Abstract
Chronic constipation affects millions of Americans, consumes significant healthcare resources, and significantly affects quality of life (QOL). Recently, several new treatment options have become available for the treatment of constipation, including intestinal secretagogues such as lubiprotone, and linaclotide, prokinetics such as prucalopride, and bile acid transporter antagonists. Plecanatide is the newest of the secretagogue class of compounds that has been approved by the US Food and Drug Administration for the treatment of adults with chronic idiopathic constipation (CIC) in the USA. It is a guanylate cyclase agonist, and a 16 amino acid synthetic peptide that is a structural analog of human uroguanylin. Two large randomized, double-blind, placebo-controlled studies assessed the efficacy and safety of plecanatide in CIC patients (Rome III). Both doses of plecanatide, 3 mg and 6 mg resulted in a significantly greater percentage of patients who were durable overall complete spontaneous bowel movement (CSBM) responders (primary endpoint) compared with those who received placebo (plecanatide 3 mg, 21.0%; plecanatide 6 mg, 19.5%; placebo, 10.2%; p < 0.001 for each drug dose versus placebo). Plecanatide treatment also significantly reduced the severity of other CIC symptoms (straining effort, stool consistency, bloating). Also, plecanatide-treated patients reported high levels of satisfaction and improved QOL and desire to continue treatment. The rate of treatment-emergent adverse events with plecanatide was low, including rates of diarrhea (5%). Plecanatide is a luminally acting secretagogue that is efficacious and safe for the treatment of CIC. This article provides an overview of plecanatide in the management of adults with CIC.
Keywords: constipation, plecanatide, treatment
Introduction
Chronic constipation is one of the most common gastrointestinal (GI) disorders, with symptoms characterized by infrequency of stools or difficulty of stool passage, or both.1,2 For many years, the definition of constipation had been restricted to infrequent bowel movements (BMs). However, this has been broadened to include other symptoms such as straining, lumpy or hard stools, the sensation of incomplete evacuation, and the need for manual removal of stool. Additionally, many patients report abdominal bloating and discomfort.
In the USA, the prevalence of chronic constipation is reported to range from 2% to 27%, with an overall average prevalence of 14.8% in the general population and 14% globally.2–6 The prevalence was reported to be lower in studies in South East Asia and those using the Rome II or III criteria.2,6 While constipation appears to increase with age,2,6 a USA survey from 2015 found that 62% of respondents were <50 years of age.5 The prevalence among women is reported as more than twice that in men.4 However, an accurate measurement of prevalence is difficult because of a variety of definitions and questionnaires used in research, as well as the subjectivity in patient reporting and the effect of cultural customs.2 It adversely affects quality of life (QOL), and consumes significant healthcare expenses.2–4 Chronic constipation is associated with 8 million visits per year, $2840.00 in medical expenses per patient and annual expenses of $11,999.00, with 45% related to outpatient services.2,7
Chronic constipation is due to secondary causes such as drugs or colonic obstruction, or primarily due to colorectal dysfunction.2 Primary constipation includes chronic idiopathic constipation (CIC), slow transit constipation, irritable bowel syndrome with constipation (IBS-C) and dyssynergic defecation.2,6,8–10
The evaluation and diagnosis of chronic constipation usually comprises clinical history and physical examination, including an abdominal and a digital rectal evaluation.2 A colonoscopy is recommended at certain ages [patients ⩾50 years of age (⩾45 years of age in African Americans)] and if alarm symptoms are present (e.g. unintentional weight loss, hematochezia, anemia). Specific tests to evaluate pathophysiology of slow transit constipation include colonic transit time (radiopaque markers or wireless motility capsule test or whole gut scintigraphy),2 for dyssynergic defecation, tests include anorectal manometry and balloon expulsion test or defecography, and for or irritable bowel syndrome with constipation, rectal sensory testing with rectal balloon distension or barostat.2,8–11
Current treatment options
Diets of high fiber, fiber supplements and a lifestyle which includes exercise are also associated with the reduced risk of constipation.2,12,13 Current guidelines recommend initial treatment with over-the-counter laxatives for episodes of constipation.14,15 The American Gastroenterological Association guidelines recommended increasing fiber intake both as foods and supplements, and using cost-effective osmotic agents such as magnesium supplements or polyethylene glycol which offer patients a viable option after fiber diets, considering the safety, efficacy and relative cost effectiveness.14,15 Mixed soluble fiber supplements such as Suprafiber® (Sunsweet Growers Inc., Yuba, CA.) and dried plums have been shown in two separate randomized controlled trials to be efficacious and safe and as useful or better than psyllium.16,17 These agents create an intraluminal osmotic gradient that encourages net water and electrolyte secretion. Stool viscosity is reduced, and fecal mass is increased with improved effects on peristalsis and constipation. Other agents such as bisacodyl or glycerol suppositories have also been shown to be helpful in relieving stool from the distal rectum. However, patients with chronic constipation frequently report dissatisfaction with traditional treatment options of fiber, supplemental bulking agents, exercise, bowel habit training, and over-the-counter laxatives.11
Patients with dyssynergic defecation are recommended undergoing biofeedback therapy.2,17,18 This treatment has been reported to improve symptoms in greater than 70% of patients with defecatory disorders. Relaxation training is noted to be successful in teaching patients to relax the pelvic floor muscles in coordination with pushing during straining to achieve defecation. The motivation and interest of the therapist and patient may contribute to the success of these types of retraining programs.18
Currently, three prescription medications are approved in the United States for the treatment of adults with CIC. These include lubiprostone (Amitiza®, Takeda Pharmaceutical Company, Osaka, Japan),17–21 linaclotide (Linzess®, Ironwood Pharmaceuticals, Inc., Cambridge, MA, USA), and plecanatide (Trulance®, Synergy Pharmaceuticals, Inc., New York, NY, USA). Lubiprostone is a chloride channel type 2 activator and prostaglandin analog.21–24 It is approved for the treatment of CIC at a dose of 24 mcg twice daily (b.i.d.) and for the treatment of IBS-C at a dose of 8 mcg b.i.d. with food.21,23 Linaclotide is a guanylate cyclase-C (GC-C) agonist and a 14 amino acid peptide containing three disulfide bonds; it acts in a pH-independent manner to stimulate fluid secretion into the intestinal lumen19,20 and is approved for the treatment of both IBS-C and CIC.19,23–24
Although there are many available and recommended treatments, patients with CIC have not been fully satisfied, and no single or combined treatment has been demonstrated to be effective in all patients.7,17,23,24 Therefore, there remains a need for new treatment options and additional therapies which are effective and well tolerated.23–28 The focus of this review article is to provide an in-depth discussion of plecanatide and its use in the treatment of chronic constipation.
Plecanatide
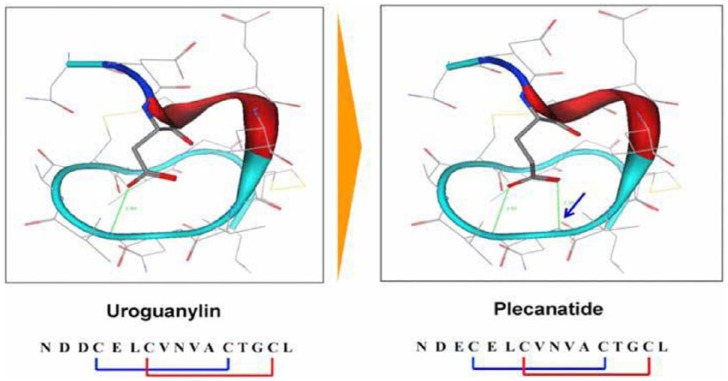
Plecanatide is a 16 amino acid peptide that has been shown in previous studies in healthy volunteers and in patients with CIC to be safe and effective in relieving symptoms of CIC.26,27,29,30 This targeted therapy is second in the GC-C agonist class of drugs for treatment of CIC.3,4 In contrast with linaclotide, plecanatide is a peptide analog of uroguanylin with two disulfide bonds31–33 (Figure 1). Plecanatide is thought to replicate the activity of human uroguanylin in binding and activating GC-C receptors in the epithelial lining of the GI mucosa in a pH-sensitive manner.29,32,33
Figure 1.
Structural configurations showing the similarities between the intrinsic uroguanylin and the synthetic plecanatide.
The arrow in the plecanatide configuration shows the two disulfide bonds.
The structure of plecanatide is nearly identical to that of uroguanylin, differing from uroguanylin only in the replacement of aspartic acid with glutamic acid at the third position near the N-terminus (Figure 1).29 Thus, plecanatide displays the same pH sensitivity in binding to the GC-C receptors as with uroguanylin.29–33 However, plecanatide has demonstrated eight times the binding potency of uroguanylin in preclinical models.30 Therefore, orally received plecanatide is anticipated to act in the same manner in binding and activating GC-C receptors within the GI tract, leading to activation of the cystic fibrosis transmembrane conductance regulator (CFTR), producing the secretion of fluid in the intestinal lumen and enabling BMs (Figure 2).26,29–33 Additionally, following oral administration, plecanatide produces biologic activity only in the intestinal tract and is not systemically absorbed. Concentrations of plecanatide and its active metabolite in plasma are below the limit of quantitation after an oral dose of 3 mg.3,4,26,31
Figure 2.
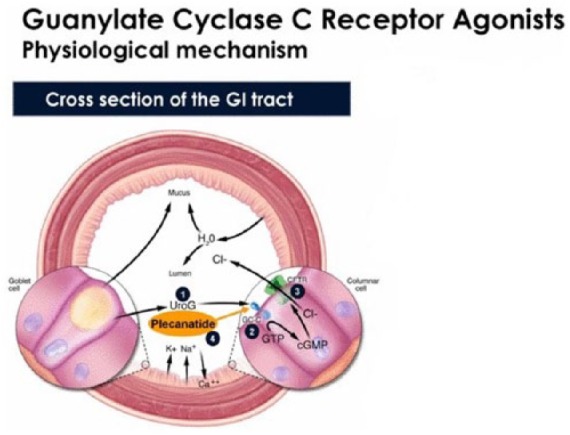
Schematic diagram illustrating the physiological mechanism of action of uroguanylin and its analog plecanatide both activating the guanylate cyclase-C receptors located on the apical cells of the brush border.
(1) Endogenously produced uroguanylin; (2) GC-C receptor activation stimulates the intracellular synthesis of cGMP; (3) cGMP activates the cystic fibrosis transmembrane conductance regulator, resulting in the secretion of chloride and bicarbonate along with fluid into the intestinal lumen; (4) plecanatide is an analog of uroguanylin that binds to GC-C receptors and induces fluid secretion.
Ca++, calcium ion; CFTR, cystic fibrosis transmembrane conductance regulator; Cl−, chloride ion; GC-C, guanylate cyclase-C; GI, gastrointestinal; GMP, guanosine monophosphate; cGMP, cyclic GMP; GTP, guanosine triphosphate; H2O, water; HCO3, bicarbonate; K+, potassium ion; Na+, sodium ion; UroG, uroguanylin.
Mechanism of action
The activation of the GC-C receptor by uroguanylin, a pH-sensitive endogenous ligand, stimulates cyclic guanosine monophosphate (cGMP) production intracellularly that in turn results in increased CFTR activity (Figure 2).32 This apically located ion channel secretes chloride and bicarbonate into the intestinal lumen. Increased cGMP also decreases the activity of the sodium hydrogen exchanger, resulting in decreased sodium absorption. The outcome is an ionic gradient that promotes fluid secretion that hydrates the stool, and produces a sufficient volume of water to facilitate BMs.33–35 GC-C activation may decrease other CIC-related symptoms by reducing visceral hypersensitivity as shown with linaclotide,28 and to relieve abdominal pain, and to facilitate BMs by enabling stool transit through the intestine. (Figure 2).28,34–37
Clinical phase III data
Two identically designed phase III, randomized, double-blind, placebo-controlled studies [ClinicalTrials.gov identifiers: NCT01982240 and NCT02122471] were conducted in the USA and Canada, each of 12 weeks in duration.3,4 In the first study, patients with CIC (n = 1394) were randomized at one of 164 clinical centers (153 in the USA and 11 in Canada) between 03 December 2013 and 23 April 2015. In the second study, patients with CIC (n = 1410) were randomized at 162 clinical centers in the USA from 16 May 2014 until 28 January 2015. After signing informed consent, patients entered the screening period, the last 2 weeks of which consisted of a pretreatment assessment to confirm eligibility and to establish each patient’s baseline for efficacy measurements. Patients were instructed to use an electronic diary to maintain a record of daily BMs (number, time, consistency, completeness of evacuation, and rescue medication use) and daily symptoms.3,4
Patients who were considered eligible at the end of the 2-week pretreatment assessment were randomized to the 12-week treatment period in a 1:1:1 ratio (stratified by gender) to one of the following three treatment groups: plecanatide 3 mg, plecanatide 6 mg, or placebo. At weeks 4, 8 and 12 of the treatment period and 2 weeks following the last dose of study medication (week 14), patients returned to the clinic to undergo efficacy and safety assessments. Patients continued to maintain daily diaries throughout the study including post-treatment periods.
Patient population
Patients eligible for inclusion were men and women (who were not pregnant or lactating) with CIC, aged 18–80 years with a body mass index (BMI) of 18–40 kg/m2 and were willing to participate in the 2-week pretreatment assessment, 12 weeks of treatment, and a 2-week post-treatment period. Patients were required to meet the Rome III functional constipation criteria (modified for this study) for at least 3 months before the screening visit and had to demonstrate symptom onset for at least 6 months before the diagnosis. This included a history of fewer than three BMs per week, no use of manual maneuvers (such as digital evacuations or support of pelvic floor) to facilitate defecations, and at least two of the following: straining during at least 25% of defecations, lumpy or hard stool for at least 25% of defecations, a sensation of incomplete evacuation for at least 25% of defecations, and a sensation of anorectal blockage/obstruction for at least 25% of defecations.3,4
Patients were excluded if they met the Rome III criteria for irritable bowel syndrome or if they reported loose stool more than rarely without the use of laxatives. Other key exclusion criteria consisted of diseases or conditions associated with constipation, diseases or conditions that could affect GI motility or defecation, medical history of cancer, or other uncontrolled medical conditions. Patients were to maintain a stable diet for at least 30 days prior to screening, use contraception, and were not to have participated in a previous plecanatide clinical trial. Patients were allowed to continue the use of fiber if they were on a high-fiber diet or supplements for the 30 days prior to screening. They could enroll in the study provided they agreed to remain on that diet or supplementation for the duration of the study.
Treatments
All treatments were given orally, once daily from day 1 through 12 weeks of the treatment period. Patients were directed to take study medication with or without food. No interruptions in daily therapy were allowed. Patients were provided bisacodyl 5 mg tablets as rescue medication and were instructed to take one or two tablets only if they had not had a BM for 3 or more days. During the pretreatment assessment period, they were not to exceed 2 days of rescue medication use in each week.3,4
Assessments and endpoints
Patients were required to report all BMs in the BM diary in real time or on a daily basis, indicating the time of the BM and without the ability to report data from the previous day. The primary US Food and Drug Administration recommended efficacy endpoint was the percentage of patients who were durable overall complete spontaneous bowel movement (CSBM) responders during the 12-week treatment period. A CSBM weekly responder was defined as a patient who had ⩾3 CSBMs for a given week and an increase from baseline of ⩾1 CSBM in that same week. An overall CSBM responder was a patient who was a weekly CSBM responder for at least 9 of the 12 treatment weeks, and a durable overall CSBM responder was also a weekly responder in at least 3 of the last 4 weeks. A spontaneous bowel movement (SBM) was defined as a BM in the absence of laxative use within 24 h of the BM. A CSBM was defined as an SBM with the sense of complete evacuation.3,4
Secondary and additional endpoints reported from the BM diary included frequency of CSBMs and SBMs within 24 h after the first dose of study medication and stool consistency from the Bristol Stool Form Scale (BSFS) score for each BM. The daily symptom diary was completed for additional endpoints. This electronic diary was completed each day in the evening and captured straining, abdominal bloating, and abdominal discomfort on a Likert scale of 0–4 (0 = none, 4 = very severe). The Patient Assessment of Constipation: Symptoms (PAC-SYM), Patient Assessment of Constipation: Quality of Life (PAC-QOL), and Patient Global Assessment questionnaires were also completed at weeks 4, 8, 12 (end of treatment), and 14 (end of study). Safety evaluations included physical examinations, electrocardiograph recordings, vital sign measurements, and standard laboratory tests. Adverse events (AEs) were captured, assessed for severity, and classified for relatedness to study medication. All AEs that were spontaneously reported or were reported in response to an open-end question were recorded verbatim.
Results
Efficacy data [ClinicalTrials.gov identifier: NCT01982240]
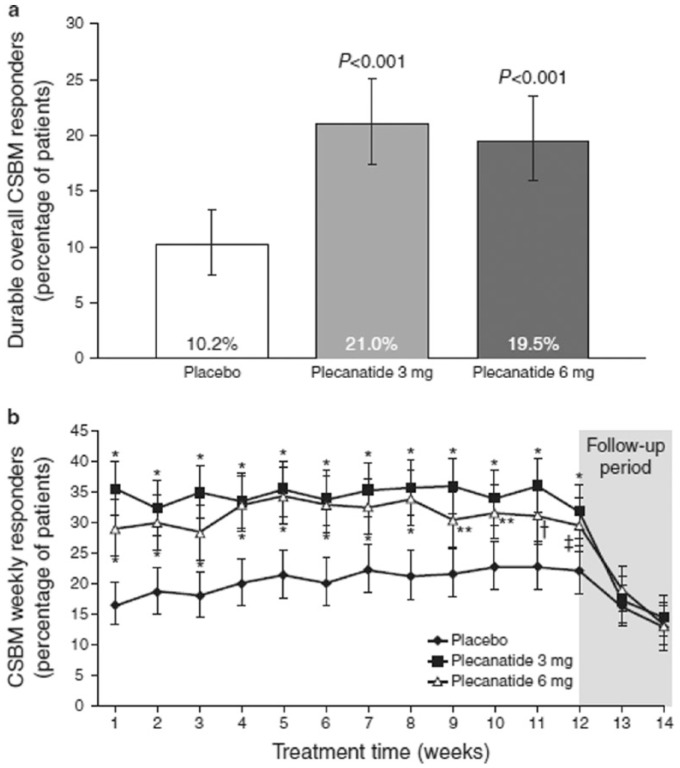
Of the 2864 patients screened, 1346 were included in the intention-to-treat (ITT) population (plecanatide 3 mg, n = 453; plecanatide 6 mg, n = 441; placebo, n = 452). The safety population included 1389 patients who received at least one dose of study drug. The primary efficacy measure was achieved with both plecanatide doses (Figure 3). Both plecanatide 3 mg and 6 mg resulted in a significantly greater percentage of patients who were durable overall CSBM responders compared with those in the placebo group (plecanatide 3 mg, 21.0%; plecanatide 6 mg, 19.5%; placebo, 10.2%; p < 0.001 for each drug dose versus placebo).3,4
Figure 3.
Effects of plecanatide and placebo on the percentage of durable overall complete spontaneous bowel movement (CSBM) responders and weekly CSBM responders after 12 weeks of drug treatment, and a 2-week drug-free follow-up period.
Effects of plecanatide and placebo on the percentage of durable overall CSBM responders (a) and weekly CSBM responders (b) after 12 weeks of drug treatment, and a 2-week drug-free follow-up period. [Clinical Trials.gov identifier: NCT01982249 SHOULD READ NCT01982240]
All error bars represent 95% confidence intervals; values in 3(b) are least squares mean; *p = 0.001, **p = 0.003, $p = 0.005, ‡p = 0.011 versus placebo.
The percentage of weekly CSBM responders in both plecanatide groups was greater than with placebo within the first week of treatment (plecanatide 3 mg, 35.8%; plecanatide 6 mg, 29.3%; placebo, 16.6%; p < 0.001 for each drug dose versus placebo), and this difference persisted for the duration of the 12-week treatment period (Figure 3). During the follow-up period after the completion of plecanatide, the proportions of CSBM weekly responders in both plecanatide dose groups decreased and were comparable with placebo.3,4
Several patient assessment tools demonstrated that both doses of plecanatide significantly improved constipation severity, stool consistency, straining, abdominal bloating, and health-related QOL in patients with CIC. Furthermore, patient satisfaction with treatment and desire to continue treatment were significantly greater with plecanatide than with placebo.
There were no unexpected safety signals and no deaths reported in this study. No plecanatide dose dependency was observed for any AE. Laboratory results, vital signs, and physical examination findings were all unremarkable, with a low incidence of any clinically important changes.
Efficacy data [ClinicalTrials.gov identifier: NCT02122471]
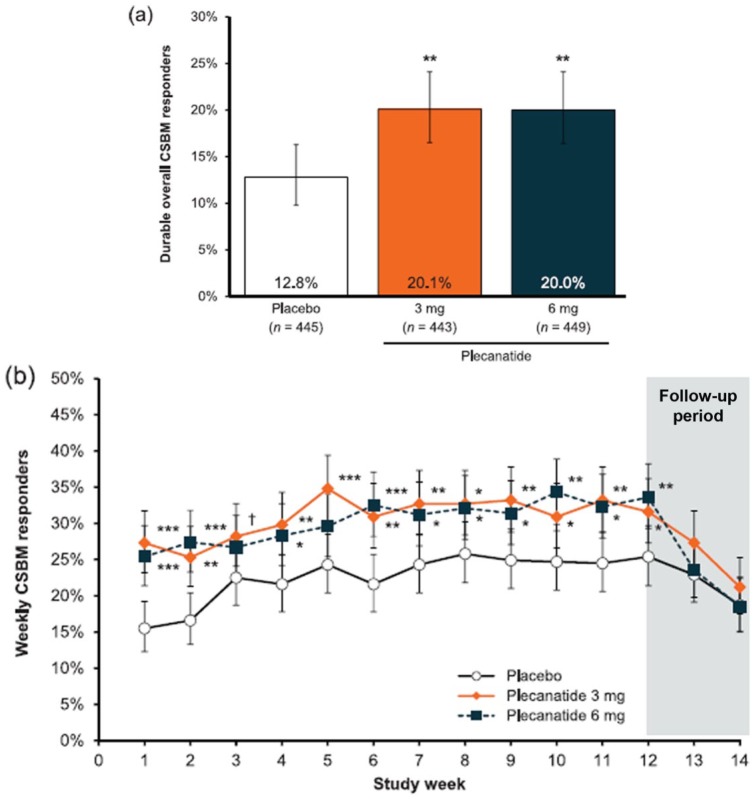
A total of 2941 patients were screened, of which 1410 patients were randomized to receive study treatment. Of these, 1402 patients received study drug and comprised the safety population (plecanatide 3 mg, n = 467; plecanatide 6 mg, n = 469; placebo, n = 466). The ITT population consisted of 1337 unique patients (plecanatide 3 mg, n = 443; plecanatide 6 mg, n = 449; placebo, n = 445). The percentage of patients who were durable overall CSBM responders after 12 weeks of treatment was statistically significantly greater for plecanatide 3 mg and 6 mg compared with placebo [3 mg = 20.1%; 6 mg = 20.0%; placebo = 12.8%; p = 0.004 for both comparisons, Figure 4(a)]. The percentage of weekly CSBM responders in each plecanatide group was statistically greater than in the placebo group as early as week 1 (p < 0.001), and this remained consistent through the 12-week treatment period [Figure 4(b)].
Figure 4.
Effects of plecanatide and placebo on the percentage of durable overall complete spontaneous bowel movement (CSBM) responders and weekly CSBM responders after 12 weeks drug treatment and a 2-week drug-free follow-up period.
(a) Effects of plecanatide (3 mg and 6 mg) and placebo on the overall rate of durable CSBM responders; (b) weekly CSBM responders. [ClinicalTrials.gov identifier: NCT02122471.] ***p < 0.001, **p < 0.01, *p < 0.05 versus placebo.
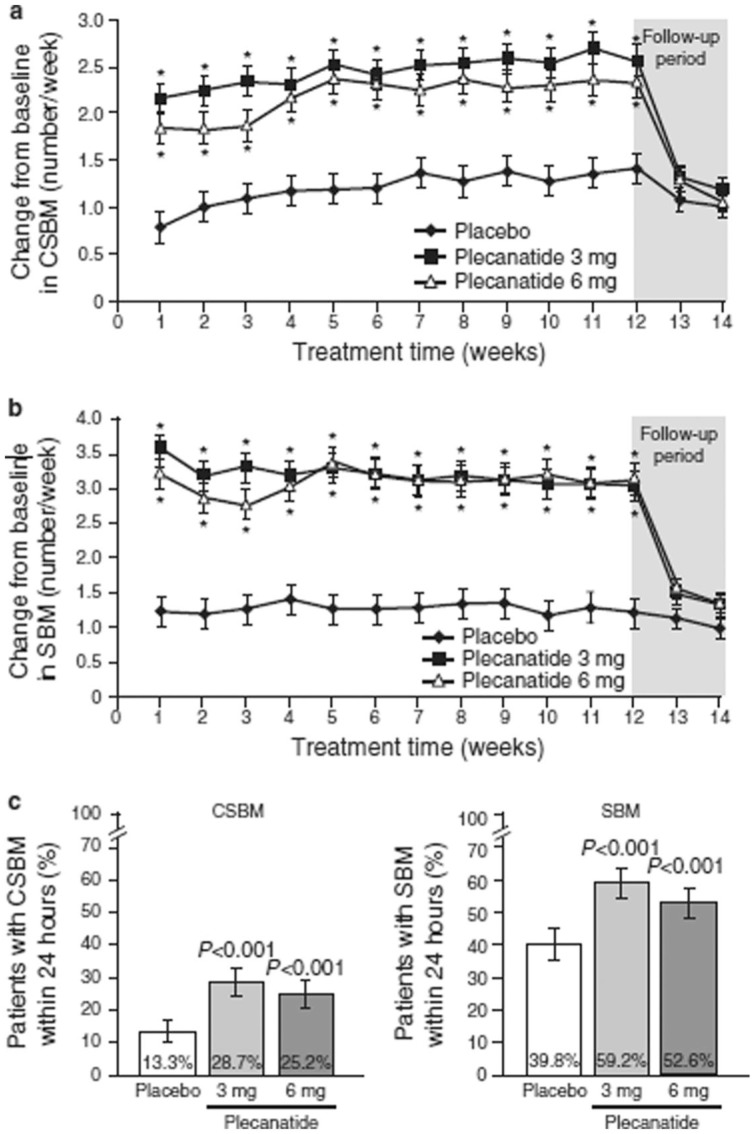
Both plecanatide doses significantly increased the weekly CSBM and SBM frequencies from baseline and increased the percentage of patients experiencing CSBMs and SBMs within 24 h compared with placebo [Figure 5(a–c)]. Significant increases in frequency from baseline were observed within the first week of treatment and continued throughout the duration of treatment. Over the 12-week treatment period, there were clinically and statistically significant least squares (LS) mean changes from baseline in weekly CSBM frequencies with 3 mg and 6 mg doses of plecanatide (2.5 and 2.2/week, respectively) as compared with placebo (1.2/week; p < 0.001 for each dose). By week 14, 2 weeks following the cessation of treatment, the values for plecanatide treatment returned toward those of placebo and were not lower than baseline levels.
Figure 5.
Effects of plecanatide and placebo on the change in complete spontaneous bowel movements (CSBMs) and spontaneous bowel movements (SBMs) per week and percentage of patients with CSBM and SBM within 24 h.
(a) Effects of plecanatide and placebo on the change in CSBM/week; and (b) SBM/week; and (c) the % patients with CSBM and SBM within 24 h. [ClinicalTrials.gov identifier: NCT01982240.]
Values are least squares mean; bars represent standard error of the mean; *p < 0.001 versus placebo.
Statistically significant changes in SBMs per week were also observed with both doses of plecanatide. The LS mean increase in weekly SBM frequency over the 12-week treatment period was 3.2 and 3.1/week for plecanatide 3 mg and 6 mg, respectively, and 1.3/week for placebo (p < 0.001 for each dose compared with placebo). The onset of plecanatide activity was rapid and occurred within the first week of treatment.
Plecanatide significantly improved stool consistency and symptom-related secondary endpoints over the 12-week treatment period compared with placebo. Stool consistency improved from baseline with both plecanatide doses by 1.5 points on the BSFS scale over the 12-week treatment period as compared with 0.8 points for placebo (p < 0.001 for each dose compared with placebo). These improvements resulted in BSFS stool scores of 4.1 (mean) over 12 weeks for both plecanatide doses.
Several patient assessment tools demonstrated that both doses of plecanatide significantly improved constipation severity, stool consistency, straining, abdominal bloating, and health-related QOL in patients with CIC. Furthermore, patient satisfaction with treatment and desire to continue treatment were significantly greater with plecanatide than with placebo.
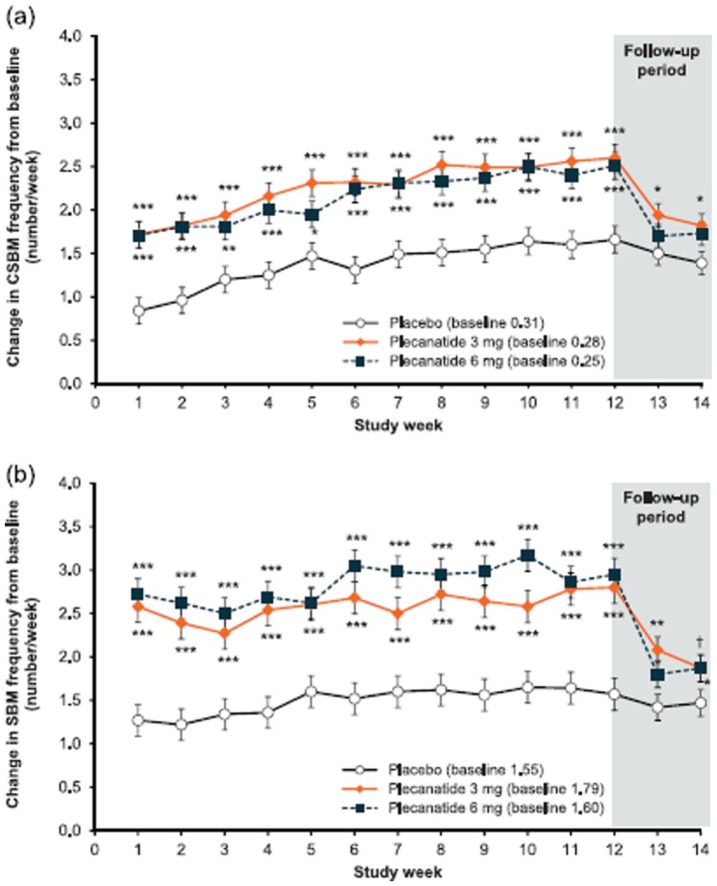
Both doses of plecanatide showed significant increases from baseline in mean weekly CSBM frequency compared with placebo, starting from week 1 and continuing throughout the 12-week treatment period [p < 0.001; Figure 6(a)]. At the end of study (week 14), mean weekly CSBM frequency had decreased toward baseline levels, with no worsening compared with baseline.
Figure 6.
Effects of plecanatide and placebo on the change in weekly complete spontaneous bowel movement frequency and weekly complete spontaneous bowel movement frequency.
(a) Effects of plecanatide and placebo on the change in weekly complete spontaneous bowel movement frequency; and (b) weekly complete spontaneous bowel movement frequency when compared with baseline over the 12-week treatment period, and during the 2-week drug-free follow-up period.
***p < 0.001, **p < 0.01, *p < 0.05 versus placebo.
CSBM, complete spontaneous bowel movement; SBM, spontaneous bowel movement.
Similar to CSBM frequency, mean weekly SBM frequency increased significantly from baseline in each plecanatide treatment group starting from week 1 (p < 0.001). The statistically significant difference from placebo continued throughout the 12-week treatment period compared with placebo (Figure 6b). However, these approached baseline levels by the end of study (week 14), with no worsening from baseline.
Improvement in stool consistency was also significantly greater in patients receiving plecanatide versus placebo (3 mg = 1.49; 6 mg = 1.50; placebo = 0.87; p < 0.001) for both doses. Since Rome IV defines CIC symptoms as BMs which are infrequent and difficult to pass, or incomplete evacuation during defecation, improvements in the frequency of weekly SBMs and stool consistency may be considered the most clinically relevant improvement for patients.16,22
The initial onset of action of plecanatide was also investigated. A significantly greater percentage of plecanatide-treated patients experienced a CSBM (p < 0.001 versus placebo for both doses) or an SBM (plecanatide 3 mg versus placebo, p < 0.05; plecanatide 6 mg versus placebo, p < 0.001) within 24 h of the first dose of study medication, and without the use of laxatives, compared with the placebo group. There was also a statistically greater improvement from baseline in the severity of abdominal bloating at week 12 in the plecanatide 3 mg treatment group (p = 0.007); and abdominal discomfort nearly reached statistical significance (p = 0.054). Constipation severity was significantly improved from baseline with plecanatide 3 mg (−1.6 ± 0.06; p = 0.001 versus placebo) and plecanatide 6 mg (−1.5 ± 0.06; p = 0.022 versus placebo) compared with placebo (−1.3 ± 0.06).
Similar to CSBM frequency, mean weekly SBM frequency increased significantly from baseline in each plecanatide treatment group starting from week 1 (p < 0.001). The statistically significant difference from placebo continued throughout the 12-week treatment period compared with placebo [Figure 6(b)]. However, these approached baseline levels by the end of study (week 14), with no worsening from baseline.
Safety data [ClinicalTrials.gov identifier: NCT01982240]
Approximately one third of patients experienced at least one treatment-emergent AE (TEAE) during the 12-week treatment period (plecanatide 3 mg, 35.4%; plecanatide 6 mg, 33.0%; placebo, 32.8%; Table 1).3 Overall, the incidence of TEAEs in any preferred term was low. The majority of TEAEs were mild to moderate in severity. Severe TEAEs occurred in ⩽3.7% of patients in all treatment groups. A total of 15 patients (1.1%) experienced a serious AE (SAE) across the treatment groups; two of the SAEs (both in the plecanatide 3 mg group) were pregnancies which were required to be reported as SAEs. Of the 13 other SAEs, four occurred with plecanatide 3 mg, five with plecanatide 6 mg, and four with placebo group. None of the SAEs were considered related to study drug.
Table 1.
Summary of treatment-emergent adverse events (safety population) for the study by Miner and colleagues [ClinicalTrials.gov identifier: NCT01982240].3
| Placebo (n = 458) n (%) |
Plecanatide 3 mg (n = 474) n (%) |
Plecanatide 6 mg (n = 457) n (%) |
|
|---|---|---|---|
| Patients with at least one TEAE | 150 (32.8) | 168 (35.4) | 151 (33.0) |
| Patients with at least one severe TEAE | 7 (1.5) | 13 (2.7) | 17 (3.7) |
| Patients with at least one TEAE leading to discontinuation | 6 (1.3) | 24 (5.1) | 24 (5.3) |
| TEAEs with incidence of >2% of plecanatide patients | |||
| Diarrhea | 6 (1.3) | 28 (5.9) | 26 (5.7) |
| Nasopharyngitis | 8 (1.7) | 4 (0.8) | 11 (2.4) |
| Sinusitis | 3 (0.7) | 10 (2.1) | 3 (0.7) |
TEAE, treatment-emergent adverse event.
Only one SAE, diverticulitis (in the placebo group), was considered possibly related to the study drug. The rates of discontinuation of study medication due to a TEAE were 5.1% with plecanatide 3 mg, 5.3% with plecanatide 6 mg, and 1.3% with placebo. The incidence rate was highest with events of diarrhea, followed by nasopharyngitis and sinusitis. The symptom of diarrhea was not defined a priori in terms of stool consistency or frequency but was recorded as reported by patients. Diarrhea occurred in 5.9% of patients in the plecanatide 3 mg, 5.7% of patients in the plecanatide 6 mg, and 1.3% of patients in the placebo treatment groups. The majority of patients who experienced diarrhea reported this as mild or moderately severe. Rates of discontinuation due to diarrhea were 2.7% for plecanatide 3 mg, 2.6% for plecanatide 6 mg, and 0.4% for placebo. For the plecanatide clinical trials, diarrhea was recorded as an AE only if the patient reported this spontaneously or as bothersome, had a BSFS score of 6 or 7 and had a sense of urgency or required hospitalization. In contrast, for the linaclotide and lubiprostone clinical trials, either spontaneous reports of diarrhea by the patient or the patient’s response to a nonleading question on changes in symptoms that suggested diarrhea, were reported as an AE. It is possible that differences in data collection may account for some of the differences in the reported incidence of diarrhea as an AE.38
There were no unexpected safety signals and no deaths reported in plecanatide studies. No plecanatide dose dependency was observed for any AE. Laboratory results, vital signs, and physical examination findings were all unremarkable, with a low incidence of any clinically important changes.
Safety data [ClinicalTrials.gov identifier: NCT02122471]
TEAEs in the plecanatide groups were similar to placebo. Overall, 372 patients (26.5%) experienced >1 TEAE (Table 2). The incidence of TEAEs on plecanatide 3 mg (25.7%) and 6 mg (29.2%) was similar to that of placebo (24.7%). Rates of diarrhea were 3.2% (plecanatide 3 mg), 4.5% (plecanatide 6 mg), and 1.3% (placebo). A total of 16 patients (4.3% of patients reporting ⩾1 TEAE) experienced 18 SAEs: six patients with seven events in the placebo group, seven patients with eight events in the plecanatide 3 mg group, and three patients with three events in the plecanatide 6 mg group. Four of the serious AEs were pregnancies reported during the treatment period, and two of these patients were receiving plecanatide.
Table 2.
Summary of treatment-emergent adverse events (safety population) for the study by DeMicco and colleagues [ClinicalTrials.gov identifier: NCT02122471].4
| Placebo (n = 466) n (%) |
Plecanatide 3 mg (n = 467) n (%) |
Plecanatide 6 mg (n = 469) n (%) |
|
|---|---|---|---|
| Patients with at least one TEAE | 115 (24.7) | 120 (25.7) | 137 (29.2) |
| Patients with at least one severe TEAE | 6 (1.3) | 8 (1.7) | 6 (1.3) |
| Discontinued study medication | |||
| Due to TEAE | 14 (3.0) | 15 (3.2) | 18 (3.8) |
| Due to diarrhea | 2 (0.4) | 5 (1.1) | 5 (1.1) |
| TEAEs with incidence of ⩾2% of patients in any treatment group | |||
| Diarrhea | 6 (1.3) | 15 (3.2) | 21 (4.5) |
| Headache | 9 (1.9) | 10 (2.1) | 10 (2.1) |
TEAE, treatment-emergent adverse event.
Only one of the serious AEs, a liver function test (LFT) abnormality, was considered possibly related to study drug, and this occurred in a patient treated with plecanatide 6 mg. In this patient, elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) started at week 4, and were considered severe and serious at week 8 (ALT 193 IU/l and AST 83 IU/l). The patient reported taking concomitant indomethacin, and elevations persisted after the discontinuation of study drug, in which case the elevated LFT may not have been related to plecanatide. None of the other SAEs were considered related to study medication.
Discontinuations due to TEAEs occurred in 3.2% (plecanatide 3 mg), 3.8% (plecanatide 6 mg), and 3.0% (placebo) of patients. Rates of discontinuation due to diarrhea were low, occurring in 1.1% of patients in each plecanatide group and 0.4% in the placebo group. No unexpected safety signals were noted, and no deaths were reported in this study. Laboratory findings, vital signs, and physical examinations were all unremarkable, with low incidence of any clinically significant changes.
Conclusions and therapeutic options
Today, there are three approved secretagogue agents for the treatment of chronic constipation and IBS-C in USA. Lubiprostone is a chloride channel agonist that has been shown to increase SBMs in patients with CIC2,21–33 at a dose of 24 mcg b.i.d., and IBS-C symptoms at a dose of 8 mcg b.i.d.21–23 Its main drawback is the occurrence of nausea (12–28%), that can be minimized by taking lubiprostone with meals.21–23 Linaclotide is a GC-C agonist that in randomized controlled trials has been shown to significantly increase the number of CSBM/week compared with placebo in CIC patients at a dose of 145 mcg, and recently at a dose of 72 mcg/day.39 Also, it improves IBS-C symptoms at a dose of 290 mcg/day.40 Its main adverse effect is diarrhea that was seen in up to 19% of patients in clinical trials, with 5% patients reporting severe diarrhea, necessitating withdrawal from clinical studies.34 Plecanatide is the newest member of intestinal secretagogues that has been shown to be efficacious and safe and has been approved in the USA for the treatment of adult patients with CIC.3,4,38,41 Also, plecanatide has just been approved in the USA for the treatment of adults with IBS-C.42 In the two phase III studies [ClinicalTrials.gov identifiers: NCT01-9822403 and NCT021224714], plecanatide 3 mg and 6 mg demonstrated a durable improvement in constipation and related CIC symptoms, compared with placebo when administered orally once daily for 12 weeks. A significantly larger percentage of patients achieved the primary durable overall CSBM responder endpoint. Improvements in BM frequency and stool consistency were rapid and sustained throughout the treatment period and were accompanied by improvements in straining and abdominal symptoms, resulting in general improvements in QOL, treatment satisfaction, and a likelihood of continuing treatment. Plecanatide was also shown to be well tolerated, exhibiting a limited AE profile with a low incidence of diarrhea.3,4 Additionally, prucalopride, a 5HT4 receptor agonist at a dose of 2–4 mg/day has been shown to be efficacious in CIC.2,17,23,25 Headache, nausea and diarrhea are its main AEs.
Together, these treatments fulfill a large unmet need and clearly enhance a clinician’s therapeutic armamentarium for providing improved relief of symptoms for patients with these chronic bowel disorders.
Acknowledgments
I sincerely appreciate the assistance of Synergy Pharmaceuticals with some of the information, including figures and tables, and Ms Helen Smith for secretarial support.
Satish SC Rao was responsible for manuscript concept and design, manuscript preparation, critical revision, and final approval.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: Dr Rao has served as an advisory board member and has received research grant support from Forest laboratories, Ironwood pharmaceuticals and Synergy Pharmaceuticals LLC.
ORCID iD: Satish SC Rao  https://orcid.org/0000-0002-4446-8452
https://orcid.org/0000-0002-4446-8452
References
- 1. Brandt LJ, Prather CM, Quigley EM, et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol 2005; 100(Suppl. 1): S5–S21. [DOI] [PubMed] [Google Scholar]
- 2. Rao SS, Camilleri M. Clinical approach to constipation. In: Podolsky D, Camilleri M, Fitz J, et al. (eds), Yamada’s text book of gastroenterology. 6th ed. Hoboken, New Jersey: John Wiley & Sons Ltd, 2016, pp.757–780. [Google Scholar]
- 3. Miner PB, Koltun WD, Wiener GJ, et al. A randomized phase III trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol 2017; 112: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeMicco M Barrow L Hickey B et al.. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Ther Adv Gastroenterol 2017: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol 2015; 110: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 1582–1591. [DOI] [PubMed] [Google Scholar]
- 7. Cai Q, Buono JL, Spaulding WM, et al. Healthcare costs among patients with chronic constipation: a retrospective claims analysis in a commercially insured population. J Med Econ 2014; 17: 148–158. [DOI] [PubMed] [Google Scholar]
- 8. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol 2011; 25(Suppl. B): 16B–21B. [PMC free article] [PubMed] [Google Scholar]
- 9. Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol 2009; 7: 537–544. [DOI] [PubMed] [Google Scholar]
- 10. Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am J Gastroenterol 1999; 94: 609–615. [DOI] [PubMed] [Google Scholar]
- 11. Nullens S, Nelsen T, Camilleri M, et al. Regional colon transit in patients with dyssynergic defaecation or slow transit in patients with constipation. Gut 2012; 61: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dukas L, Willett WC, Giovannucci EL. Association between physical activity, fiber intake, and other lifestyle variables and constipation in a study of women. Am J Gastroenterol 2003; 98: 1790–1796. [DOI] [PubMed] [Google Scholar]
- 13. Tuteja AK, Talley NJ, Joos SK, et al. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol 2005; 100: 124–129. [DOI] [PubMed] [Google Scholar]
- 14. Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013; 144: 21–27. [DOI] [PubMed] [Google Scholar]
- 15. Bharucha AE, Pemberton JH, Locke GR. American Gastroenterological Association technical review on constipation. Gastroenterology 2013; 144: 218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erdogan A Rao SS Thiruvaiyaru D et al.. Randomized clinical trial: mixed soluble/insoluble fibre vs. psyllium for chronic constipation. Alimen Pharmacol Ther 2016; 44: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol 2016; 13: 295–305. [DOI] [PubMed] [Google Scholar]
- 18. Rao SS, Benninga M, Bharucha A, et al. ANMS-ESMN – position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil 2015; 27: 594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao SS, Lembo A, Shiff S, et al. A 12-week randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu WB, Rao SS. Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide. Ther Adv Gastroenterol 2014; 7: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schey R, Rao SSC. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci 2011; 56: 1619–1625. [DOI] [PubMed] [Google Scholar]
- 22. Ambizas EM, Ginzburg R. Lubiprostone: a chloride channel activator for treatment of chronic constipation. Ann Pharmacother 2007; 41: 957–964. [DOI] [PubMed] [Google Scholar]
- 23. Wald A. Constipation: advances in diagnosis and treatment. JAMA 2016; 315: 185–191. [DOI] [PubMed] [Google Scholar]
- 24. Manabe N, Rao AS, Wong BS, et al. Emerging pharmacologic therapies for irritable bowel syndrome. Curr Gastroenterol Rep 2010; 12: 408–416. [DOI] [PubMed] [Google Scholar]
- 25. Camilleri M. Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clin Pharmacol Ther 2012; 91: 44–59. [DOI] [PubMed] [Google Scholar]
- 26. Hamra FK, Eber SL, Chin DT, et al. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci USA 1997; 94: 2705–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brierley SM. Guanylate cyclase-C receptor activation: unexpected biology. Curr Opin Pharmacol 2012; 12: 632–640. [DOI] [PubMed] [Google Scholar]
- 28. Castro J, Harrington AM, Hughes PA, et al. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3ʹ,5ʹ-monophosphate. Gastroenterology 2013; 145: 1334–1346. [DOI] [PubMed] [Google Scholar]
- 29. Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanylate cyclase C antagonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci 2013; 58: 2580–2586. [DOI] [PubMed] [Google Scholar]
- 30. Shailubhai K, Palejwala V, Arjunan KP, et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther 2015; 6: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kita T, Smith CE, Fok KF, et al. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol 1994; 266: F342–F348. [DOI] [PubMed] [Google Scholar]
- 32. Forte LR., Jr. Uroguanylin: physiological role as a natriuretic hormone. J Am Soc Nephrol 2005; 16: 291–292. [DOI] [PubMed] [Google Scholar]
- 33. Forte LR., Jr. Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 2004; 104: 137–162. [DOI] [PubMed] [Google Scholar]
- 34. Camilleri M. Guanylate cyclase C agonists: emerging gastrointestinal therapies and actions. Gastroenterology 2015; 148: 483–487. [DOI] [PubMed] [Google Scholar]
- 35. Vaandrager AB, Smolenski A, Tilly BC, et al. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator C1-channel activation. Proc Natl Acad Sci USA 1998; 95: 1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toriano R, Ozu M, Politi MT, et al. Uroguanylin regulates net fluid secretion via the NHE2 isoform of the Na+/H+ exchanger in an intestinal cellular model. Cell Physiol Biochem 2011; 28: 733–742. [DOI] [PubMed] [Google Scholar]
- 37. Shailubhai K. Therapeutic applications of guanylate cyclase-C receptor agonists. Curr Opin Drug Discov Devel 2002; 5: 261–268. [PubMed] [Google Scholar]
- 38. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonist for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol 2018; 113: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schoenfeld P, Lacy BE, Chey WD, et al. Low-dose linaclotide (72 mg) for chronic idiopathic constipation: a 12-week, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol 2018; 113: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rao SSC, Lembo A, Shiff SJ, et al. A 12-week randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. TRULANCE [package insert]. New York, NY: Synergy Pharmaceuticals Inc, 2017. [Google Scholar]
- 42. US Food and Drug Administration approval of Plecanatide for IBS-C. https://www.accessdata.fda.gov/drugs@TFDA-doc/appletter/…/200745org/500/ltr.pdf (accessed 27 January 2018).