Short abstract
Aims
The main objective was to investigate the effects of the transient receptor potential cation channel subfamily V member 1 (TRPV1) on nerve regeneration following sciatic transection injury by functional blockage of TRPV1 using AMG-517, a specific blocker of TRPV1.
Methods
AMG-517 was injected into the area surrounding ipsilateral lumbar dorsal root ganglia 30 min after unilateral sciatic nerve transection. The number of sciatic axons and the expression of growth-associated protein-43 (GAP-43) and glial fibrillary acidic protein was examined using semithin sections, Western blot, and immunofluorescence analyses.
Results
Blockage of TRPV1 with AMG-517 markedly promoted axonal regeneration, especially at two weeks after sciatic injury; the number of axons was similar to the uninjured control group. After sciatic nerve transection, expression of glial fibrillary acidic protein was decreased and GAP-43 was increased at the proximal stump. However, the expression of both glial fibrillary acidic protein and GAP-43 increased significantly in AMG-517-treated groups.
Conclusions
TRPV1 may be an important therapeutic target to promote peripheral nerve regeneration after injury.
Keywords: Peripheral nerve injury and regeneration, transient receptor potential cation channel subfamily member V1, calcitonin gene-related peptide, glial fibrillary acidic protein, growth-associated protein-43
Introduction
Nerve injury leads to physical and mental damage. Comparatively, peripheral nerves have the capacity to regenerate after injury. Based on that characteristic, it may be possible to cure various types of peripheral nerve damage or diseases. However, the regeneration of peripheral nerves is usually unsatisfactory and delayed. To promote/accelerate regeneration after peripheral nerve injury, many previous efforts have focused on improving surgical procedures, alleviating local inflammatory reactions, and supplementing nerve growth factors locally. Severe pain can develop after various peripheral nervous system injuries. Transient receptor potential cation channel subfamily V member 1 (TRPV1) is a well-known nociception-mediating ion channel that is abundantly expressed in peripheral sensory system, especially in the terminals and cell bodies of sensory dorsal root ganglion (DRG) neurons, trigeminal ganglion neurons, and vagal neurons.1–3 It is activated by several physical stimuli, including temperature, voltage, protons, pH, and chemical ligands such as resiniferatoxin and capsaicin.2–5 TRPV1 is regarded as a nociceptor, meaning that it plays an important role in peripheral neuropathic pain.2,6,7 In addition, TRPV1 was shown to be involved in neural plasticity, cancer prevention, regulation of obesity, cardiovascular protection, and gastrointestinal mucosa integrity maintenance.6–10 These different functions suggest that TRPV1 is involved in transmission of a variety of intracellular signals.
Our previous study showed that blockage of TRPV1 before sciatic crush injury prevented the overactivation of TRPV1 and accelerated nerve repair.11 However, whether the attenuation of TRPV1 after nerve injury can exert the same effects on nerve regeneration, which is a plausible clinical strategy, is not yet clear. In this study, TRPV1 was blocked by AMG-517 applied ipsilaterally 30 min after unilateral sciatic nerve transection injury. We then investigated the functional activity of TRPV1, axonal regeneration in the proximal stump of the injured sciatic nerve, expression of growth-associated protein-43 (GAP-43), and glial fibrillary acidic protein (GFAP), which is regarded as a marker of Schwann cells12,13, to determine whether attenuation of TRPV1 after nerve injury promotes nerve regeneration.
Materials and methods
Animal and the model of rat sciatic nerve transection injury
This is an exploratory research designed to assess the relationship between TRPV1 receptor function and nerve regeneration after sciatic injury.
Forty-two Sprague-Dawley rats, male, 220–240 g, were provided by the Experimental Animal Center of Shanxi Medical University, China (license No. SCXK (Jin) 2009–0001). This study was approved by the Shanxi Medical University Animal Ethics Committee in China. All experiments were performed in accordance with the use of Laboratory Animals. Animals were kept in a clean environment where temperature and humidity are appropriate.
Animals were divided into seven groups randomly(n = 6): (1) control group, (2) postoperative 1 week group, (3) postoperative 2 weeks group, (4) postoperative 1 week + AMG-517 150 μg/kg group, (5) postoperative 2 weeks + AMG-517 150 μg/kg group, (6) postoperative 1 week + AMG-517 300 μg/kg group, (7) postoperative 2 weeks + AMG-517 300 μg/kg group. The groups (2), (3) and (4) were divided into two time periods: one week and two weeks after injury.
Establishment of sciatic nerve transection injury was executed according to the literature1,14. Briefly, Animals were anesthetized with 1% sodium pentobarbital (4 mL/kg, intraperitoneally), the two hind limbs were stretched and fixed in position. One to two centimeter incision was made lateral to the left knee, locate, and expose the sciatic nerve below muscles. The sciatic nerve was transversely cut off using fine scissors just at 3 mm from the bifurcations of sural, fibular, and tibial nerve. The distal stump was stitched to the surrounding syndesm, far away from the proximal stump. After surgery, animals were housed in temperature (20°C–24°C) with 45%–50% of humidity and 12-h light cycles. Normal food and water were ad libitum.
Application of AMG-517 around L3-L5 DRGs
The stock solution of AMG-517 (5 mM/L, Selleckchem, USA) was prepared with appropriate amount of dimethyl sulfoxide and sterile saline and stored at −80°C until use. Two doses of AMG-517 (150 μg/kg or 300 μg/kg) were selected, and the required volume of stock solution was calculated for each animal depending on body weight.
Before the model was established, the required stock solution for each animal was diluted with sterile saline and brought the volume up to 90 μL.
After 30 min of the sciatic nerve section, a dorsal median incision was made between L2 and S1 vertebra. AMG-517 dilution with a total volume of 90 μL was injected into three sites surrounding lumbar DRGs using a microinjector, 30 μL for each site and 0.3 mm of needle insertion depth. The three locations were the interstitial tissue located between two spinal transverse processes of the left L3-L4, L4-L5, and L5-L6. The same volume of sterile saline was applied into the injury-only group at the same injection site.
Detection of the TRPV1 function by assessment of calcitonin gene-related peptide release from the dorsal horn of spinal cord
The release of calcitonin gene-related peptide (CGRP) from dorsal horn of spinal cord was regarded as one of the indicators of periphery TRPV1 activation.15 The quantification of lumbar dorsal spinal CGRP release was assayed by enzyme-linked immunosorbent assay (ELISA). The CGRP ELISA kit was provided by Heng Yuan Biological Technology (Shanghai, China). The operation was executed according to the manufacture’s manual. Summarily, lumbar spinal dorsal horns corresponding to L3, L4, and L5 DRGs were harvested after one week and two weeks of sciatic nerve transection. Tissues were grinded and suspended in cold phosphorylate buffer saline (0.01 mmol/L). Total protein concentration of each sample was measured using Bradford method. Thirty micrograms of protein for each sample were added into a 96-well plate. The absorbance values at 450 nm wavelength were measured, and CGRP concentration was calculated using SpectraMax® 190 Microplate Reader (Molecular Devices, USA).
Quantification of the number of sciatic axons and karyocytes
The quantification of the number of sciatic axons and karyocytes was done at semithin section with toluidine blue staining.16 Briefly, the proximal stumps (about 1 cm) of sciatic nerve were harvested at the time points described earlier, three rats for each group. The harvested nerve stems were quickly immersed into precold 2.5% glutaraldehyde and fixed for 4 h, and then the samples were postfixed in 1% osmium tetroxide for 2 h. After dehydration with acetone, the samples were embedded with epoxy resin (Araldite Epon-812) and cut into 1 μm thickness (semithin) of transverse sections by an Ultra-Microtome (LKB, Sweden), then the sections were stained with 1% of toluidine blue. Eight to nine sections were collected for each nerve, which were selected by the appearance of complete epineurium under microscope. Sections were checked under upright light microscopy (Olympus BX43, Japan). Five high-power images (100×, including the up, down, left, right, and center areas of the nerve) for each section were captured randomly. The number of axons was counted in each image and then the average number of axons in each group was calculated. In addition, the counts of axons and karyocytes on each section were finished by the way of double-blind trial.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis/Western blot analysis
Sciatic nerves and left L3-L5 DRGs were dissected and homogenized rapidly on ice at the time point of one week and two weeks after sciatic injury. The protein concentration of each sample was determined by Bradford assay (Sangon Biotech, China). Twenty micrograms homogenate of DRGs and 30 μg homogenate of sciatic nerves were uploaded onto 10% acrylamide gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis. The protein was then transferred onto polyvinylidene fluoride membranes. Afterward, the membranes were blocked with 5% no-fat milk solution and then incubated with mouse anti-TRPV1 (1:4000, Biosensis, USA), mouse anti-GFAP (1:2500, Novus Biologicals, USA), or rabbit anti-GAP-43 (1:5000, Bioworld, USA) overnight at 4°C. On the second day, the membranes were washed with 0.1% Tris-buffered saline and Tween 20, 3× 5 min and then incubated for 1 h in horseradish peroxidase (HRP)-conjugated goat antimouse IgG (1:5000, Sangon Biotech, China) or HRP-conjugated goat antirabbit IgG (1:5000, Sangon Biotech, China). Finally, the protein bands were detected after incubated in Easy See Western Blot ECL reagent (Sangon Biotech, China) and exposed onto X-ray film in dark room. Meanwhile, rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5000, Bioworld, USA) was used as a loading control.
Immunofluorescence of sciatic nerve
Sciatic nerves were dissected rapidly at the time point of one week and two weeks after sciatic nerve transection and then were freshly embedded with optimal cutting temperature compound and fixed in the liquid nitrogen. Samples were stored at −80°C till use. Nerves were longitudinally cut into sections of 12 μm thickness. The slices were kept onto the glass slides, which were precoated with 0.1% polylysine. The sections were dried in the air completely and immersed into 4% paraformaldehyde for 12 h of fixation. Sections were first washed three times with 0.01 M phosphate-buffered solution (PBS) and incubated in 0.1% Triton for 10 min. After 1 h of incubation with 10% normal serum, two primary antibodies (mouse anti-GFAP (1:1000, Novus Biologicals, USA) and rabbit anti-GAP-43 (1:500, Bioworld, USA)) were applied onto slices, incubated overnight at 4°C. On the following day, the secondary antibodies of Alexa Fluor® conjugated with 488 or 594 (1:500, Life Technology, USA) were loaded onto slices after substantially washing with 0.01 M PBS. Finally, the sections were mounted with an antifade-mount medium (Life Technology). Immunofluorescence images of GFAP and GAP-43 were observed under upright immunofluorescence microscope (Olympus BX43, Japan).
Statistical analyses
All of the data were expressed as mean ± standard deviation. The data were analyzed using Graph-pad prism 5.0 software, two-way analysis of variance. The value of p < 0.05 was regarded as statistically significant.
Results
AMG-517 blockage of TRPV1 decreases CGRP release from spinal dorsal horn
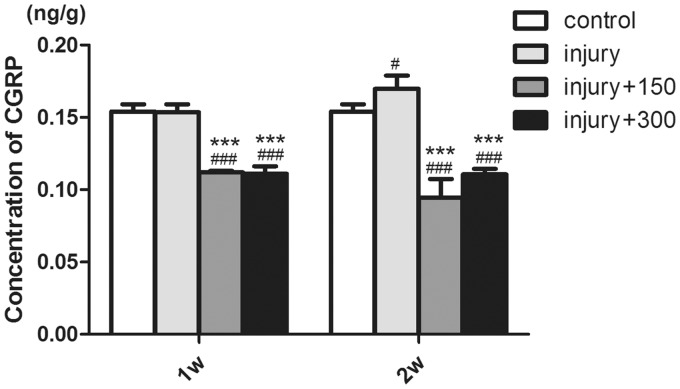
ELISA showed that there was no significant difference in lumbar dorsal CGRP release between one week after sciatic transection injury and the control group (Figure 1, the concentration of CGRP (CCGRP) in these two groups was approximately 0.15 ng/g, p > 0.05). However, the release of CGRP was increased two weeks after sciatic injury (Figure 1, CCGRP = 0.17 ng/g, p < 0.05), which indicated that TRPV1 was overactivated two weeks after peripheral nerve injury.
Figure 1.
Quantization for the release of CGRP from dorsal horn of lumbar spinal cord by ELISA method (#p < 0.05 vs. control group,. ###p < 0.001 vs. control group, ***p< 0.001 vs. injury-only groups).
The administration of AMG-517 to both one-week and two-week groups after injury induced a decrease in CGRP release (Figure 1, one week: CCGRP-150 μg/kg = 0.11 ng/g, CCGRP–300 μg/kg = 0.11 ng/g, two weeks: CCGRP-150 μg/kg = 0.095 ng/g, CCGRP-300 μg/kg = 0.11 ng/g, p < 0.001). This suggested that the injection of AMG-517 around DRGs was efficient to inhibit the function of TRPV1. Further, both doses of AMG-517 (150 μg and 300 μg/kg) were sufficient to block TRPV1, and there was no linear dose response for inhibition of CGRP release (Figure 1, p > 0.05).
Expression of TRPV1 in DRGs
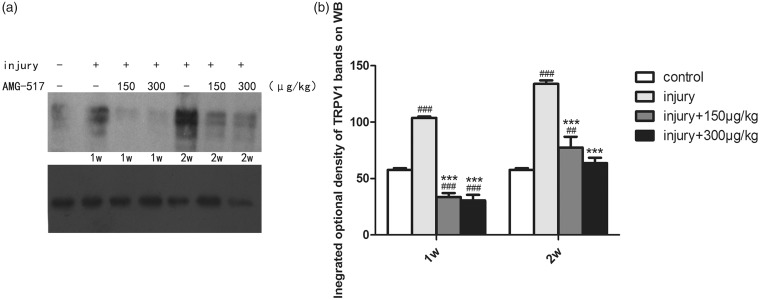
Western blot for TRPV1 expression in DRGs showed that the expression of TRPV1 increased significantly after sciatic injury, which was semiquantitatively determined to be almost twice of the expression in the control group (Figure 2, p < 0.001), with much highly increased expression at two weeks. However, a strong decrease in expression was seen in tissue samples treated with AMG-517 (Figure 2, p < 0.001). This result indicated that TRPV1 was upregulated both functionally and structurally after sciatic injury. The activation of TRPV1 after nerve injury may be regarded as overexpression, which was inhibited by injecting AMG-517 in the area surrounding DRGs.
Figure 2.
The expression of TRPV1 on DRG in each group. (a) Bands of Western blot for TRPV1. (b) Statistical graph for integrated optical density of TRPV1 in DRG (##p < 0.01 vs. control group, ###p < 0.001 vs. control group, ***p< 0.001 vs. injury-only groups).
Attenuation of TRPV1 function facilitated axonal regeneration
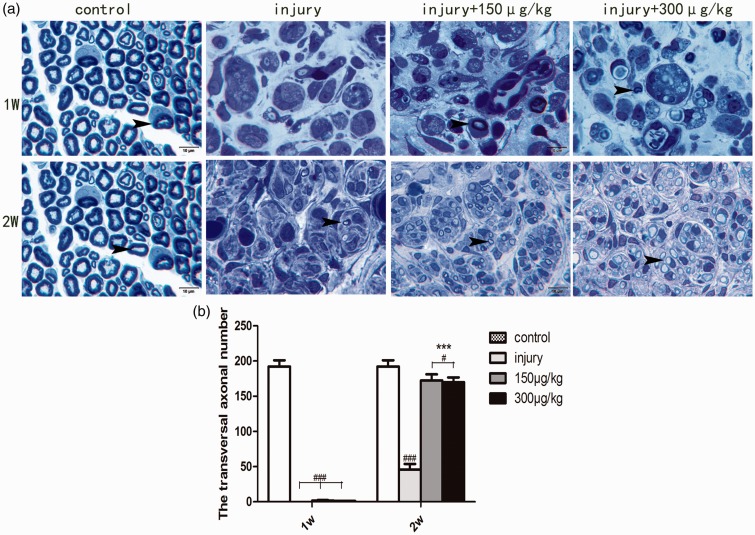
Semithin transverse sections (1-µm thickness) from the proximal stump of transected sciatic nerves were stained by toluidine blue. Sections from the different experimental groups exhibited some interesting morphological patterns, providing evidence that TRPV1 is involved in nerve regeneration. Normal sciatic nerves mainly consist of bundles of myelinated axons, with few cells that can be seen under the light microscope (Figure 3(a)). One week after sciatic nerve injury, the normal structures disappeared, a lot of debris was observed at the terminus of the proximal stump and few axons could be found (Figure 3(a)). At a longer time after injury (two weeks), some myelinated axons were found among many cells and tissue debris (Figure 3(a)). There was no obvious difference in the number of axons in the one-week injury-only group compared with the group treated with AMG-517. However, two weeks of AMG-517 treatment induced strong regeneration of axons in the proximal stump compared with the untreated control group (Figure 3, p < 0.001). Moreover, the sciatic nerve axons with two weeks of AMG-517 treatment were well organized and included both myelinated and nonmyelinated regenerating axons (Figure 3(a)).
Figure 3.
Semithin transverse sections of sciatic nerves and axon quantification analysis. (a) The transverse sections of sciatic nerve in each group. (b) Statistical graph for the axonal number in each group. #p<0.05 compared to the control group, ###p<0.001 compared to the control group, ***p< 0.001, compared to the injury-only groups.  indicating axons, Scale bar =10 μm.
indicating axons, Scale bar =10 μm.
Blockage of TRPV1 function modified the cellular response after sciatic nerve injury
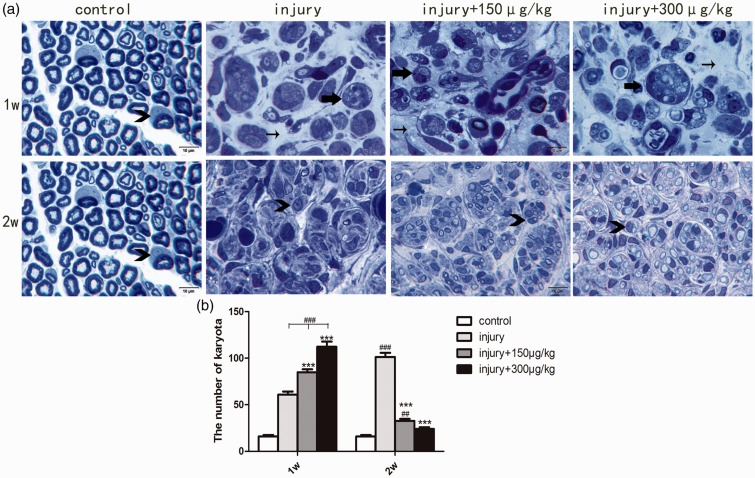
Interstitial cellular reactions were another feature of injured sciatic nerves. There were a few karyocytes in normal sciatic nerve transverse sections (Figure 4, the number of karyocytes (Nkaryocyte) was approximately 16). Quantitation of karyocytes in injured sciatic nerve transverse sections showed an obvious interstitial cellular response in each group at one week, which was higher in the AMG-517 intervention groups (Figure 4, injury-only group: Nkaryocyte = 61, AMG-517 150 µg/kg: Nkaryocyte = 85, AMG-517 300 µg/kg: Nkaryocyte= 112, p < 0.001). Based on the presence of intracellular engulfed components, most of the cells appeared to be phagocytes (Figure 4(a)). Two weeks after sciatic nerve injury, there was a decrease in the cellular response in nerves from the AMG-517 treatment groups (Figure (4), injury-only group: Nkaryocyte = 101, AMG-517 150 µg/kg: Nkaryocyte = 33, AMG-517 300 µg/kg: Nkaryocyte = 24). Further, tissue debris was scattered and the number of phagocytes decreased. Instead, Schwann cells enclosed regenerating axons in well-organized axon bundles, although myelination was not as good as in the noninjured controls (Figure 4). Overall, the sciatic nerve transverse structure two weeks after injury with AMG-517 treatment appeared more normal. The results suggested that AMG-517 accelerated nerve regeneration by promoting elimination of disintegrated axons and myelin, benefiting remyelination by Schwann cells and increasing axon regeneration.
Figure 4.
Semithin transverse sections of sciatic nerves and karyocyte quantification analysis. (a) Observation of the sciatic transverse sections in each group. (b) Statistical graph for the number of karyota in each group.  ##p<0.01 compared to the control group, ###p<0.001 compared to the control group, *** p< 0.001 compared to the injury-only groups.
##p<0.01 compared to the control group, ###p<0.001 compared to the control group, *** p< 0.001 compared to the injury-only groups.  the macrophages,
the macrophages,  the Schwann cells,
the Schwann cells,  the interstitial tissue, Scale bar = 10 μm.
the interstitial tissue, Scale bar = 10 μm.
Increased Schwann cell responses by blockage of TRPV1
The cellular response described earlier indicated that Schwann cells were modulated by the inhibitory effect of AMG-517 on TRPV1. Morphologically, the cells that were found around regenerating axons looked like Schwann cells. To verify the cell type, immunofluorescence analyses and Western blot for GFAP were performed. The results revealed that most of the cells two weeks after injury with AMG-517 treatment were Schwann cells. Western blot for GFAP showed that the expression of GFAP was low one week after injury (p < 0.001) but was almost recovered two weeks after injury (Figure 5(b)). GFAP was upregulated by TRPV1 blockage using AMG-517 (both one week and two weeks, Figure 5). Apart from this, the GFAP immunofluorescence formed a trimmed cord-like pattern, similar to the uninjured control (Figure 5(a)). There is no doubt that the proliferation and reorganization of Schwann cells facilitated the axonal regeneration.
Figure 5.
Immunofluorescence and Western blot for GFAP. (a) Immunofluorescence of longitudinal section of sciatic nerve in each group. The green fluorescence represents GFAP-positive fibers. (b) Bands of Western blot for GFAP. (c) The statistical graph for integrated optical density of Western blot bands (###p<0.01 compared to the control group, **p<0.01 compared to the injury-only groups, ***p < 0.001 compared to the injury-only groups. Scale bar = 50 μm). GFAP: glial fibrillary acidic protein.
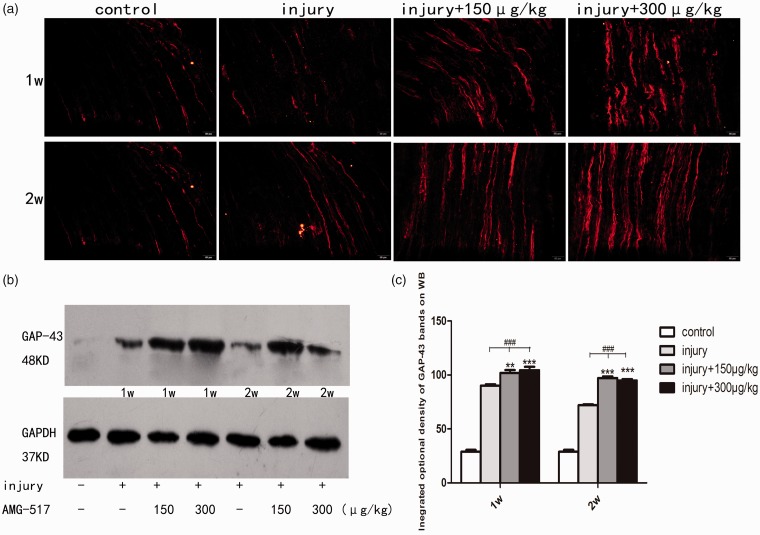
Blockage of TRPV1 upregulates the expression of GAP-43
To verify the role of TRPV1 blockage in nerve regeneration, immunofluorescence and Western blot of GAP-43 were also applied after sciatic transection injury with/without AMG-517 application. Semiquantitative analysis showed that the GAP-43 intensity at the proximal stump of the sciatic nerve was eventually increased after injury. However, the administration of AMG-517 made the increased intensity more marked (Figure 6, p < 0.001). GAP-43-positive fibers eventually became well-ordered with AMG-517 treatment, which coincided with the regenerated axons shown in Figure 3. The two dosages selected in this study showed no significant differences. These results indicate that inhibition of TRPV1 may activate regeneration-associated signaling pathways to overcome insufficient nerve repair resulting from microenvironmental alteration caused by the overactivation of TRPV1.
Figure 6.
Immunofluorescence and Western blot for GAP-43. (a) Immunofluorescence of longitudinal section of sciatic nerve in each group. The red fluorescence represents GAP-43-positive fibers. (b) Bands of Western blot for GAP-43. (c) The statistical graph for integrated optical density of Western blot bands (###p<0.01 compared to the control group, **p<0.01 compared to the injury-only groups, ***p < 0.001, compared to the injury-only groups; Scale bar = 50 μm).
Discussion
The peripheral nervous system is susceptible to a variety of injuries but has the ability to regenerate after injury. Successful regeneration or increased speed of injury repair would positively impact on patients’ quality of life. Prompt and well-established nerve regeneration following peripheral injury promotes nervous function recovery. Peripheral nerve regeneration involves the regrowth of axons and the proliferation and myelination of Schwann cells. Alterations in the local environment after injury can interrupt the regenerative procedure and delay repair. The local microenvironment changes include accumulation of tissue debris, an excessive inflammatory reaction, and activation of TRPV1.1,17 Therefore, a variety of factors can impact nerve regeneration after peripheral nerve injury. TRPV1, a receptor that mainly plays an important role in the transmission of pain signals, has long been regarded as one of the targets for pain therapeutics. The mechanism by which TRPV1 induces hyperalgesia or allodynia is by contributing to an increase in the release of pain neuropeptides (substance-P, CGRP) from the dorsal horn of the spinal cord. Apart from this, TRPV1 is involved in other intracellular signaling pathways through increasing calcium influx and interacting with other membranous receptors involved in neural plasticity, cancer prevention, cardiovascular protection,6–10 inflammation, regeneration, and infarcts.18 In our previous study,11 TRPV1 was first blocked by the subcutaneous injection of AMG-517 into the ipsilateral plantar 60 min prior to sciatic nerve crush. Two weeks after injury, the number of axons after TRPV1 blockage was increased compared with no TRPV1 inhibition. The results indicated that inhibition of TRPV1 prior to injury aided nerve rehabilitation following injury. However, clinically, therapeutics for nerve injury are generally administered after the event. Thus, more clinically relevant confirmation that the functional attenuation of TRPV1 facilitates nerve repair after injury was required. As we have already known, TRPV1 is also abundantly expressed in the neuronal membrane of DRGs as what its expression in peripheral nerve terminals. Thus, the blockage of TRPV1 in L3∼L5 DRGs by injection of AMG-517 surrounding these ganglia will affect both the intracellular signal pathways in both afferent and efferent processes from DRGs. The decrease in CGRP release can be regarded as the result of the intracellular signal pathway alteration by TRPV1 blockage to the afferent process of DGR. On the other hand, the difference in sciatic nerve regeneration between the groups of sciatic injury-only and injury + AMG-517 can be contributed to the peripheral effect of TRPV1 blockage in DRGs. In this study, the blockage of TRPV1 commenced 30 min after sciatic transection injury, which increased the number of sciatic axons and the expression of GAP-43 and GFAP over the same time periods after injury. This investigation provides further evidence that TRPV1 is actively involved in nerve regeneration after injury, beyond its role in nociception signaling pathways. The results also indicate that the severe pain induced by the overactivation of TRPV1 after peripheral nerve injury can delay nerve repair and rehabilitation. Therefore, specific strategies for blocking TRPV1 after nerve injury are suggested and should be examined clinically in the future.
AMG-517 is a potent and selective TRPV1 antagonist that has been extensively applied in many investigations for interruption of TRPV1 function.19,20 Based on dosages mentioned in the literature, two doses (150 µg/kg and 300 µg/kg) were selected in this study and injection of either dose around DRGs was very effective at inhibiting spinal CGRP release, showing that local AMG-517 injection around DRG can efficiently block overactivation of TRPV1. No dose dependence was found in the parameters explored; therefore, 150 µg/kg of AMG-517 is suggested as the better dosage for research. It is interesting that the application of AMG-517 in this study not only inhibited TRPV1 function but also reduced its expression in DRGs. But this decrease in TRPV1 expression needs to be verified in future studies.
Peripheral nerve regeneration after injury involves the regeneration of axons and the proliferation of Schwann cells. Moreover, the proliferation and rearrangement of Schwann cells are essential for the accomplishment of complete structural and functional nerve repair. This study showed that functional block of TRPV1 increased the elimination of degraded debris by infiltration of macrophages and pinocytosis, providing a good environment for nerve branching and Schwann cell proliferation.
With lasting TRPV1 blockage, the number of Schwann cells was increased and they appeared to be wrapped around newly regenerated axons. It is well known that Schwann cells can produce various neurotrophic factors.12,13 Generally, Wallerian degeneration and nerve structure collapses occur after nerve transection injury, leading to the loss of neurotrophic factors from Schwann cells. Any therapeutic that can promote Schwann cell recovery after Wallerian degeneration will therefore likely promote nerve repair after injury. From the results of this study, we speculated that prevention of TRPV1 overactivation will benefit not only pain relief but also promote nerve regeneration after peripheral injury.
Peripheral nerve injury leads to the upregulation of many growth factors and regeneration-associated proteins, including actin, tubulin, and GAP-43.21,22 GAP-43, a phosphoprotein in the axoplasm, is mainly expressing in the immature brain and in regenerating neurites as part of the neuronal growth cone.22–24 GAP-43 is regarded as a marker for regeneration of axons,22,25,26 and increased expression of axonal GAP-43 is important evidence of nerve regeneration. This study showed an increase in axonal expression of GAP-43 in the sciatic nerve after AMG-517 intervention. We propose that inhibition of the excessive activation/overexpression of TRPV1 after injury by AMG-517 injection around DRGs accelerates nerve regeneration by promoting both axonal GAP-43 expression and Schwann cell proliferation.
In summary, our study revealed a direct effect of TRPV1 on peripheral nerve regeneration after injury. Therapeutic studies on various peripheral nerve injuries should be aimed at the functional modulation of TRPV1 to relieve severe pain and promote faster nerve repair. However, the specific mechanisms by which TRPV1 promotes nerve regeneration need more detailed investigation to be fully elucidated.
Acknowledgments
The authors would like to thank the staff from Department of Pathophysiology, Shanxi Medical University in China.
Author Contributions
Xia-Qing Li was responsible for the study design. Juan Bai drafted manuscript, collected data, and performed analysis. Fu Liu, Li-fei Wu, and Ya-Fang Wang reviewed and inspected the manuscript. All authors read and approved the final manuscript.
Ethical Approval
Research involving animals were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. This study was approved by the Shanxi Medical University Animal Ethics Committee in China. In this study, all efforts were made to minimize the number of animals used and their suffering. All experiments were performed in accordance with the use of Laboratory Animals.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (Grant No. 81171178).
References
- 1.Hsu ST, Yao CH, Hsu YM, Lin JH, Chen YH, Chen YS. Effects of taxol on regeneration in a rat sciatic nerve transection model. Sci Rep 2017; 7: 42280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SI, Lim JY, Yoo S, Kim H, Hwang SW. Emerging role of spinal cord TRPV1 in pain exacerbation. Neural Plast 2016: 5954890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007; 76: 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat activated ion channel in the pain pathway. Nature 1997; 389: 816–824. [DOI] [PubMed] [Google Scholar]
- 5.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 1999; 19: 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo XJ, Peng J, Li YJ. Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 2011; 650: 1–7. [DOI] [PubMed] [Google Scholar]
- 7.Ramírez-Barrantes R, Cordova C, Poblete H, Muñoz P, Marchant I, Wianny F, Olivero P. Perspectives of TRPV1 function on the neurogenesis and neural plasticity. Neural Plast 2016; 2016: 1568145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Andaloussi-Lilja J, Lundqvist J, Forsby A. TRPV1 expression and activity during retinoic acid-induced neuronal differentiation. Neurochem Int 2009; 55: 768–774. [DOI] [PubMed] [Google Scholar]
- 9.Guo SY, Yang GP, Jiang DJ, Wang F, Song T, Tan XH, Sun ZQ. Protection of capsaicin against hypoxia-reoxygenation-induced apoptosis of rat hippocampal neurons. Can J Physiol Pharmacol 2008; 86: 785–792. [DOI] [PubMed] [Google Scholar]
- 10.Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 2009; 40: 89–97. [DOI] [PubMed] [Google Scholar]
- 11.Ren F, Zhang H, Qi C, Gao ML, Wang H, Li XQ. Blockade of transient receptor potential cation channel subfamily V member 1 promotes regeneration after sciatic nerve injury. Neural Regen Res 2015; 8: 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayers ST, Khan N, Ahmed Y, Shahid R, Khan T. Preparation of brain-derived neurotrophic factor- and neurotrophin-3-secreting Schwann cells by infection with a retroviral vector. J Mol Neurosci 1998; 10: 143–160. [DOI] [PubMed] [Google Scholar]
- 13.Carenini S, Montag D, Schachner M, Martini R. Subtle roles of neural cell adhesion molecule and myelin-associated glycoprotein during Schwann cell spiralling in P0-deficient mice. Glia 1999; 27: 203–212. [PubMed] [Google Scholar]
- 14.Liao CF, Yang TY, Chen YH, Yao CH, Way TD, Chen YS. Effects of swimming exercise on nerve regeneration in a rat sciatic nerve transection model. Biomedicine (Taipei) 2017; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, Wilson DM, Dillon WP. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med 2015; 7: 305ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y, MacEwan MR, Hunter DA, Farber S, Newton P, Tung TH, Mackinnon SE, Johnson PJ. Nerve regeneration in rat limb allografts: evaluation of acute rejection rescue. Plast Reconstr Surg 2013; 131: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol 2001; 24: 577–583. [DOI] [PubMed] [Google Scholar]
- 18.Hakimizadeh E, Shamsizadeh A, Roohbakhsh A, Arababadi MK, Hajizadeh MR, Shariati M, Fatemi I, Moghadam-Ahmadi A, Bazmandegan G, Rezazadeh H, Allahtavakoli M. TRPV1 receptor-mediated expression of Toll-like receptors 2 and 4 following permanent middle cerebral artery occlusion in rats. Iran J Basic Med Sci 2017; 20: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X. Trisubstituted pyrimidines as transient receptor potential vanilloid 1 (TRPV1) antagonists with improved solubility. Bioorg Med Chem Lett 2007; 17: 6539–6545. [DOI] [PubMed] [Google Scholar]
- 20.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev 2008; 60: 267–277. [DOI] [PubMed] [Google Scholar]
- 21.Sulaiman W, Gordon T. Neurobiology of peripheral injury, regeneration, and functional recovery: from bench top research to beside application. Ochsner J 2013; 13: 100–108. [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 1997; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 23.Caprini M, Gomis A, Cabedo H, Planells-Cases R, Belmonte C, Viana F, Ferrer-Montiel A. GAP-43 stimulates inositol trisphosphate-mediated calcium release in response to hypotonicity. EMBO J 2003; 22: 3004–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp 46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA 1986; 83: 3537–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein Induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science 1986; 233: 783–786. [DOI] [PubMed] [Google Scholar]
- 26.Gamby C, Waage MC, Allen RG, Baizer L. Analysis of the role of calmodulin binding and sequestration in neuromodulin (GAP-43) function. J Biol Chem 1996; 271: 26698–26705. [DOI] [PubMed] [Google Scholar]