Summary
The influenza viruses have the ability to agglutinate erythrocytes by binding to sialic acid receptors on the host cell. Human influenza viruses preferentially bind to sialic acid linked to galactose by α 2.6 linkage, while avian influenza viruses preferentially bind to sialic acid linked to Gal by α 2.3 linkage. There is a close correlation between the ability of influenza A viruses to agglutinate erythrocytes from different animal species and their receptor specificity. The haemagglutination and haemagglutination inhibition assays are influenced by the species of erythrocytes. To provide an overview of the expression of sialic acid receptors on different erythrocytes, avian (turkey, chicken, pigeon) and mammalian (sheep, horse, human) species have been analysed by flow cytometry. Chicken, turkey and human erythrocytes display both types of linkages. Horse and sheep erythrocytes show almost exclusively α 2.3 Gal linkages, while pigeon erythrocytes express almost exclusively α 2.6 Gal linkages. The erythrocytes from the same avian and mammalian species have been evaluated by haemagglutination and haemagglutination inhibition assays with seasonal and avian strains. Chicken and turkey erythrocytes seem to be the most appropriate for both assays with seasonal influenza strains, in addition to pigeon erythrocytes, particularly for the B strains. In the case of the avian strain, chicken erythrocytes are suitable for haemagglutination assay and horse erythrocytes for haemagglutination inhibition assay. The choice of erythrocytes has a significant impact on the titres measured by both assays.
Keywords: Avian and mammalian erythrocytes, Sialic acid receptors, Haemagglutination assay, Haemagglutination inhibition assay
Introduction
The influenza viruses have the ability to agglutinate erythrocytes by binding to sialic acid (SA) receptors on the host cell [1, 2]. Human influenza viruses preferentially bind to SA linked to galactose (Gal) by α 2.6 linkage, while avian influenza viruses preferentially bind to SA linked to Gal by α 2.3 linkage [3].
There is a close correlation between the ability of influenza A viruses to agglutinate erythrocytes from different animal species and their receptor specificity. The agglutination of cells by viruses with specific linkage preferences depends on the amount of SA α 2.3 and α 2.6 Gal linkages on the erythrocytes surface [4]. Several studies have shown that human, chicken, pig and guinea pig erythrocytes express both linkages; pig erythrocytes present a high proportion of SA α 2.6 Gal linkage, while chicken erythrocytes display greater SA α 2.3 Gal linkage than those of guinea pigs and humans. Sheep, mouse erythrocytes display SA α 2.3 Gal linkage only; horse and cow mainly exhibit this linkage, while rabbit shows low amounts of SA α 2.6 Gal linkage [1, 4-6].
The haemagglutination assay (HA) and haemagglutination inhibition assay (HI) are influenced by the species of erythrocytes. The HI assay is currently the most widely used to measure immune responses to influenza vaccines, and is considered the gold standard as a correlate of protection [7]. However, the assay has limitations including differences in the sensitivity of erythrocytes from individual animals of the same species, a high degree of variability among laboratories owing to many factors (including the source of erythrocytes) and low sensitivity to B strains [8-10]. Traditionally the assay is performed with turkey erythrocytes, which are small, nucleated cells, displaying rapid sedimentation [11]. However, these erythrocytes could significantly underestimate antibody responses to avian viruses, as they express a mixture of SA α 2.3 and α 2.6 Gal linkages, while avian viruses prefer the SA α 2.3 Gal linkage [6, 11, 12]. The use of horse erythrocytes has improved the detection of H5 antibody responses, owing to their higher proportion of SA α 2.3 Gal linkage [6, 11, 13, 14] and are routinely used to measure HI responses to avian viruses. Furthermore, goose and guinea pig erythrocytes can enhance the sensitivity of the assay for avian strains [8].
The aim of this study was to provide an overview of the expression of SA receptors on avian (turkey, chicken, pigeon) and mammalian (sheep, horse, human) species and possible intra-species variation for chicken and horse erythrocytes. The erythrocytes from the same avian and mammalian species have been analysed by HA and HI assays with seasonal and avian strains.
Methods
INFLUENZA VIRUSES, SERUM SAMPLES AND ERYTHROCYTES
The influenza viruses were the live, seasonal and pandemic, strains from NIBSC and CBER: A/California/07/2009 (H1N1, 15/252), A/Victoria/361/2011 (H3N2, 11/226) B/Wisconsin/01/2010 (Yamagata lineage) (B, 12/198) and A/Indonesia/05/2005 (H5N1, H5-Ag-0904). The live viruses were propagated in 10-days-old embryonated chicken eggs and stored at – 80°C until use.
The human serum samples from the serum bank of the University of Siena had been drawn from adults aged 17-59 years, in compliance with Italian ethics law.
All serum samples were pre-treated with receptor destroying enzyme (RDE) from Vibrio Cholerae at 1:5 ratio (Sigma Aldrich, Italy) for 18 h at 37°C in a water bath and then heat-inactivated for 1 h at 56°C in a water bath with 8% sodium citrate at 1:4 ratio before testing in the HI assay.
The sources of erythrocytes for HA and HI assays and flow cytometry were: turkey, chickens (different batches), sheep, horses (different breeds: Italian (IT), Argentinian (AG), German Mecklenburger Kaltblut (GM), French Trait Percheron (TP F) and French Cheval de Trait (CT F), pigeon (Emozoo S.N.C., Italy) and humans (group 0) (Tab. I).
Tab. I.
The erythrocytes used for the HA, HI assays and flow cytometry (FC). Argentinian horse (AG), French horse (Cheval de Trait (CT F), German horse (Mecklenburger Kaltblut GM), Italian Horse (IT), French horse (Trait Percheron (TP F).
| Erythrocytes | HA assay | HI assay | FC analysis |
|---|---|---|---|
| Chicken | X | X | X |
| AG horse | X | X | X |
| CT F horse | X | ||
| GM horse | X | ||
| IT horse | X | X | X |
| TP F horse | X | ||
| Human | X | X | X |
| Sheep | X | X | X |
| Pigeon | X | X | X |
| Turkey | X | X | X |
HAEMAGGLUTINATION ASSAY
Erythrocytes were used at a 0.35% (for chicken, pigeon and turkey - seasonal strains), 0.50% (for humans, horses, sheep - seasonal strains and turkey - avian strain), 0.75% (sheep - avian strain) concentrations suspended in saline solution (0.9%).
The HA assay was performed as previously described [15].
The HA titre was expressed as the reciprocal of the highest influenza virus dilution showing complete agglutination [15].
The HA assay was performed in triplicate. Geometric mean titres (GMTs) of seasonal and avian strains were calculated in order to compare the different erythrocytes analyzed.
HAEMAGGLUTINATION INHIBITION ASSAY
Erythrocytes were used at the same concentration of the HA assay.
The HI assay was performed as previously described [15].
The antibody titre was expressed as the reciprocal of the highest serum dilution showing complete inhibition of agglutination. Since the starting dilution was 1:10, the lower limit of detectable antibody titre was 10. When the titre was below the detectable threshold, the results were conventionally expressed as 5 for calculation purposes, half the lowest detection threshold.
The HI assay was performed in triplicate with human serum samples. GMTs of seasonal and avian strains were calculated in order to compare the different erythrocytes analyzed.
FLOW CYTOMETRY
The expression pattern of SA α 2.3 and 2.6 Gal linkages on erythrocytes was evaluated by flow cytometry using digoxigenin-conjugated specific lectins Sambucus nigra (SNA) and Maackia amurensis (MAA), which recognize the α 2.6 and the α 2.3 Gal linkages, respectively (Digoxigenin Glycan Differentiation Kit, Roche) as previously described [5]. Sheep anti-digoxigenin fluorescein antibody was used to detect the lectins. Negative controls without lectins were evaluated. Flow cytometry was carried out on a GUAVA EasyCyte 6-2L flow cytometer (Millipore) and the data were analyzed and plotted by means of FlowJo software (TreeStar Inc).
STATISTICS
Geometric Coefficient of Variation was calculated from the SD per sample across erythrocytes species. GCV = 100 x Sqrt (2SDlog2-1). Ratio’s between HI titres for different erythrocytes combinations were calculated per sample. The geometric mean ratio with 95% CI is presented in the ratio graphs.
Results
The expression of SA α 2.3 and α 2.6 Gal linkages was investigated by flow cytometry in avian species (chicken, turkey, pigeon) and mammalian species (sheep, horse and human). Chicken, turkey and human erythrocytes displayed both types of linkages. Notably, chicken erythrocytes expressed higher amounts of SA α 2.3 Gal linkage, and turkeys higher amounts of SA α 2.6 Gal linkage; human erythrocytes expressed both linkages in similar proportions. Horses showed almost exclusively SA α 2.3 Gal linkage, while pigeon displayed almost exclusively SA α 2.6 Gal linkage. Sheep erythrocytes expressed only α 2.3 Gal linkage (Fig. 1).
Fig. 1.
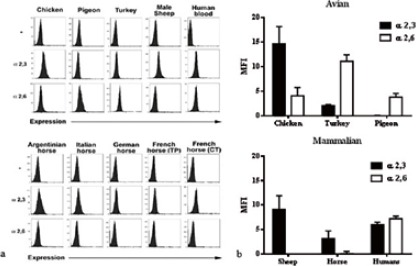
Expression of SA α 2,3 and α 2,6 Gal linkages in erythrocytes from avian (turkey, chicken, pigeon) and mammalian (sheep, horse, humans) species. Data originated from replicates of the same samples. a) Erythrocytes were incubated with DIG-labeled SNA (α 2,6 Gal linkage), DIG-labelled MAA (α 2,3 Gal linkage) or without lectins (-) and subsequently incubated with anti-digoxigenin antibody conjugated with fluorescein. Cells were analysed by flow cytometry and the results were expressed as the log10 relative fluorescence versus number of cells. The graphs show the Mean Fluorescence Intensity (MFI). Data are presented as mean ± SD (n ≥ 2). Representative histograms are shown. b) Distribution of α 2,3 and α 2,6 Gal linkages among animal species. The graphs show the MFI. Data are presented as mean ± SD (n ≥ 2).
To assess intra-species variation, erythrocytes from different batches of chickens and different breeds of horses – Italian, Argentinian, French (two breeds) and German – were tested (Fig. 2).
Fig. 2.
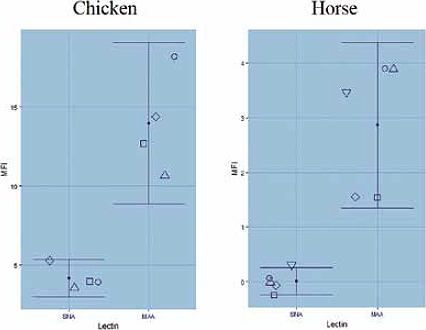
Variation of SA α 2,3 (MAA) and α 2,6 (SNA) Gal linkages within different chickens and horses (Mean MFI with 95% confidence intervals). Data are presented as mean SD (n ≥ 4). Different symbols indicate chickens (4 individuals) and horses (circle = Cheval de Trait – French –; square = Mecklenburger Kaltblut (German); diamond = Percheron (French); up triangle = Italian horse and down triangle = Argentinian horse).
Chicken and horses erythrocytes showed much higher α 2.3 Gal linkage than α 2.6 Gal linkage with variation in the expression among the same species.
In addition to the flow cytometry, the HA and HI assays were performed with erythrocytes from chicken, turkey, sheep, pigeon, humans and horses for both, seasonal (A/California/07/2009 H1N1, A/Victoria/361/2011 H3N2, B/Wisconsin/01/2010 - Yamagata lineage) and avian (A/Indonesia/05/2005 H5N1) strains to investigate the influence of different erythrocytes on seasonal and avian titres.
Seasonal viruses did not agglutinate horse and sheep erythrocytes. Regarding the A/California/07/2009 H1N1 strain, the highest geometric mean (GM) HA titre was displayed by chicken erythrocytes (2016), followed by turkey (1600) and pigeon (1270). Turkey erythrocytes (1600) were more sensitive to the A/Victoria/361/2011 H3N2 strain than those from pigeons (1008), chickens (800) and humans (716). In the case of the B/Wisconsin/01/2010 strain, turkey erythrocytes (716) yielded the highest HA titre, whereas other erythrocytes species displayed much lower HA titres. Human erythrocytes showed relatively low HA titres for all three seasonal strains (Tab. II).
Tab. II.
HA GMTs of influenza seasonal and avian strains with different erythrocytes.
| Influenza strain | Erythrocytes | ||||||
|---|---|---|---|---|---|---|---|
| A/California/07/2009 H1N1 |
Chicken 2016 |
Turkey 1600 |
Sheep no HA titer |
Pigeon 1270 |
Human 800 |
IT horse no HA titer |
AG horse no HA titer |
| A/Victoria/361/2011 H3N2 |
Chicken 800 |
Turkey 1600 |
Sheep no HA titer |
Pigeon 1008 |
Human 716 |
IT horse no HA titer |
AG horse no HA titer |
| B/Wisconsin/01/2010 | Chicken 358 |
Turkey 716 |
Sheep no HA titer |
Pigeon 179 |
Human 89 |
IT horse no HA titer |
AG horse no HA titer |
| A/Indonesia/05/2005 H5N1 |
Chicken 12800 |
Turkey 8063 |
Sheep 800 |
Pigeon 6400 |
Human 1600 |
IT horse 6400 |
AG horse 4032 |
The avian H5N1 strain elicited the highest HA titres in chicken erythrocytes (12800) (Tab. II).
Considerable variations in HI titres were detected when using different sources of erythrocytes for seasonal and avian strains. Overall, chicken erythrocytes displayed the highest titres for all the seasonal strains. Horse and sheep erythrocytes added much variation for H5N1 strain and horse erythrocytes yielded the highest titres (Fig. 3).
Fig. 3.
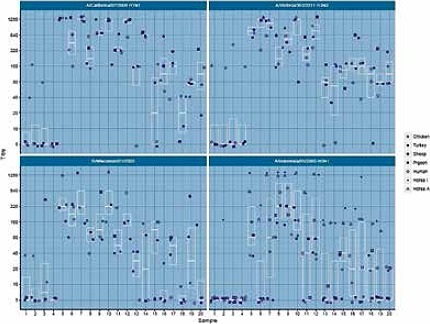
HI titres of human serum samples with different erythrocytes.
Regarding the H1N1 and H3N2 strains, the highest HI titres were displayed by chicken erythrocytes followed by pigeon and turkey erythrocytes showing the same titres. The only statistically significant difference is between chicken and human erythrocytes for H1N1 strain. For the H3N2 strain, turkey and human yield lower HI titres than chicken and human gives lower titres than turkey (Fig. 4). Human erythrocytes showed low HI titres for both strains (Fig. 4). For the B strain turkey yields lower titres than chicken.
Fig. 4.
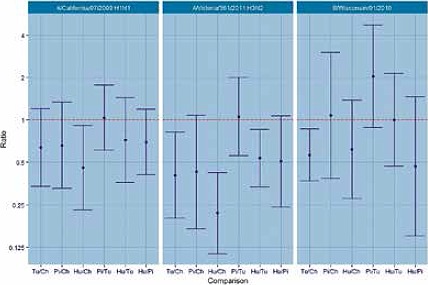
HI titre ratio’s for erythrocytes comparisons (a ratio of 1 indicates equality, when the 95% CI do not include 1 the titre ratio is statistically significant p #x003C; 0.05) with seasonal influenza strains.
Samples 1-4 yielded positive titres with chicken erythrocytes while all other erythrocytes were negative.
The avian strain showed the highest HI titres when horses erythrocytes were used. In particular, Italian horse gave significantly higher titres than Argentinian horse. Turkey and chicken displayed similar HI titres but lower than the other species. The HI titres with sheep erythrocytes were lower than human and horse and higher than chicken, turkey and pigeon (Fig. 5).
Fig. 5.
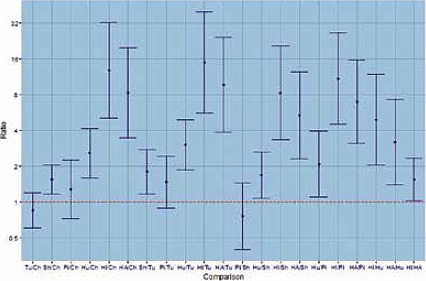
HI titre ratio’s for erythrocytes comparisons with avian H5N1 strain.
Discussion
The agglutination of cells by viruses with specific linkage preferences depends on the amount of SA α 2.3 and α 2.6 Gal linkages on the erythrocytes surface. The human influenza viruses preferentially bind to SA linked to Gal by α 2.6 linkage, while avian influenza viruses prefer SA linked to Gal by α 2.3 linkage [13].
The HA and HI assays are based on the ability of influenza viruses to agglutinate erythrocytes and are influenced by the erythrocytes used, resulting in different HA and HI titres; these may explain some of the variation observed in the titres of both assays between different laboratories [8, 16-18].
This study provides a comprehensive investigation of avian (turkey, chicken, pigeon) and mammalian (sheep, horse, humans) erythrocytes analysed by flow cytometry in order to investigate the expression of SA receptors. Moreover, the HA and HI titres with seasonal and avian strains have been evaluated to investigate the influence of different erythrocytes on both assays.
As reported in previous studies [1, 4-6], the results confirm that chicken erythrocytes showed higher amounts of SA α 2.3 Gal linkage, while turkey SA α 2.6 Gal linkage, sheep SA α 2.3 Gal linkage only and humans both linkages. In addition, a deeper investigation has been performed with the analysis of different batches of chickens’ erythrocytes. The results provide evidence of the variability in the expression of SA α 2.3 Gal linkages. This finding is of relevance as chicken with turkey erythrocytes are the most commonly used in HA and HI assays. It would therefore be interesting to analyse different batches of chicken in both assays and different batches of turkey by flow cytometry and HA/HI assays in order to evaluate possible intra-species variability. The data seem to support that chicken and turkey erythrocytes, which are characterized by both linkages, are suitable for use in the HA assays with seasonal strains. For the avian strain, the chicken erythrocytes seem to be the best choice. The results are in good agreement with those of other studies, which have demonstrated the advantage of goose, guinea pig and turkey erythrocytes for avian strain in both assays and turkey erythrocytes for the HI assay with the human pandemic virus H1N1 [8, 16, 18, 19].
As expected, sheep erythrocytes were not agglutinated by seasonal influenza strains due to the lack of SA α 2.6 Gal linkage. This is the only species analysed in this study that expresses exclusively SA α 2.3 Gal linkage. Nevertheless, they did not show high HA and HI titres for the avian strain.
Human erythrocytes seem not to be very useful for both assays with seasonal and avian strains despite expressing both linkages.
The novelty of the study is the characterization of pigeon erythrocytes displaying almost exclusively SA α 2.6 Gal linkage. As for the chicken and turkey, which show appreciable expression level of SA α 2.6 Gal linkage, pigeon erythrocytes yielded good HA titres with seasonal strains. Unexpected results were obtained with the avian strain where an appreciable HA titre has been observed. The HA data from sheep and pigeon erythrocytes may suggest that other mechanisms, in addition to a preference for SA α 2.3 or 2.6 Gal linkages, could be involved in the assay and need to be further investigated. The pigeon erythrocytes resulted in appreciable HI titres with H1N1 and H3N2 strains, comparable to those of turkey, and a high titre with influenza B strain. These data suggest that this animal species may constitute an interesting alternative, particularly for the B strains, and additional data should be gathered on pigeon erythrocytes.
Horse erythrocytes showed almost exclusively SA α 2.3 Gal linkages and therefore were not agglutinated by seasonal strains. A deeper investigation has been performed analysing different breeds of horse’ erythrocytes. The data displayed diverse expression of SA α 2.3 Gal linkages among the breeds. It would be interesting to perform the HA and HI assays with different breeds of horses in order to evaluate possible differences in the titres.
The HA titre of the horses was lower than that seen in erythrocytes from other species with the avian strain. These results are in line with those of a previous study, in which horse erythrocytes were the least sensitive with the HA assay but provided the highest antibody titre in the HI assay [17]. The use of horse erythrocytes significantly improved the sensitivity of the HI assay for the detection of antibodies against avian H5N1 virus, thus overcoming the initial drawback of using avian strains and supporting the HI assay as an effective means of evaluating pandemic vaccines [11, 13, 14, 20, 21].
Conclusions
The data from the present study provide evidence that the choice of erythrocytes has large impact on the HI titres for seasonal and avian strains. Collectively, chicken and turkey erythrocytes seem to be the most appropriate for both assays with seasonal influenza strains, in addition to pigeon erythrocytes, particularly for the B strains. In the case of the avian strain, chicken erythrocytes are suitable for HA assay and horse erythrocytes for HI assay. Especially the horse erythrocytes showed a deep impact for the HI titres. However, the differences in HA and HI titres highlight the need to harmonize the choice of erythrocytes for both assays, in order to define the most suitable species and conditions for assays involving seasonal and avian strains. The choice of erythrocytes has a significant impact on the titres measured by both assays. The selection and standardization of appropriate erythrocytes could significantly increase the sensitivity and reproducibility of the assays. The cooperation among regulatory authorities, academia, public health institutions and manufacturers is aimed at standardizing assays such as HI [22].
Acknowledgments
We thank Ilaria Manini, Alessandro Manenti, Elisa Llorente Pastor and Otfried Kistner for their technical support.
The authors declare that there is no conflict of interest.
Footnotes
Authors’ Contributions
EM and CMT conceived and designed the experiments; CMT, CU, CC, DP, GP, SM performed the experiments; CMT, EJR and SR analysed the data; CMT wrote the paper; RJC and EJR revised the paper.
References
- [1].Medeiros R, Escriou N, Naffakh N, Manuguerra JC, van der Werf S. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 2001;289(1):74-85. [DOI] [PubMed] [Google Scholar]
- [2].WHO. Manual for the laboratory diagnosis and virological surveillance of influenza. 2011:1-137. [Google Scholar]
- [3].WHO. WHO recommendation on influenza A(H7N9) vaccine virus. 2013: p. 1-2. [Google Scholar]
- [4].Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 1997;227(2):493-9. [DOI] [PubMed] [Google Scholar]
- [5].Takemae N, Ruttanapumma R, Parchariyanon S, Yoneyama S, Hayashi T, Hiramatsu H, Sriwilaijaroen N, Uchida Y, Kondo S, Yagi H, Kato K, Suzuki Y, Saito T. Alterations in receptor-binding properties of swine influenza viruses of the H1 subtype after isolation in embryonated chicken eggs. J Gen Virol 2010;91(Pt 4): 938-48. [DOI] [PubMed] [Google Scholar]
- [6].Jia N, Wang SX, Liu YX, Zhang PH, Zuo SQ, Lin Z, Dang RL, Ma YH, Zhang C, Zhang L, Lu S, Cao WC. Increased sensitivity for detecting avian influenza-specific antibodies by a modified hemagglutination inhibition assay using horse erythrocytes. J Virol Methods 2008;153(1):43-8. [DOI] [PubMed] [Google Scholar]
- [7].Trombetta CM, Montomol E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines 2016. [DOI] [PubMed] [Google Scholar]
- [8].Pawar SD, Parkhi SS, Koratkar SS, Mishra AC. Receptor specificity and erythrocyte binding preferences of avian influenza viruses isolated from India. Virol J 2012;9: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Montomoli E, Capecchi B, Hoschler K. Correlates of Protection Against Influenza, in Influenza Vaccines for the Future S.B. AG, Editor. 2011, Springer. [Google Scholar]
- [10].Wood JM, Montomoli E, Newman RW, Daas A, Buchheit KH, Terao E. Collaborative study on influenza vaccine clinical trial serology - part 2: reproducibility study. Pharmeur Bio Sci Notes 2011;2011(1):36-54. [PubMed] [Google Scholar]
- [11].Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004;103(1-2):91-5. [DOI] [PubMed] [Google Scholar]
- [12].Stephenson I, Wood JM, Nicholson KG, Zambon MC. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol 2003;70(3):391-8. [DOI] [PubMed] [Google Scholar]
- [13].WHO. Serological detection of avian influenza A(H7N9) virus infections by modified horde red blood cells haemagglutination-inhibition assay. 2013:1-11. [Google Scholar]
- [14].Kayali G, Setterquist SF, Capuano AW, Myers KP, Gill JS, Gray GC. Testing human sera for antibodies against avian influenza viruses: horse RBC hemagglutination inhibition vs. microneutralization assays. J Clin Virol 2008;43(1):73-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].WHO. WHO Manual on Animal Influenza Diagnosis and Surveillance. 2002. [Google Scholar]
- [16].Wiriyarat W, Lerdsamran H, Pooruk P, Webster RG, Louisirirotchanakul S, Ratanakorn P, Chaichoune K, Nateerom K, Puthavathana P. Erythrocyte binding preference of 16 subtypes of low pathogenic avian influenza and 2009 pandemic influenza A (H1N1) viruses. Vet Microbiol 2010;146(3-4):346-9. [DOI] [PubMed] [Google Scholar]
- [17].Louisirirotchanakul S, Lerdsamran H, Wiriyarat W, Sangsiriwut K, Chaichoune K, Pooruk P, Songserm T, Kitphati R, Sawanpanyalert P, Komoltri C, Auewarakul P, Puthavathana P. Erythrocyte binding preference of avian influenza H5N1 viruses. J Clin Microbiol 2007;45(7):2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ovsyannikova IG, White SJ, Albrecht RA, Garcia-Sastre A, Poland GA. Turkey versus guinea pig red blood cells: hemagglutination differences alter hemagglutination inhibition responses against influenza A/H1N1. Viral Immunol 2014;27(4):174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Makkoch J, Prachayangprecha S, Payungporn S, Chieochansin T, Songserm T, Amonsin A, Poovorawan Y. Erythrocyte binding preference of human pandemic influenza virus a and its effect on antibody response detection. Ann Lab Med 2012;32(4):276-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wibawa H, Henning J, Waluyati DE, Usman TB, Lowther S, Bingham J, Junaidi A, Meers J. Comparison of serological assays for detecting antibodies in ducks exposed to H5 subtype avian influenza virus. BMC Vet Res 2012;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol 2009;16(4):558-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].FLUCOP. in http://www.flucop.eu/.


