Summary
Japanese encephalitis (JE) is a vector-borne disease caused by the Japanese encephalitis virus (JEV). JEV is transmitted by mosquitoes to a wide range of vertebrate hosts, including birds and mammals. Domestic animals, especially pigs, are generally implicated as reservoirs of the virus, while humans are not part of the natural transmission cycle and cannot pass the virus to other hosts. Although JEV infection is very common in endemic areas (many countries in Asia), less than 1% of people affected develop clinical disease, and severe disease affects about 1 case per 250 JEV infections. Although rare, severe disease can be devastating; among the 30,000-50,000 global cases per year, approximately 20-30% of patients die and 30-50% of survivors develop significant neurological sequelae. JE is a significant public health problem for residents in endemic areas and may constitute a substantial risk for travelers to these areas. The epidemiology of JE and its risk to travelers have changed, and continue to evolve. The rapid economic growth of Asian countries has led to a surge in both inbound and outbound travel, making Asia the second most-visited region in the world after Europe, with 279 million international travelers in 2015. The top destination is China, followed by Thailand, Hong Kong, Malaysia and Japan, and the number of travelers is forecast to reach 535 million by 2030 (+ 4.9% per year). Because of the lack of treatment and the infeasibility of eliminating the vector, vaccination is recognized as the most efficacious means of preventing JE. The IC51 vaccine (IXIARO®) is a purified, inactivated, whole virus vaccine against JE. It is safe, well tolerated, efficacious and can be administered to children, adults and the elderly. The vaccination schedule involves administering 2 doses four weeks apart. For adults, a rapid schedule (0-7 days) is available, which could greatly enhance the feasibility of its use. Healthcare workers should inform both short- and long-term travelers of the risk of JE in each period of the year and recommend vaccination. Indeed, it has been shown that short-term travelers are also at risk, not only in rural environments, but also in cities and coastal towns, especially in tourist localities where excursions to country areas are organized.
Keywords: Japanese encephalitis disease, Japanese encephalitis virus, IC51 vaccine (IXIARO®), Travel medicine
Introduction
Japanese encephalitis (JE) is a vector-borne disease caused by the Japanese encephalitis virus (JEV), which is a single-stranded RNA virus belonging to the genus Flavivirus (Flaviviridae family) and is closely related to the West Nile encephalitis virus [1].
There are five JEV genotypes in the world, but genotype 1 circulates much more than the others [2, 3]. JEV is transmitted by mosquitoes to a wide range of vertebrate hosts, including birds and mammals. With regard to human infection, domestic animals, especially pigs, are generally implicated as reservoirs of the virus. As humans are not part of the natural transmission cycle, they cannot pass the virus to other hosts [4].
While JEV infection is very common in endemic areas (many countries in Asia), less than 1% of people affected develop clinical disease [2], and severe disease affects about 1 case per 250 JEV infections. Although rare, severe disease can be devastating; among the 30,000-50,000 global cases per year, approximately 20-30% of patients die and 30-50% of survivors develop significant neurological sequelae [5].
JE is a significant public health problem for residents in endemic areas and may constitute a substantial risk for travelers to endemic areas. Because of the lack of treatment and the infeasibility of eliminating the vector, vaccination is recognized as the most efficacious means of preventing JE [4].
This overview focuses on the epidemiology and clinical features of JE and describes the use of the IC51 vaccine (IXIARO®) in travel medicine.
Epidemiology of japanese encephalitis
The vector of the JEV is the Culex mosquito, specifically Culex tritaeniorhyncus, which lives and breeds in water pools and flooded rice fields. This type of mosquito bites during night, with two peaks in biting time: a few hours after sunset and around midnight [6].
Humans and some kinds of animals, such as cattle or horses, are dead-end hosts of JEV, as they cannot develop a sufficient level of viremia to re-infect other mosquitoes. Transmission among humans is not possible, although transfusion-related JEV transmission was reported in two immunocompromised lung transplant recipients in Hong Kong, resulting in one case of severe encephalitis and one case of asymptomatic infection with seroconversion [7].
Culex tritaeniorhyncus lives throughout South-East Asia and tropical areas. However, its presence also extends to the Middle East and Africa, and has recently been reported even in Europe. Within endemic areas, transmission varies according to climatic conditions: in temperate areas of Asia, transmission is seasonal, especially during summer and fall; in subtropical and tropical areas, transmission occurs during the monsoon season, but can be prolonged throughout the year [2].
The global incidence of JE is unknown, as the intensity and quality of JE surveillance systems and the availability of diagnostic laboratory testing vary throughout the world [8]. JE surveillance was not well established until 2012 and a 2011 systematic review of JE disease burden [8] estimated that approximately 68,000 cases occurred globally each year, and that only about 10% of these cases were reported to the WHO [9]. In 2011, Campbell et al. [8] reported that, owing to the lack of efficacious surveillance systems, it was difficult obtain a precise estimate of the incidence of JE. Nevertheless, it was estimated that 3 billion people living in 24 countries in the WHO’s South-East Asian and Western Pacific regions are at risk of JE [8, 3]. The authors estimated that about 67,900 cases per year occurred in JE-endemic countries, with half of these cases in China alone. They also classified countries in terms of their incidence (high, medium and low) and the availability of vaccination (vaccination implemented, vaccination program expanding, and no vaccination) (Figure 1). Although Japan, the Republic of Korea and Taiwan are “high-incidence” countries, their routine vaccination programs have enabled them to limit JE incidence to 0.003 per 100,000 in the overall population. Vaccination began in Japan in 1954, in Taiwan in 1968, and in Korea in 1970. In Japan, however, following the withdrawal of the recommendation in 2005, vaccination coverage declined; although the recommendation was reinstated in 2009, coverage remains low. By contrast, China, which is also a high-incidence country, does not have a quality vaccination program (vaccination was implemented from 1981 to 2007, but non-uniformly in children; a routine vaccination program was started in 2008). Consequently, the incidence of JE is 3.3 overall, 12.7 in children aged 0-14 years and 1.0 in subjects aged > 15 years. Some countries where JE incidence is high do not implement any, or are only now developing, vaccination programs: Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines and East Timor. Moreover, countries such as India and Nepal are improving their routine vaccination programs.
Fig. 1.
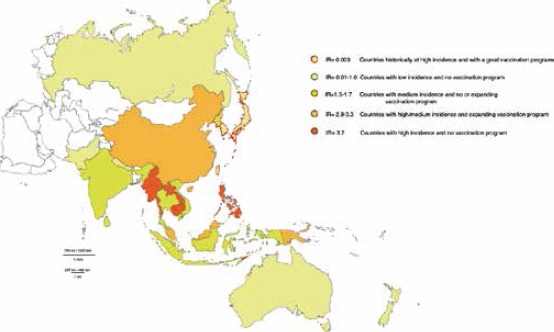
Incidence of JE and vaccination policies in Asian countries [8].
Medium-incidence countries (such as Indonesia and Papua New Guinea) have a total incidence rate of 1.7 per 100,000, while those with low incidence have a rate of 1.0 per 100,000 (Fig. 1).
Annual incidence varies by age-group, with children being the most affected [8]. Most adults are immune to infection, as natural infection with JEV (or other Flaviviruses that share antigens inducing cross-protection) is probably able to confer lifelong immunity. This explains why children are the most susceptible subjects [3, 4]. It is noteworthy that, where childhood vaccination programs are implemented, there is a shift to a greater proportion of cases in other unvaccinated age-groups [3].
In 2016, 22 of 24 (92%) countries at risk of JE ran surveillance systems, compared with 75% in 2012; some of these systems are national, some are sub-national and others use sentinel surveillance. In 12 of the 24 countries (50%), an immunization program was in place in 2016. Some of these immunization programs are national, others are sub-national in all risk areas, and several are sub-national but do not include all risk areas [9].
Recently, several factors have changed the geographical distribution of JE. First of all, international travel, especially for reasons of work, has increased. Indeed, the growth of US-based multinational corporations in Asia in the last twenty years has led to a significant rise in the number of business travelers. The business travelers frequently have to spend short or long periods in Asia, where they acquire the same risk of JE as the local population. Furthermore, changes have taken place in agriculture; in many areas of Asia, both rice production and pig farming have been intensified, which could lead to the development of a high risk of JE even in suburban areas, which were not at risk some years ago. The epidemiology of JE is also influenced by ecological and climatic factors. The appearance of JEV in Tibet (a cold, high-altitude region) and the emergence of two new serotypes of JEV in Australia provide confirmation that the spread of JEV is unpredictable. Moreover, global warming may lead to a change in the peak and duration of JEV transmission [5].
Clinical features and treatment
Most infections are asymptomatic or cause non-specific febrile symptoms [4]. Incubation time ranges from 5 to 15 days; the non-specific febrile illness that frequently constitutes the clinical presentation of JE may be the only manifestation in some patients [10]. Other symptoms can occur: coryza, diarrhea and rigor, followed by the onset of altered consciousness and seizures (convulsions occur in 75% of children) [3] or vomiting after several days [11]. When the disease is severe, affecting the central nervous system, the clinical features are mental status changes, focal neurological deficits, weakness and, after a few days, even movement disorders. Moreover, classical symptoms associated with severe JE are Parkinsonian syndrome, with mask-like face, tremor, cogwheel rigidity and choreoathetoid movements. Rarely, the clinical presentation can include acute facial paralyses (similar to poliomyelitis paralyses) [2]. Blood tests reveal neutrophilia, hyponatremia, elevated liver enzymes and cerebrospinal fluid (CSF) pleocytosis, with lymphocytic predominance [12, 2]. JE is most commonly diagnosed by testing for JEV IgM in the CSF, which is reliably positive if the CSF sample is taken at least one week into illness [13]. Other laboratory tests are molecular methods [14].
The treatment of JE usually consists of supportive care, as there are no specific antiviral therapies. The case fatality rate is high, and it is estimated that, in endemic areas, approximately one third of patients admitted to hospital die [11]. JE-related deaths are due to raised intracranial pressure, hypoglycemia and seizures [2, 3]. JE disease also causes serious brain damage in about 25-50% of survivors, the highest rate of sequelae being reported in children [3, 15]. Moreover, in pregnant women, the infection can cause harm to the fetus.
Even though JE is rare, it is potentially a severe life-changing illness and requires prevention. Vaccination is the cornerstone of JE control.
International travelers
The epidemiology of JE and its risk to travelers have changed, and continue to evolve. Estimates of JE incidence among Western travelers to tropical and subtropical destinations show that the usual itineraries followed by tourists or businesspeople are considered at low risk of infection (#x003C; 1 per 1,000,000 travelers per month) [16].
The risk of travelers contracting JE varies according to their travel itinerary. The overall risk of about 1/400,000 visits [17] may, however, increase for certain high-risk groups, such as those who stay for long periods in rural areas, approaching the risk of 1/50,000 per week seen in the local population [18, 19]. From 1973 to 2008, 55 cases were reported in travelers, most of whom were “high-risk travelers” who had spent a month or more in endemic regions (especially rural areas) and had acquired the same risk of JE as the local population. The case-fatality rate was 18% and 24 survivors developed sequelae. Even though long stays have always been considered a risk factor, the authors found that 13 of the 55 cases occurred in travelers who had stayed for less than 1 month: 10 for 2-4 weeks and 3 for 10-12 days trip [20]. This demonstrates that short-term travelers also have a JE disease risk.
Kollaritsch H. et al. [21] also reported JE cases in travelers, and from 1973 to 2015 the Centers for Disease Control and Prevention reported 79 cases among travelers or expatriates from non-endemic countries [2]. Some of these cases could have been avoided if travelers had been vaccinated in accordance with current recommendations.
More recently, a fatal case of JEV infection following short-term travel to Thailand was reported. In this case, JE viral RNA was detected in urine and whole blood 26 and 28 days, respectively, after the onset of symptoms. Furthermore, live virus was isolated from a urine specimen taken on day 14. This suggests that testing whole blood and urine could offer additional diagnostic information in certain situations [22].
It is well known that most cases of JE are asymptomatic. Consequently, a large number of infections may go unrecognized. Moreover, in the near future, JE could become a major public health issue even in non-endemic countries, as a result of cultural, economic and climatic changes [16].
Travel is changing; the increased speed and availability of transport (especially by air) encourages inter-regional and international tourism and business trips. In terms of population, Asia and the Pacific are the world’s largest regions, with 4.1 billion inhabitants in 2015, about 56% of the world’s total population [23]. The rapid economic growth of Asian countries has led to a surge in both inbound and outbound travel, making Asia the second most-visited region in the world after Europe, with 279 million international travelers in 2015. The top destination is China, followed by Thailand, Hong Kong, Malaysia and Japan, and the number of travelers is forecast to reach 535 million by 2030 (+4.9% per year). According to the United Nations Word Tourism Organization (UNWTO), this unprecedented growth in travel is the consequence of the Asian economic boom. Further, a large and growing number of “free and independent travelers”, identifiable as a “consumer class”, especially young Asian travelers aged 15-34 years (“millennials”), use online travel agencies and mobile technology. Their destinations are often secondary and tertiary cities, which could be potentially at risk of JE [24]. Furthermore, if they travel alone or simply organize their travel on their own, they are unlikely to be informed about JE, its symptoms and prevention. These changes in travel habits should prompt different information strategies, e.g. the use of social media, and collaboration between public health organizations and the most widely used travel websites.
Prevention of japanese encephalitis
Travelers to JE-endemic areas can reduce their exposure to vectors by adopting personal protective measures: using mosquito-repellent agents, wearing appropriate clothing, avoiding outdoor activities in the evening, using permethrin-impregnated mosquito nets and staying in rooms with air conditioning. In combination with these protective measures, vaccination against JEV infection can provide travelers with safe and effective protection [21, 25].
Different vaccines against JE have been available since the 1950s [14]. First-generation JE vaccines were of various types; mouse brain-derived inactivated vaccines, inactivated vaccines cultivated on primary hamster kidney cells, live attenuated vaccines based on strain SA 14-14-2 and cultivated on primary hamster kidney cells have been widely used for the past few decades [26]. Although mouse brain-derived vaccine was used for several decades in the US and Europe, its production has been discontinued, owing to its unsatisfactory safety profile [24, 25]. A formalin-inactivated vaccine developed in China in 1968 was cultivated in Vero cells and used the P-3 strain; this was widely used and displayed a good safety and effectiveness profile [25, 26]. A live-attenuated SA14-14-2 vaccine authorized in 1988 is currently used in Nepal, India, Sri Lanka, Thailand and South Korea, and has proved to be a valid tool in terms of effectiveness and safety [25].
More interestingly, second-generation JE vaccines are available, which use a lower dosage scheme and exhibit a better safety profile; these constitute a noteworthy option for the protection of residents of endemic areas and travelers. They are the IC51 Japanese encephalitis vaccine (IXIARO®) and the chimeric Japanese encephalitis vaccine.
Overall, vaccination has dramatically reduced the number of JE cases in several Asian countries [8]. However, as the virus is maintained in animal reservoirs, non-immune travelers remain at risk of infection in all endemic areas of South-East Asia.
DEVELOPMENT OF THE IC51 VACCINE (IXIARO®)
The IC51 vaccine (IXIARO®) is a purified, inactivated, whole virus vaccine against JE. Developed at the Walter Reed Army Institute of Research, it is based on an SA14-14-2 virus strain cultivated in Vero cells and formulated with 0.1% aluminum hydroxide [27]. Each 0.5 ml dose of IC51 vaccine comes in a ready-to-use liquid format that contains 0.1% aluminum hydroxide as an adjuvant; it does not contain stabilizers such as porcine gelatin or preservatives such as thimerosal.
Several clinical trials have positively evaluated the immunogenicity and safety of the IC51 vaccine. The first studies were carried out to evaluate its immunogenicity and safety in comparison with placebo or mouse-brain JE vaccines. The encouraging results, in terms of immune response and low reactogenicity, of a phase I study [21] that evaluated different doses of vaccine (0.4 micrograms and 2.0 micrograms following a 0 and 28, or a 0, 7 and 28 schedule) in 25 adult subjects prompted subsequent studies.
Lyons et al. reported the results of a phase II study, conducted from 2001 to 2003, which enrolled a total of 94 subjects. The vaccine was administered in two or three intramuscular doses (6.0 or 12.0 micrograms each) with observation over 8 weeks. No serious adverse reactions were observed. The vaccine was well tolerated and conferred high seroconversion rates [day 56 (primary endpoint): 95-100%] and induced persisting immune responses up to 2 years after vaccination [28].
Subsequent phase III trials were conducted. In a multi-center, observer-blinded, randomized controlled phase III trial, 867 adult volunteers received either two intramuscular doses the JEV test vaccine (on days 0 and 28; n = 430) or the licensed vaccine (JE-VAX®) subcutaneously according to its recommended three-dose schedule (on days 0.7, and 28; n = 437). The safety profile of the test vaccine was good, and its local tolerability profile was more favorable than that of the licensed vaccine. The frequency of adverse events was similar in both treatment groups and adverse events were generally mild. The seroconversion rate of the test vaccine was 98%, compared with 95% for the licensed vaccine, on day 56. The geometric mean titer in recipients of the test vaccine was 244, compared with 102 for the licensed vaccine [ratio 2.3 (95% CI 1.967-2.75)] [29].
Later, the same research group published the results of a randomized multi-center phase III trial. Healthy subjects were randomized to receive 2 doses of IC51 vaccine (n = 2012) or placebo (n = 663) four weeks apart. Adverse events were documented over a period of 2 months. The rate of severe events was similar in both the IC51 group (0.5%) and the placebo group (0.9%) demonstrating a good safety profile of IC51 vaccine. These data, together with the immunogenicity data from the previous phase III clinical trial, were the basis for the authorization of the IC51 vaccine [29, 30].
In another study, adult subjects were followed up in order to compare the immunogenicity of the IC51 vaccine and the vaccine JE-VAX®. At 6 months, immunogenicity was higher with the IC51 vaccine (seroconversion rate [SCR]: 95%; geometric mean titer [GMT]: 84) than with JE-VAX® (SCR: 74%; GMT: 34). With regard to IC51, the SCR at 12 months was 83% and the GMT remained above the protective titer of 1:10. This phase III follow-up study confirmed that the immune response following IC51 vaccination was more robust than the immune response following JE-VAX® [31].
The standard administration of IC51 is of 2 doses of 6 micrograms 28 days apart. However, one study investigated the immunogenicity of a single-shot, high-dose regimen (1 x 12 micrograms) in comparison with the 2-injection, standard regimen. The single-shot, high-dose regimen resulted in about 60% SCR 10 days after administration, but did not reach the almost 100% SCR achieved by the 2-dose standard administration on day 35. The standard regimen conferred essentially 100% seroconversion 7 days after the second injection [32].
On December 18, 2008, the European Committee for Medicinal Products for Human Use (CHMP) recommended granting marketing authorization for the IC51 vaccine (IXIARO®) in the 27 countries of the European Union and in Norway and Iceland. In the US, IXIARO® was authorized and recommended for use in persons aged ≥ 17 years in 2009 [33]. The product is also approved and available in Canada, Switzerland and Australia.
In the first 12 months after licensing, the safety profile of IXIARO® was reviewed on the basis of clinical trial results and post-marketing safety data. The clinical safety profile was derived from a pooled analysis that included safety data from 10 phase III trials carried out in 4,043 subjects who had received at least one dose of IXIARO® and were followed up for 3 years after the primary immunization. The local and systemic tolerability of IXIARO® was similar to that seen in the previous evaluation at the time of licensure of the vaccine. On post-marketing surveillance, no serious allergic reactions were observed. This comprehensive safety review confirmed the good safety profile of IXIARO® in clinical and post-marketing use [34].
A phase III study investigated the immunogenicity and safety of IC51 and HAVRIX1440 (hepatitis A vaccine) when administered alone or concomitantly to healthy subjects. The immune response elicited by single administration was compared with that of concomitant vaccination in terms of GMT and SCR on days 28 and 56. The results indicated that the immunogenicity of the two vaccines was comparable, whether they were administered together or separately. On the basis of these data, the authors affirmed that travelers to endemic regions could receive both vaccines concomitantly [35].
Eder S. et al. assessed the effect of a booster dose on neutralizing JE antibody titers up to 12 months after boosting. The booster dose of IXIARO® was administered 15 months after the primary immunization to 198 subjects who had previously been immunized in a randomized trial [36]. Neutralizing antibody titers were assessed by means of the plaque-reduction neutralization test (PRNT). Prior to the booster dose, 69.2% (137/198) of subjects had PRNT50 titers ≥ 1:10. One month after the booster, the rate of subjects with PRNT50 ≥ 1:10 (recognized as a protective titer) had increased to 100%. The evaluation of PRNT50 showed a high rate (98.5%) at 6 and 12 months. GMTs were 22.5 before the booster and 900, 487 and 361 at 1, 6 and 12 months, respectively, after the booster. The booster dose of IXIARO® 15 months after primary immunization proved highly immunogenic; GMTs were > 5-fold higher than those seen immediately after primary immunization, and remained at high levels for at least 12 months after the booster [37].
IXIARO® IN CHILDREN
In 2009, phase III studies to evaluate the immunogenicity and safety of administering the IC51 vaccine to children began. A study involving 60 healthy Indian children aged between 1 and 3 years evaluated the immunogenicity and safety of 3 and 6 micrograms of IXIARO® in comparison with the licensed vaccine JenceVac. Immunogenicity was assessed by measuring antibodies at the baseline and 28 days after the first and second administrations. On day 56, SCRs in the 3- and 6-microgram IXIARO® groups and the JenceVac group were 95.7%, 95.2% and 90.9%, respectively, and GMTs were 201, 218 and 230, respectively. Local and systemic tolerability was registered in a diary 7 days post-vaccination. No apparent difference was seen in the safety profiles of the two vaccines. These first immunogenicity and safety data in children supported the use of a 3-microgram dose in children under 3 years of age [38].
A very recent uncontrolled, open-label, phase III trial was carried out in order to assess the safety and immunogenicity of IXIARO® in a population of previously JEV-unexposed travelers to JE-endemic regions [39]. The vaccine was administered to 100 travelers aged ≥ 2 months to #x003C; 18 years. Solicited adverse events were observed for 7 days after each injection, and unsolicited adverse events for a total of 7 months. Furthermore, JE neutralizing antibodies were investigated in 64 subjects. Two different vaccine doses were studied: 0.25 and 0.50 ml IXIARO®. The most common solicited local adverse events were redness, induration and tenderness with 0.25 ml IXIARO®, and tenderness and pain with 0.5 ml IXIARO®. Common solicited systemic adverse events were diarrhea and loss of appetite with 0.25 ml IXIARO® and muscle pain and excessive fatigue with 0.5 ml IXIARO®. In total, up to day 56, adverse events were reported by 83.3% of subjects who had received the 0.25 ml dose and 76.1% of those vaccinated with the 0.5 ml dose. All subjects had developed protective levels of JE neutralizing antibodies by day 56, and 91.2% retained protective titers at month 7. IXIARO® was generally well tolerated in children, with an overall safety profile similar to that seen in adults. Moreover, it was highly immunogenic in both dose-groups.
Another recent study [40] monitored the safety profile of IXIARO® in a pediatric population of 1869 children aged between 2 months and 17 years, who were recruited and randomized in an age-stratified manner to receive IXIARO® or one of the control vaccines: Prevenar® and HAVRIX®. Adverse events, serious adverse events and medically attended adverse events were assessed up to day 56 and month 7 after the first dose. This study showed that incidences of adverse events, serious adverse events or medically attended adverse events did not differ significantly between the groups. Adverse events were most frequent in children #x003C; 1 year of age and decreased with age. Adverse events of special interest, such as hypersensitivity/allergy or neurological disorders up to day 56, were reported in 4.6% (IXIARO®) versus 6.3% (Prevenar®) in the ≥ 2 months to #x003C; 1 year age-group and in 3.4% (IXIARO®) versus 3.3% (HAVRIX®) in the ≥ 1 to #x003C; 18 years age-group. Fever, the most frequent systemic reaction, was observed in 23.7% of infants and 3.8% of adolescents, and decreased with age. The authors concluded that the safety profile of IXIARO® was comparable to that of the control vaccines.
Another study also evaluated the immunogenicity of IXIARO®. Age-stratified cohorts of children between 2 months and 17 years of age received 2 doses of IXIARO® administered 28 days apart [#x003C; 3 years, 0.25 mL (half adult dose); ≥ 3 years, 0.5 mL (full adult dose)]. The endpoints were seroconversion rate, 4-fold increase in JE neutralizing antibody titer and geometric mean titer assessed 56 days and 7 months after the first administration. A total of 496 subjects were enrolled and the immune response to JE virus at both time-points was also analyzed according to pre-vaccination JE virus and dengue virus serostatus. On day 56, seroconversion had been obtained in ≥ 99.2% of subjects, 4-fold increases in titer were reported in 77.4-100% in various age-groups, and geometric mean titers ranged from 176 to 687, with younger children displaying the greatest immune response. At month 7, seroconversion was maintained in 85.5-100% of subjects. Pre-existing JE virus immunity did not impact on the immune response at day 56; however, it yielded better persistence of protective antibody titers at month 7 [41]. The above findings revealed that IXIARO® was highly immunogenic in the pediatric population at both doses tested, eliciting protective antibody titers by day 56 in > 99% of subjects who received the age-appropriate dose.
In May 2013, the Food and Drug Administration (FDA) licensed IXIARO® for use in children aged 2 months through 16 years [42], and in the same year the vaccine was also indicated for children by the European Medicines Agency (EMA) [43].
IXIARO® IN THE ELDERLY
The safety and immunogenicity of IXIARO® were also evaluated in a clinical trial involving elderly subjects. An open-label, single-arm, multi-center study was conducted in 200 healthy subjects aged 64-83 years, including those with adequately controlled chronic conditions, who received two doses of IXIARO® 28 days apart. Antibody levels were tested 42 days after the second dose. Systemic and local adverse events were monitored for 7 days after each dose, and unsolicited adverse events were recorded up to day 70 and subsequently at month 7. Although 19% of subjects had serious or medically attended adverse events up to day 70 (primary endpoint), none of these was associated with the vaccine. Solicited local adverse events were reported by 33.5% of participants, the most common being local tenderness; solicited systemic adverse events were reported by 27% of participants, the most common being headache. A seroprotection rate of 65%, with a GMT of 37, was found. Subjects who had received a tick-borne encephalitis (TBE) vaccine in the previous 5 years had an SCR of 90% and GMT of 65. The authors concluded that IXIARO® was generally well tolerated in the elderly, and that its safety profile was largely comparable to that seen in younger adults. While SCR and GMT were lower than in younger adults, SCR was in the range reported in the elderly for other vaccines, such as TBE, hepatitis-A virus (HAV)/hepatitis-B virus (HBV), and influenza vaccines. Another aspect to be taken into account was the duration of protection, which is uncertain in the elderly. The authors therefore suggested that a booster dose (third dose) should be considered before any further exposure to JEV [44].
DOSAGE, ADMINISTRATION AND SCHEDULE OF IXIARO®
IXIARO® is injected into a muscle, preferably the shoulder muscle, or, in young children, into the thigh muscle. In adults, including those aged over 65 years, and children aged three years and older, a full dose of IXIARO® (0.5 ml) should be administered, and an additional 0.5 ml dose should be given four weeks later. In adults aged 18-65 years, a rapid vaccination course may also be implemented, in which the second dose is given seven days after the first. In children aged between two months and three years, half the adult dose of IXIARO® (0.25 ml) should be given, and an additional 0.25 ml dose should be given four weeks later. It is recommended that individuals who receive the first dose of IXIARO® should receive both doses, and that the second dose be given at least one week before potential exposure to the virus. In adults, the second dose can be given up to 11 months after the first. Adults aged 18-65 years who are likely to be exposed to the JEV again, or who are at continuous risk of the disease, should receive a booster dose of IXIARO® one to two years later and a second booster dose 10 years after the first booster. Children and adolescents may also receive a booster dose one to two years after the initial vaccination. A booster dose should also be considered for adults aged over 65 years before any further exposure to JEV. IXIARO® can be injected under the skin in people who have a bleeding disorder, such as low blood platelet counts or hemophilia [43].
A randomized, observer-blind, phase 3 study evaluated the immunogenicity of IXIARO® administered according to a rapid schedule [43]. Two cohorts were recruited: 217 subjects aged 18-65 years received IXIARO® together with rabies vaccine in a rapid immunization schedule (0-7 days); 56 subjects received IXIARO® according to the common immunization schedule (0-28 days). Seroconversion was similar in both cohorts and seroconversion rates and antibody titers remained high in both groups up to 12 months after immunization. With the rapid schedule, seroconversion rates on days 14 and 21 and after one year were 99%, 100% and 94%, respectively; at the same time-points, GMT values were 715, 1255 and 117, respectively. A third dose (first booster dose) was given within the second year (12-24 months) after the primary vaccination course. Long-term seroprotection data following a first booster dose suggested that a second booster should be given 10 years after the first, prior to potential exposure to JEV.
Subsequently, antibody persistence after booster immunization in adults was assessed in an uncontrolled, open-label extension, in which 67 subjects were followed up to determine JEV neutralizing antibody titers approximately 6 years after a booster dose. In 96% of subjects (64/67), protective antibody levels (PRNT50 ≥ 1:10) persisted, with a GMT of 148 (95% CI:107-207). Mathematical modeling was applied to evaluate the average duration of protection. On the basis of this model, it was estimated that the average duration of protection would be 14 years and that 75% of vaccinees would retain protective antibody levels (PRNT50 ≥ 1:10) for 10 years. A second booster should therefore be given 10 years after the first, prior to potential exposure to JEV [43]. Some authors have speculated that the protection conferred by the booster dose could last for a lifetime.
Conclusions
JE is the main cause of viral encephalitis in many Asian countries, and causes significant morbidity and mortality in autochthonous populations. Survivors often suffer permanent severe neurological sequelae that require lifelong support and care. The burden of the disease is heavy not only in terms of its clinical impact, but also on account of its socio-economic consequences in endemic areas. JE epidemics have also been documented, such as the outbreaks in India, which caused about 400 cases in 2014 and 1700 deaths in 2005 [45, 46].
As mentioned above, JE virus transmission is seasonal. However, climate changes and increasingly extreme weather conditions could lengthen the infective period of the JE virus. Moreover, the areas affected by JE have gradually expanded; indeed, growing numbers of JE cases have been observed further north in Asia, owing to the rise in global temperatures. For example, cases have spread to Nepal from Northern India, and the disease has now become widespread in hill and mountain districts [47]. JE cases have also been associated with increasingly frequent floods in China [48].
Travelers to endemic areas are exposed to the risk of acquiring a serious JE infection, and the rise in international travel to Southeast Asia has exacerbated this risk. Published recommendations for travelers underscore the importance of considering the travel destination, the high morbidity and mortality rates of JE, the lack of available treatment, travel-related factors (itinerary, duration, season and activity) and the benefits of JE vaccination [26, 33]. With regard to the risk of JE infection, the activity of the traveler is more significant than the length of the stay. Indeed, activities such as camping, trekking, biking, fishing and hunting increase the risk of exposure to the mosquitoes that transmit the JE virus. The fact that holiday-makers are now more inclined to engage in outdoor activities and to stay in unconventional tourist lodgings may have contributed to the recent increase in cases among travelers [49].
Travelers should be aware of the risk of contracting JE and how it can be prevented by vaccination, as this disease can have devastating effects. IXIARO® can be administered to children, adults and the elderly. It is safe, well tolerated and efficacious. The vaccination schedule involves 2 administering doses four weeks apart. For adults, a rapid schedule (0-7 days) is available, which could greatly enhance the feasibility of its use.
Healthcare workers should inform both short- and long-term travelers of the risk of JE in each period of the year and recommend vaccination. Indeed, it has been shown that short-term travelers are also at risk, not only in rural environments, but also in cities and coastal towns, especially in tourist localities where excursions to country areas are organized. In rural areas, the risk is linked to potential contact with poultry.
Acknowledgments
The University of Genoa received a grant by PaxVax Italy to conduct this overview. The authors declare no conflict of interest. The authors thank Dr. Bernard Patrick for revising the manuscript.
Footnotes
Author contributions
DA and DP conceived and designed the overview. FZ, PLL and MI performed a search of the literature on epidemiology of Japanese encephalitis. DA carried out a search of literature on the immunogenicity, efficacy and safety of the IC51 vaccine (IXIARO®). All authors contributed to the draft of the article. DA, PLL and DP revised critically the manuscript. All authors read and approved the final version of the manuscript.
References
- [1].Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol 2003;77:3091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hills S, Rabe I, Fischer M. Japanese Encephalitis. CDC Yellow Book, 2016. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/japanese-encephalitis. [Accessed on 10 October 2017].
- [3].WHO Report. Japanese encephalitis vaccines: WHO position paper, February 2015- Recommendations. Vaccines 2016;34:302-3. [DOI] [PubMed] [Google Scholar]
- [4].Schiøler KL, Samuel M, Wai KL. Vaccines for preventing Japanese encephalitis. Cochrane Database Syst Rev 2007;(3):CD004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Connor B, Bunn WB. The changing epidemiology of Japanese encephalitis and New data: the implications for New recommendations for Japanese encephalitis vaccine. Trop Dis Travel Med Vaccines 2017;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Longbottom J, Browne AJ, Pigott DM, Sinka ME, Golding N, Hay SI, Moyes CL, Shearer FM. Mapping the spatial distribution of the Japanese encephalitis vector, Culex tritaeniorhynchus Giles, 1901 (Diptera: Culicidae) within areas of Japanese encephalitis risk. Parasit Vectors 2017;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cheng V, Sridhar S, Wong S, Wong S. C. Y., Chan J. F. V, Yip C, Chau C, Au T.W.K, Hwang Y, Yau C, Lo J, Lee C, Yuen K. Japanese encephalitis virus transmitted via blood transfusion, Hong Kong, China. Emerging Infectious Diseases 2018;24(1). doi: 10.3201/eid2401.171297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011;89:766-74, 774A-774E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heffelfinger JD, Li X, Batmunkh N, Grabovac V, Diorditsa S, Liyanage JB, Pattamadilok S, Bahl S, Vannice KS, Hyde TB, Chu SY, Fox KK, Hills SL, Marfin AA. Japanese Encephalitis Surveillance and Immunization - Asia and Western Pacific Regions, 2016. MMWR Morb Mortal Wkly Rep 2017;66:579-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Watt G, Jongsakul K. Acute undifferentiated fever caused by infection with Japanese encephalitis virus. Am J Trop Med Hyg 2003;68:704-6. [PubMed] [Google Scholar]
- [11].Griffiths MJ, Turtle L, Solomon T. Japanese encephalitis virus infection. Handb Clin Neurol. 2014;123:561-76. [DOI] [PubMed] [Google Scholar]
- [12].Kumar R, Tripathi P, Singh S, Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis 2006;43:123-31. [DOI] [PubMed] [Google Scholar]
- [13].Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis 1985;151:1093-9. [DOI] [PubMed] [Google Scholar]
- [14].Halstead SB, Jacobson J. Japanese Encephalitis Vaccines. Plotkin SA, Orenstein WA, Offit P, (eds). Vaccines. 4th edn. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- [15].Halstead SB, Thomas SJ. Japanese encephalitis: new options for active immunization. Clin Infect Dis 2010;50:1155-64. [DOI] [PubMed] [Google Scholar]
- [16].Steffen R, Behrens RH, Hill DR, Greenaway C, Leder K. Vaccine-preventable travel health risks: what is the evidence - what are the gaps? J Travel Med 2015;22:1-12. [DOI] [PubMed] [Google Scholar]
- [17].Buhl MR, Lindquist L. Japanese encephalitis in travelers: review of cases and seasonal risk. J Travel Med 2009;16:217-9. [DOI] [PubMed] [Google Scholar]
- [18].Halstead SB, Jacobson J, Dubischar-Kastner K. Japanese encephalitis vaccines. Plotkin S, Orenstein W, Offit P. (eds). Vaccines. 6th edn. Amsterdam: Elsevier; 2012, pp. 201-205. [Google Scholar]
- [19].Lindquist L. Recent and historical trends in the epidemiology of Japanese encephalitis and its implication for risk assessment in travellers. J Travel Med 2018;25(suppl 1):S3-S9. [DOI] [PubMed] [Google Scholar]
- [20].Hills S, Griggs A, Fischer M. Japanese encephalitis in travelers from non-endemic countries, 1973-2008. Am J Tro Med Hyg 2010;82:930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kollaritsch H, Paulke-Korinek M, Dubischar-Kastner K. IC51 Japanese encephalitis vaccine. Expert Opin Biol Ther 2009;9:921-31. [DOI] [PubMed] [Google Scholar]
- [22].Huang GKL, Tio SY, Caly L, Nicholson S, Thevarajan I, Papadakis G, Catton M, Tong SYC, Druce J. Prolonged detection of japanese encephalitis virus in urine and whole blood in a returned short-term traveler. Open Forum Infectious Disease. 2017;4:ofx203. doi.org/10.1093/ofid [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].World Tourism Organization and Global Tourism Economy Research Centre (2016), UNWTO/GTERC Annual Report on Tourism Trends - 2016 Edition, Executive Summary, UNWTO, Madrid. [Google Scholar]
- [24].Pavli A, Maltezou HC. Travel-acquired Japanese encephalitis and vaccination considerations. J Infect Dev Ctries 2015;9:917-24. [DOI] [PubMed] [Google Scholar]
- [25].Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines 2011;10:355-64. [DOI] [PubMed] [Google Scholar]
- [26].Wilder-Smith A, Halstead SB. Japanese encephalitis: update on vaccines and vaccine recommendations. Curr Opin Infect Dis 2010; 23:426-431. [DOI] [PubMed] [Google Scholar]
- [27].Eckels KH, Yu YX, Dubois DR, Marchette NJ, Trent DW, Johnson AJ. Japanese encephalitis virus live-attenuated vaccine, Chinese strain SA14-14-2; adaptation to primary canine kidney cell cultures and preparation of a vaccine for human use. Vaccine 1988;6:513-8. [DOI] [PubMed] [Google Scholar]
- [28].Lyons A, Kanesa-thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, Burge R, Towle AC, Wilson P, Tauber E, Vaughn DW. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine 2007;25:3445-53. [DOI] [PubMed] [Google Scholar]
- [29].Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, Klade CS. Safety and immunogenicity of a Vero cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet 2007;370:1847-53. [DOI] [PubMed] [Google Scholar]
- [30].Tauber E, Kollaritsch H, von Sonnenburg F, Lademann M, Jilma B, Firbas C, Jelinek T, Beckett C, Knobloch J, McBride WJ, Schuller E, Kaltenböck A, Sun W, Lyons A. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis 2008;198:493-9. doi: 10.1086/590116. Erratum in: J Infect Dis. 2010;201:1278. [DOI] [PubMed] [Google Scholar]
- [31].Schuller E, Jilma B, Voicu V, Golor G, Kollaritsch H, Kaltenböck A, Klade C, Tauber E. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine 2008;26:4382-6. [DOI] [PubMed] [Google Scholar]
- [32].Schuller E, Klade CS, Wölfl G, Kaltenböck A, Dewasthaly S, Tauber E. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine 2009;27:2188-93. [DOI] [PubMed] [Google Scholar]
- [33].CDC. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010;59(No. RR-1). [PubMed] [Google Scholar]
- [34].Schuller E, Klingler A, Dubischar-Kastner K, Dewasthaly S, Müller Z. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO®. Vaccine 2011;29:8669-76. [DOI] [PubMed] [Google Scholar]
- [35].Kaltenböck A, Dubischar-Kastner K, Eder G, Jilg W, Klade C, Kollaritsch H, Paulke-Korinek M, von Sonnenburg F, Spruth M, Tauber E, Wiedermann U, Schuller E. Safety and immunogenicity of concomitant vaccination with the cell culture-based Japanese Encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX1440 in healthy subjects: a single-blind, randomized, controlled Phase 3 study. Vaccine 2009;27:4483-9. [DOI] [PubMed] [Google Scholar]
- [36].Dubischar-Kastner K, Eder S, Buerger V, Gartner-Woelfl G, Kaltenboeck A, Schuller E, Tauber E, Klade C. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO®, IC51. Vaccine 2010;28:5197-202. [DOI] [PubMed] [Google Scholar]
- [37].Eder S, Dubischar-Kastner K, Firbas C, Jelinek T, Jilma B, Kaltenboeck A, Knappik M, Kollaritsch H, Kundi M, Paulke-Korinek M, Schuller E, Klade CS. Long-term immunity following a booster dose of the inactivated Japanese Encephalitis vaccine IXIARO®, IC51. Vaccine 2011;29:2607-12. doi: 10.1016/j.vaccine.2011.01.058. [DOI] [PubMed] [Google Scholar]
- [38].Kaltenböck A, Dubischar-Kastner K, Schuller E, Datla M, Klade CS, Kishore TS. Immunogenicity and safety of IXIARO® (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine 2010;28:834-9. [DOI] [PubMed] [Google Scholar]
- [39].Jelinek T, Cromer MA, Cramer JP, Mills DJ, Lessans K, Gherardin AW, Barnett ED, Hagmann SHF, Askling HH, Kiermayr S, Kadlecek V, Eder-Lingelbach S, Taucher C, Dubischar KL. Safety and immunogenicity of an inactivated Vero cell-derived Japanese encephalitis vaccine (IXIARO®, JESPECT®) in a pediatric population in JE non-endemic countries: an uncontrolled, open-label phase 3 study. Travel Med Infect Dis 2018. pii: S1477-8939(18)30046-2. doi: 10.1016/j.tmaid.2018.03.003* [DOI] [PubMed] [Google Scholar]
- [40].Dubischar KL, Kadlecek V, Sablan B, Jr, Borja-Tabora CF, Gatchalian S, Eder-Lingelbach S, Mueller Z, Westritschnig K. Safety of the inactivated japanese encephalitis virus vaccine IXIARO® in children: an open-label, randomized, active-controlled, phase 3 study. Pediatr Infect Dis J 2017;36:889-97. [DOI] [PubMed] [Google Scholar]
- [41].Dubischar KL, Kadlecek V, Sablan JB, Borja-Tabora CF, Gatchalian S, Eder-Lingelbach S, Kiermayr S, Spruth M, Westritschnig K. Immunogenicity of the inactivated japanese encephalitis virus vaccine IXIARO® in children from a japanese encephalitis virus-endemic region. Pediatr Infect Dis J 2017;36:898-904. [DOI] [PubMed] [Google Scholar]
- [42].Centers for Disease Control and Prevention (CDC). Use of Japanese encephalitis vaccine in children: recommendations of the advisory committee on immunization practices, 2013. MMWR Morb Mortal Wkly Rep 2013;62(:898-900. [PMC free article] [PubMed] [Google Scholar]
- [43].European Medicine Agency (EMA). EMA/18321/2013 EMEA/H/C/963. Available at: http://www.eespof.gr/sites/default/files/EPAR_IXIARO_ENG.pdf [Accessed on 10 December 2017].
- [44].Cramer JP, Dubischar K, Eder S, Burchard GD, Jelinek T, Jilma B, Kollaritsch H, Reisinger E, Westritschnig K. Immunogenicity and safety of the inactivated Japanese encephalitis vaccine IXIARO® in elderly subjects: open-label, uncontrolled, multi-center, phase 4 study. Vaccine 2016;34:4579-85. [DOI] [PubMed] [Google Scholar]
- [45].Gurav YK, Bondre VP, Tandale BV, Damle RG, Mallick S, Ghosh US, Nag SS. A large outbreak of Japanese encephalitis predominantly among adults in northern region of West Bengal, India. J Med Virol 2016;88:2004-11. [DOI] [PubMed] [Google Scholar]
- [46].Lawrence J. Japanese encephalitis outbreak in India and Nepal. Euro Surveill 2005;10:E050922.4. [DOI] [PubMed] [Google Scholar]
- [47].Dhimal M, Ahrens B, Kuch U. Climate change and spatiotemporal distributions of vector-borne diseases in nepal - a systematic synthesis of literature. PLoS One 2015;10:e0129869 http://dx.doi.org/10.1371/journal.pone.0129869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang F, Liu Z, Zhang C, Jiang B. Short-term effects of floods on Japanese encephalitis in Nanchong, China, 2007-2012: a time-stratified case-crossover study. Sci Total Environ 2016;563-564:1105-10. doi: 10.1016/j.scitotenv.2016.05.162. [DOI] [PubMed] [Google Scholar]
- [49].Centers for Disease Control and Prevention (CDC). Japanese encephalitis in two children - United States, Morbidity Mortality Weekly Report. 2011; 60(9):276-8. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6009a3.htm [Accessed 10 November 2017]. [PubMed] [Google Scholar]


