Abstract
Background and Aims
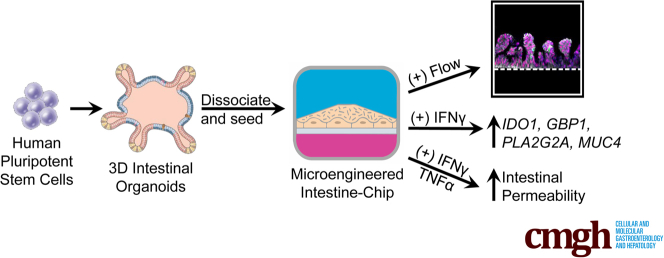
Human intestinal organoids derived from induced pluripotent stem cells have tremendous potential to elucidate the intestinal epithelium’s role in health and disease, but it is difficult to directly assay these complex structures. This study sought to make this technology more amenable for study by obtaining epithelial cells from induced pluripotent stem cell–derived human intestinal organoids and incorporating them into small microengineered Chips. We then investigated if these cells within the Chip were polarized, had the 4 major intestinal epithelial subtypes, and were biologically responsive to exogenous stimuli.
Methods
Epithelial cells were positively selected from human intestinal organoids and were incorporated into the Chip. The effect of continuous media flow was examined. Immunocytochemistry and in situ hybridization were used to demonstrate that the epithelial cells were polarized and possessed the major intestinal epithelial subtypes. To assess if the incorporated cells were biologically responsive, Western blot analysis and quantitative polymerase chain reaction were used to assess the effects of interferon (IFN)-γ, and fluorescein isothiocyanate–dextran 4 kDa permeation was used to assess the effects of IFN-γ and tumor necrosis factor-α on barrier function.
Results
The optimal cell seeding density and flow rate were established. The continuous administration of flow resulted in the formation of polarized intestinal folds that contained Paneth cells, goblet cells, enterocytes, and enteroendocrine cells along with transit-amplifying and LGR5+ stem cells. Administration of IFN-γ for 1 hour resulted in the phosphorylation of STAT1, whereas exposure for 3 days resulted in a significant upregulation of IFN-γ related genes. Administration of IFN-γ and tumor necrosis factor-α for 3 days resulted in an increase in intestinal permeability.
Conclusions
We demonstrate that the Intestine-Chip is polarized, contains all the intestinal epithelial subtypes, and is biologically responsive to exogenous stimuli. This represents a more amenable platform to use organoid technology and will be highly applicable to personalized medicine and a wide range of gastrointestinal conditions.
Keywords: Human Intestinal Organoids, Induced Pluripotent Stem Cells, Small Microengineered Chips
Abbreviations used in this paper: GBP1, guanylate binding protein 1; HIOs, human intestinal organoids; IDO1, indolamine 2,3-dioxygenase 1; IFN-γ, interferon-γ; iPSCs, induced pluripotent stem cells; PDMS, poly(dimethylsiloxane); TNF-α, tumor necrosis factor-α
Graphical abstract
See editorial on page 634.
Summary.
The 3-dimensional structure of human intestinal organoids makes them challenging to use. Here we describe how organoids, derived from induced pluripotent stem cells, can be incorporated into small microengineered Chips making them more amenable for study.
Studies examining human intestinal epithelial cell function have been severely hampered because primary intestinal epithelial cells rapidly undergo apoptosis when cultured ex vivo.1, 2 Although adenocarcinoma lines, such as Caco-2 cells, recapitulate some aspects of intestinal function, namely barrier function, a substantial breakthrough in the intestinal epithelial field occurred when it was reported that 3-dimensional human intestinal “organoids” (HIO) could be generated from either human biopsy samples3, 4 or induced pluripotent stem cells (iPSCs).5 Irrespective of how these organoids are derived, they contain all the intestinal epithelial subtypes, are polarized toward the lumen, and can be maintained for prolonged periods of time in a tightly controlled milieu. However, there are substantial technical challenges associated with this technology. Organoids are heterogeneous both in shape and size, which may lead to inconsistent findings. Access to the lumen, which is crucial for assessing intestinal permeability, microbial-epithelial interactions, and drug absorption are technically challenging. Coculture with other cell types, such as immune cell subtypes or endothelial cells, is also difficult given that organoids are typically embedded in a 3-dimensional matrix.
One potential way to overcome such challenges is to combine intestinal organoid culture with microengineering technology. Small microengineered Chips are integrated systems that place living human cells in precisely microengineered environments that can more accurately recapitulate human physiology and disease states. They allow unprecedented control over key physiological aspects, such as interactions between tissues, mechanical forces, blood and immune components, and the biochemical milieu.6 This engineering also allows the tuning and control of the microenvironment. Indeed, lung7, 8 and Caco-2 cells9, 10, 11 have previously been incorporated into such Chips and studies examining epithelial-immune cell interactions, prolonged epithelial-microbial interactions, and permeability experiments have all successfully been performed.
Given that iPSCs can be generated from any individual,12 iPSC-derived HIOs were chosen for incorporation into this Chip system, thereby permitting the study of intestinal epithelial cells from virtually any patient or nondiseased control. It also allows for the generation of other patient-specific cell types, such as macrophages,13 dendritic cells,14 and neutrophils,15 that can also be incorporated into the Chip and used to study multicellular interactions. Furthermore, we have previously reported that lymphoblastoid cell lines can be reliably reprogrammed to form iPSCs.16 Given there are numerous lymphoblastoid cell lines available in well-characterized worldwide repositories that are linked to patient clinical history and long-term genotype-phenotype data,17 studies into epithelial cells and their interactions with other cell types from well-characterized patient cohorts are now possible.
Materials and Methods
Ethics Statement
All the cell lines and protocols in the present study were carried out in accordance with the guidelines approved by the stem cell research oversight committee and institutional review board at the Cedars-Sinai Medical Center under the auspice of the institutional review board stem cell research oversight committee protocols Pro00027264 (Derivation of Intestinal Stem Cells). All authors had access to the study data and have reviewed and approved the final manuscript.
Cell Lines and Culturing Conditions
Two iPSC lines (CS83iCTR-33n1 and CS688iCTR-n5) were obtained from the iPSC Core at Cedars-Sinai. Both lines were fully characterized and were confirmed to be karyotypically normal. All iPSC lines were maintained in an undifferentiated state on Matrigel-coated plates in mTeSR1 media (Stem Cell Technologies, Vancouver, British Columbia, Canada) under feeder-free conditions. All iPSC cultures were tested monthly for mycoplasma contamination. Caco-2 (ATCC HTB-37) cells were cultured in Eagle's Minimum Essential Medium supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD).
Small Microengineering Chip Microfabrication
Chips were fabricated using modified methods for Chip microfabrication as previously described.18 Briefly, poly(dimethylsiloxane) (PDMS) prepolymer was mixed at a 10:1 ratio of PDMS base to curing agent, wt/wt using a planetary mixer (Thinky ARE-310). PDMS prepolymer was then cast onto molds forming the microchannels of the upper layer (1000 μm wide × 1000 μm high) and lower layer (1000 μm wide × 200 μm high). The membrane was cast onto a silicon mold that was fabricated using photolithography and deep reactive ion etching, resulting in 7-μm pores. The components were cured overnight and removed from the mold. The upper layer, membrane, and lower layer were permanently bonded via plasma bonding to form the complete Chip.
Generation of Human Intestinal Organoids From Induced Pluripotent Stem Cells
The generation of HIOs from iPSCs involves a multistep technique whereby iPSCs were directed to form definitive endoderm, epithelial structures, and ultimately organoids. To induce definitive endoderm formation, iPSCs were cultured with a high dose of Activin A (100 ng/mL, R&D Systems, Minneapolis, MN) with increasing concentrations of fetal bovine serum over time (0%, 0.2%, and 2% on Days 1, 2, and 3, respectively). Wnt3A (25 ng/mL, R&D Systems) was also added on the first day of endoderm differentiation. To induce hindgut formation, cells were cultured in Advanced DMEM/F12 with 2% fetal bovine serum and FGF4 (500 ng/mL, R&D Systems) and CHIR99021 (3 μM, Tocris, Minneapolis, MN). After 3–4 days, free-floating epithelial spheres and loosely attached epithelial tubes became visible and were harvested. These epithelial structures were subsequently suspended in Matrigel and then overlaid in intestinal medium containing CHIR99021 (2 μM, Tocris), noggin, and epidermal growth factor (both 100 ng/mL, all R&D Systems) and B27 (1X, Invitrogen, Carlsbad, CA). HIOs were passaged every 7–10 days thereafter.
Cell Sorting, Seeding, and Maintenance of Intestine-Chip
To seed intestinal epithelial cells into the Chip, HIOs were first dissociated and the intestinal epithelial cells were positively selected using fluorescent activated cell sorting. At 24 hours before sorting, ROCK inhibitor (10 μM, Tocris) was added to HIO culture media. The following day, HIOs were removed from Matrigel and subsequently incubated in TrypLE Express (Life Technologies, Camarillo, CA) for 15–30 minutes until the organoids were completely disassociated to a single cell suspension. These cells were then passed through a 30-μm filter and stained with CD326 (Biolegend, San Diego, CA) for 30 minutes. A minimum of 5 × 106 cells were then positively sorted for CD326. Cells were collected and resuspened to a density of 6.25 × 106 cells/mL in intestinal media containing ROCK inhibitor (10 μM, Tocris), SB202190 (10 μM, Tocris), and A83-01 (500 nM, Tocris). Before cell seeding, Chips were treated with plasma in 100% oxygen for 2 minutes using a CUTE series plasma system (Femto Science, Gyeonggi-Do, South Korea) and immediately coated with Matrigel (83 μg/mL). Dead/nonadhered cells were removed after 3–6 hours by flushing media through the Chip. Flow was started 3–6 hours later at a rate of 30 μL/h. ROCK inhibitor was removed from media 24 hours postseeding. To induce flow, gas-permeable tubing (PharMed BPT, 1/32 × 5/32 inch tubing, Cole Palmer, Vernon Hills, IL) with a connector (Right angle tube 18RW, Fourslide Spring and Stamping, Bristol, CT) was inserted into the microchannels to supply cell culture medium. A Fusion 200 Touch Syringe Pump (Chemyx, Stafford, TX) fitted with a modular 10 syringe rack was used to induce and maintain flow. All of the apparatus, the Intestine-Chip, and syringe pumps were stored in an incubator and all experiments were carried out at 37°C.
Quantitative Real-Time Polymerase Chain Reaction
Phosphate-buffered saline was flushed through the upper and lower channels and total RNA from the Intestine-Chip was extracted in situ with the RNeasy mini kit (Qiagen, Germantown, MD). cDNA was generated from 1 μg of RNA using the Omniscript RT Kit (Qiagen). Quantitative real-time polymerase chain reaction was performed using SYBR Select Master Mix (Applied Biosystems, Carlsbad, CA) on a BioRad CFX384 Real-Time System. Primer sequences as follows; IDO1 F-ACACTTTGCTAAAGGCGCTG, IDO1 R-TGCCTTTCCAG CCAGACAAA, GBP1 F-AAACTTCAGGAACAGGAGCAAC, GBP1 R-GGTACATGCCTTTCGTCGTCT, PLA2G2A F-CAAGTTTAGCAACTCGGGGA, PLA2G2A R-TCTAGCAAAACAGGTGGC AG, MUC4 F-AGACGCACAGCCACATCAC, MUC4 R-GGGAAACTCCTCTCTCAGGC, LYZ F-TTTCTGTTACGGTCCAGGGC, LYZ R-ACACATCCAGTTTGCTAGGCT.
Immunohistochemistry and Microscopy
HIOs were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), transferred to 30% sucrose overnight at 4°C, embedded in Tissue-Tek O.C.T Compound (VWR, Radnor, PA), and cut into 10-μm sections. Sections were blocked in 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) with 0.5% Triton X-100 and incubated with primary antibodies (Supplementary Table 1) for either 3 hours at room temperature or overnight at 4°C. Sections were then rinsed and incubated in species-specific AF488, AF594, or AF647-conjugated secondary antibodies (Life Technologies, Carlsbad, CA) followed by DAPI (0.5 μg/mL; Life Technologies) to counterstain nuclei, and were imaged using a Leica DM6000 B microscope. Intestine-Chips were flushed through the upper and lower channels, and cells were fixed with 4% paraformaldehyde for 15 minutes without flow. Intestine-Chips cultured under static conditions were immunostained and imaged in a similar manner as previously mentioned. To obtain cross-section images, a Leica VT1200S vibratome or Leica CM3050S cryostat was used to obtain sections of the Intestine-Chip. These sections were blocked in 10% normal donkey serum with 0.5% Triton X-100 and incubated with primary antibodies (Supplementary Table 1) for 24 hours at 4°C. Sections were rinsed; incubated in species-specific AF488, AF594, and AF647 followed by DAPI; and were imaged using a Nikon A1R Eclipse Ti Confocal Microscope.
In Situ Hybridization
Intestine-Chips were fixed as previously mentioned in 4% paraformaldehyde for 15 minutes without flow. To obtain cross-section images, Intestine-Chips were sectioned with a Leica CM3050S cryostat at 10 μm per section. These sections were prepared and treated using RNAscope In Situ Hybridization 2.5 HD brown assay kit (Advanced Cell Diagnostic, Newark, CA). In brief, the tissue underwent target retrieval, permeabilization, hybridization of LGR5 (Hs-LGR5 311021) and WDR43 (Hs-WDR43 472711), amplification, and visualization using DAB-A and DAB-B. Sections were imaged using a Leica DM6000 B microscope.
Permeability and Cytotoxicity Assay
Intestine-Chips were prepared as previously mentioned, and maintained under flow conditions (30 μL/h) for 10 days. Each Intestine-Chip was microscopically assessed to confirm barrier integrity before experimentation. After 10 days, 10 ng/mL interferon (IFN)-γ and 10 ng/mL tumor necrosis factor (TNF)-α (both from R&D Systems) was added to the lower channel of the Intestine-Chip for 3 days, whereas the control group was untreated. After 3 days, the media was removed and the system was flushed with transport buffer (HBSS supplemented with 12.5 mM glucose and 25 mM HEPES) and equilibrated for 1.5 hours. At time zero, fluorescein isothiocyanate–dextran 4 kDa was added to the top channel (10 μg/mL) in transport buffer, and the lower channel transport buffer was either untreated or contained 10 ng/mL IFN-γ and 10 ng/mL TNF-α. Samples from the lower channel were taken every 1.5 hours for 6 hours and samples from the top channel were taken at 0 and 6 hours to determine the area under the curve. Fluorescence was measured in a spectrofluorometer (EnVision 2104, Perkin Elmer, Waltham, MA) with an excitation wavelength of 490 nm and an emission wavelength of 525 nm. Area under the curve was calculated using Prism-7 software (GraphPad, La Jolla, CA). After the final time point for the permeability experiments, the Intestine-Chip was flushed with transport buffer and the epithelium was treated with MTS (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium) to assess cytotoxicity induced by IFN-γ and TNF-α exposure. Intestine-Chips were incubated with MTS for 4 hours at 37°C in a humidified incubator with 5% CO2 and 95% O2. Optical density was measured at 490 nm. Each value presented was normalized against untreated control. Permeability and cytotoxicity experiments were calculated from 2 independent experiments, each of which included 6 replicates.
Western Blotting
Phosphate-buffered saline was flushed through both the upper and lower channels and total protein was extracted in situ using cell lysis buffer supplemented with protease and phosphatase inhibitor (# MSSAFE, Sigma, Carlsbad, CA). Protein concentration was then determined and equal amounts of protein lysates were heat denatured and separated on a 10% mini-protean precast gel (Bio-Rad, Hercules, CA). Gel was then transferred on to midi format 0.2 μm Nitrocellulose Membrane (Bio-Rad). Blot was blocked in Odyssey Blocking Buffer (LI-COR, Lincoln, NE) for 1 hour and then incubated in primary antibody for STAT1, pSTAT1, and glyceraldehyde-3-phosphate dehydrogenase (Supplementary Table 1) overnight at 4ºC. After incubation with infrared conjugated secondary antibodies (LI-COR) for 1 hour, blots were washed with TBST and bands were visualized by scanning membrane on Odyssey Infrared Imaging System (Li-COR). Image J analysis was used to quantify levels of phosphorylated STAT1 compared with glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analyses
All data are represented as mean ± standard error of the mean. An unpaired Student’s t test was completed using Excel, and control and IFN-γ treated Chips were compared with each other in each condition. Differences between groups were considered statistically significant when P < .05. No statistical method was used to predetermine sample size. The investigators were not blinded to allocation during experiments and outcome assessment. No randomization was made.
Results and Discussion
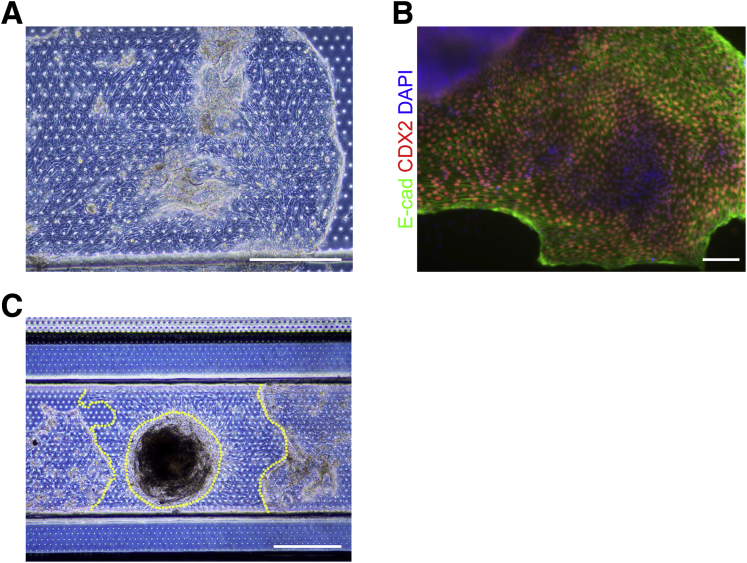
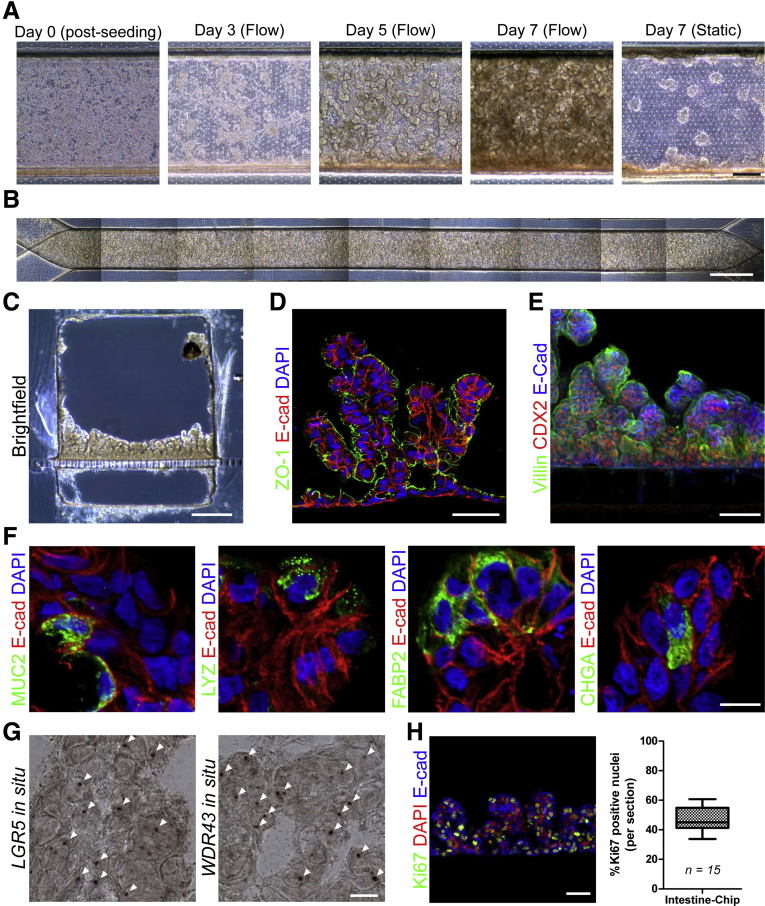
To develop the Intestine-Chip, we established a multistep technique whereby iPSCs were differentiated into HIOs, dissociated to single cells, sorted on an epithelial cell marker, and subsequently incorporated into small microengineered Chips (Figure 1A). iPSCs were directed to form 3-dimensional HIOs by culturing them with Activin A to induce definitive endoderm formation; CHIR99021 and fibroblast growth factor 4 to induce hindgut formation; and ultimately epidermal growth factor, Noggin, and CHIR99021 to induce and maintain organoid formation. This protocol resulted in the robust generation of intestinal organoids that were polarized towards the lumen, were CDX2/E-cadherin-immunopositive and, in contrast to biopsy-derived organoids, contained mesenchymal cells as previously reported (Figure 1B).5 The Chips used in this study were fabricated as previously described.18 Briefly, the Chips are made of PDMS and contain a top channel with a height and width of 1 mm separated from a parallel bottom channel (0.2 mm high × 1 mm wide) by a thin (50 μm), porous (7-μm pores) PDMS membrane coated on the upper side with Matrigel (Figure 1C). Initially, we attempted to place intact HIOs into the top channel of the Chip. Although some HIOs adhered to the PDMS membrane and formed a partial monolayer of intestinal epithelial cells (Supplementary Figure 1A and B), most did not adhere and some HIOs gave rise to partial monolayers of mesenchymal cells (Supplementary Figure 1C) that physically impeded the expansion of the epithelial cells (data not shown). Given that the number of epithelial and mesenchymal cells within HIO cultures can vary enormously and the presence of mesenchymal cells seems to impede the expansion of epithelial cells, we sought to refine our approach. HIOs were dissociated to a single cell suspension and epithelial cell adhesion molecule (EpCAM/CD326) was used to positively select for HIO-derived epithelial cells using fluorescent activated cellular sorting. Concentrations of positively selected cells ranging from 2.5–7.5 × 106 cells/mL were added to the Matrigel-coated top channel of each Chip and it was found that 6.25 × 106 cells/mL gave a near continuous monolayer after 24 hours (Figure 1D). After 3 days, we observed a fully confluent monolayer of intestinal epithelial cells along the entire top channel of the Chip and thus this cell concentration was added in all future experiments to incorporate HIO-derived epithelial cells into the Intestine-Chip (Figure 1E).
Figure 1.
(A) Schematic of workflow for incorporation of iPSC-derived HIOs into the Intestine-Chip. Scale bar = 200 μm. (B) Representative images showing polarized HIOs immunopositive for E-cadherin (red) and ZO-1 (green), and HIOs immunopositive for E-cadherin/CDH1 (red), CDX2 (gray), and vimentin (green) all counterstained with DAPI (blue). Scale bar = 50 μm. (C) Schematic of the small microengineered Chip used in this study. (D) HIO-epithelial cells ranging in concentrations from 2.5–7.5 × 106 cells/mL were seeded into the Chip and representative phase contrast images were obtained after 24 hours. Yellow lines denote areas of the Chip not covered by HIO-derived epithelial cells. Scale bar = 200 μm. (E) HIO-epithelial cells were seeded at 6.25 × 106 cells/mL into the Intestine-Chip and E-cadherin/CDH1+ cells (red) counterstained with DAPI (blue) were imaged 3 days later. Images were stitched together to show a fully confluent monolayer of intestinal epithelial cells along the entire channel of the Chip. Scale bar = 1 mm.
Supplementary Figure 1.
(A) Phase contrast image (scale bar = 500 μm) and (B) fluorescent image (scale bar = 100 μm) showing E-cadherin/CDH1 (green), CDX2 (red), counterstained with DAPI (blue) of intact HIO that was incorporated into the Chip and imaged 3 days later. (C) Phase contrast image showing epithelial and mesenchymal cells that arose from intact HIOs that were incorporated into the Chip and imaged 4 days later. Yellow dotted lines represent mesenchymal cells in Chip. Scale bar = 500 μm.
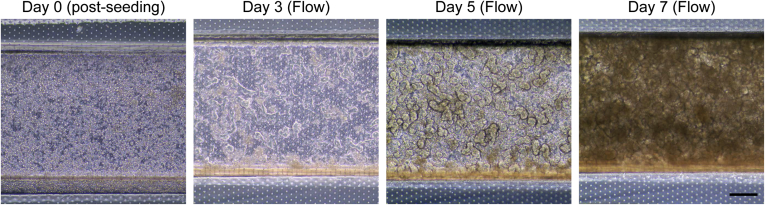
After establishing the conditions to successfully incorporate HIO-derived epithelial cells into the Intestine-Chip, we then ascertained the optimal rate that media should continuously be flowed through the Chip. Continuous media flow over cells mimics some of the in vivo microenvironmental conditions and not only permits prolonged exposure to microbes, but enhances the differentiation of cells.6, 9, 10, 11 Indeed, it was previously shown that continuous media flow caused Caco-2 cells to spontaneously develop intestinal folds.9, 10, 11 We first tested a similar flow rate of 30 μL/h that was previously reported9, 10, 11 and found that after 72 hours, the HIO-derived epithelial cells exhibited a more crenulated appearance along the Intestine-Chip, which became more pronounced after 5 and 7 days (Figure 2A). Under static conditions, some small folds were observed after 7 days, although they primarily remained as a monolayer. Similarly, cells seeded at a lower density (2.5–5 × 106) failed to generate intestinal folds despite exposure to continual flow (data not shown). A stitched phase contrast image of HIO-epithelial cells that were exposed to continual media flow at 30 μL/h for 5 days demonstrated homogenous and near complete coverage of the Intestine-Chip (Figure 2B). An increased flow rate of 60 μL/h did not seem to accelerate the process and so a flow rate of 30 μL/h was used in all subsequent studies (Supplementary Figure 2). After 14 days, a cross-section of the Intestine-Chip revealed a substantially thickened layer that seemed to have organized into villous-like projections (Figure 2C). The presence of E-cadherin and polarized ZO-1 immunostaining toward the apical surface demonstrates these are polarized epithelial cells and illustrates that the top channel is representative of the luminal aspect of the intestine (Figure 2D). Additionally, the presence of CDX2 and villin staining demonstrates that these cells are of intestinal lineage with brush borders (Figure 2E). Given that it was previously reported that HIOs contained the major epithelial subtypes,5, 16 we confirmed the presence of lysozyme+ cells, MUC2+ cells, chromogranin A+ cells and FABP2+ in the Intestine-Chip (Figure 2F), which suggests that this platform may be useful for studying Paneth cells, goblet cells, enterocytes, and enteroendocrine cells. We also confirmed the presence of LGR5+ stem cells and WDR43+ transit-amplifying cells via in situ hybridization (Figure 2G).19 Although the number of differentiated cells were relatively low, we did find a large percentage of proliferative (Ki67+) cells, which were distributed throughout the intestinal folds (Figure 2H).
Figure 2.
(A) Representative phase contrast image of HIO-derived epithelial cells seeded into the Chip. Cells were exposed to continuous media flow at 30 μL/h and imaged after 0, 3, 5, and 7 days. Static Chip was imaged after 7 days. Scale bar = 200 μm. (B) Stitched phase contrast image of HIO-epithelial cells that were exposed to continual media flow at 30 μL/h for 5 days. Scale bar = 1 mm. (C) Representative brightfield image of cross-section of Chip that was exposed to continual media flow at 30 μL/h for 14 days. Scale bar = 250 μm. Representative fluorescent images showing (D) E-cadherin/CDH1 (red), ZO-1 (green), DAPI (blue), and (E) E-cadherin (blue), CDX2 (red), and Villin (green), in cross-section of Chip under conditions similar to C. Both scale bars = 50 μm. (F) Representative fluorescent images showing E-cadherin/CDH1 (red), and MUC2, lysozyme, FABP2, and chromogranin A (all green), counterstained with DAPI (blue), in cross-section of Chips that were exposed to continual media flow at 30 μL/h for 7 days. Scale bar = 10 μm. (G) Representative images of in situ hybridization for LGR5+ and WDR43+ (white arrows) in conditions similar to F. Scale bar = 10 μm. (H) Representative fluorescent image showing E-cadherin/CDH1 (blue), Ki67 (green), and DAPI (blue) in conditions similar to F. Scale bar = 100 μm. Graph shows percent Ki67+ nuclei per section.
Supplementary Figure 2.
Representative phase contrast images of HIO-derived epithelial cells seeded into the chip. Cells were exposed to continuous media flow of 60 μL/h and imaged after 0, 3, 5, and 7 days. Scale bar = 200 μm.
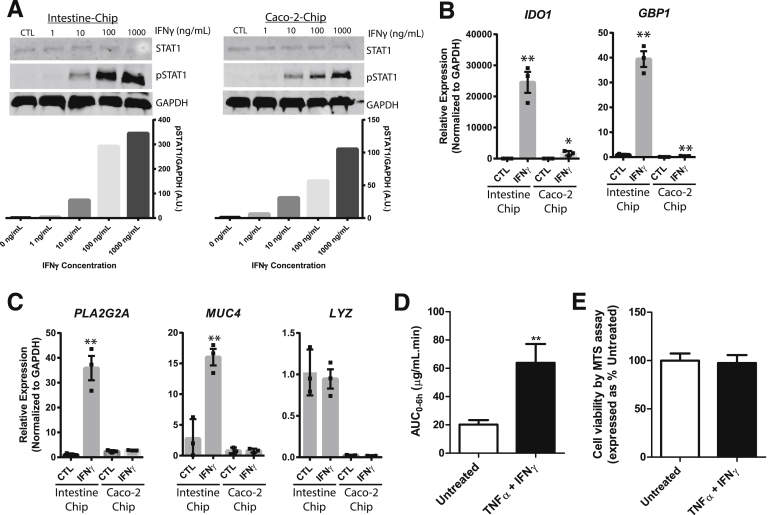
To demonstrate that the intestinal epithelial cells in the Intestine-Chip were biologically responsive to exogenous stimuli and therefore suitable for modeling epithelial responses, we chose to examine the response to IFN-γ, a cytokine known to be upregulated in patients with inflammatory bowel disease.20, 21, 22 Because Caco-2 cells are routinely used to assess intestinal epithelial responses and have previously been incorporated into Chips,9, 10, 11 we included these cells as a direct comparison with the new Intestine-Chip. Exposure to IFN-γ at concentrations ranging from 10–1000 ng/mL resulted in the robust phosphorylation of STAT1 in both cell types when added to the bottom channel for 1 hour (Figure 3A). Thus, both cell types were incubated with 10 ng/mL of IFN-γ for 3 days and assessed for upregulation of indoleamine 2,3-dioxygenase 1 (IDO1) and guanylate binding protein 1 (GBP1). These proteins were chosen because they are both upregulated in various cell lines in response to IFN-γ exposure and are also upregulated in the inflamed intestinal epithelium of patients with inflammatory bowel disease.23, 24 We found that IDO1 and GBP1 were significantly increased in IFN-γ-treated Intestine-Chips as compared with respective untreated controls, validating results from these previous reports (Figure 3B). Although IDO1 and GBP1 were upregulated in Caco2-Chips, this biologic affect was attenuated in comparison with the Intestine-Chip. We then looked for changes in expression of various genes that are specific to Paneth and goblet cells. There was significant upregulation of the antimicrobial, PLA2G2A, and the mucin, MUC4, although no differences were observed in LYZ (Figure 3C). Although Caco-2 cells in the Intestine-Chip were immunopositive for the intestinal epithelial subtypes (Supplementary Figure 3), corroborating a previous study,10 there was no significant upregulation of the aforementioned genes. These findings indicate that HIO-derived intestinal epithelial cells may provide a more faithful model to predict human intestinal epithelial responses. We then performed barrier function studies on the Intestine-Chip whereby IFN-γ and TNF-α were added to the bottom channel for 3 days and the permeability of fluorescein isothiocyanate–dextran 4 kDa was subsequently assessed over 6 hours. The area under the curve of fluorescein isothiocyanate–dextran 4 kDa was significantly increased in Intestine-Chips exposed to IFN-γ and TNF-α compared with those untreated (Figure 3D). Following the permeation assay, a cytotoxicity assay (MTS) was carried out and revealed that there was no change in the viability between groups, which demonstrates that this increase in permeability was not a result of increased cell death (Figure 3E).
Figure 3.
(A) Western blot analyses and quantification of phosphorylation of STAT1 in Chips containing either HIO-derived intestinal epithelial or Caco2 cells in response to exposure of increasing concentrations (0–1000 ng/mL) of IFN-γ in the lower channel for 1 hour. Glyceraldehyde-3-phosphate dehydrogenase and STAT1 were used as loading controls. Image J analysis was used to quantify levels of phospho-STAT1 compared with glyceraldehyde-3-phosphate dehydrogenase. (B) Quantitative reverse-transcription polymerase chain reaction analyses of IDO1 and GBP1 and (C) PLA2G2A, MUC4, and LYZ in Chips incorporating either HIO-derived epithelial or Caco-2 cells in response to exposure of 10 ng/mL of IFN-γ in the lower channel for 3 days. Three independent experiments were carried out; data represent mean ± SEM; *P < .05, **P < .01 compared with respective untreated controls. (D) Area under the curve of fluorescein isothiocyanate–dextran 4 kDa permeation over 6 hours was statistically higher (P < .01) in Intestine-Chip treated with IFN-γ and TNF-α compared with untreated. (E) Following the permeability studies, MTS cytotoxicity assay was carried out and showed no statistical difference between Intestine-Chips treated with IFN-γ and TNF-α or untreated. Two independent experiments were carried out; data represent mean ± SEM. **P < .01. AUC, area under the curve; CTL, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Supplementary Figure 3.
Representative fluorescent images showing E-cadherin (gray), CDX2 (red), and MUC2, lysozyme, FABP2, and chromogranin A (all green) in cross-section of Chips incorporating Caco-2 cells that were exposed to continual media flow of 30 μL/h and imaged after 8 days. Scale bar = 50 μm.
Conclusions
The Intestine-Chip combines the strengths of both human iPSC-derived intestinal organoids and small microengineering technologies. The intestinal organoid component permits the incorporation of biologically responsive intestinal epithelial cells from almost any individual into the system. Previous studies have shown that various immune cell types and microbes can be incorporated into Chips,7, 8, 9, 10, 11 and the microengineering component now allows for an examination of how an almost unlimited combination of cytokines, microbes, and immune cells affects the functioning of intestinal epithelial cells in a patient-specific manner. The Chips further allow the establishment and control of a more physiologically relevant microenvironment, such as recapitulating the in vivo Wnt and BMP signaling gradients that regulate the location and differentiation of intestinal epithelial cells,25 or establishing an anaerobic upper channel model to potentiate epithelial-microbial studies.18 All of these approaches would be highly applicable in the gastrointestinal field and will likely have major implications for personalized medicine.
Acknowledgements
The authors thank Dr Nur Yucer for her technical assistance and Dr Soshana Svendsen for assistance in editing this manuscript.
Footnotes
Author contributions Study concept and design: Michael J. Workman, Stephan R. Targan, Clive N. Svendsen, and Robert J. Barrett. Acquisition of data: Michael J. Workman, John P. Gleeson, Elissa J. Troisi, Hannah Q. Estrada, and Robert J. Barrett. Statistics: Michael J. Workman and John P. Gleeson. Analysis and interpretation of data: Michael J. Workman, John P. Gleeson, S. Jordan Kerns, Christopher D. Hinojosa, Stephan R. Targan, Clive N. Svendsen, and Robert J. Barrett. Drafting of the manuscript: Robert J. Barrett. Review and editing: Michael J. Workman, Geraldine A. Hamilton, S. Jordan Kerns, Stephan R. Targan, Clive N. Svendsen, and Robert J. Barrett. Study supervision: Robert J. Barrett.
Conflicts of interest These authors disclose the following: Michael J. Workman, Stephan R. Targan, Clive N. Svendsen, and Robert J. Barrett are named as inventors on a patent application (PCT/US2017/016098) entitled “Systems And Methods For Growth Of Intestinal Cells In Microfluidic Devices” and owned by Emulate Inc and Cedars-Sinai Medical Center. Cedars-Sinai is an investor in Emulate Inc. The remaining authors disclose no conflicts.
Funding Supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R56DK106202-01, S.R.T. and C.N.S.). Funded by the Board of Governors Regenerative Medicine Institute, the F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, and the Drown Foundation. The study sponsors played no role in the study design, collection, analysis, or interpretation of data.
Supplementary Material
Supplementary Table 1.
Table of Antibodies Used in Study
| Antigen | Dilution | Catalogue # | Isotype | Manufacturer |
|---|---|---|---|---|
| CDX2 | 1:500 | NBP1-40553 | Rabbit IgG | Novus Biologicals |
| Chromogranin A(CGA414) | 1:500 | NBP2-29428 | Mouse IgG1 | Novus Biologicals |
| E-cadherin | 1:1000 | AF648 | Goat IgG | R&D Systems |
| FABP2/1-FABP(9A9B7B3) | 1:500 | NBP1-51589 | Mouse IgG1 | Novus Biologicals |
| GAPDH | 1:1000 | sc-25778 | Rabbit IgG1 | Santa Cruz |
| Ki67 (SP6) | 1:200 | RM-9106-S0 | Rabbit IgG | Thermo Fisher |
| Lysozyme (BGN/0696/5B1) | 1:500 | NB100-63062 | Mouse IgG2A | Novus Biologicals |
| Mucin-2 (CCP58) | 1:500 | NBP2-25221 | Mouse IgG1 | Novus Biologicals |
| P-Stat1(Y701) (58D6) | 1:1000 | 9167S | Rabbit mAb | Cell Signaling |
| Stat1(9H2) | 1:1000 | 9176S | Mouse IgG1 | Cell Signaling |
| Villin 1 (1DC2C3) | 1:500 | NB600-1349 | Mouse IgG1 | Novus Biologicals |
| Vimentin | 1:1000 | 550513 | Mouse IgG1 | BD Biosciences |
| ZO-1 (ZO1-1A12) | 1:500 | 33-9100 | Mouse IgG1 | Life Technologies |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Strater J., Wedding U., Barth T.F., Koretz K., Elsing C., Moller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology. 1996;110:1776–1784. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann J., Mohr S., Lapentina E.G., Fiocchi C., Levine A.D. Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am Physiol. 1998;274(6 Pt 1):G1117–G1124. doi: 10.1152/ajpgi.1998.274.6.G1117. [DOI] [PubMed] [Google Scholar]
- 3.Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 4.VanDussen K.L., Marinshaw J.M., Shaikh N., Miyoshi H., Moon C., Tarr P.I., Ciorba M.A., Stappenbeck T.S. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., Shroyer N.F., Wells J.M. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 7.Huh D., Leslie D.C., Matthews B.D., Fraser J.P., Jurek S., Hamilton G.A., Thorneloe K.S., McAlexander M.A., Ingber D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra47. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh D., Matthews B.D., Mammoto A., Montoya-Zavala M., Hsin H.Y., Ingber D.E. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H.J., Huh D., Hamilton G., Ingber D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12 doi: 10.1039/c2lc40074j. 2165–1274. [DOI] [PubMed] [Google Scholar]
- 10.Kim H.J., Ingber D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb) 2013;5:1130–1140. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.J., Li H., Collins J.J., Ingber D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A. 2016;113:E7–E15. doi: 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y., Inoue H., Wu J.C., Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Wilgenburg B., Browne C., Vowles J., Cowley S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senju S., Haruta M., Matsumura K., Matsunaga Y., Fukushima S., Ikeda T., Takamatsu K., Irie A., Nishimura Y. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–883. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- 15.Morishima T., Watanabe K., Niwa A., Fujino H., Matsubara H., Adachi S., Suemori H., Nakahata T., Heike T. Neutrophil differentiation from human-induced pluripotent stem cells. J Cell Physiol. 2011;226:1283–1291. doi: 10.1002/jcp.22456. [DOI] [PubMed] [Google Scholar]
- 16.Barrett R., Ornelas L., Yeager N., Mandefro B., Sahabian A., Lenaeus L., Targan S.R., Svendsen C.N., Sareen D. Reliable generation of induced pluripotent stem cells from human lymphoblastoid cell lines. Stem Cells Transl Med. 2014;3:1429–1434. doi: 10.5966/sctm.2014-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler H.E., Dolan M.E. Lymphoblastoid cell lines in pharmacogenomic discovery and clinical translation. Pharmacogenomics. 2012;13:55–70. doi: 10.2217/pgs.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh D., Kim H.J., Fraser J.P., Shea D.E., Khan M., Bahinski A., Hamilton G.A., Ingber D.E. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 19.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T., Hiwatashi N., Yamazaki H., Noguchi M., Toyota T. The role of interferon gamma in the pathogenesis of Crohn's disease. Gastroenterol Jpn. 1992;27:29–36. [PubMed] [Google Scholar]
- 21.Noguchi M., Hiwatashi N., Liu Z., Toyota T. Enhanced interferon-gamma production and B7-2 expression in isolated intestinal mononuclear cells from patients with Crohn's disease. J Gastroenterol. 1995;30(Suppl 8):52–55. [PubMed] [Google Scholar]
- 22.Camoglio L., Te Velde A.A., Tigges A.J., Das P.K., Van Deventer S.J. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:285–290. doi: 10.1002/ibd.3780040406. [DOI] [PubMed] [Google Scholar]
- 23.Ciorba M.A. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–152. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnoor M., Betanzos A., Weber D.A., Parkos C.A. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bloemendaal A.L., Buchs N.C., George B.D., Guy R.J. Intestinal stem cells and intestinal homeostasis in health and in inflammation: a review. Surgery. 2016;159:1237–1248. doi: 10.1016/j.surg.2016.01.014. [DOI] [PubMed] [Google Scholar]