Abstract
Background & Aims
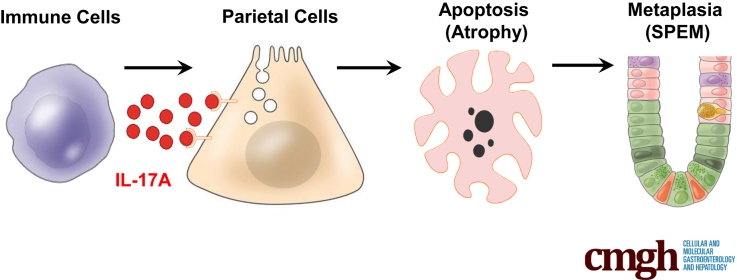
Atrophic gastritis caused by chronic inflammation in the gastric mucosa leads to the loss of gastric glandular cells, including acid-secreting parietal cells. Parietal cell atrophy in a setting of chronic inflammation induces spasmolytic polypeptide expressing metaplasia, a critical step in gastric carcinogenesis. However, the mechanisms by which inflammation causes parietal cell atrophy and spasmolytic polypeptide expressing metaplasia are not well defined. We investigated the role of interleukin-17A (IL-17A) in causing parietal cell atrophy.
Methods
A mouse model of autoimmune atrophic gastritis was used to examine IL-17A production during early and late stages of disease. Organoids derived from corpus glands were used to determine the direct effects of IL-17A on gastric epithelial cells. Immunofluorescent staining was used to examine IL-17A receptors and the direct effect of signaling on parietal cells. Mice were infected with an IL-17A-producing adenovirus to determine the effects of IL-17A on parietal cells in vivo. Finally, IL-17A neutralizing antibodies were administered to mice with active atrophic gastritis to evaluate the effects on parietal cell atrophy and metaplasia.
Results
Increased IL-17A correlated with disease severity in mice with chronic atrophic gastritis. IL-17A caused caspase-dependent gastric organoid degeneration, which could not be rescued with a necroptosis inhibitor. Parietal cells expressed IL-17A receptors and IL-17A treatment induced apoptosis in parietal cells. Overexpressing IL-17A in vivo induced caspase-3 activation and terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling staining in parietal cells. Finally, IL-17A neutralizing antibody decreased parietal cell atrophy and metaplasia in mice with chronic atrophic gastritis.
Conclusions
These data identify IL-17A as a cytokine that promotes parietal cell apoptosis during atrophic gastritis, a precursor lesion for gastric cancer.
Keywords: IL-17A, Atrophy, Metaplasia, Apoptosis
Abbreviations used in this paper: ADV, adenovirus; IL-17A, interleukin 17A; rIL-17A, recombinant interleukin 17A; SPEM, spasmolytic polypeptide-expressing metaplasia; Th, T helper; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
Graphical abstract
See editorial on page 636.
Summary.
This study reports that interleukin-17A (IL-17A) is an important contributor to parietal cell atrophy and metaplasia during chronic atrophic gastritis. IL-17A induces parietal cell apoptosis, while IL-17A neutralization in the setting of gastritis limits atrophy and metaplasia.
Gastric cancer is a leading cause of cancer-related mortality in the world.1 Conditions that induce chronic gastritis, such as Helicobacter pylori infection or autoimmune gastritis, increase the risk for gastric cancer.2, 3 Most gastric cancers are adenocarcinomas that develop over time because gastric epithelial cells are exposed to chronic inflammation comprising various cytokines and DNA-damaging compounds released by immune cells in the gastric mucosa.4 A number of cytokine genes are associated with an increased risk of gastric cancer;5, 6, 7 however, relatively little is known about the pathophysiology of how cytokines regulate the initiation and progression of the disease.
The Correa pathway proposes that gastric cancer develops via a stepwise progression through a sequence of histopathologic changes8, 9: gastritis, oxyntic atrophy (loss of parietal cells), metaplasia, dysplasia, and eventually neoplasia.8 More recent studies have led to a molecular understanding of how the gastric epithelium responds to oxyntic atrophy. The loss of parietal cells leads to increased proliferation by gastric stem and progenitor cells10 and is associated with metaplasia that is likely to arise from zymogenic chief cells recruited back into the cell cycle.11, 12 These metaplastic changes occur along with or in response to parietal cell death and inflammation, and are referred to as spasmolytic polypeptide-expressing metaplasia (SPEM) because of the expression of spasmolytic polypeptide (also known as trefoil factor 2) by the metaplastic cells. SPEM, which may represent a repair response to acute injury, also is believed to be a precursor to gastric cancer when present for long periods in chronically inflamed gastric mucosa.13, 14
We previously have shown that suppressing inflammation was effective at reducing parietal cell atrophy using the TxA23 mouse model of autoimmune gastritis.15, 16, 17, 18 However, it is unclear which cytokines are responsible for SPEM and parietal cell atrophy both in this and other models. In this study we focused on IL-17A, a proinflammatory cytokine secreted by CD4+ T helper 17 cells (Th17) and other immune cells such as CD8+ T cells, natural killer cells, and γ-δ T cells.19, 20, 21 The receptor for IL-17A is composed of two protein monomers: IL-17 Receptor A (IL17RA) and IL-17 Receptor C (IL17RC). The IL-17 receptor complex is expressed on many cell types, including various types of epithelial cells.22 Signals received through IL-17R are known to induce genes involved in antimicrobial responses, such as chemokines and antimicrobial peptides.23, 24 Importantly, IL-17A is secreted in response to H pylori infection and in patients with autoimmune gastritis, but how chronic exposure to IL-17A may affect gastric epithelial cell biology is unknown.25, 26 Recent studies have reported that IL-17A-producing cells are present in the gastric mucosa in human beings with gastric cancer, and that high frequencies of IL-17A-producing cells correlated with more severe disease and a poor prognosis, implicating a previously unrecognized role for this cytokine in promoting gastric cancer.27, 28, 29
To determine the role IL-17A plays in promoting metaplasia and parietal cell atrophy we used the TxA23 mouse model in which gastritis is induced by CD4+ T cells that are autoreactive against the H+/K+ adenosine triphosphatase expressed by parietal cells. The TxA23 model mimics many aspects of atrophic gastritis and metaplasia in human beings. Similarities include chronic inflammation and parietal cell atrophy, mucous neck cell hyperplasia, SPEM, and, eventually, gastric intraepithelial neoplasms.30, 31 We identified immune cells in the gastric mucosa that secrete IL-17A and observed that, similar to human beings, high frequencies of IL-17A-producing cells correlated with the degree of parietal cell atrophy and SPEM. Three-dimensional organoid cultures derived from gastric corpus glands showed that IL-17A acts directly on epithelial cells to induce organoid cell death, and that IL-17A-induced organoid death could be inhibited with a caspase-inhibiting compound (Z-VAD-FMK). We showed that parietal cells express IL-17A receptors and undergo apoptosis when treated with IL-17A. Infecting mice with an adenoviral vector that increased systemic levels of IL-17A induced caspase-3 activation and parietal cell apoptosis in vivo. Finally, we tested the hypothesis that IL-17A plays a role in oxyntic atrophy and SPEM development by administering an IL-17A-neutralizing antibody to TxA23 mice with active autoimmune gastritis. Anti-IL-17A treatment significantly reduced the extent of parietal cell atrophy and SPEM in mice with autoimmune gastritis. Together, these findings showed that IL-17A acts directly on parietal cells to trigger caspase-mediated apoptosis and that neutralizing IL-17A can limit the extent of parietal cell atrophy and the development of metaplasia, two critical steps in gastric carcinogenesis.
Methods
Mice
TxA23 mice express a transgenic T-cell–receptor specific for a peptide from H+/K+ adenosine triphosphatase α chain on a BALB/c background, and have been described previously.16, 30, 31, 32 BALB/c mice were purchased from Jackson Laboratories. All mice were maintained in our animal facility and cared for in accordance with institutional guidelines. Studies were performed on a mixed group of male and female mice with co-housed littermate controls. For neutralization studies, mice were treated with 150 μg anti-mouse IL-17A (BE0173, Clone 17F3; BioXCell, Lebanon, NH) or corresponding isotype control (BE0083; BioXCell) biweekly for 4 weeks after weaning via intraperitoneal injection. For IL-17A adenovirus infection experiments, a recombinant adenovirus expressing murine IL-17A, originally developed by Dr Jay Kolls (University of Pittsburgh, Pittsburgh, PA)33 and generously provided by Dr Shabaana Khader (Washington University, Saint Louis, MO), was propagated and purified as previously described.34 A control recombinant adenovirus expressing β-galactosidase also was used. BALB/c mice were given 5 × 109 50% Tissue Culture Infective Dose of recombinant adenovirus encoding either recombinant murine IL-17A (IL-17A-ADV) or β-gal (control) by tail vein injection. Mice were killed 7–14 days after infection and serum IL-17A was measured by Th1/Th2/Th17 cytometric bead array according to the manufacturer’s recommendations (560283; BD Biosciences, San Jose, CA).
Histopathology
Stomachs were removed from mice, rinsed in saline, immersion fixed in 10% neutral-buffered formalin, paraffin embedded, sectioned, and stained with H&E. For scoring, investigators were blinded, and sections from individual mice were assigned scores between 0 (absent) and 4 (severe) to indicate the severity of inflammation, oxyntic atrophy, and neck cell hyperplasia.
Immunofluorescence/Immunohistochemistry
Stomachs were prepared, stained, and imaged using methods modified from Ramsey et al.35 The primary antibodies used for immunostaining were as follows: rabbit anti-IL-17RA (1:100, bs-2606R; BIOSS, Woburn, MA), goat anti–vascular endothelial growth factor B (VEGF-B) (1:100, sc-13083; Santa Cruz Biotechnology, Dallas, TX), and anti-CD44v9 (1:10,000, LKGM002; Cosmo Bio, Carlsbad, CA). Secondary antibody labeling was as described. For activated caspase-3 immunohistochemistry, a Cell Signaling Technologies (Danvers, MA) SignalStain Apoptosis Kit (12692S) was used according to the manufacturer’s protocol. For terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, an In Situ Death Detection Kit (11684795910; Millipore-Sigma, St. Louis, MO) was used according to the manufacturer’s specifications. Slides of glands for immunofluorescence were generated using a Cytospin 4 centrifuge (A78300003; ThermoFisher, Waltham, MA). Cells were fixed on the slide in 4% paraformaldehyde for 20 minutes at room temperature and permeabilized (0.5% bovine serum albumin, 0.1% Triton (VWR International, Radnor, PA), and 2 mmol/L EDTA in phosphate-buffered saline) for 30 minutes at room temperature before blocking and staining according to the earlier-described protocols.
Image Cytometry Analysis
Immunofluorescent images were segmented and individual cells were enumerated using CellProfiler (The Broad Institute, Cambridge, MA) software. Resulting data files were analyzed using FCS Express Image 6 (De Novo Software, Glendale, CA).
Isolation of Immune Cells
The method for isolating cells from the stomach tissue has been described previously.17, 36 Briefly, the gastric lymph nodes were removed from the stomachs, homogenized, and passed through a 40-μmol/L pore nylon filter. To detect secreted cytokines, 1 × 106 cells were cultured in vitro in 15-mL conical tubes containing 1 mL of supplemented RPMI. Supernatants from cell cultures were collected after 48 hours, and cytokines were measured using a Th1/Th2/Th17 cytometric bead array according to the manufacturer’s recommendations (560485; BD Biosciences).
Flow Cytometry
Cell surface staining was performed according to standard procedures using antibodies against CD4 (562891; BD Pharmingen, San Diego, CA). Intracellular cytokine staining was performed using antibodies against IL-17A (559502; BD Pharmingen). All flow cytometry was performed on a BD LSRII (BD Biosciences) or BD FACSCanto (BD Biosciences) and analyzed using FlowJo (FlowJo, Ashland, OR). For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate and ionomycin for 4 hours at 37°C. Golgi-stop (BD Biosciences) was added after 1 hour. Cells then were washed, fixed in 4% formyl saline, washed, and permeabilized (0.5% bovine serum albumin, 0.1% Triton, and 2 mmol/L EDTA in phosphate-buffered saline) for 30 minutes at room temperature. After washing, cells were incubated overnight with the anticytokine antibodies, and then washed and analyzed by flow cytometry.
Gastroid/Gland Culture
Whole gastric glands isolated from the corpus of healthy BALB/c mice were cultured in Matrigel (356234; Corning, Tewksbury, MA) using gland culture media: Dulbecco's modified Eagle medium-F12 supplemented with EGF (PHG0313; ThermoFisher), insulin-selenium-transferrin (I3146; Sigma-Aldrich, St. Louis, MO), and hydrocortisone (H0888; Sigma-Aldrich). Gastroids formed over a 48-hour period. At this time, recombinant murine IL-17A (576002; Biolegend, San Diego, CA) at 3 ng/mL was added to the cultures and gastroids were followed up for size and viability over a 3-day culture period. For Z-VAD-FMK inhibition assays, Z-VAD-FMK (FMK001; R&D Systems, Minneapolis, MN) was added to gastroids at 3 μmol/L. For necrostatin-1 inhibition assays, necrostatin-1 (ab141053; Abcam, Cambridge, UK) was added to gastroids at 20 μmol/L. Gastroids were followed up for viability over a 3-day culture period using photomicroscopy and ImageJ software version 1.49 (National Institutes of Health, Bethesda, MD).
For cultures of gastric glands, glands were suspended in gland culture media and cultured for 24 hours. For IL-17A gland treatment assays, recombinant IL-17A (rIL-17A) was added to the treated conditions at 10 ng/mL.
Statistical Analysis
Data are expressed as means of individual determinations ± SE. Statistical analysis was performed by either the Mann–Whitney U test or an unpaired Student t test using GraphPad Prism 5 (GraphPad Software, San Diego, CA).
Results
IL-17A Production Correlates With Disease Severity in Mice With Autoimmune Gastritis
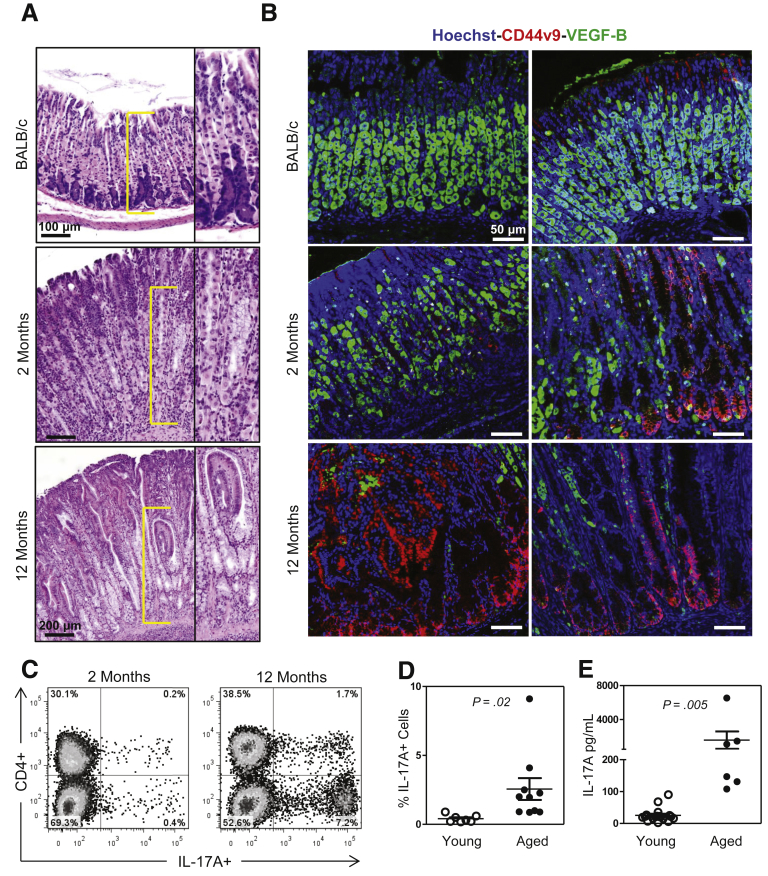
Our first goal was to determine the extent of IL-17A production in a mouse model of autoimmune gastritis (TxA23 T cell receptor transgenic mice) that develops parietal cell atrophy and SPEM. At 2 months of age, these mice have inflammation but mild degrees of atrophy, mucous neck cell hyperplasia, and metaplasia, compared with 8- to 12-month-old mice, which have severe atrophy, hyperplasia, and metaplasia (Figure 1A). Immunofluorescent staining was used to assess the development of SPEM using antibodies against CD44v9, a splice variant form of CD44 that has been established as a reliable marker of SPEM,37 showing that 2-month-old mice develop focal SPEM lesions, whereas 12-month-old mice have widespread and severe SPEM lesions (Figure 1B). Immune cells were isolated from the gastric lymph nodes of 2-month-old and 12-month-old mice and the percentage of IL-17A-producing immune cells were determined by intracellular cytokine staining. IL-17A-producing cells were present in both young and old mice, however, the frequency of IL-17A+ cells was >6 times higher in the gastric lymph nodes of older mice compared with younger mice (2.6 ± 0.8 vs 0.4 ± 0.1; P = .02) (Figure 1C and D). The amounts of IL-17A protein secreted by cells isolated from younger and older mice also was determined, and cells from older mice secreted greater than 50-fold more IL-17A than cells isolated from younger mice (1582 ± 1018 vs 25 ± 5 pg/mL; P = .01) (Figure 1E). These data show that IL-17A production in this mouse model correlates with the progression of gastritis toward gastric neoplasia, a finding that also was reported in recent studies of human beings with gastric cancer.27, 28, 29
Figure 1.
IL-17A production correlates with the severity of atrophic gastritis in TxA23 mice. (A) Representative H&E sections showing the degree of parietal cell atrophy in healthy BALB/c (2 months old), 2-month-old TxA23, and 12-month-old TxA23 mice. Yellow brackets indicate location of high magnification inset image. (B) Immunofluorescent staining with anti–VEGF-B (green), anti-CD44v9 (red), and Hoechst (blue) to illustrate the extent of parietal cell atrophy and SPEM development in healthy mice, mice early in disease, and mice with advanced disease. (C) Representative flow cytometry plots showing the percentage of IL-17A+ cells in the gastric lymph nodes of young and aged TxA23 mice. (D) The means ± SEM showing significantly higher percentages of IL-17A+ cells in older TxA23 mice compared with younger mice. (E) Results of enzyme-linked immunosorbent assays showing significantly higher amounts of IL-17A secreted by cells isolated from older TxA23 mice compared with younger mice. Each dot represents 1 mouse, 6–18 mice per group.
IL-17A Acts Directly on Gastric Epithelium and Induces Caspase-Dependent Apoptosis
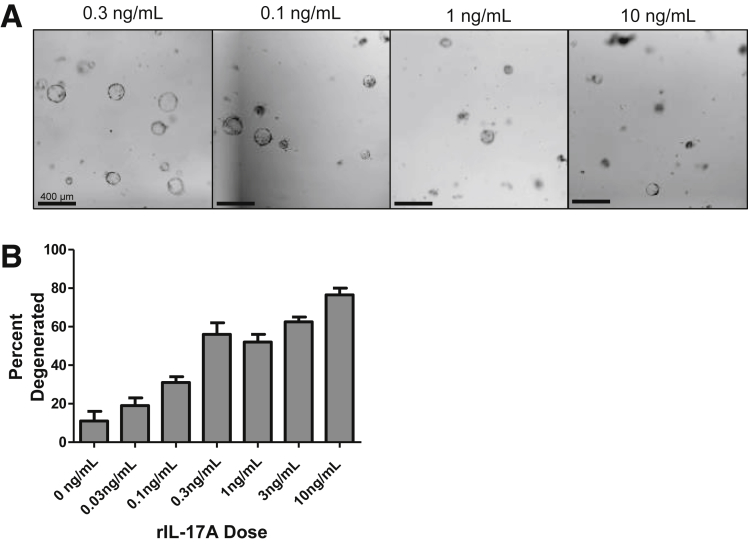
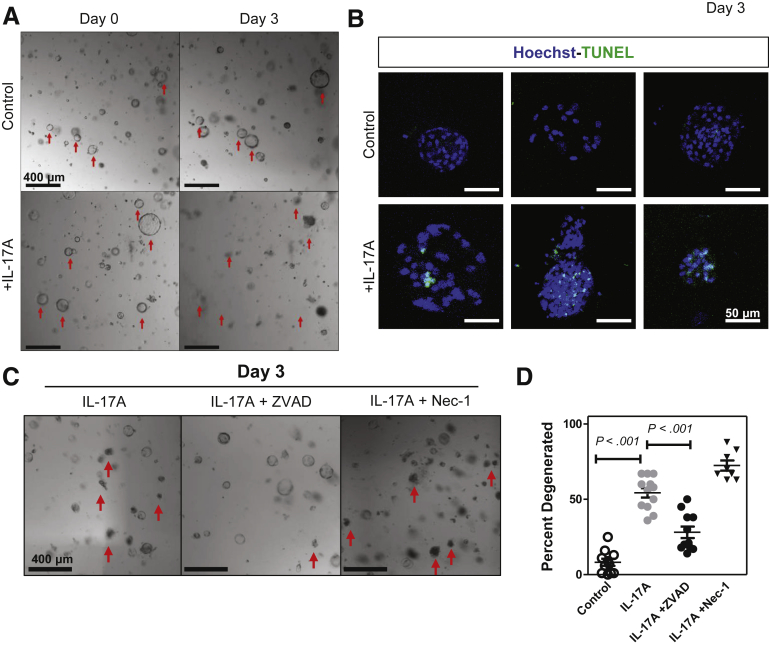
The previous results indicated that increases in disease severity during ongoing chronic inflammation could be owing to effects of increased IL-17A in the local cytokine milieu. We hypothesized that these effects were caused by IL-17A signaling directly into gastric epithelium. To test this we generated 3-dimensional spheroids from glands dissociated from the gastric corpus mucosa, known as gastric organoids or gastroids.38 These gastroids allowed us to examine the direct effect(s) of IL-17A on gastric epithelial cells. Recombinant IL-17A (rIL-17A) was added to gastroids that had been cultured without stem cell growth factors. Gastroids were analyzed for degeneration using photomicroscopy. The addition of rIL-17A to these epithelial cell cultures caused organoid degeneration in a dose-dependent manner (Supplementary Figure 1). By using the dose determined to cause 50% degeneration, 3 ng/mL, we observed a significant increase in organoid degeneration on day 3 (Figure 2A). We hypothesized that IL-17A might be inducing programmed cell death, leading to organoid degeneration, and used TUNEL to assess this hypothesis. We observed that organoids induced to degenerate by IL-17A contained a larger number of TUNEL+ cells (Figure 2B). Next, we wanted to determine whether the IL-17A-induced cell death in the organoids was caused by apoptosis (caspase-dependent) or necroptosis (Receptor-interacting serine/threonine-protein kinase 3-dependent). To distinguish between these 2 death pathways we cultured organoids with an irreversible pan-caspase inhibitor (Z-VAD-FMK) or necrostatin-1 (Nec-1), a compound shown to inhibit enzymes necessary for the necroptotic pathway.39 Z-VAD-FMK significantly reduced the amount of IL-17A-induced organoid degeneration but Nec-1 did not (Figure 2C and D). Together, these data show that IL-17A acts directly on gastroids, and that a primary effect of IL-17A in the gastroid cultures was to induce caspase-dependent apoptosis.
Supplementary Figure 1.
Dose-dependent gastroid killing by rIL-17A. Primary gastroids were cultured for 3 days in various concentrations of IL-17A and assessed for viability. (A) Representative images of gastroids on day 3 at differing concentrations of IL-17A. (B) Percentage of gastroid death per culture per day. Data are means ± SEM of 2 experiments with 4 cultures per group.
Figure 2.
IL-17A induces the caspase-dependent death of corpus gastroids in vitro. Corpus glands were isolated and cultured in Matrigel in gastroid-forming conditions. Red arrows denote degenerated organoids. (A) Gastroids were cultured for 48 hours and then media was supplemented with IL-17A. Gastroid death was compared between IL-17A supplemented and controls. (B) Representative immunofluorescent images of control and IL-17A-treated organoids stained with Hoechst (blue) and TUNEL (green). (C) Gastroids were cultured for 48 hours and then media was supplemented with IL-17A or IL-17A + a pan-caspase inhibitor, ZVAD-FMK, or IL-17A + a necroptosis inhibitor, necrostatin-1. Red arrows denote dead organoids in each field of view. (D) Percentage of degenerated organoids in control, IL-17A-treated, IL-17A + ZVAD-FMK, and IL-17A + necrostatin-1 conditions. Data are the means ± SEM of 3 experiments with 8–12 cultures per group. Significance was calculated using the Student t test.
Parietal Cells Express IL-17A Receptors and Undergo Apoptosis When Treated With IL-17A
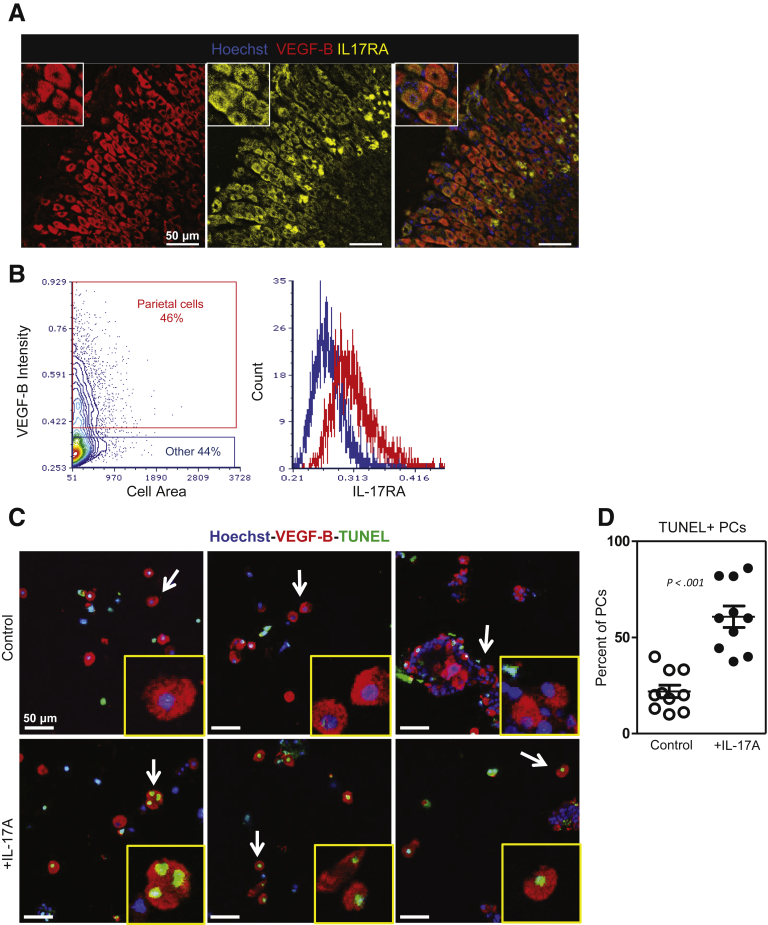
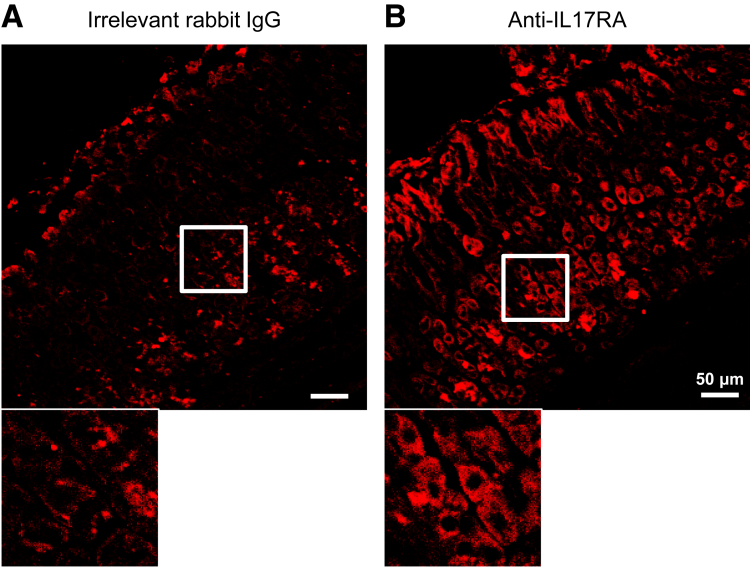
There is a lack of information about which cytokine receptors are expressed by epithelial cells in the gastric mucosa. Our observations using in vitro organoid cultures confirmed that IL-17A has effects on gastric epithelial cells, but did not elucidate which cell type(s) were responsible for the observed degeneration. We wanted to determine if parietal cells were capable of responding to IL-17A and if this response was promoting atrophy directly. We used immunofluorescence to examine IL-17RA expression in the gastric mucosa of healthy BALB/c mice. Tissues were stained with antibodies against IL-17RA and VEGF-B to detect parietal cells (Figure 3A), and positive cells were determined using isotype controls (Supplementary Figure 2). These studies identified parietal cells as expressing the IL-17A receptor. Next, we used image cytometry software to quantify the staining intensity of VEGF-B+ parietal cells relative to all other cells (Figure 3B). Image cytometry software uses data compiled by a cell segmentation software platform to analyze fluorescence intensity of immunofluorescent images on a per-cell basis. Shown in Figure 3B is the gating strategy for identifying parietal cells (red) and all other cells (blue) in an immunofluorescent image based on VEGF-B intensity. This analysis identified parietal cells as staining for IL-17RA at a higher intensity than nearly all other cell types as shown in the representative histograms. We next sought to determine if IL-17A induced parietal cell death. To do this, we cultured freshly isolated gastric glands from healthy BALB/c mice in the presence or absence of IL-17A for 24 hours. After 24 hours in culture, slides were generated using a Cytospin centrifuge and immunofluorescently stained for VEGF-B and TUNEL (Figure 3C). Although there is some parietal cell apoptosis associated with the experimental controls, we observed a significant increase in the percentage of TUNEL+ parietal cells above this background in the IL-17A-treated cultures (60.8 ± 5.6 vs 22.0 ± 3.2; P < .001) (Figure 3D). TUNEL+ non–parietal cells were relatively rare in both control and IL-17A-treated conditions (data not shown).
Figure 3.
Parietal cells express IL-17A receptor. (A) Representative immunofluorescent staining of healthy BALB/c (2 months old) mouse gastric corpus for Hoechst (blue), VEGF-B (red), and IL-17RA (yellow). A magnification pane is outlined in white showing the expression of IL-17RA on VEGF-B–expressing parietal cells. (B) Image cytometry software was used to quantify the intensity of IL-17RA fluorescence on parietal cells using immunofluorescent images. Shown is a representative plot of VEGF-B–positive parietal cells (red) and VEGF-B–negative cells (blue) with histograms of IL-17RA staining intensity in each of these groups. Epithelial cells isolated from the gastric corpus of BALB/c mice (2 months old) were cultured in media or media supplemented with IL-17A for 24 hours. Cytospin slides were generated from control and treated glands for microscopic analysis. (C) Representative immunofluorescent staining of Cytospins generated from cultured gastric epithelial cells stained with Hoechst (blue), anti–VEGF-B (red), and TUNEL (green). (D) TUNEL+ parietal cells were quantified as a percentage of total parietal cells (PCs). Data are means ± SEM of 10 representative images prepared from 6 slides over 2 separate experiments.
Supplementary Figure 2.
IL-17RA staining compared with isotype control. (A) Staining of healthy BALB/c gastric corpus tissue with irrelevant rabbit IgG (left) and (B) rabbit anti-IL-17RA (right). Images were acquired using identical microscope settings and subjected to identical brightness/contrast settings.
IL-17A Production Is Sufficient to Cause Parietal Cell Apoptosis In Vivo
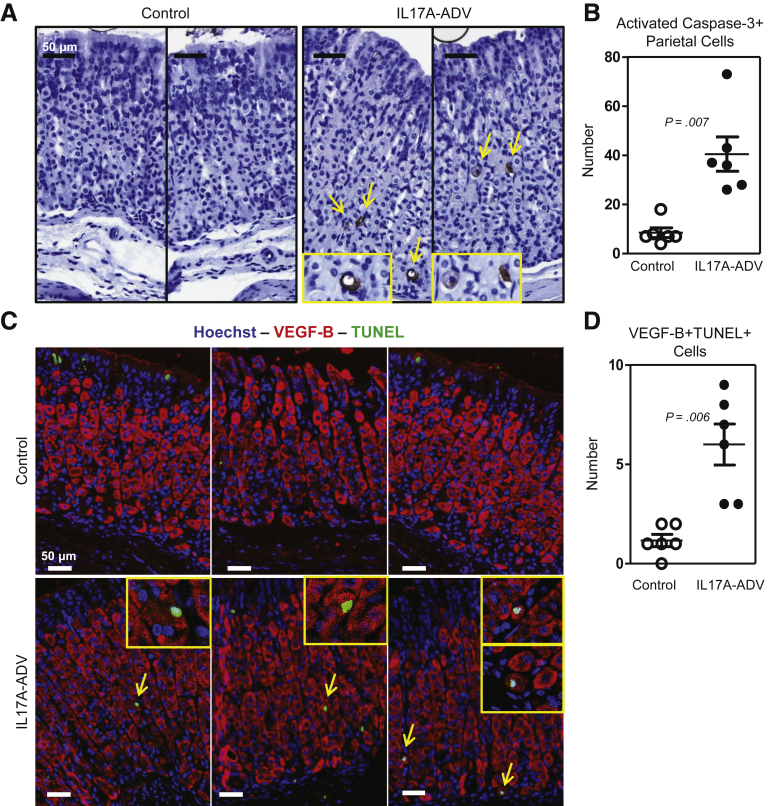
Having shown that IL-17A acted directly on the gastroid cultures and induced caspase-dependent death, and that IL-17A induced parietal cell death in gastric gland cultures, we sought to determine whether IL-17A could induce apoptosis of gastric epithelial cells in vivo. To do this, we infected cohorts of BALB/c mice with a recombinant IL-17A-producing adenovirus (IL-17A-ADV) or with β-galactosidase–expressing adenovirus as a control (β-gal–ADV). Infection with the IL-17A-ADV increased serum levels of IL-17A to 20–70 ng/mL compared with less than 1 ng/mL in the serum of β-gal–ADV–infected mice. Levels of interferon-γ, IL2, IL4, tumor necrosis factor α, IL6, or IL10 were not significantly different between IL-17A-ADV compared with controls (data not shown). After confirming increased serum IL-17A in IL-17A-ADV–infected mice, we evaluated gastric tissue sections using an antibody that recognizes the activated form of caspase-3 to determine the number of epithelial cells undergoing apoptosis in both groups of mice 1–2 weeks after the infections. We observed many more activated caspase-3–positive parietal cells in IL-17A-ADV–treated mice compared with controls (Figure 4A). We also observed that the majority of activated caspase-3–positive cells in the IL-17A-ADV–treated stomachs were parietal cells based on morphology. A quantification of the number of activated caspase-3–positive parietal cells from 3 complete cross-sections of gastric corpus in each mouse showed a significant (4-fold) increase in the IL-17A-ADV mice compared with controls (mean ± SE, 40.5 ± 6.9 for IL-17A-ADV, and 8.5 ± 4.8 for controls; P = .001) (Figure 4B). To ensure accurate identification of apoptotic parietal cells, we used immunofluorescent staining to identify VEGF-B+ cells that also stained TUNEL+ (Figure 4C). We quantified VEGF-B+ TUNEL+ double-positive cells in 3 representative 20× fields of view from each treated mouse and observed a significant increase in the absolute number of TUNEL+ parietal cells in the IL-17A-ADV cohort compared with the controls (6.0 ± 1.0 vs 1.2 ± 0.3; P = .006) (Figure 4D). These results show that IL-17A induces caspase-3 activation and parietal cell atrophy in vivo.
Figure 4.
IL-17A induces caspase activation in parietal cells in vivo. Two-month-old BALB/c mice were infected with 109 TCID50 of ADV via tail vein injection. The ADV expressed either β-galactosidase (control) or rIL-17A (IL-17A-ADV). (A) Representative immunohistochemical staining for activated caspase-3 in control and IL-17A-ADV–infected mice. Yellow arrows denote activated caspase-3 staining. (B) Quantification of all activated caspase-3–positive parietal cells present in 3 complete sections of gastric corpus from each infected mouse (6 mice per group). (C) Representative immunofluorescent staining of control and IL-17A-ADV mouse stomachs for Hoechst (blue), VEGF-B (red), and TUNEL (green). Yellow arrows denote VEGF-B+TUNEL+ cells with high magnification inset images located in the yellow boxes in the top right corner. (D) TUNEL+ parietal cells were quantified in 3 representative images from each mouse, showing increased numbers of parietal cell–specific caspase activation in IL-17A-ADV mice. Significance was determined using an unpaired Student t test.
Administering Anti-IL-17A Reduces Parietal Cell Atrophy and SPEM During Chronic Gastritis
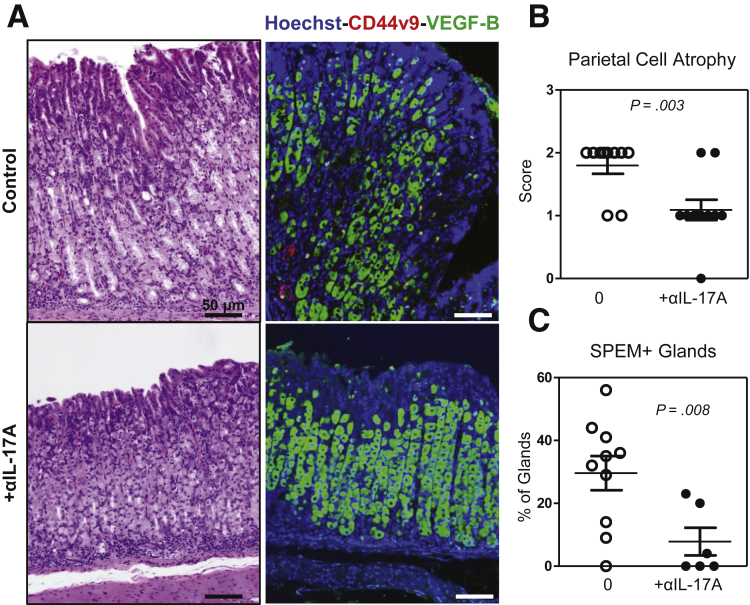
Previous results showed that in vitro gastric epithelial cell cultures degenerate in a caspase-dependent manner in the presence of IL-17A; that parietal cells express IL-17RA, specifically under apoptosis when cultured with IL-17A; and that parietal cells respond to in vivo overexpression of IL-17A with increased frequency of caspase-3–mediated apoptosis. Because of these data, we hypothesized that if IL-17A was inducing parietal cell apoptosis, then neutralizing IL-17A in mice with ongoing autoimmune gastritis might be an effective means to reduce the degree of parietal atrophy and subsequent metaplasia. To test this hypothesis, a cohort of TxA23 mice received injections of a monoclonal antibody to neutralize IL-17A over a 4-week period of time beginning at weaning and concluding at 2 months of age. The severity of atrophy and SPEM was compared between anti-IL-17A–treated TxA23 and control TxA23 mice after 4 weeks of treatment. An analysis of stomach sections stained with H&E showed that mice treated with the IL-17A-neutralizing antibody had significantly less severe parietal cell atrophy in the anti-IL-17A mice compared with control mice (1.1 ± 0.1 vs 1.7 ± 0.2; P = .015) (Figure 5A and B). In addition, staining gastric tissue sections with a parietal cell–specific antibody (anti–VEGF-B) confirmed that mice treated with anti–IL-17A had significantly less CD44v9+ gastric units compared with untreated mice (8 ± 4 vs 30 ± 5; P = .008) (Figure 5D). Together, these data show that neutralizing IL-17A is an effective means to reduce the extent of parietal cell atrophy and SPEM in mice with active and ongoing gastritis.
Figure 5.
Neutralizing IL-17A reduces parietal cell atrophy and SPEM. TxA23 mice were treated biweekly with αIL-17A beginning at 4 weeks and concluding at 8 weeks of age. (A) Representative H&E and immunofluorescently stained sections show decreased parietal cell atrophy and SPEM in the stomachs of anti-IL-17A–treated mice compared with control TxA23 mice. Immunofluorescent sections stained with Hoechst (blue), anti-CD44v9 (red), and anti–VEGF-B (green). (B) Scoring individual stomachs for the degree of atrophy showed significantly less atrophy in treated mice compared with control mice. Each dot represents 1 mouse combined from 4 separate experiments, 7–11 mice per group. Significance was determined using a Mann–Whitney U test. (C) Quantification of SPEM+ (CD44v9+) glands in control and IL-17A-neutralized mice. Each dot represents the percentage of positive glands in 3 representative images from 1 mouse. Significance was calculated using the Student t test.
Discussion
Chronic inflammation that causes parietal cell atrophy and metaplasia increases the risk for gastric cancer.8, 9 Although the importance of parietal cell loss in the development of SPEM is well established, the signals that cause parietal cell death and induce metaplastic changes in surrounding epithelium are currently not well understood. The mechanisms causing parietal cell death are critical because the key cellular event in increased gastric cancer risk seems to be whether chronic inflammation causes parietal cell death; in other words, whether superficial gastritis progresses to chronic atrophic gastritis. By using the TxA23 mouse model of autoimmune gastritis and inflammation-induced metaplasia, we showed that IL-17A-producing immune cell abundance in the gastric mucosa and the concentration of IL-17A produced by these cells correlated with the degree of atrophy and metaplasia. The recent reports that IL-17A levels correlate with gastric cancer progression and poor prognosis in human beings motivated us to examine a direct atrophy/metaplasia-inducing role of IL-17A on the gastric epithelial cells themselves. The data presented in this study show that IL-17A induces caspase-dependent death of gastric organoids, induces caspase-3 activation and parietal cell apoptosis in vitro and in vivo, and can be neutralized to reduce the extent of parietal cell atrophy and SPEM in mice with ongoing atrophic gastritis.
We previously used the TxA23 model of chronic atrophic gastritis to show that CD4+ Th17 cells, which produce many cytokines in addition to IL-17A, were present and contributed to disease. Furthermore, Th17 cells induced a more severe atrophic gastritis phenotype compared with CD4+ T cells differentiated into Th1 or Th2 cells.25 The current study shows that IL-17A is involved directly in the loss of parietal cells from the gastric mucosa, providing mechanistic insight into the pathogenicity of Th17 cells during chronic atrophic gastritis.
IL-17A is a pleotropic cytokine that is widely known for its ability to induce proinflammatory genes by various types of epithelial cells. In our study, direct administration of IL-17A to epithelial organoids caused TUNEL+ degeneration, and this degeneration subsequently was inhibited by Z-VAD. This degeneration was not prevented by necrostatin-1, a known inhibitor of the necroptotic pathway,39 indicating apoptosis as the mechanism of organoid degeneration. In addition, in vivo studies using the IL-17A-producing adenoviral vector induced caspase-3 activation and TUNEL+ staining that was detected almost exclusively in parietal cells. Finally, we observed that IL-17A was critical to the progression of chronic atrophic gastritis in which many different cytokines are active. We determined that neutralizing IL-17A in mice with autoimmune gastritis reduced the degree of parietal cell atrophy and SPEM. From these data, we conclude that parietal cells respond to IL-17A by undergoing apoptosis, which promotes the development of atrophy and metaplasia during chronic inflammatory processes in which IL-17A is produced in excess. Although it is conceivable that the effect of IL-17A on parietal cells is the indirect result of actions on other gastric epithelial cell types that express the receptor, it is most likely the result of direct interaction with parietal cells. Reports of IL-17A inducing apoptosis are not common, but IL-17A has been reported to activate caspase-3/9 and induce apoptosis of vascular endothelial cells and cardiomyocytes.40, 41
Although the homeostatic function of IL-17A–induced apoptosis of parietal cells is not clear, it is possible that parietal cells, which secrete large amounts of acid as a part of their physiologic role, respond to some inflammatory signals such as IL-17A by undergoing apoptosis to protect the tissue from additional damage during a disease process. Under acute circumstances the parietal cells are likely to be replaced as inflammation is resolved and the gastric mucosa regenerates. The metaplastic proliferation seen in acute atrophy is likely the attempt to generate new parietal cells, but in a setting of chronic inflammation it is plausible that parietal cells are constantly signaled to undergo apoptosis and the metaplasia never resolves. This hypothesis is strengthened by the observation that inflammatory signals in addition to parietal cell death are required for metaplastic transformation.10
IL-17A has been reported to have protective effects in mouse models of H pylori infection.42, 43 Because IL-17A-deficient mice develop less severe gastritis in response to infection,44 protective effects may depend on whether the infection is viewed from the acute or chronic standpoint. IL-17A may play a protective role in limiting acute infection while also contributing to gastric pathology under chronic inflammatory conditions, such as H pylori infection and autoimmune gastritis. Knowing that IL-17A acts directly on gastric epithelial cells and promotes parietal cell atrophy is an important piece of a complicated puzzle: the mechanism by which the complex milieu of cytokines in any individual patient influences whether gastritis progresses to atrophic gastritis, metaplasia, dysplasia, and neoplastic transformation. This provides key mechanistic insight into the mounting evidence in both human beings and in mouse models that IL-17A levels are associated with the severity of gastritis and gastric cancer development.27, 28, 29
This study identifies IL-17A as a critical component of the inflammatory microenvironment that induces parietal cell death, but certainly not the only pathogenic cytokine driving disease progression. We hypothesize a scenario in which a complex milieu of cytokines participates in a cross-talk between immune and gastric epithelial cells, some of which serve to drive disease progression and others that inhibit preneoplastic epithelial cell changes. The types and amounts of cytokines made by any individual could play a role in determining the severity of inflammation, degree of parietal cell atrophy, the type of metaplasia, and ultimately whether disease progresses to neoplasia. This view is supported by our finding that neutralizing IL-17A significantly inhibits but does not completely reverse parietal cell atrophy in the context of ongoing gastritis, as well as data from multiple laboratories showing the importance of a number of soluble signaling molecules such as sonic hedgehog,45, 46 IL-33,47 and IL-1148 in atrophic gastritis and gastric preneoplasia. Additional studies are required to provide a better understanding of the interface between the immune system and the gastric epithelium in both homeostatic and disease settings. The complex interactions between cytokine signals that promote and inhibit the progression of gastritis to preneoplasia are just beginning to be elucidated. Human studies also are needed to properly assess the role of IL-17A on parietal cells in both the steady state and in response to acute and chronic inflammation. An understanding of what cytokines inhibit the actions of IL-17A also are important to understanding the key regulatory mechanisms governing the atrophy of parietal cells and the development of SPEM during chronic inflammation. This information may help develop strategies to diagnose individuals with a high risk of progressing from atrophic gastritis to gastric cancer and perhaps even to develop new treatments that target cytokines such as IL-17A.
Acknowledgments
The authors thank Joy Eslick and Sherri Koehm for assistance with flow cytometry; Dr Grant Kolar, Barbara Nagel, and Katie Phelps from the Saint Louis University Research Microscopy and Histology Core for generation of tissue sections; and the Saint Louis University Comparative Medicine Department for assistance in maintaining mouse colonies. The authors also thank the Advanced Imaging and Tissue Analysis Core of the Washington University Digestive Disease Research Cores Center.
Footnotes
Author contributions Kevin Bockerstett was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and revision of the manuscript; Luciana Osaki was responsible for the acquisition, analysis, and interpretation of data; Christine Petersen was responsible for the acquisition, analysis, and interpretation of data, and revision of the manuscript; Catherine Cai was responsible for the study concept and design, acquisition of data, and revision of the manuscript; Chun Fung Wong was responsible for the acquisition of data and technical support; Thanh-Long Nguyen was responsible for the study concept and design, acquisition of data, and revision of the manuscript; Eric Ford was responsible for the acquisition of data and technical support; Daniel Hoft was responsible for the study concept and design, and interpretation of data; Jason Mills was responsible for the study concept and design, and revision of the manuscript; James Goldenring was responsible for revision of the manuscript; and Richard DiPaolo was responsible for the study concept and design, revision of the manuscript, interpretation of data, and obtained funding.
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the American Cancer Society (RSG-12-171-01-LIB) and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK110406) (R.J.D. and J.C.M.); a grant from the Digestive Diseases Research Core Center of the Washington University School of Medicine (National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK52574) and by the American Gastroenterological Association Funderburg Research Award (R.J.D.); a VA Merit Review (1I01BX000930) and National Institutes of Health grant DK101332 (J.R.G.); a National Institutes of Health National Research Service Award Predoctoral Fellowship (F31 DK104600 to C.P.P.); and a pre-Program Projects Grants award from the Siteman Cancer Center and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK094989 and R01 DK105129 to J.C.M.).
Supplementary Material
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19(Suppl 1):S37–S43. [PubMed] [Google Scholar]
- 3.Landgren A.M., Landgren O., Gridley G., Dores G.M., Linet M.S., Morton L.M. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117:1163–1171. doi: 10.1002/cncr.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox J.G., Wang T.C. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Omar E.M., Rabkin C.S., Gammon M.D., Vaughan T.L., Risch H.A., Schoenberg J.B., Stanford J.L., Mayne S.T., Goedert J., Blot W.J., Fraumeni J.F., Jr., Chow W.H. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 6.Machado J.C., Pharoah P., Sousa S., Carvalho R., Oliveira C., Figueiredo C., Amorim A., Seruca R., Caldas C., Carneiro F., Sobrinho-Simoes M. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 7.Dai Z.M., Zhang T.S., Lin S., Zhang W.G., Liu J., Cao X.M., Li H.B., Wang M., Liu X.H., Liu K., Li S.L., Dai Z.J. Role of IL-17A rs2275913 and IL-17F rs763780 polymorphisms in risk of cancer development: an updated meta-analysis. Sci Rep. 2016;6:20439. doi: 10.1038/srep20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 9.Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 10.Burclaff J., Osaki L.H., Liu D., Goldenring J.R., Mills J.C. Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology. 2017;152:762–766 e7. doi: 10.1053/j.gastro.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam K.T., Lee H.J., Sousa J.F., Weis V.G., O'Neal R.L., Finke P.E., Romero-Gallo J., Shi G., Mills J.C., Peek R.M., Jr., Konieczny S.F., Goldenring J.R. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037 e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills J.C., Sansom O.J. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennerz J.K., Kim S.H., Oates E.L., Huh W.J., Doherty J.M., Tian X., Bredemeyer A.J., Goldenring J.R., Lauwers G.Y., Shin Y.K., Mills J.C. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi H., Goldenring J.R., Kaminishi M., Lee J.R. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 2002;47:573–578. doi: 10.1023/a:1017920220149. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T.L., Sullivan N.L., Ebel M., Teague R.M., DiPaolo R.J. Antigen-specific TGF-beta-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187:1745–1753. doi: 10.4049/jimmunol.1004112. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T.L., Makhlouf N.T., Anthony B.A., Teague R.M., DiPaolo R.J. In vitro induced regulatory T cells are unique from endogenous regulatory T cells and effective at suppressing late stages of ongoing autoimmunity. PLoS One. 2014;9:e104698. doi: 10.1371/journal.pone.0104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPaolo R.J., Glass D.D., Bijwaard K.E., Shevach E.M. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 18.DiPaolo R.J., Brinster C., Davidson T.S., Andersson J., Glass D., Shevach E.M. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 19.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 21.Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge D., You Z. Expression of interleukin-17RC protein in normal human tissues. Int Arch Med. 2008;1:19. doi: 10.1186/1755-7682-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley T.A., Haudenschild D.R., Rose L., Reddi A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 24.Kolls J.K., Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Stummvoll G.H., DiPaolo R.J., Huter E.N., Davidson T.S., Glass D., Ward J.M., Shevach E.M. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caruso R., Pallone F., Monteleone G. Emerging role of IL-23/IL-17 axis in H pylori-associated pathology. World J Gastroenterol. 2007;13:5547–5551. doi: 10.3748/wjg.v13.i42.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B., Rong G., Wei H., Zhang M., Bi J., Ma L., Xue X., Wei G., Liu X., Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y., Saito H., Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012;178:685–691. doi: 10.1016/j.jss.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 29.Li Q., Li Q., Chen J., Liu Y., Zhao X., Tan B., Ai J., Zhang Z., Song J., Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013;30:1215–1222. doi: 10.3892/or.2013.2570. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen T.L., Khurana S.S., Bellone C.J., Capoccia B.J., Sagartz J.E., Kesman R.A., Jr., Mills J.C., Dipaolo R.J. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 2013;73:2117–2126. doi: 10.1158/0008-5472.CAN-12-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen T.L., Dipaolo R.J. A new mouse model of inflammation and gastric cancer. Oncoimmunology. 2013;2:e25911. doi: 10.4161/onci.25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh R.S., Shevach E.M., Margulies D.H., Natarajan K.A. T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol. 2001;31:2094–2103. doi: 10.1002/1521-4141(200107)31:7<2094::aid-immu2094>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenberger P., La Russa V., Miller A., Ye P., Huang W., Zieske A., Nelson S., Bagby G.J., Stoltz D., Mynatt R.L., Spriggs M., Kolls J.K. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 34.Hervas-Stubbs S., Lasarte J.J., Sarobe P., Prieto J., Cullen J., Roggendorf M., Borras-Cuesta F. Therapeutic vaccination of woodchucks against chronic woodchuck hepatitis virus infection. J Hepatol. 1997;27:726–737. doi: 10.1016/s0168-8278(97)80090-6. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey V.G., Doherty J.M., Chen C.C., Stappenbeck T.S., Konieczny S.F., Mills J.C. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 36.Alderuccio F., Toh B.H., Gleeson P.A., van Driel I.R. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity. 1995;21:215–221. doi: 10.3109/08916939509008018. [DOI] [PubMed] [Google Scholar]
- 37.Wada T., Ishimoto T., Seishima R., Tsuchihashi K., Yoshikawa M., Oshima H., Oshima M., Masuko T., Wright N.A., Furuhashi S., Hirashima K., Baba H., Kitagawa Y., Saya H., Nagano O. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher M.A., Aihara E., Feng R., Engevik A., Shroyer N.F., Ottemann K.M., Worrell R.T., Montrose M.H., Shivdasani R.A., Zavros Y. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–1827. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 40.Zhu F., Wang Q., Guo C., Wang X., Cao X., Shi Y., Gao F., Ma C., Zhang L. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin Immunol. 2011;141:152–160. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Liao Y.H., Xia N., Zhou S.F., Tang T.T., Yan X.X., Lv B.J., Nie S.F., Wang J., Iwakura Y., Xiao H., Yuan J., Jevallee H., Wei F., Shi G.P., Cheng X. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLyria E.S., Redline R.W., Blanchard T.G. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136:247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velin D., Favre L., Bernasconi E., Bachmann D., Pythoud C., Saiji E., Bouzourene H., Michetti P. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology. 2009;136:2237–2246. doi: 10.1053/j.gastro.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 44.Varga M.G., Piazuelo M.B., Romero-Gallo J., Delgado A.G., Suarez G., Whitaker M.E., Krishna U.S., Patel R.V., Skaar E.P., Wilson K.T., Algood H.M., Peek R.M., Jr. TLR9 activation suppresses inflammation in response to Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol. 2016;311:G852–G858. doi: 10.1152/ajpgi.00175.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merchant J.L., Ding L. Hedgehog signaling links chronic inflammation to gastric cancer precursor lesions. Cell Mol Gastroenterol Hepatol. 2017;3:201–210. doi: 10.1016/j.jcmgh.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding L., Hayes M.M., Photenhauer A., Eaton K.A., Li Q., Ocadiz-Ruiz R., Merchant J.L. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126:2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzzelli J.N., Chalinor H.V., Pavlic D.I., Sutton P., Menheniott T.R., Giraud A.S., Judd L.M. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell Mol Gastroenterol Hepatol. 2015;1:203–221 e3. doi: 10.1016/j.jcmgh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howlett M., Chalinor H.V., Buzzelli J.N., Nguyen N., van Driel I.R., Bell K.M., Fox J.G., Dimitriadis E., Menheniott T.R., Giraud A.S., Judd L.M. IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut. 2012;61:1398–1409. doi: 10.1136/gutjnl-2011-300539. [DOI] [PMC free article] [PubMed] [Google Scholar]