (NS); + 10 d: 0.03 ± 0.02 (RPO), 0.04 ± 0.02 (supplement), 0.03 ± 0.02 (placebo control) (NS) | N/A | 5–10 mL collected by manual expression midmorning; samples pretreated with bile salts, protease, and lipase; saponified with KOH; and extracted with hexane; retinol measured by HPLC per method of Liu et al. (19) | Significant changes in milk α- and β-carotene, more in RPO than supplement group, remained after controlling for baseline values; no significant differences in milk retinol between groups | Large intra- and interindividual variability |
| da Silva Ribeiro et al., 2010 (20) | Brazil | Yes | 86, >50% intake from preformed VA (group A, n = 37); >50% intake from pro-VA carotenoids (group B, n = 49) | Within 16 h postpartum | Cross-sectional | Not reported | Overall: 3.5 ± 1.9 (group A: 3.4 ± 1.7, group B: 3.6 ± 1.9) (NS) | Overall: 0.131 ± 0.070 (group A: 0.140 ± 0.100; group B: 0.125 ± 0.034) (NS) | Overall: 33.9 ± 22.4 (group A: 32.8 ± 20.3; group B, 35.0 ± 24.0) (NS) | 1–3 mL collected by manual expression from a single breast not suckled in the previous feeding, first milk ejection discarded; HPLC per method of Giuliano et al. (15) | No significant difference in colostrum retinol between groups, despite significantly higher serum retinol and retinol intake in group A (P < 0.05) | Large CVs in retinol and retinol:fat |
| de Azeredo and Trugo, 2008 (21) | Brazil | Yes (low middle class) | 72 | 30–120 d | Cross-sectional | Not reported | 0.62 ± 0.42 | 0.021 ± 0.002 | 35.6 ± 15.5 | Single full expression from breast not suckled in previous feeding, obtained between 0800 and 1100 after overnight fast; HPLC per method of Liu et al. (19) | No significant correlation between milk and plasma retinol; 3% deficient by plasma retinol <0.7 μmol/L, but 87.5% had HM retinol <1.05 μmol/L | Adolescent mothers |
| Dijkhuizen et al., 2004 (22) | Indonesia | No | 124 | 1 and 6 mo | RCT; supplementation during pregnancy (>20 wk gestation) with daily iron/folate and 1) Ø (n = 31), 2) β-carotene (n = 29), 3) Zinc (n = 29), or 4) zinc + β-carotene (n = 35) | — | N/A | Respectively, at 1 mo [median (IQR)]: 0.051 (0.044, 0.089), 0.033 (0.034, 0.073), 0.051 (0.041, 0.080), 0.054 (0.031, 0.106); at 6 mo: 0.028 (0.019, 0.037), 0.031 (0.020, 0.047), 0.030 (0.020, 0.048), 0.041 (0.025, 0.057) | Respectively, at 1 mo: 30.1 ± 12.0, 34.4 ± 12.2, 33.9 ± 15.8, 31.6 ± 9.6; at 6 mo: 33.8 ± 14.0, 32.5 ± 16.2, 34.2 ± 16.5, 30.5 ± 14.2 | Full expression of right breast 45–60 min after last feeding by using manual pump; samples saponified with KOH in ethanol, extracted with hexane, retinol measured by HPLC per method of Jackson et al. (23) | HM retinol significantly higher at 6 mo in zinc + β-carotene group compared with group 1 (control); significant decrease in HM retinol from 1 to 6 mo of lactation across groups (P < 0.001) | Samples representing first month of lactation were collected at different weeks, possibly reflecting different stages of milk composition and maturity |
| Engle-Stone et al., 2014 (24) | Cameroon | Yes | 419 | Median: 37.8 wk (IQR: 35.0, 40.6 wk) | Cross-sectional (prenational oil fortification program) | 48.1 | Median (IQR): 3.79 (3.53, 4.05) | Median (IQR): 0.082 (0.077, 0.088) | Median (IQR): 50.4 (47.7, 53.1) | 5–10 mL expressed manually exactly 30 s after infant finished feeding from same (fuller) breast; samples saponified with ethanolic KOH, extracted with ethanol, retinol measured with HPLC per methods of Tanumihardjo and Penniston (25) and Lunetta et al. (26) | HM VA was negatively associated with pCRP concentrations only after controlling for milk-fat concentration, possibly mediated by pRBP | 48% of women received high-dose VA supplements, although average time since supplementation was ∼9 mo and supplement was not a predictor of HM retinol:fat |
| Engle-Stone et al., 2017 (27) | Cameroon | Yes | 154 | Mixed | Cross-sectional (post–national oil fortification program) | Not reported | 4.38 ± 0.25 | 0.095 ± 0.004 | 51.7 ± 3.5 | 5–10 mL expressed manually exactly 30 s after infant finished feeding from same (fuller) breast; samples saponified with ethanolic KOH, extracted with ethanol, retinol measured with HPLC | Distribution of HM VA did not differ before and after initiation of national oil fortification program after controlling for age of the infant, milk-fat concentration, and a square term for milk fat | 47% of women received high-dose VA supplements |
| Klevor et al., 2016 (28) | Ghana | No | 756 | 6 mo | RCT; from ≤ 20 wk gestation to 6 mo postpartum: 1) 800 μg RE/d in lipid-based nutrient supplement (n = 253), 2) 800 μg/d RE in multimicronutrient supplement (n = 260), and 3) control (n = 243) | — | Lipid-based nutrient supplement: 2.5 ± 0.1, multimicronutrient supplement: 2.5 ± 0.1; control: 2.5 ± 0.1 (NS) | Lipid-based nutrient supplement: 0.055 ± 0.003; multimicronutrient supplement: 0.055 ± 0.003; control: 0.059 ± 0.003 (NS) | Lipid-based nutrient supplement: 44.7 ± 1.9; multimicronutrient supplement: 45.2 ± 1.9; control: 41.9 ± 1.8 (NS) | 10 mL expressed manually after feeding infant for 1 min on same breast; retinol measured with HPLC per the method of Tanumihardjo and Penniston (25) | 17% of participants had HM retinol:g fat ≤28 nmol/g; 41% of infants estimated to have daily VA intakes above the Tolerable Upper Intake Level of 600 μg RE/d | 58% of women received high-dose pospartum VA supplements; adherence to study supplement was 76.6% ± 22.7%; women receiving lipid-based supplements were not blinded |
| Liyanage et al., 2008 (29) | Sri Lanka | Mixed | 88 (2–3 mo: n = 19; 4–6 mo: n = 47; ≥7 mo: n = 22) | 2–9 mo (median: 5.2 mo) | Cross-sectional | Not reported | 2–3 mo: 0.56 (95% CI: 0.39, 0.79); 4–6 mo: 0.49 (95% CI: 0.42, 0.58), ≥7 mo: 0.36 (95% CI: 0.29, 0.46) (P < 0.05 comparing ≥7 mo with other groups) | 2–3 mo: 0.025 ± 0.02; 4–6 mo: 0.030 ± 0.02; ≥7 mo: 0.021 ± 0.02 (NS) | 2–3 mo: 31.54 ± 10.99; 4–6 mo: 26.78 ± 13.07; ≥7 mo: 24.55 ± 12.62 (P < 0.05 comparing ≥7 mo with other groups) | 5 mL collected by pump from right breast ≥60 min after last feeding; analytical methods of Tanumihardjo and Penniston (25) | Despite mixed socioeconomic status, 92% of mothers with HM retinol <1.05 μmol/L | Wide range in lactational stage, mixed socioeconomic status |
| Lopez-Teros et al., 2017 (30) | Mexico | N/A | 56 (urban: n = 26; agricultural: n = 30) | 3 ± 2 mo | Cross-sectional | Not reported | Overall: 1.1 ± 0.6 (urban: 1.3 ± 0.69; agricultural: 0.92 ± 0.51; P = 0.03) | Overall: 0.056 ± 0.029 (urban: 0.057 ± 0.030; agricultural: 0.055 ± 0.029; NS) | Overall: 23 ± 14 | 5 mL extracted by manual pump collected after 5 s of production, at 1130 ± 2 h; samples saponified with ethanolic KOH, extracted with hexane, retinol measured by HPLC | 57% and 16% of mothers deficient defined as HM VA <1.05 μmol/L and <0.028 μmol/g fat, respectively; HM VA not associated with BMI | Small n within each demographic; range in lactational stage |
| Macias and Schweigert, 2001 (31) | Cuba | Yes | 21 | 24–48 h, 7 d, and 15 d | Prospective cohort | 95 | 24–48 h: 1.02 ± 0.56; 7 d: 0.50 ± 0.21; 15 d: 0.33 ± 0.14 (P < 0.001 between time points) | 24–48 h: 0.09 ± 0.05; 7 d: 0.02 ± 0.02; 15 d: 0.02 ± 0.01 (NS) | Reported as grams per liter (likely off by factor of 10); 24–48 h: 1.54 ± 1.55; 7 d: 3.64 ± 2.53; 15 d: 3.31 ± 2.61 (P < 0.001 between time points) | 10–12 mL foremilk collected from one breast by manual expression; samples saponified with ethanolic KOH, extracted with hexane, retinol measured by HPLC per the method of Schweigert et al. (32) | Total lipids increased from colostrum to transitional milk | Small n |
| Meneses and Trugo, 2005 (33) | Brazil | Yes | 49 | 30–120 d (54.9 ± 27.3 d) | Cross-sectional | Not reported | 1.4 ± 0.7 | 0.050 ± 0.010 | 33.0 ± 18.3 | Full breast collection by hand pump from breast not suckled in last feeding; analyzed with method of Liu et al. (19) | Retinol:fat higher in multiparous than in primiparous women | Range in lactational stage |
| Muslimatun et al., 2001 (34) | Indonesia | No | 85 | 4–7 d and 3 mo | RCT; beginning at 16–20 wk gestation, weekly: 1) 120 mg Fe + 500 mg folic acid + 4800 IU VA (n = 39) and 2) iron + folic acid (n = 46) | — | 4–7 d: 3.37 (95% CI: 2.47, 4.26) (VA); 2.29 (95% CI: 1.80, 2.79) (control); 3 mo: 1.24 (95% CI: 1.03, 1.45) (VA); 1.06 (95% CI: 0.89, 1.22) (control) (P < 0.001 for 4–7 d vs. 3 mo; P < 0.05 between groups at 4–7 d) | 4–7 d: 0.113 (95% CI: 0.093, 0.132) (VA), 0.097 (95% CI: 0.078, 0.115) (control); 3 mo: 0.053 (95% CI: 0.044, 0.063) (VA), 0.044 (95% CI: 0.037, 0.052) (control) (P < 0.001 for 4–7 d vs. 3 mo; P < 0.05 between groups at 3 mo) | 4–7 d: 29.4 ± 2.5 (VA), 25.8 ± 2.0 (control); 3 mo: 26.3 ± 2.1 (VA), 27.2 ± 1.7 (control) (NS) | Full breast expression between 0800 and 1100 by pump from right breast ≥60 min after last feeding; fat determined by Roese-Gottlieb method (35); samples saponified with KOH, ethanol and ascorbic acid; no details about extraction, retinol measured by HPLC | HM retinol significantly higher in VA supplementation group at 4–7 d; HM retinol:fat significantly higher in VA group at 3 mo | VA-deficient population |
| Olafsdottir et al., 2001 (8) | Iceland | Yes | 77 | 2 or 4 mo | Cross-sectional | Not reported | 2.15 ± 1.05 RE | 0.046 ± 0.021 | 51 ± 21 | 40 mL collected manually or by pump at different times of day; lipid measured by method of Zöllner and Kirsch (36); samples not saponified; retinol and esters measured with HPLC by using the method of Jakob and Elmadfa (37) | Retinyl palmitate:fat correlated with VA intake | High average VA intake (1700 μg/d) |
| Palmer et al., 2016 (38) | Zambia | No | 139 | 4–12 mo | RCT; 6 d/wk × 3 wk: 1) OM containing 600 μg RE/d + placebo (n = 48) and 2) white maize + 600 μg RE/d retinyl palmitate (VA; n = 46), or 3) control (white maize + placebo; n = 45) | — | Geometric mean (95% CI); baseline: 0.95 (0.78, 1.16) (OM); 1.10 (0.85, 1.20) (VA); 0.93 (0.76, 1.14) (control); final: 1.15 (0.96, 1.39) (OM); 1.17 (0.99, 1.38) (VA); 0.91 (0.72, 1.14) (control); P < 0.05, VA vs. control group | Geometric mean (95% CI); baseline: 0.026 (0.022, 0.030) (OM), 0.029 (0.025, 0.034) (VA), 0.026 (0.022, 0.030) (control); final: 0.031 (0.027, 0.036) (OM), 0.033 (0.030, 0.037) (VA), 0.027 (0.023, 0.032) (control) (NS) | Geometric mean (95% CI); baseline: 36.4 (32.7, 40.4) (OM), 34.7 (32.0, 37.7) (VA), 36.4 (33.0, 40.1) (control); final: 36.8 (32.9, 41.1) (OM), 34.9 (31.4, 38.9) (VA), 33.5 (29.9, 37.6) (control) (NS) | 10 mL collected manually from right breast after 1 min of feeding; 1 h after end of feeding, right breast fully expressed by using manual pump; retinol measured with HPLC per the method of Tanumihardjo and Penniston (25) | VA group had significantly lower odds of HM retinol <1.05 μmol/L compared with control at study end (P = 0.03); trend toward greater HM retinol across groups (P < 0.01); effects consistent in casual and full sample, but prevalence estimate for HM VA <1.05 μmol/L higher in full expression (55% vs. 30%) | Actual β-carotene content of OM may have been lower than anticipated; short duration of intervention |
| Ribeiro et al., 2007 (39) | Brazil | Yes | 24 | 0 and 24 h | Prospective cohort | Not reported | 0 h: 3.30 ± 2.06; 24 h: 4.50 ± 2.74 (P = 0.02) | 0 h: 0.154 ± 0.134; 24 h: 0.163 ± 0.134 (NS) | 0 h: 29 ± 17; 24 h: 35 ± 23 (NS) | 1–3 mL collected by manual expression from breast not suckled at last feeding, discarding first ejection; samples saponified with KOH in water, extracted with hexane, retinol measured by HPLC | Great variability in HM retinol between samples collected at different times, less when expressed per fat | Small n; dependence on sample volume |
| Rice et al., 1999 (40) | Bangladesh | No | 220 | 2 wk and 3, 6, and 9 mo | RCT; At 1–3 wk postpartum: 1) 200,000 IU VA followed by daily placebo (n = 73), 2) 7.8 mg β-carotene/d through 9 mo (n = 74), and 3) placebo (n = 73) daily through 9 mo | — | Respectively, at 2 wk: 1.71 ± 1.34, 1.39 ± 1.19, 1.51 ± 1.08; at 3 mo: 1.20 ± 1.00, 0.85 ± 0.55, 0.83 ± 0.43; at 6 mo: 0.85 ± 0.53, 0.99 ± 0.62, 0.87 ± 0.61; at 9 mo: 0.91 ± 0.68, 1.00 ± 0.58, 0.79 ± 0.44 (significantly higher in VA vs. placebo at 3 mo; P < 0.01; significantly higher in β-carotene vs. placebo at 9 mo; P < 0.05) | Respectively, at 2 wk: 0.037 ± 0.019, 0.032 ± 0.017, 0.034 ± 0.018; at 3 mo: 0.028 ± 0.014, 0.022 ± 0.008, 0.023 ± 0.011; at 6 mo: 0.024 ± 0.011, 0.024 ± 0.010, 0.024 ± 0.016; at 9 mo: 0.024 ± 0.013, 0.026 ± 0.008, 0.021 ± 0.001 (significantly higher in VA vs. placebo at 3 mo; P < 0.05; significantly higher in β-carotene vs. placebo at 9 mo; P < 0.01) | N/A | 5 mL milk expressed manually from fuller breast between 0800 and 1700 with median collection time 1050; samples saponified with KOH in methanol, sodium chloride added, extracted with hexane/ether, retinol measured by HPLC | >50% deficiency based on both retinol and retinol:fat in all groups from 3 mo on (despite interventions) | Women with underlying subclinical VA deficiency |
| Stoltzfus et al., 1993 (41, 42) | Indonesia | No | 147 | Baseline (before treatment) and 1, 2, 3, 4, 5, 6, and 8 mo | RCT; 1) 300,000 IU VA at 1–3 wk postpartum (n = 74) and 2) placebo (n = 73) | — | VA vs. control at baseline: 2.30 vs. 2.79; 1 mo: 3.10 vs. 192; 2 mo: 2.61 vs. 1.76; 3 mo: 2.45 vs. 1.82; 4 mo: 2.80 vs. 1.81; 5 mo: 2.38 vs. 1.68; 6 mo: 2.36 vs. 1.77; 8 mo: 2.04 vs. 1.56 (P < 0.01 between groups at all times) | VA vs. control at 1 mo: 0.066 ± 0.027 vs. 0.040 ± 0.021; 3 mo: 0.050 ± 0.019 vs. 0.037 ± 0.020 (NS); not measured at other time points | N/A | Full breast expression manually or by pump between 0900 and 1100 from breast that had not been suckled for ≥60 min; no saponification; sample mixed with ethanol, extracted with hexane, retinol measured by HPLC | HM retinol remained relatively stable from 2 to 8 mo of lactation in both groups | Mothers in VA supplementation group had slightly lower HM and serum retinol at baseline |
| Turner et al., 2013 (43) | Bangladesh | No | 135 | 5 mo | RCT; 2 times/d for 3 wk: 1) orange-fleshed sweet potato (n = 34), 2) 0.5 mg VA (n = 34), 3) tangerine (n = 34) and 4) control (n = 33) | 44–56, by group | Baseline: 0.85 ± 0.04, 0.95 ± 0.05, 0.88 ± 0.05, 0.83 ± 0.03; final: 0.96 ± 0.04, 1.21 ± 0.05, 0.93 ± 0.04, 0.86 ± 0.03 (significantly higher at VA endpoint vs. baseline and at VA endpoint vs. control endpoint) (P < 0.05) | RE/g fat postintervention: 0.022 ± 0.002, 0.029 ± 0.002, 0.022 ± 0.002, 0.020 ± 0.002 | N/A | Full breast expression by electric pump from breast not suckled for ≥60 min; sample saponified with KOH in methanol, extracted with hexane, retinol measured by HPLC | HM retinol increased by 36% in VA group (16% when expressed per fat); HM retinol decreased in all but VA group over course of intervention | Short length of intervention |
| Villard and Bates, 1987 (44) | The Gambia | Yes (low intake, but plasma retinol above “high risk” range) | 55 (supplemented: n = 37; unsupplemented: n = 18) | Weekly from 3 to 15 wk | In pregnancy and lactation: 1) MV-fortified tea drink containing average of 2167 IU RE/d and 2) control | — | Mean of samples: 3.03 ± 0.53 vs. 2.47 ± 0.76 (P < 0.01) | 0.103 ± 0.023 vs. 0.080 ± 0.027 (NS) | 30 vs. 32 (likely error in reported units) | 5–10 mL collected by manual expression from the right breast in the morning, between feedings; milk fat measured by the method of Bucolo and David (45); samples saponified with KOH in water and ascorbic acid, extracted with hexane, retinol measured fluorometrically (46) | Significant difference in HM retinol between groups at 5–9 wk postpartum | Group allocation by village of residence |
1HM, human milk; KOH, potassium hydroxide; MV, multivitamin; N/A, not available; OM, orange maize; pCRP, plasma C-reactive protein; pRBP, plasma retinol-binding protein; RCT, randomized controlled trail; RE, retinol equivalent; ref, reference; RPO, red palm oil; S1, first supplementation protocol; S2, second supplementation protocol; VA, vitamin A; Ø, nothing.
2Values are means ± SDs unless otherwise noted.
3Unless otherwise noted, milk fat was measured by using the creamatocrit method (47).
Data were analyzed by using SAS 9.4 (SAS Institute). With the use of the SAS MIXED procedure, random-effects models weighted by the inverse of the square of the SE or sample size were constructed to examine the time trajectory of human-milk retinol:fat and retinol with the use of post hoc contrasts to identify when retinol:fat and retinol stabilized. Comparisons between retinol and retinol:fat in colostrum and mature milk were generated by including a fixed effect for colostrum (sample collection at <7 d lactation). Pearson correlation coefficients were used to evaluate the correlation between retinol and milk fat. Polynomial models were fitted to investigate the effect of supplementation over time, controlling for fixed and random effects.
Results
Description of studies included in the systematic review and meta-analysis
Seven of the 24 included studies were conducted in South or Southeast Asia (Bangladesh, India, Indonesia, and Sri Lanka) (13, 22, 29, 34, 40, 42, 43), 8 in Central or South America (Mexico, Honduras, Brazil, and Peru) (13, 14, 18, 20, 21, 30, 33, 39), 7 in Africa (Cameroon, Ghana, Kenya, Zambia, and The Gambia) (12, 13, 24, 44), 2 in the United States (16, 17), 1 in Cuba (31), and 1 in Iceland (8). One multicenter trial included 3 sites in India, Peru, and Ghana (13). Both human-milk retinol:fat and retinol were reported in 22 of the 24 included studies. Seven studies were conducted in rural populations with poor socioeconomic status (12, 22, 34, 40, 42–44), whereas 2 were conducted in middle- or upper-middle class populations (16, 17). The remaining studies included women of mixed socioeconomic backgrounds. One study included only adolescent mothers (21).
Nine of the 24 studies were longitudinal in design, measuring human-milk vitamin A at 2–8 time points from colostrum through 9 mo postpartum (12–14, 22, 31, 34, 40, 42, 44). Of the longitudinal trials, 5 included intervention groups who received high-dose vitamin A supplementation (200,000–400,000 IU) at delivery or in the early postpartum period (12–14, 40, 42), 3 included intervention groups supplemented with β-carotene or vitamin A daily or weekly during the second half of gestation (22, 34, 44), and 1 did not involve an intervention (31). Five additional studies involved short-term supplementation protocols in which human-milk retinol was measured before and after a single- or multidose intervention with β-carotene supplements, vitamin A supplements, or food sources of β-carotene (16–18, 38, 43). In the remaining 10 studies, milk was collected at one point during lactation (8, 20, 21, 24, 27–30, 33, 39).
Methods for milk collection varied widely. In 9 studies, milk collection protocols involved full manual or pumped expression of a breast not suckled for ≥1 h or not suckled in the previous feeding (16, 17, 21, 22, 33, 34, 38, 42, 43). In other studies, 1–3 mL colostrum (14, 20, 39) or 2–40 mL transitional or mature milk was collected from one breast either with or without regard to time elapsed since last feeding. Milk was collected in the morning in the protocols of 7 studies (13, 18, 21, 30, 34, 42, 44).
The fat content of milk was measured by using the creamatocrit method, which is based on centrifugation of samples in hematocrit capillary tubes (47), in all but 3 studies (8, 34, 44). The remaining studies measured fat by using the Roese-Gottlieb (35), Zöllner and Kirsch (36), or Bucolo and David (45) methods. The CV for fat was reported in 5 studies and ranged from 1.3% to <10% (8, 22, 24, 27, 28).
Milk retinol concentration was measured by HPLC in all but one study, in which retinol was quantified fluorometrically (44). The techniques used for human-milk sample preparation varied (15, 19, 23, 25, 26, 32, 48), but most involved alkaline saponification with potassium hydroxide in ethanol or methanol to hydrolyze retinyl esters to retinol, followed by hexane extraction. In 3 studies, human-milk samples were pretreated with bile salts, protease, and lipase by using the method of Liu et al. (18, 19, 21, 33). Reported CVs for retinol were ≤10% in all but 3 studies, in which interassay CVs were <12%, 13.3%, and 13.4% (27, 33, 43).
Potential sources of bias in individual studies are described in Table 1. These include a small sample size, method and timing of milk collection, and selection bias based on known conditions.
Retinol:fat and retinol concentration in human milk
Both mean retinol:fat and retinol concentration were significantly higher in colostrum or early milk samples (<7 d lactation) than in mature milk (>21 d lactation; mean retinol:fat: 0.161 ± 0.082 compared with 0.038 ± 0.016 μmol/g; P < 0.0001; mean retinol: 3.49 ± 0.70 compared with 1.34 ± 0.75 μmol/L; P < 0.0001). After an initial sharp decline, human-milk retinol:fat reached statistical stability (nonsignificant variation between subsequent time points) after 2 wk of lactation, whereas human-milk retinol stabilized after 4 wk of lactation.
Bahl et al. (13) found a slight upward trend in retinol:fat from 6 to 9 mo at all 3 study sites. The same trend was not apparent in the only other longitudinal study that continued through 9 mo (40), nor was it corroborated through the present statistical analysis that included cross-sectional data. In mature milk, retinol was positively correlated with milk fat (r = 0.61, P = 0.008).
Factors affecting human-milk vitamin A concentration
Human-milk retinol:fat and retinol were positively associated with plasma RBP among Cameroonian women in the lower 2 vitamin A intake tertiles (β = 0.81–0.84, P < 0.01), but not among women in the highest intake tertile (24). In the study by Muslimatun et al. (34) in a vitamin A–deficient population in Indonesia, milk retinol:fat and retinol were significantly correlated with serum retinol only in subjects who were not receiving vitamin A supplements (r = 0.487 and 0.304, P < 0.01). No association was found between milk retinol:fat or retinol and plasma or serum retinol in 3 studies in which mothers were well nourished per the author descriptions (21, 33, 44).
Engle-Stone et al. (24) found a positive association between vitamin A intake the previous day and both human-milk retinol:fat and retinol in Cameroon (R2 = 0.13 and 0.07, respectively; P < 0.001 for both). Olafsdottir et al. (8) also found a significant correlation between dietary vitamin A intake and retinyl palmitate:fat in milk (r = 0.23, P < 0.05). The investigators concluded that the concentration of retinyl palmitate reflected the esterification of dietary retinol. Habitual vitamin A intake and human-milk retinol were not significantly associated in healthy, well-nourished Brazilian women (33). However, the proportion of women with vitamin A intakes below the Estimated Average Requirement was consistent with the proportion whose milk retinol concentrations were below the cutoff of 1.05 μmol/L.
Colostrum retinol was not associated with serum C-reactive protein (CRP) in the study by da Silva Ribeiro et al. (20) in Brazil, nor was human-milk retinol:fat or retinol associated with plasma α1-acid glycoprotein in the study by Engle-Stone et al. (24) in Cameroon. In the Cameroonian study, both human-milk retinol:fat and retinol were inversely associated with plasma CRP only after controlling for maternal BMI, milk-fat content, time postpartum, time of day, and time since last breastfeeding (β = − 0.03 ± 0.01, P < 0.02). However, when plasma RBP was added as a term in the simple linear regression for retinol:fat, plasma CRP was no longer a significant predictor. There was no evidence of confounding or effect modification by baseline CRP in the study by Palmer et al. (38) in Zambia.
Sri Lankan women living in urban environments had significantly lower milk-fat content and higher retinol:fat than did women living in rural areas (P = 0.03) (29); however, the opposite was found between rural and urban communities in Cameroon (P < 0.05) (24) and in Mexico (P = 0.03) (30). Retinol:fat was positively correlated with the weight (r = 0.274, P = 0.01) and height (r = 0.328, P < 0.001) of the participants in the Sri Lankan study (29), and both human-milk retinol:fat and retinol were predicted by BMI in Cameroon [β = 0.74 ± 0.29 (P = 0.012) and β = 0.75 ± 0.29 (P = 0.011), respectively, for log-transformed variables] (24). However, human-milk retinol was not correlated with BMI in 2 Brazilian studies in healthy, well-nourished adolescents and women (21, 33), nor in a study in urban and rural Mexican women (30). Parity and gravidity have been associated with human-milk retinol:fat or retinol in some studies (21, 33) but not in others (14, 42). Low maternal socioeconomic status and education were associated with lower human-milk retinol concentration in one study (24).
Effect of supplementation
Postpartum supplementation with high-dose vitamin A (200,000–400,000 IU) in 5 studies increased human-milk retinol:fat and retinol significantly compared with controls through 12 and 24 wk of lactation, respectively (P < 0.05) (12–14, 40, 42). The difference between retinol:fat in the intervention and control groups was linearly and inversely correlated with time (r = 0.74, P = 0.002), with an x-intercept of 28.6 wk, which suggested no further effect of high-dose supplementation on human-milk retinol:fat beyond this time. The difference in human-milk retinol between supplementation and control groups also diminished as lactation progressed, although the correlation with time was not significant.
Maternal supplementation with β-carotene or carotenoid-rich food rapidly increased human-milk carotenoid concentrations. Human-milk retinol was unchanged in studies that supplemented pregnant or lactating women of varying vitamin A status with cumulative doses of 60–900 mg β-carotene (16–18, 22). Bangladeshi mothers who received 7.8 mg β-carotene/d from delivery through 9 mo (cumulative dose >2000 mg) had higher human-milk retinol concentrations than did a placebo group at 9 mo postpartum (P < 0.05), but not at 3 or 6 mo (40).
Discussion
Because human infants are born with meager vitamin A reserves regardless of maternal nutritional status, adequate retinol in milk is critical for ensuring healthy infant growth and development. The present analysis considered 2 indexes of human-milk vitamin A: 1) retinol adjusted for milk fat and 2) direct retinol concentration. Despite their different interpretations, human-milk retinol:fat and retinol behaved similarly in terms of time trends, association with maternal factors, and response to supplementation.
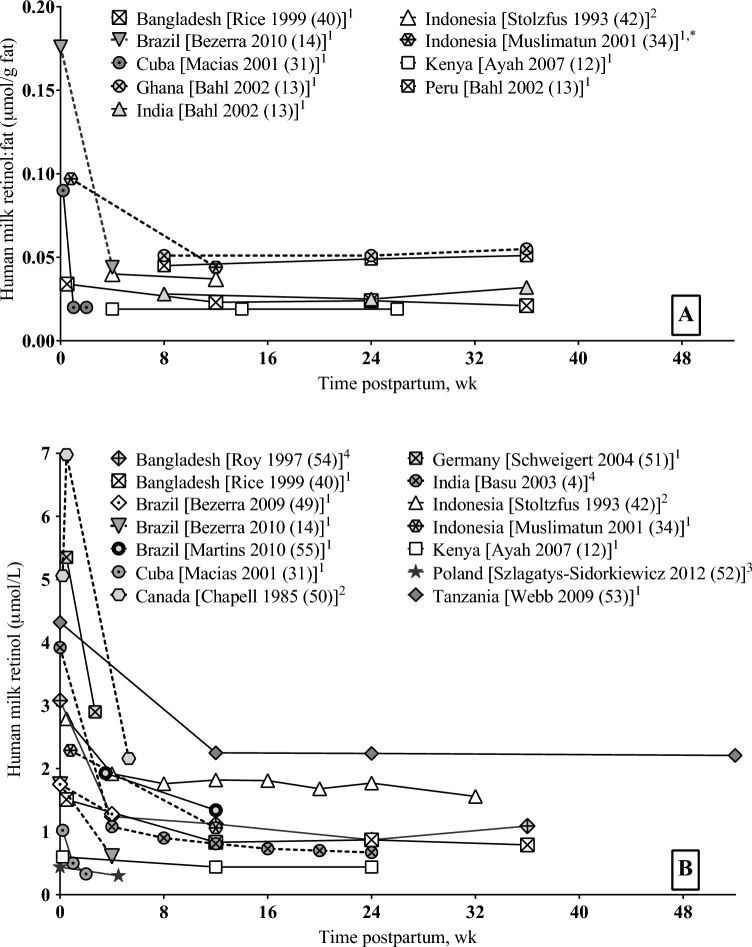
Both retinol:fat and retinol peaked in colostrum and reached statistical equilibrium in mature milk, corroborating data from earlier longitudinal studies of human-milk retinol in unsupplemented women (49–53) (Figure 2). Because colostrum is low in milk fat, adjusting colostrum retinol for milk fat amplifies its peak. High concentrations of retinol in colostrum rapidly replete tissue stores in the newborn after limited placental transfer during gestation (5).
FIGURE 2.
Human-milk retinol:fat (A) and retinol concentrations (B) in longitudinal studies (unsupplemented women). Analytical methods for measuring vitamin A are indicated by superscript numbers. Unless otherwise noted by an asterisk (*), milk fat was measured by using the creamatocrit method (47). 1HPLC after saponification to obtain free retinol from retinyl esters; 2HPLC without saponification; 3HPLC with the use of a commercial kit for sample preparation (validated for plasma but not milk); 4colorimetry after saponification.
In the present analysis, human-milk retinol was directly correlated with fat in mature milk. It should be noted that this correlation was based on study means of human-milk retinol and fat rather than individual values and should be interpreted with caution given the known heterogeneity within and between studies. However, the result is supported by 2 studies that found significant correlations between human-milk retinol or retinol equivalents and milk fat with the use of individual data points (8, 24).
Retinol, mainly as retinyl palmitate, is found primarily in milk-fat globules. A large part of the fluctuation in milk-fat concentration within an individual can be accounted for by breast fullness at the point of sampling, which is related to time of day, interfeeding interval, and point of sampling during a feeding (56). Diurnal variations in milk fat are mimicked by human-milk retinol (24). As a result, retinol concentration in casual milk samples of lactating women may be used as a population indicator of vitamin A status or as a marker of response to an intervention, provided that the samples are collected at random or expressed relative to milk fat (2, 57).
In addition to diurnal variation, the fat content of human milk is affected by the general nutritional status of the mother, as well as recent dietary fat intake (29, 58). Human-milk vitamin A is inconsistently correlated with maternal anthropometric measurements; this relation may be confounded by maternal nutritional status and milk fat.
The biological sources of human-milk retinol depend on maternal intake and vitamin A status. On the basis of models in lactating rats, when maternal status is adequate, circulating RBP-retinol contributes a relatively constant amount of vitamin A to the lactating mammary gland, whereas the contribution of chylomicron vitamin A varies directly with recent intake (59). Because of this relation, a correlation between human-milk and circulating retinol concentrations is more likely when vitamin A intake is low, as shown in the present review. Circulating RBP-retinol is under homeostatic regulation by the liver and draws upon liver retinol stores, maintaining milk retinol concentration at the expense of maternal reserves.
Possibly because of the buffer of dietary retinol, there is some indication that human-milk retinol is less sensitive to acute infection than circulating retinol, which is depressed in response to inflammation (60, 61). In general agreement with the results of the present review, a previous study found no association between human-milk retinol and plasma CRP or α1-acid glycoprotein at 2 wk postpartum in HIV-negative Malawian women (62).
Maternal supplementation with high-dose oral vitamin A in the first weeks postpartum results in an increase in human-milk retinol:fat and retinol that diminishes over time. On the basis of our statistical models, supplementation significantly increases human-milk retinol:fat and retinol compared with controls through 3–6 mo of lactation and continues to have a nonsignificant but measurable effect through 7.5 mo. In poorly nourished populations, the effect of high-dose vitamin A supplementation on human milk concentrations may be sustained for a longer period (49, 55). More research is needed to understand the mechanism by which human-milk retinol is repleted over the long term.
There are mixed results with regard to the effect of maternal β-carotene consumption on human-milk retinol concentration. Although a number of studies found no effect of increased dietary intake or supplementation with β-carotene (16, 18, 22, 38, 63), others found a modest but significant increase in human-milk retinol after a β-carotene intervention (40, 64). The discrepancy may be related to the underlying vitamin A status of the population and to the cumulative dose received through the intervention.
A previous review found that despite increases in human-milk retinol, maternal supplementation with high-dose vitamin A failed to affect infant vitamin A status, morbidity, and mortality (65). The WHO rescinded its recommendation for postpartum high-dose vitamin A supplementation of at-risk populations after these findings (66). However, a benefit of supplementation may be that of offsetting maternal depletion, as shown with the modified relative dose-response method in the study of Rice et al. (40) in Bangladesh.
A minimum acceptable concentration of 1.05 μmol retinol/L in human milk has been suggested on the basis of empirical calculations of accumulated liver reserves necessary to sustain physiologic needs in the latter half of infancy (67) as well as on data from an Indonesian cohort in whom milk retinol concentrations <1.05 μmol/L and low infant liver reserves measured by modified relative dose-response at 6 mo were equally prevalent (42). The standard cutoff for retinol:fat is 0.028 μmol/g fat (57), likely derived from the value for human-milk retinol and the average fat content of human milk. Additional research is needed to verify the validity of these cutoffs for defining maternal and infant adequacy.
The present analysis has several limitations. Of the studies that measured retinol in human milk, only approximately one-third reported retinol:fat and were included in the data set. Because baseline maternal vitamin A status was not available in the majority of studies, it could not be controlled for in analysis. Furthermore, it was not possible to ascertain how variability in sample collection, milk volume, feeding duration, and analytical methods may have contributed to observed differences. Because the National Institute of Standards and Technology offers no standard for human milk micronutrient analysis, established methods for retinol assay in human milk have not been validated using a standardized reference material, and the accuracy and precision of published results depends on internal method validation and standardization.
In conclusion, accurate measurement and interpretation of human-milk vitamin A are imperative to understanding how lactation interfaces with maternal and infant vitamin A status. Vitamin A adequacy is critical for maternal well-being and reproductive success as well as for optimal infant immune function, growth, and development. In a human-milk sample, adjusting retinol for fat is a means of dissociating temporal variations in milk fat from its substrate of interest. At the population level, direct retinol concentrations of human milk in samples with variable fat concentrations can function as a general indicator of maternal vitamin A status and, in concert with milk volume, provide a measure of maternal-infant transfer of the vitamin. The present review and analysis confirm that human-milk retinol:fat and retinol showed similar trends over the course of lactation and respond in parallel fashion to maternal intake, status, and supplementation. Both indexes decrease from colostrum to mature milk and increase temporarily with high-dose postpartum maternal supplementation. To better define retinol adequacy in human milk as a component of overall vitamin A nutrition, additional research is needed to verify the validity of established cutoffs for defining maternal and infant adequacy. It would also be prudent to investigate the relative contribution of circulating and dietary retinol to human-milk retinol in the presence and absence of acute infection. Furthermore, maternal vitamin status should be monitored to determine how uptake of retinol into the mammary gland affects maternal vitamin reserves, particularly in women who are at high risk or who are known to be vitamin A deficient.
Acknowledgments
Both authors read and approved the final manuscript.
Notes
Published in a supplement to Advances in Nutrition. Supplement funding was provided by the Bill & Melinda Gates Foundation. The Supplement Coordinators for this supplement were Lindsay H Allen and Daphna K Dror. Supplement Coordinator disclosure: Lindsay H Allen has no conflict of interest. Daphna K Dror has no conflict of interest. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the author(s) and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported by the Bill & Melinda Gates Foundation (OPP1061055) and intramural USDA–Agricultural Research Service projects 5306-51000-003-00D and 5306-51000-004-00D.
Author disclosures: DKD and LHA, no conflicts of interest.
The USDA, Agricultural Research Service, is an equal opportunity provider and employer.
References
- 1. Institute of Medicine, Food and Nutrition Board Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 2. Stoltzfus RJ, Underwood BA. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull World Health Organ 1995;73:703–11. [PMC free article] [PubMed] [Google Scholar]
- 3. Ross JS, Harvey PW. Contribution of breastfeeding to vitamin A nutrition of infants: a simulation model. Bull World Health Organ 2003;81:80–6. [PMC free article] [PubMed] [Google Scholar]
- 4. Basu S, Sengupta B, Paladhi PK. Single megadose vitamin A supplementation of Indian mothers and morbidity in breastfed young infants. Postgrad Med J 2003;79:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Debier C, Larondelle Y. Vitamins A and E: metabolism, roles and transfer to offspring. Br J Nutr 2005;93:153–74. [DOI] [PubMed] [Google Scholar]
- 6. Ross AC, Davila ME, Cleary MP. Fatty acids and retinyl esters of rat milk: effects of diet and duration of lactation. J Nutr 1985;115:1488–97. [DOI] [PubMed] [Google Scholar]
- 7. O'Byrne SM, Kako Y, Deckelbaum RJ, Hansen IH, Palczewski K, Goldberg IJ, Blaner WS. Multiple pathways ensure retinoid delivery to milk: studies in genetically modified mice. Am J Physiol Endocrinol Metab 2010;298:E862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olafsdottir AS, Wagner KH, Thorsdottir I, Elmadfa I. Fat-soluble vitamins in the maternal diet, influence of cod liver oil supplementation and impact of the maternal diet on human milk composition. Ann Nutr Metab 2001;45:265–72. [DOI] [PubMed] [Google Scholar]
- 9. Allen LH, Haskell MJ. Vitamin A requirements of infants under six months of age. Food Nutr Bull 2001;22:214–34. [Google Scholar]
- 10. Mitoulas LR, Kent JC, Cox DB, Owens RA, Sherriff JL, Hartmann PE. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br J Nutr 2002;88:29–37. [DOI] [PubMed] [Google Scholar]
- 11. Hassiotou F, Hepworth AR, Williams TM, Twigger AJ, Perrella S, Lai CT, Filgueira L, Geddes DT, Hartmann PE. Breastmilk cell and fat contents respond similarly to removal of breastmilk by the infant. PLoS One 2013;8:e78232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayah RA, Mwaniki DL, Magnussen P, Tedstone AE, Marshall T, Alusala D, Luoba A, Kaestel P, Michaelsen KF, Friis H. The effects of maternal and infant vitamin A supplementation on vitamin A status: a randomised trial in Kenya. Br J Nutr 2007;98:422–30. [DOI] [PubMed] [Google Scholar]
- 13. Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK. Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J Nutr 2002;132:3243–8. [DOI] [PubMed] [Google Scholar]
- 14. Bezerra DS, de Araujo KF, Azevedo GM, Dimenstein R. A randomized trial evaluating the effect of 2 regimens of maternal vitamin A supplementation on breast milk retinol levels. J Hum Lact 2010;26:148–56. [DOI] [PubMed] [Google Scholar]
- 15. Giuliano AR, Neilson EM, Kelly BE, Canfield LM. Simultaneous quantitation and separation of carotenoids and retinol in human milk by high-performance liquid chromatography. Methods Enzymol 1992;213:391–9. [DOI] [PubMed] [Google Scholar]
- 16. Canfield LM, Giuliano AR, Neilson EM, Yap HH, Graver EJ, Cui HA, Blashill BM. beta-Carotene in breast milk and serum is increased after a single beta-carotene dose. Am J Clin Nutr 1997;66:52–61. [DOI] [PubMed] [Google Scholar]
- 17. Canfield LM, Giuliano AR, Neilson EM, Blashil BM, Graver EJ, Yap HH. Kinetics of the response of milk and serum beta-carotene to daily beta-carotene supplementation in healthy, lactating women. Am J Clin Nutr 1998;67:276–83. [DOI] [PubMed] [Google Scholar]
- 18. Canfield LM, Kaminsky RG, Taren DL, Shaw E, Sander JK. Red palm oil in the maternal diet increases provitamin A carotenoids in breastmilk and serum of the mother-infant dyad. Eur J Nutr 2001;40:30–8. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Xu M, Canfield L. Enzymatic hydrolysis, extraction, and quantitation of retinol and major carotenoids in mature human milk. J Nutr Biochem 1998;9:178–83. [Google Scholar]
- 20. da Silva Ribeiro KD, de Araujo KF, de Souza HH, Soares FB, da Costa Pereira M, Dimenstein R. Nutritional vitamin A status in northeast Brazilian lactating mothers. J Hum Nutr Diet 2010;23:154–61. [DOI] [PubMed] [Google Scholar]
- 21. de Azeredo VB, Trugo NM. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 2008;24:133–9. [DOI] [PubMed] [Google Scholar]
- 22. Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus β-carotene supplementation of pregnant women is superior to β-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am J Clin Nutr 2004;80:1299–307. [DOI] [PubMed] [Google Scholar]
- 23. Jackson JGLE, White SJ, Bruns NJ, Kuhlman CF. Major carotenoids in mature human milk: longitudinal and diurnal patterns. J Nutr Biochem 1998;9:2–7. [Google Scholar]
- 24. Engle-Stone R, Haskell MJ, Nankap M, Ndjebayi AO, Brown KH. Breast milk retinol and plasma retinol-binding protein concentrations provide similar estimates of vitamin A deficiency prevalence and identify similar risk groups among women in Cameroon but breast milk retinol underestimates the prevalence of deficiency among young children. J Nutr 2014;144:209–17. [DOI] [PubMed] [Google Scholar]
- 25. Tanumihardjo SA, Penniston KL. Simplified methodology to determine breast milk retinol concentrations. J Lipid Res 2002;43:350–5. [PubMed] [Google Scholar]
- 26. Lunetta JM, Zulim RA, Dueker SR, Lin Y, Flaig V, Schneider PD, Wolfe BM, Clifford AJ. Method for the simultaneous determination of retinol and beta-carotene concentrations in human tissues and plasma. Anal Biochem 2002;304:100–9. [DOI] [PubMed] [Google Scholar]
- 27. Engle-Stone R, Nankap M, Ndjebayi A, Gimou MM, Friedman A, Haskell MJ, Tarini A, Brown KH. Vitamin A status of women and children in Yaounde and Douala, Cameroon, is unchanged one year after initiation of a national vitamin A oil fortification program. Nutrients 2017;9:E522–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klevor MK, Haskell MJ, Lartey A, Adu-Afarwuah S, Zeilani M, Dewey KG. Lipid-based nutrient supplements providing approximately the recommended daily intake of vitamin A do not increase breast milk retinol concentrations among Ghanaian women. J Nutr 2016;146:335–42. [DOI] [PubMed] [Google Scholar]
- 29. Liyanage C, Hettiarachchi M, Mangalajeewa P, Malawipathirana S. Adequacy of vitamin A and fat in the breast milk of lactating women in south Sri Lanka. Public Health Nutr 2008;11:747–50. [DOI] [PubMed] [Google Scholar]
- 30. Lopez-Teros V, Limon-Miro AT, Astiazaran-Garcia H, Tanumihardjo SA, Tortoledo-Ortiz O, Valencia ME. “Dose-to-mother” deuterium oxide dilution technique: an accurate strategy to measure vitamin A intake in breastfed infants. Nutrients 2017;9:E169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macias C, Schweigert FJ. Changes in the concentration of carotenoids, vitamin A, alpha-tocopherol and total lipids in human milk throughout early lactation. Ann Nutr Metab 2001;45:82–5. [DOI] [PubMed] [Google Scholar]
- 32. Schweigert FJ, Hurtienne A, Bathe K. Improved extraction procedure for carotenoids from human milk. Int J Vitam Nutr Res 2000;70:79–83. [DOI] [PubMed] [Google Scholar]
- 33. Meneses F, Trugo NMF. Retinol, β-carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: associations with plasma concentrations and influences of maternal characteristics. Nutr Res 2005;25:443–51. [Google Scholar]
- 34. Muslimatun S, Schmidt MK, West CE, Schultink W, Hautvast JG, Karyadi D. Weekly vitamin A and iron supplementation during pregnancy increases vitamin A concentration of breast milk but not iron status in Indonesian lactating women. J Nutr 2001;131:2664–9. [DOI] [PubMed] [Google Scholar]
- 35. Association of Official Analytical Chemists Official methods of analysis. 14th ed.S Williams, editor Arlington (VA): Association of Analytical Communities; 1984. [Google Scholar]
- 36. Zöllner N, Kirsch K. Über die quantitative Bestimmung von Lipiden (Micromethode) mittels der von vielen natürlichen Lipoiden (allen Plasmalipiden) bekannten Sulphophosphovanilin-Reaktion. [The quantitative determination of lipids (micromethod) by means of the sulfo-phospho-vanillin reaction common to many natural lipids (all plasma lipids).]Z Gesamte Exp Med 1962;135(Suppl):545–61(in German). [Google Scholar]
- 37. Jakob E, Elmadfa I. Rapid HPLC assay for the assessment of vitamin K1, A, E and beta-carotene status in children (7-19 years). Int J Vitam Nutr Res 1995;65:31–5. [PubMed] [Google Scholar]
- 38. Palmer AC, Chileshe J, Hall AG, Barffour MA, Molobeka N, West KP Jr., Haskell MJ. Short-term daily consumption of provitamin A carotenoid-biofortified maize has limited impact on breast milk retinol concentrations in Zambian women enrolled in a randomized controlled feeding trial. J Nutr 2016;146:1783–92. [DOI] [PubMed] [Google Scholar]
- 39. Ribeiro KD, Araújo KF, Pereira MC, Dimenstein R. Evaluation of retinol levels in human colostrum in two samples collected at an interval of 24 hours. J Pediatr (Rio J) 2007;83:377–80. [DOI] [PubMed] [Google Scholar]
- 40. Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed M. Maternal vitamin A or β-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr 1999;129:356–65. [DOI] [PubMed] [Google Scholar]
- 41. Stoltzfus RJ, Habicht JP, Rasmussen KM, Hakimi M. Evaluation of indicators for use in vitamin A intervention trials targeted at women. Int J Epidemiol 1993;22:1111–8. [DOI] [PubMed] [Google Scholar]
- 42. Stoltzfus RJ, Hakimi M, Miller KW, Rasmussen KM, Dawiesah S, Habicht JP, Dibley MJ. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr 1993;123:666–75. [DOI] [PubMed] [Google Scholar]
- 43. Turner T, Burri BJ, Jamil KM, Jamil M. The effects of daily consumption of β-cryptoxanthin-rich tangerines and β-carotene-rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial. Am J Clin Nutr 2013;98:1200–8. [DOI] [PubMed] [Google Scholar]
- 44. Villard L, Bates CJ. Effect of vitamin A supplementation on plasma and breast milk vitamin A levels in poorly nourished Gambian women. Hum Nutr Clin Nutr 1987;41:47–58. [PubMed] [Google Scholar]
- 45. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973;19:476–82. [PubMed] [Google Scholar]
- 46. Neeld JB Jr., Pearson WN. Macro- and micromethods for the determination of serum vitamin A using trifluoroacetic acid. J Nutr 1963;79:454–62. [DOI] [PubMed] [Google Scholar]
- 47. Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. BMJ 1978;1:1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turner T, Burri BJ. Rapid isocratic HPLC method and sample extraction procedures for measuring carotenoid, retinoid, and tocopherol concentrations in human blood and breast milk for intervention studies. Chromatographia 2012;75:241–52. [Google Scholar]
- 49. Bezerra DS, Araujo KF, Azevedo GM, Dimenstein R. Maternal supplementation with retinyl palmitate during immediate postpartum period: potential consumption by infants. Rev Saude Publica 2009;43:572–9. [DOI] [PubMed] [Google Scholar]
- 50. Chappell JE, Francis T, Clandinin MT. Vitamin A and E content of human milk at early stages of lactation. Early Hum Dev 1985;11:157–67. [DOI] [PubMed] [Google Scholar]
- 51. Schweigert FJ, Bathe K, Chen F, Buscher U, Dudenhausen JW. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Nutr 2004;43:39–44. [DOI] [PubMed] [Google Scholar]
- 52. Szlagatys-Sidorkiewicz A, Zagierski M, Jankowska A, Luczak G, Macur K, Baczek T, Korzon M, Krzykowski G, Martysiak-Zurowska D, Kaminska B. Longitudinal study of vitamins A, E and lipid oxidative damage in human milk throughout lactation. Early Hum Dev 2012;88:421–4. [DOI] [PubMed] [Google Scholar]
- 53. Webb AL, Aboud S, Furtado J, Murrin C, Campos H, Fawzi WW, Villamor E. Effect of vitamin supplementation on breast milk concentrations of retinol, carotenoids and tocopherols in HIV-infected Tanzanian women. Eur J Clin Nutr 2009;63:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roy S, Islam A, Molla A, Akramuzzaman S, Jahan F, Fuchs G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. Eur J Clin Nutr 1997;51:302–7. [DOI] [PubMed] [Google Scholar]
- 55. Martins TM, Ferraz IS, Daneluzzi JC, Martinelli CE Jr., Del Ciampo LA, Ricco RG, Jordao AA Jr., Patta MC, Vannucchi H. Impact of maternal vitamin A supplementation on the mother-infant pair in Brazil. Eur J Clin Nutr 2010;64:1302–7. [DOI] [PubMed] [Google Scholar]
- 56. Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006;117:e387–95. [DOI] [PubMed] [Google Scholar]
- 57. WHO Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes. Geneva (Switzerland): WHO; 1996. [Google Scholar]
- 58. Nommsen LA, Lovelady CA, Heinig MJ, Lonnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING study. Am J Clin Nutr 1991;53:457–65. [DOI] [PubMed] [Google Scholar]
- 59. Ross AC, Pasatiempo AM, Green MH. Chylomicron margination, lipolysis, and vitamin a uptake in the lactating rat mammary gland: implications for milk retinoid content. Exp Biol Med (Maywood) 2004;229:46–55. [DOI] [PubMed] [Google Scholar]
- 60. Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Miotti PG, Eisinger W, Hoover D, Chiphangwi JD. Plasma and breast milk vitamin A as indicators of vitamin A status in pregnant women. Int J Vitam Nutr Res 2000;70:271–7. [DOI] [PubMed] [Google Scholar]
- 61. Thurnham DI, McCabe GP. Influence of Infection and Inflammation on Biomarkers of Nutritional Status with an Emphasis on Vitamin A and Iron: Priorities in the Assessment of Vitamin A and Iron Status in Populations. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 62. Dancheck B, Nussenblatt V, Ricks MO, Kumwenda N, Neville MC, Moncrief DT, Taha TE, Semba RD. Breast milk retinol concentrations are not associated with systemic inflammation among breast-feeding women in Malawi. J Nutr 2005;135:223–6. [DOI] [PubMed] [Google Scholar]
- 63. de Pee S, West CE, Hautvast J, Karyadi D. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet 1995;346:75–81. [DOI] [PubMed] [Google Scholar]
- 64. Khan NC, West CE, de Pee S, Bosch D, Phuong HD, Hulshof PJ, Khoi HH, Verhoef H, Hautvast JG. The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: a randomized controlled trial. Am J Clin Nutr 2007;85:1112–20. [DOI] [PubMed] [Google Scholar]
- 65. Oliveira-Menegozzo JM, Bergamaschi DP, Middleton P, East CE. Vitamin A supplementation for postpartum women. Cochrane Database Syst Rev 2010;10:CD005944. [DOI] [PubMed] [Google Scholar]
- 66. WHO Guideline: Vitamin A Supplementation in Postpartum Women. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 67. Underwood BA. Maternal vitamin A status and its importance in infancy and early childhood. Am J Clin Nutr 1994;59:517S–22S; discussion 22S–24S. [DOI] [PubMed] [Google Scholar]