Abstract
The glycoprotein sclerostin inhibits activation of the canonical Wnt pathway and thereby suppresses bone formation by inhibiting the osteoblasts. Additionally, sclerostin increases bone resorption by stimulating the production of receptor activator of nuclear factor kappa-β-ligand (RANKL). Romosozumab (ROMO) is a monoclonal antibody against sclerostin. Phase III clinical trials in postmenopausal women with osteoporosis have shown that ROMO increases bone mineral density at the lumbar spine and hip and reduces the risk of vertebral and clinical fractures in comparison with placebo. In women with severe osteoporosis, ROMO reduces the risk of vertebral, nonvertebral and clinical fractures in comparison with alendronate. ROMO is the first treatment for osteoporosis with dual action, and may become a valuable tool for improving the treatment of osteoporosis. At present, the approval of ROMO by the authorities is awaiting further investigations of a potential increased risk of cardiovascular events associated with ROMO treatment.
Keywords: fracture, review, romosozumab, sclerostin, osteoporosis
Introduction
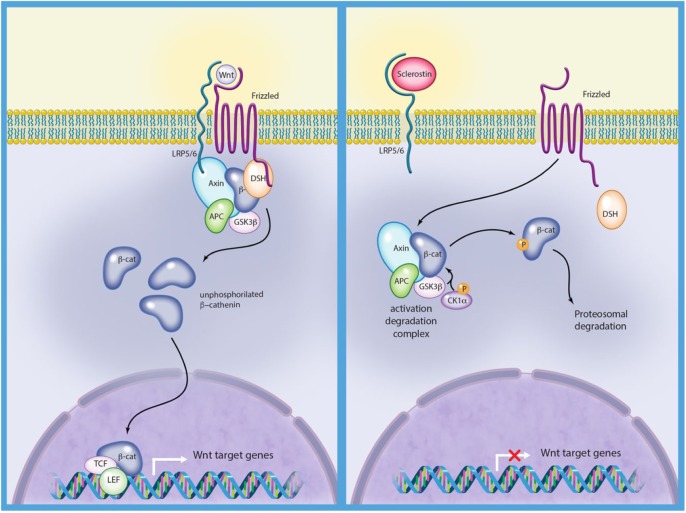
Activation of the canonical Wnt pathway leads through a series of intracellular events to translocation of β-catenin to the nucleus of osteoblasts and subsequently to gene transcription that stimulates bone formation through stimulation of osteoblast differentiation, proliferation and survival (Figure 1).1 ‘The glycoprotein, sclerostin encoded by the SOST gene is expressed in osteocytes, and recent data have also shown, that sclerostin is expressed in other cells types like for example chondrocytes.’2,3 When sclerostin binds to the low-density lipoprotein receptor protein 5 and 6 (LRP5/6) and Frizzled coreceptors on the osteoblast surface, the canonical Wnt pathway is inhibited (Figure 1).4,5 Accordingly, sclerostin inhibits bone formation by inhibiting the osteoblasts. In addition, sclerostin increases bone resorption by increasing the production of receptor activator of nuclear factor kappa-β-ligand (RANKL) by the osteocytes.6
Figure 1.
The Wnt signalling pathway.
Left: the Wnt pathway is activated by the interaction between LRP5/6, Wnt and Frizzled. Beta-catenin is released and enters the nucleus and activates transcription from Wnt target genes. Right: the Wnt pathway is inactivated due to sclerotin binding to LRP5/6 and beta-catenin is phosphorylated and degraded.
Illustration courtesy of Alessandro Baliani© 2018.
LRP5/6, low-density lipoprotein receptor protein 5 and 6; β-cat, beta-catenin; Axin, a scaffold protein; APC, adenomatous polyposis coli (tumor suppressor protein); GSK3β, glycogen synthase kinase 3 beta; P, phosphate; CK1α, casein kinase 1 alpha; DSH, disheveled (cytoplasmic protein).
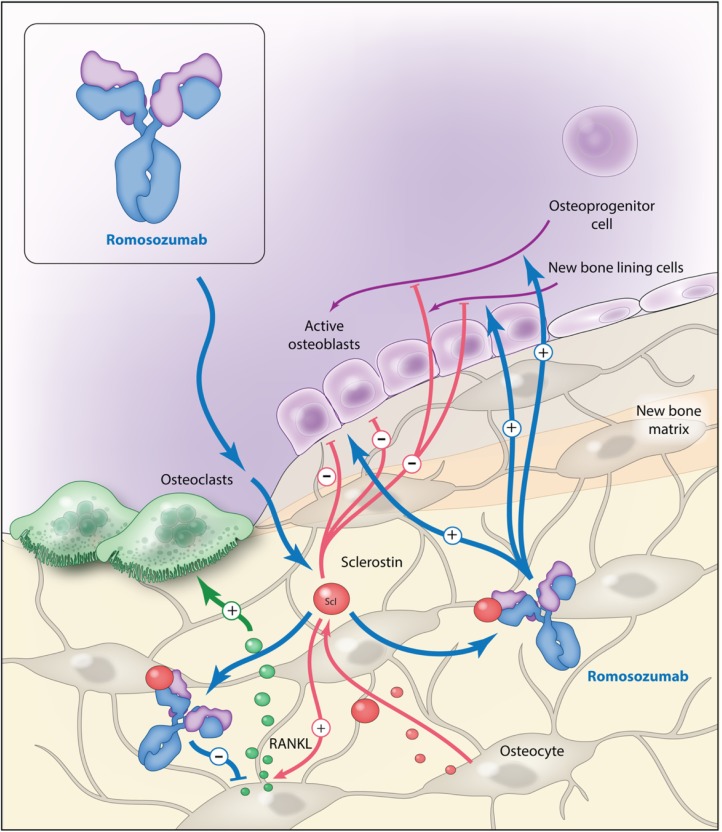
The clinical effects of sclerostin have been unraveled by two autosomal recessive disorders. Sclerosteosis is caused by a loss of function mutations in the SOST gene7,8 and patients with sclerosteosis have increased bone mineral density (BMD) and a very low risk of fractures.8–10 Van Buchem disease, another rare, autosomal recessive disease, is caused by a mutation in the regulatory region of SOST. Like sclerosteosis, the patients have high BMD, but the condition is milder than sclerosteosis.11,12 Recognition of the effects of sclerostin has inspired the development of sclerostin inhibitors as a potential treatment of osteoporosis. Romosozumab (ROMO) is a monoclonal antibody against sclerostin13 (Figure 2). The antibody is humanized, meaning that it is nonhuman but the amino-acid sequence is modified to increase similarity with a human antibody. ROMO is administrated subcutaneously with an absorption of 50–70% and a half-life of 6–7 days.13,14
Figure 2.
Romosozumab mode of action.
Illustration courtesy of Alessandro Baliani© 2018.
RANKL, receptor activator of nuclear factor kappa-β-ligand; Scl, sclerostin.
Animal studies
The effect of inhibition of sclerostin was first investigated in animals. Some 19-month-old female ovariectomized or sham-operated mice were randomized to either treatment with a sclerostin antibody (Scl-Ab) 25 mg/kg twice weekly or placebo for 5 weeks.15 BMD in the Scl-Ab treated mice increased by 26% at the lumbar spine and 17% at the femur–tibia. Histomorphometric analyses of cancellous and cortical bone showed increased bone volume in both compartments and elevated bone formation indices on trabecular, periosteal, endocortical, and intracortical surfaces. Similar findings were seen in a short-term study comprising aged, gonad-intact male rats.16 Investigations of the longer-term effects were then undertaken. A 26-week study with Scl-Ab treatment in ovariectomized 6-month-old female rats found increased bone mass and strength compared with vehicle-treated animals.17 Interestingly, the bone formation rate peaked after 6 weeks’ treatment and thereafter, declined towards baseline, whereas bone resorption remained suppressed throughout the 26 weeks.17
The effects of previous or concomitant treatment with alendronat (ALN) were investigated in 10-month-old ovariectomized rats. Neither pretreatment nor pre- and concomitant treatment with ALN negatively affected the outcome of treatment with Scl-Ab for 6 weeks.18
Monkeys have, unlike rodents, a bone remodeling process very similar to bone remodeling in humans and therefore the effect of inhibition of sclerostin has been investigated in female, gonad-intact cynomolgus monkeys. The monkeys were randomized to Scl-Ab 3, 10, or 30 mg/kg or placebo monthly for 2 months.19 Bone mineral content (BMC) and BMD increased dose dependently (11–29%) and bone strength was increased in the monkeys treated with 30 mg/kg. Histomorphometric analyses of bone samples revealed increased bone formation on trabecular, periosteal, endocortical and intracortical surfaces. Serum levels of serum type 1 aminoterminal propeptide (s-P1NP) and osteocalcin increased dose dependently, whereas serum C-telopeptide (s-CTX) decreased temporarily, suggesting an uncoupling of bone formation and resorption.19 Similar effects of ROMO were seen in ovariectomized monkeys, a model of postmenopausal osteoporosis.20 In this study, it was demonstrated that the effects of ROMO are reversible when stopping the treatment. The authors also demonstrated that the material properties of bone were maintained during treatment and that bone strength was positively correlated to bone mass.
Finally, a study comprising male cynomolgus monkeys was undertaken to investigate the mechanisms underlying the continued increase in bone mass, despite the attenuation in bone formation markers seen with continued ROMO treatment. This study demonstrated that the early phase of treatment (10 weeks) is characterized by increased modeling-based bone formation, increased wall thickness at remodeling sites and reduction in remodeling space secondary to reduction in erosion surfaces at cancellous bone. In addition, periosteal and endocortical bone formation was increased at the cortex of the vertebral bodies. Later on (28 weeks), the effect on bone modeling at cancellous bone attenuates, but the reduction in remodeling is maintained and combined with a positive remodeling balance due to reduction in resorption depth and increase in wall thickness. The increased bone formation at periosteal and endocortical surfaces was maintained.21
Clinical trials
See Table 1 for an overview of the clinical trials investigating the effects of ROMO. Two phase I studies have been undertaken. The first was a single-dose study demonstrating highly significant increases in markers of bone formation, s-P1NP, bone-specific alkaline phosphatase (s-BAP) and osteocalcin, 85 days after administration of ROMO.13 Interestingly, the bone resorption marker, s-CTX, decreased significantly over the same period of time. BMD increased dose dependently, 5.3% and 2.8% at the lumbar spine and total hip, respectively, 85 days after a single administration of 10 mg/kg subcutaneously.
Table 1.
Clinical trials investigating the effects of ROMO.
| Study | Year | Length | Treatment | Participants (n) | Results: BTM | Results: BMD | Results: fracture |
|---|---|---|---|---|---|---|---|
| Phase I | |||||||
| Padhi et al.13 | 2011 | 85 days | ROMO subcutaneously (0.1, 0.3, 1, 3, 5, or 10 mg/kg) single dose; ROMO intravenously (1 or 10 mg/kg) single dose; placebo |
72 men and postmenopausal women | Dose dependently; the maximum changes from baseline: subcutaneously 10 mg/kg: P1NP +184%, BAP +126%, osteocalcin +176%, CTX −54%; intravenously 5 mg/kg: P1NP +167%, BAP +125%, osteocalcin +143%, CTX −49% |
Dose dependently; the largest increases: subcutaneously 10 mg/kg: LS +5.3%, TH +2.8%; intravenously 5 mg/kg: LS +5.2%, TH +1.1% |
– |
| Padhi et al.14 | 2014 | 12 weeks | ROMO: women: six doses of 1 or 2 mg/kg every 2 weeks, or three doses of 2 or 3 mg/kg every 4 weeks; men: six doses of 1 mg/kg every 2 weeks or three doses of 3 mg/kg every 4 weeks; placebo |
48 men and postmenopausal women | Dose dependently; P1NP: +66–147% CTX: −15–50% |
Dose dependently. LS: +4–7%, TH: +2–3% |
– |
| Phase II | |||||||
| McClung et al.25 | 2014 | 12 months; extension: 12 months |
ROMO monthly (70 mg, 140 mg, 210 mg); ROMO 3 monthly (140 mg, 210 mg); ALN 70 mg/week; TPTD 20 µg/day; placebo |
419 postmenopausal women | ROMO 210 mg/month: P1NP + 91% after 1 month, −20% below baseline level after 12 months; CTX −41% after 1 week, −26% below baseline after 12 months; ALN (12 months): P1NP −64%, CTX −66% TPTD (12 months): P1NP +84%, CTX +80% |
ROMO (12 months): LS +11.3%, TH +4.1%; ROMO (24 months): LS +15.7%, TH +6.0%; ALN (12 months): LS +4.1%, TH +1.9%; TPTD (12 months): LS 7.1%, TH +1.3%; placebo (12 months): LS +0.1%, TH −0.7% |
|
| Phase III | |||||||
| Cosman et al.,22
FRAME study |
2016 | 12 months extension: 12 months |
ROMO 210 mg/month; placebo Extension: DMAB 60 mg/6 months for all patients |
7180 postmenopausal women | ROMO: P1NP +150%* (maximum at day 14), returned to baseline levels by 9 months; CTX −50%* (maximum at day 14); DMAB: reduced the levels of P1NP and CTX similarly in each group |
ROMO (12 months): LS +13.3%, TH +6.8% ROMO + DMAB (24 months): LS +17.6%, TH +8.8% Placebo + DMAB (24 months): LS +5.0%, TH +29% |
ROMO (12 months): RR (VFx) −73% RR (CFx) −36%, RR (NVFx) −25% (p = 0.06, interaction between geographical regions); ROMO + DMAB (24 months): RR reduction for fracture was maintained although only significantly for VFx |
| Saag et al.,23
ARCH study |
2017 | 12 months extension: 12 months |
ROMO 210 mg/month; ALN 70 mg/week Extension: ALN 70 mg/week for all patients |
4093 postmenopausal women | ROMO: P1NP +80%*, CTX −40%*; after the transition to ALN, P1NP (−60%*) and CTX (−60%*) decreased and remained below baseline levels at 36 months; ALN: P1NP and CTX decreased within 1 month and remained below baseline levels at 36 months |
ROMO–ALN (24 months): LS +15.2%, TH +7.1% ALN-ALN (24 months): LS+7.1%, TH +3.4% |
ROMO–ALN (24 months): RR (VFx) −48% RR (CFx) −27% RR (NVFx) −19 % RR (HFx) −38% compared with the ALN–ALN group |
| Langdahl et al.,24
STRUCTURE study |
2017 | 12 months | ROMO 210 mg/month; TPTD 20 µg/day |
436 postmenopausal women | ROMO: P1NP +180%* (maximum at 1 month); returned towards baseline during the 12 months; CTX −30%* (maximum at day 14); returned to baseline by month 3; TPTD: P1NP and CTX concentrations increased during the first 6 months of treatment; remained significantly higher than baseline values for the subsequent 6 months of treatment |
ROMO (12 months) LS +9.8%, TH −2.9%; TPTD (12 months): LS +5.4%, TH −0.5% |
– |
| ClinicalTrials.gov identifier: NCT02186171 | 2018 | 12 months | ROMO 210 mg/month; placebo |
245 men | Pending | Pending | – |
The precise changes in BTM were not reported in the publications and the changes are estimated from the figures of the publication.
ROMO, romosozumab; TPTD, teriparatide; ALN, alendronate; DMAB, denosumab; BMD, bone mineral density; LS, lumbar spine; TH, total hip; BTM, bone turnover marker; CTX, serum C-telopeptide; P1NP, serum type 1 aminoterminal propeptide; BAP, bone-specific alkaline phosphatase; RR, relative risk; VFx, vertebral fracture; CFx, clinical fracture; NVFx, nonvertebral fracture; HF, hip fracture.
The second phase I study investigated repeated dosing of ROMO and confirmed the prominent increases in markers of bone formation, moderate decreases in the bone resorption marker, and increases in BMD.14 Thus, the phase I studies demonstrated that treatment with ROMO in humans appears to be dual action, stimulating bone formation and at the same time, inhibiting bone resorption.
Next, a phase II study comprising 419 postmenopausal women with low bone mass was initiated. The study investigated the effect of treatment with one of five different doses of ROMO either monthly (70 mg, 140 mg, 210 mg) or 3 monthly (140 mg, 210 mg) as well as ALN 70 mg weekly, teriparatide (TPTD) 20 µg daily or placebo.25 Patients treated with ROMO 210 mg monthly had a 91% increase in s-P1NP after 1 month, however, after 12 months, s-P1NP was 20% below baseline level. s-CTX decreased by 41% after 1 week; the suppression of s-CTX leveled of over the treatment duration, but remained 26% below baseline after 12 months. As expected, ALN suppressed the bone turnover markers (P1NP −64%, CTX −66%) and TPTD increased turnover (P1NP +84%, CTX +80%). As for BMD, the effect of ROMO was significantly better than the effect seen for the other treatment regimens. Thus, ROMO increased lumbar spine BMD by 11.3% compared with 0.1% in the placebo group and 4.1% and 7.1% in patients treated with ALN or TPTD (p < 0.001 for all three comparisons), respectively.25 The same pattern was seen at the total hip (ROMO 4.1%, TPTD 1.3%, ALN 1.9%, and placebo −0.7%, p < 0.001 for all three comparisons). Quantitative computed tomography (QCT) was performed in a subset of participants receiving placebo, TPTD, or ROMO 210 mg monthly.26 Trabecular volumetric BMD (vBMD) increased similarly with ROMO and TPTD at the spine, however, at the hip, trabecular vBMD increased 10.8% with ROMO compared with 4.2% with TPTD. Finally, cortical vBMD at the total hip increased with ROMO (+1.1%), but decreased with TPTD (–0.9%). Finite element analyses revealed that ROMO increased vertebral and femoral neck strength more than TPTD and placebo. At the vertebrae, the increase was +27.3% in women treated with ROMO compared with +18.5% and −3.9% in women treated with TPTD and placebo, respectively. At the femoral neck, the pattern was similar, although the changes were smaller: +3.6%, −0.7%, and −0.1% in women treated with ROMO, TPTD and placebo, respectively.27 An extension of the study led to further increase in BMD (lumbar spine 15.7%, total hip 6.0%) in women treated with 210 mg ROMO for another 12 months.28 After 2 years, the participants we re-randomized to placebo or denosumab (DMAB) 60 mg/6 months.28 Participants treated with DMAB during the third year had cumulative increases of 19.4% and 7.1% in BMD at the spine and total hip, respectively. Women treated with placebo during the third year lost bone, and BMD returned towards pretreatment levels.
The antifracture efficacy of ROMO in women with osteoporosis was demonstrated in two phase III trials. In the FRAME study, 7180 postmenopausal women with osteoporosis were randomized to ROMO 210 mg monthly or placebo for 12 months, followed by an open-label extension during which all patients received DMAB 60 mg every 6 months for 12 months.22 During the first 12 months, ROMO reduced the risk of vertebral fracture by 73% [ROMO group: 0.5% (16 of 3321 patients); placebo group: 1.8% (59 of 3322 patients); p < 0.001] and clinical fractures by 36% [ROMO group: 1.6% (58 of 3589 patients); placebo group: 2.5% (90 of 3591 patients); p = 0.008]. A 25% nonsignificant reduction in nonvertebral fractures was noted [ROMO: 1.6% (56 of 3589 patients); placebo group: 2.1% (75 of 3591); p = 0.10]. Interestingly, an interaction between geographical region and the effect of ROMO on nonvertebral fractures was seen; apparently, there was no effect among women from Latin America [ROMO: 1.5% (24 of 1550 patients); placebo group: 1.2% (19 of 1534)], but a 42% reduction in nonvertebral fractures among women from the rest of the world [ROMO: 1.6% (32 of 2039); placebo group: 2.7% (56 of 2057); p = 0.04 for the treatment-by-region interaction]. The authors state that the unexpected low rate of nonvertebral fractures among women from Latin America is in accordance with a post hoc estimation of fracture risk using FRAX® and more recent epidemiology data, revealing a very low risk of nonvertebral fractures despite low nonspine BMD.22 During the extension, the risk reduction for fracture was maintained, although this was only formally significant for vertebral fractures. In addition, ROMO increased BMD at the spine by 13.3% and at the total hip by 6.8% after 12 months compared with 0.0% at both regions in women treated with placebo. During the extension, BMD increased in both the ROMO + DMAB and placebo + DMAB groups. By 24 months, BMD at the spine had increased by 17.6% and 5.0% in the ROMO + DMAB and placebo + DMAB groups, respectively and at the total hip by 8.8% and 2.9%, respectively meaning that the absolute difference in BMD between the two groups was maintained during the extension.22
In the ARCH study, 4093 women with severe osteoporosis (T score ⩽ −2.5 and a prevalent vertebral fracture) were randomized to ROMO 210 mg monthly or ALN 70 mg/week for 12 months followed by ALN 70 mg/week for all patients.23 At 24 months, the risk of new vertebral fractures, clinical fractures, nonvertebral fractures, and hip fractures was reduced by 48%, 27%, 19%, and 38%, respectively, in the ROMO–ALN group compared with the ALN–ALN group. In addition, at 24 months, BMD increased by 15.2% at the lumbar spine and 7.1% at the total hip in women treated with ROMO–ALN compared with 7.1% and 3.4%, respectively, in women treated with ALN–ALN.
In real life, most patients do not have the option of bone-forming treatment as first-line treatment, and most patients starting bone-forming treatment will therefore previously have been treated with antiresorptives, most often, bisphosphonates. The aim of the STRUCTURE study was therefore to compare the effects of ROMO with TPTD in postmenopausal women previously treated with bisphosphonates.24 The study enrolled 436 postmenopausal women who had been treated with bisphosphonates for more than 3 years. The women were randomized to 1 year of treatment with either ROMO 210 mg monthly or TPTD 20 µg daily. BMD increased significantly more with ROMO than with TPTD at both the lumbar spine (9.8% versus 5.4%, p < 0.0001) and total hip (2.9% versus −0.5%, p < 0.0001).24 Bone strength was estimated by finite element analysis of hip QCT and bone strength increased by 2.5% in women treated with ROMO compared with a change of −0.7% in women treated with TPTD.
Safety
Approximately 10% of individuals administered ROMO in the phase I single-dosing study developed antibodies against ROMO.13 The antibodies were predominantly neutralizing. In the phase II and the FRAME studies, antibodies against ROMO were found in 20% of the ROMO treated women. A few percent was neutralizing in vitro but did not affect pharmacodynamics, pharmacokinetics, or the clinical response.22,25 The clinical relevance of these antibodies in relation to long-term treatment is currently not known.
The adverse events in the FRAME22 and the STRUCTURE studies24 were generally balanced between the two treatment groups. Adverse events in the STRUCTURE study were nasopharyngitis, hypercalcemia, arthralgia and injection-site reactions. There was a numeric imbalance in serious adverse events affecting the cardiovascular system during the first 12 months in the ARCH study (2.5% of the women in the ROMO group compared with 1.9% of the women in the ALN group). After 24 months, this subgroup of serious adverse events had occurred in 6.5% and 6.1% of women treated with ROMO–ALN and ALN–ALN, respectively.23 Further adjudication of these events is ongoing. Interestingly, the incidences of death, adjudicated cardiovascular and serious cardiovascular events in the FRAME study were well balanced between the women treated with ROMO and placebo; 0.6% versus 0.8%, 0.4% versus 0.5%, and 1.1% versus 1.2%, respectively.
Preclinical studies have not demonstrated any increased risk of osteosarcoma, as is the case for long-term treatment with TPTD and abaloparatide.29
Discussion
The current treatment options for osteoporosis are mostly antiresorptives, bisphosphonates or DMAB. The antiresorptives have strong antifracture efficacy, especially against vertebral fractures but less so against nonvertebral fractures. In the clinic, we see patients with mild or even moderate osteoporosis in whom we can prevent fractures with the existing antiresorptive treatments available. However, we do also see patients with severe osteoporosis; they have very low bone mass and multiple fractures. These patients need bone-forming treatment to improve bone mass and architecture in order to prevent future fractures. Currently, we have the option of TPTD or abaloparatide. Both are strong stimulators of osteoblasts and bone formation, but the effect is limited, to some extent, by the concomitant increase in bone resorption and because these treatments can only be used once for up to 24 months due to safety concerns. In this context, ROMO, with its dual-action mode of action and the potential for retreatment, is a very interesting new treatment modality.
Treatment of postmenopausal women with osteoporosis with ROMO increases BMD of the lumbar spine and hip areas, in addition to reducing the risk of vertebral and clinical fractures. The BTM response after treatment with ROMO is well described.13,14,25 Generally, the markers of bone formation increase, whereas the markers of bone resorption decrease, indicating a dual action of ROMO. It has previously been reported that activation of the Wnt pathway in osteoblasts not only stimulates bone formation, but also inhibits bone resorption by increasing osteoprotegerin (OPG) production.30 The reduced bone resorption might also be caused by deceased production of RANKL by osteocytes due to inhibition of sclerostin.31 In the phase II study, s-P1NP increased by 91% after 1 month in women treated with ROMO 210 mg monthly, however, the increase was temporary and s-P1NP was back to baseline after 6 months and even 20% below baseline level after 12 months.25 It is somewhat surprising that the increase in bone formation measured by bone turnover markers is only temporary. It might be caused by depletion of osteoblast progenitors or a compensatory increase in other inhibitors of bone formation such as dickkopf.32 In the same study, s-CTX also returned towards baseline at 12 months, after the initial decrease.25
Despite the above-described changes in the markers of bone turnover, BMD continues to increase throughout the 12 months. The possible underlying mechanisms have been investigated in male monkeys. It was demonstrated that the early treatment phase is characterized by increased modeling-based bone formation, as well as an increased wall thickness at remodeling sites and a reduction in the remodeling space. In the later phase, the effect on bone modeling at cancellous bone attenuates, but reduction in remodeling is maintained and now combined with a positive remodeling balance due to reduction in resorption depth and increase in wall thickness. In addition, periosteal and endocortical bone formation at the cortex of the vertebral bodies was increased throughout the study period.21 The strong positive effect on bone mass therefore seems to be explained by an initial stimulation of bone modeling at trabecular surfaces, a more continued positive effect on bone remodeling, reduction in remodeling space and a positive remodeling balance, and an ongoing stimulation of bone formation at the cortex.
During the extension of the phase II study, the women were treated with either DMAB or placebo during year 3. Women treated with ROMO during the first 24 months followed by placebo during the third year lost bone mass, and BMD returned towards pretreatment levels during the third year.28 Thus, the effect of ROMO is reversible, but does not seem to be characterized by the very steep rise in bone resorption seen after stopping DMAB.33,34 BMD continued to increase in the women who were treated with DMAB in the third year. This effect was confirmed in the FRAME study.22 Furthermore, the ARCH study demonstrated that treatment with ALN following ROMO also leads to further increases in bone mass.23
The ARCH study is the first trial to demonstrate superiority of one treatment of osteoporosis in a head-to-head comparison with fractures as the primary endpoint. Treatment for 12 months with ROMO reduced the risk of vertebral and clinical fractures by 37% and 28% in comparison with treatment with ALN, respectively. After an additional year, where both groups were treated with ALN, the reduction of vertebral, clinical and nonvertebral fractures in the women treated with ROMO–ALN compared with women treated with placebo–ALN was 48%, 27% and 19%, respectively.
There is no head-to-head comparison between ROMO and TPTD or abaloparatide with fracture as the endpoint. The first year of the phase II study included a comparison of ROMO with TPTD on BMD and bone turnover. The increases in bone mass was superior with ROMO.25 The STRUCTURE study compared the effect of ROMO with TPTD on BMD and bone strength in patients previously treated with long-term bisphosphonates.24 The study demonstrated that while the changes seen at the lumbar spine were comparable, the effects of ROMO at the hip sites, BMD and estimated bone strength, were more prominent. The effect of the two treatments on bone formation is similar, although bone formation markers remain increased for a longer duration with TPTD compared with ROMO. The most important difference is probably related to the effect on bone resorption; ROMO inhibits resorption, whereas, bone resorption is stimulated by TPTD secondarily to the stimulation of bone formation. This difference may explain the differences seen in BMD.
The mode of administration, a subcutaneous injection once a month with ROMO, may be easier for patients than the daily subcutaneous injection with TPTD or abaloparatide. Some patients may be able to administer the injection of ROMO themselves others may need assistance from a healthcare professional. This needs to be taken into account when considering choice of treatment.
While we are waiting for the adjudication of the serious adverse events seen in the ARCH study, possible explanations for the potential increased risk of cardiovascular events with ROMO compared with ALN has been discussed. One possible explanation is that the difference seen between the groups is not caused by ROMO increasing the risk, but by ALN reducing the risk of cardiovascular events. The reduced risk of mortality seen in the zoledronic acid clinical trials35 led to reanalysis of the clinical trials investigating bisphosphonates; however, meta-analyses of clinical trials investigating ALN failed to confirm a cardio-protective role of ALN.36 Another possible explanation is that sclerostin plays a role in the cardiovascular system.37 Sclerostin is expressed outside the bone; it has been found in aortic vascular smooth muscle and inhibition of sclerostin could therefore potentially affect the Wnt pathway in these cells, and thereby, vascular remodeling. Furthermore, upregulation of sclerostin at sites of vascular calcification has been demonstrated; the pathogenic role of this is currently unknown. Finally, the difference could be a chance finding, as the number of events is small.
An interesting future research topic will be the effect of sclerostin inhibition in patients treated with glucocorticoids. Glucocorticoids stimulate osteocyte release of sclerostin and this may be part of the explanation for the reduced bone formation seen with long-term glucocorticoid treatment.38 Concomitant treatment of mice with glucocorticoids and Scl-Abs prevented the glucocorticoid-induced reduction in osteoblast activity and subsequently trabecular and cortical bone mass and strength.39
If approved for treatment of osteoporosis, ROMO will be an appropriate therapy for patients with severe osteoporosis. In these patients, we aim to increase bone mass, restore bone architecture and quality, thereby improve bone strength and reduce fracture risk. Two studies have now showed that in this group of patients, bone-forming treatments are superior to antiresorptives. In the VERO study, it was demonstrated that TPTD prevented vertebral and clinical fractures better than risedronate.40 The ARCH study demonstrated that ROMO prevented vertebral, clinical, and nonvertebral fractures better than ALN.23 Based on the clinical trials, ROMO is most likely being approved for a treatment duration of 12 months. As the treatment effects are reversible, it will be important to continue with an antiresorptive treatment thereafter. Some patients may need retreatment with ROMO and it will therefore be interesting to see more data on this from the extension of the phase II study where retreatment with ROMO was tried.25
In conclusion, the clinical potential for ROMO, if approved by the authorities for the treatment of osteoporosis, is great. ROMO is the first treatment for osteoporosis with dual action, ROMO stimulates bone formation, modeling and remodeling based, and inhibits bone resorption. This leads to impressive increases in bone mass and bone strength and most importantly, significant reductions in the risk of fractures. It has also been demonstrated that the fracture risk reductions are more prominent than the reductions seen with a strong antiresorptive treatment.
Acknowledgments
The authors thank Alessandro Baliani (Managing Editor, SAGE Publications Ltd.) for drawing Figures 1 and 2.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Torben Harsløf received lecture fees from Amgen, Astra Zeneca, and Eli Lilly.
Bente Langdahl serves on advisory boards for Amgen, Eli Lilly, and UCB and has received lecture fees from Merck, Eli Lilly, TEVA and Amgen.
Anne Sophie Koldkjær Sølling has nothing to disclose.
ORCID iD: Anne Sophie Koldkjær Sølling  https://orcid.org/0000-0001-7252-8128
https://orcid.org/0000-0001-7252-8128
Contributor Information
Anne Sophie Koldkjær Sølling, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Torben Harsløf, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Bente Langdahl, Department of Endocrinology and Internal Medicine, THG, Aarhus University Hospital, Tage-Hansens Gade 2, 8000 Aarhus C, Denmark.
References
- 1. Baron R, Rawadi G. Minireview: targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton Endocrinology 2007; 148: 2635–2643. [DOI] [PubMed] [Google Scholar]
- 2. Thompson ML, Jimenez-Andrade JM, Mantyh PW. Sclerostin immunoreactivity increases in cortical bone osteocytes and decreases in articular cartilage chondrocytes in aging mice. J Histochem Cytochem 2016; 64: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roudier M, Li X, Niu QT, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum 2013; 65: 721–731. [DOI] [PubMed] [Google Scholar]
- 4. Poole KE, Van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation FASEB J 2005; 19: 1842–1844. [DOI] [PubMed] [Google Scholar]
- 5. Van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004; 199: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 2011; 10: e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 2001; 10: 537–543. [DOI] [PubMed] [Google Scholar]
- 8. Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 2001; 68: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner JC, Van Bezooijen RL, Mervis B, et al. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab 2005; 90: 6392–6395. [DOI] [PubMed] [Google Scholar]
- 10. Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet 2003; 63: 192–197. [DOI] [PubMed] [Google Scholar]
- 11. Balemans W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 2002; 39: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Lierop AH, Hamdy NA, Van Egmond ME, et al. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers J Bone Miner Res 2013; 28: 848–854. [DOI] [PubMed] [Google Scholar]
- 13. Padhi D, Jang G, Stouch B, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 2011; 26: 19–26. [DOI] [PubMed] [Google Scholar]
- 14. Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol 2014; 54: 168–178. [DOI] [PubMed] [Google Scholar]
- 15. Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 2009; 24: 578–588. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Warmington KS, Niu QT, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 2010; 25: 2647–2656. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Niu QT, Warmington KS, et al. Progressive increases in bone mass and bone strength in an ovariectomized rat model of osteoporosis after 26 weeks of treatment with a sclerostin antibody. Endocrinology 2014; 155: 4785–4797. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Ominsky MS, Warmington KS, et al. Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology 2011; 152: 3312–3322. [DOI] [PubMed] [Google Scholar]
- 19. Ominsky MS, Vlasseros F, Jolette J, et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 2010; 25: 948–959. [DOI] [PubMed] [Google Scholar]
- 20. Ominsky MS, Boyd SK, Varela A, et al. Romosozumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys. J Bone Miner Res 2017; 32: 788–801. [DOI] [PubMed] [Google Scholar]
- 21. Boyce RW, Niu QT, Ominsky MS. Kinetic reconstruction reveals time-dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone 2017; 101: 77–87. [DOI] [PubMed] [Google Scholar]
- 22. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532–1543. [DOI] [PubMed] [Google Scholar]
- 23. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017; 377: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 24. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017; 390: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 25. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014; 370: 412–420. [DOI] [PubMed] [Google Scholar]
- 26. Genant HK, Engelke K, Bolognese MA, et al. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 2017; 32: 181–187. [DOI] [PubMed] [Google Scholar]
- 27. Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res 2017; 32: 1956–1962. [DOI] [PubMed] [Google Scholar]
- 28. McClung M, Chines A, Brown J, et al. Effects of 2 years of treatment with romosozumab followed by 1 year of denosumab or placebo in postmenopausal women with low bone mineral density. J Bone Miner Res 2014; 29: abstract 1152. [DOI] [PubMed] [Google Scholar]
- 29. Chouinard L, Felx M, Mellal N, et al. Carcinogenicity risk assessment of romosozumab: a review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol 2016; 81: 212–222. [DOI] [PubMed] [Google Scholar]
- 30. Glass DA, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 2005; 8: 751–764. [DOI] [PubMed] [Google Scholar]
- 31. Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int 2012; 23: 2067–2079. [DOI] [PubMed] [Google Scholar]
- 32. Ferrari S. Future directions for new medical entities in osteoporosis. Best Pract Res Clin Endocrinol Metab 2014; 28: 859–870. [DOI] [PubMed] [Google Scholar]
- 33. Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008; 43: 222–229. [DOI] [PubMed] [Google Scholar]
- 34. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011; 96: 972–980. [DOI] [PubMed] [Google Scholar]
- 35. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim DH, Rogers JR, Fulchino LA, et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gay A, Towler DA. Wnt signaling in cardiovascular disease: opportunities and challenges. Curr Opin Lipidol 2017; 28: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone 2017; 96: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yao W, Dai W, Jiang L, et al. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos Int 2016; 27: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kendler DL, Marin F, Zerbini CA, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. Epub ahead of print 9 November 2017. DOI: 10.1016/S0140-6736(17)32137-2 [DOI] [PubMed] [Google Scholar]