Abstract
Many endogenous gene expressions in the liver are well known to be predominant in males, compared with those of females. In contrast, the fate of hepatic transgene expression between sexes is not fully understood. Here we studied whether sex hormones changed hepatic transgene expression in the ubiquitous CAG promoter-driven lacZ transgenic (Tg) rat. Both sexes of CAG-lacZ Tg rats received gonadectomy. Liver biopsy was taken weekly to determine the change of transgene expression. Histological result of adult males showed mosaic lacZ expression but it was negative in adult females, while livers in neonatal stage showed comparable expression of lacZ. Other organs exhibited equal expression in both sexes. At 2 weeks after castration, lacZ expression in male liver was significantly decreased and became negative after 4 weeks while no significant difference was observed in the lacZ expression pattern in other organs. After ovariectomy, lacZ expression in female liver remained undetectable. Moreover, testosterone treatment to gonadectomized rats of both sexes could enhance lacZ expression in the liver. In summary, we report that CAG-lacZ Tg rats demonstrate sexual dimorphism of transgene expression specifically only in the liver. Testosterone administration mediated upregulation of liver lacZ expression. Our findings suggested that androgen, especially testosterone, plays an important role in the hepatic transgene expression.
Key words: Transgene expression, lacZ, CAG promoter, Testosterone, Gonadectomy
SEX-DEPENDENT genetic regulatory events have been known for decades to occur in the liver (7,11). Sexually dimorphic susceptibility of the liver to disease (e.g., hepatocellular adenomas and carcinomas) occurs in humans (12) and rodents (4,17). Furthermore, the liver contains a broad range of proteins, which are predominantly present in males, such as urinary protein pheromone carriers, steroids, drug-metabolizing cytochrome P450s (CYPs), and proteins that function in reproduction (21).
On the other hand, exogenous gene expression has been conducted by using various genetic techniques. However, transgene expression is dependent on each transgenic (Tg) animal by chance. In some Tg animals, liver transgene expression in males has been reported to be greater than in females (2,6,13,14,16,24). Expressions of given transgenes are likely to be cumulative outcomes from complex regulatory mechanisms of genomes (e.g., a positional effect, epigenetic modification, cis-upstream element, and tissue-specific regulation) (9). The precise mechanism that controls sex-dependent transgene expression remains unclear.
In a previous report, we addressed the idea that exogenous transgene expression was dependent on each organ and developmental stage in the green fluorescent protein (GFP) Tg rat (23). The CAG (cytomegalovirus intermediate early enhancer, chicken β-actin promoter, and chicken β-actin/rabbit β-globin composite intron) promoter used directs the ubiquitous expression of foreign genes at high levels (19). Interestingly, the newly established CAG-lacZ Tg rat (22) showed tissue-, age-, and sex-dependent expression patterns. Because protein of the lacZ reporter gene, β-galactosidase (β-gal), displays distinct sexual dimorphism only in the liver, we manipulated the sex hormones in adult male and female rats. The results of the present study suggest that testosterone plays an important role in transgene expression in CAG-lacZ Tg rats and could be used for reactivating silenced transgene expression in the liver.
MATERIALS AND METHODS
Experimental Animals
The generation of CAG-lacZ Tg rats was described previously (22). Wild DA rats (initial body weight, 150–220 g), purchased from Charles River Japan Inc. (Yokohama, Japan), were used as normal controls for CAG-lacZ Tg rats. All animals had free access to standard food and water and were maintained on a 12-h light/dark cycle. All experiments were performed in accordance with the Jichi Medical School Guide for Laboratory Animals.
X-Gal Staining and Immunohistochemistry in CAG-lacZ Tg Rats
All tissue samples were obtained after the animals were given ether anesthesia. The presence of β-gal was determined by X-gal staining, a standard histochemical method. Liver tissue samples were embedded in an OCT compound (Miles laboratories, Elkhardt, IN) and snap-frozen in liquid nitrogen. Cryostat sections (10 μm) were fixed with 100% acetone for 10 min and incubated with X-gal solution for 2–3 h after the procedure previously described (22). To confirm the presence of β-gal protein, immunohistochemical study of the liver was performed. Liver tissue samples were fixed in 4% paraformaldehyde and embedded in paraffin. The sections (5 μm) were deparaffinized and incubated with a polyclonal rabbit anti-β-gal antibody (Chemicon, Temecula, CA), diluted in phosphate-buffered saline, and then incubated with biotinylated goat anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA) and horseradish peroxidase-conjugated streptavidin (Vector Laboratories). Reactions were visualized using diaminobenzidine (Sigma, St. Louis, MO) as a chromogen in the presence of 0.003% H2O2.
Quantification of β-Gal Protein in Liver
The presence of β-gal in 200 μl of tissue homogenates (containing 500 μg of total protein) was quantitatively analyzed using a sandwich enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim, Mannheim, Germany) procedure. The β-gal ELISA kit (detection limit, 30 pg/ml) was used according to the manufacturer’s instructions.
Gonadectomy
To ascertain the effect of gonadal hormones on transgene expression, 8–10-week-old rats (body weight 120–150 g) were divided into four groups of three animals each: group 1, males that had a sham operation; group 2, females that had a sham operation; group 3, males that were bilaterally castrated; and group 4, females that had a bilateral ovariectomy. Castration and ovariectomy in animal groups 3 and 4 were performed via a lower midline abdominal incision with the animals under ether anesthesia. The sham operation consisted of only cutting and suturing of the lower midline abdomen with the animals under ether anesthesia. After surgery, the rats were returned to cages under normal controlled conditions. Liver biopsies were performed weekly at the same predetermined site.
Administration of Testosterone and Estradiol (E2)
In females, 12-week-old rats weighing about 130–170 g were randomly selected and divided into three groups (four animals each) of control, ovariectomized, and intact female rats, respectively. The group 2 animals were ovariectomized 1 week before the start of hormone manipulation. The control rats were treated with vehicle (corn oil) by intraperitoneal (IP) injection, and both experimental groups were injected with testosterone proprionate (Wako, Japan) 10 mg/kg body weight IP in corn oil daily. Liver biopsies were performed at the same predetermined site 1 week after the start of hormone administration.
In males, the same age of Tg rats were divided into three groups (three animals each). The first two groups of animals were castrated 4 weeks before starting the experiment; these rats were then treated with corn oil (control) and testosterone (same dose/route as in females) to each group, respectively. The third group, intact male rats, received an IP injection of 17β-estradiol (E2) (Sigma Chemical) 10 mg/kg in propylene glycol (Wako Chemical, Japan) every day for 1 week. Liver biopsies were done the same as in female rats.
All liver tissue samples were stained with X-gal to determine β-gal expression, and the number of β-gal-positive cells was counted in five randomly selected fields (100 mm2 area each) under an AX80TR microscope (Olympus, Tokyo, Japan). The testosterone concentration in the blood was also measured for confirmation at the same time the liver biopsy was performed (SRL Company, Tokyo, Japan).
Reverse Transcription-Polymerase Chain Reaction Assay
Total liver RNAs from the Tg males, castrated males, females, and ovariectomized females were isolated using RNAgents kit (Promega, Madison WI) according to the manufacturer’s protocol. cDNA was synthesized using total RNA (5 μg), oligo(dT)12-18 primer (0.5 μg), and 200 units of SuperScript II (Invitrogen) at 42°C for 1 h in 25 μl of buffer, which consisted of 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 0.5 mM each dATP, dGTP, dCTP, and dTTP, and 10 mM dithiothreitol. The transcription products were then treated with RNase H. A negative control was carried out in the same manner, but with the exclusion of reverse transcriptase enzyme. The synthesized cDNA was stored at −20°C until use.
PCR amplification of lacZ reporter gene, endogenous male-predominant genes in rat liver from cytochrome P450s family, CYP4A2 and CYP3A18 gene, and rat glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH; internal control) was performed in this study. The following primers were used for reverse transcription-PCR (RT-PCR) verification of sex hormone-induced gene expression changes: lacZ (forward primer, 5′-TGC CAC TCG CTT TAA TGA TG-3′; reverse primer, 5′-CGT TTC ACC CTG CCA TAA AG-3′) (GenBank Accession No. U89671); GAPDH (forward, 5′-GCT GTG GGC AAG GTC ATC C-3′; reverse, 5′-CTT CAC TAC CTT CTT GAT GTC-3′) (GenBank Accession No. X02231); CYP4A2 (forward, 5′-CCA GCA GGT TCT TAC GTG GG-3′; reverse, 5′-AGC CAC TGT AAG CAG GCA CC-3′) (GenBank Accession No. M33936); CYP3A18 (forward 5′-TTT GGA GTG AAC GTC GAT TCC-3′; reverse, 5′-TCT GCA CGA AGG GAT CCT GT-3′) (Gen Bank Accession No. X79991).
Each 20-μl reaction mixture contained 1× PCR buffer with MgCl2 (Promega, Madison, WI), 200 μm dNTP mix, 1 μl cDNA, 400 nM each of the forward and reverse primers, and 0.5 U of Taq polymerase (Promega, Madison, WI). PCR amplification was initiated by 95°C for 3 min; this was followed by 35 cycles of the following: 30 s for denaturation at 95°C, 30 s for annealing at 55°C, 30 s for extension at 72°C. Final extension step was performed at 72°C for 3 min. PCR reactions were carried out in GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA). To visualize the PCR products, 10 μl of each preparation was subjected to agarose (3%) gel electrophoresis in the presence of ethidium bromide (0.08 (μg/ml).
Statistical Analysis
Quantitative analyses to determine the significance of differences between sexes or between control and experimental groups from target livers were done using the paired Student’s t-test. A level of p < 0.05 was considered statistically significant.
RESULTS
Postnatal Gene Expression of CAG-lacZ Tg Rat in the Liver
Establishment of the CAG-lacZ Tg rat and the expression profile in adult males were described previously (22). In brief, strong lacZ expression was observed in skeletal muscles, myocardium, pancreas, and skin, while moderate expression was observed in the liver, spleen, and kidney. After evaluating postnatal transgene expression, we found that the neonatal liver of these Tg rats exhibited a comparable amount of positive β-gal expression in males (Fig. 1A, a) and females (Fig. 1A, c). The different β-gal expression levels in the liver were based on sex, and persisted until adulthood in the male rats (Fig. 1A, b). In contrast, β-gal expression was downregulated during development in female rats, resulting in no expression in adulthood (Fig. 1A, d). A positive expression pattern in the liver showed random scattered clusters of positive hepatocytes stained with X-gal solution. Immunostained data were used to confirm the presence of β-gal protein in the liver (insets in Fig. 1A, b and d). In particular, we found that the β-gal levels in nonhepatic organs were well matched between males and females (Table 1). These findings strongly suggest that a sex-dependent mechanism regulates hepatic transgene expression.
Figure 1.
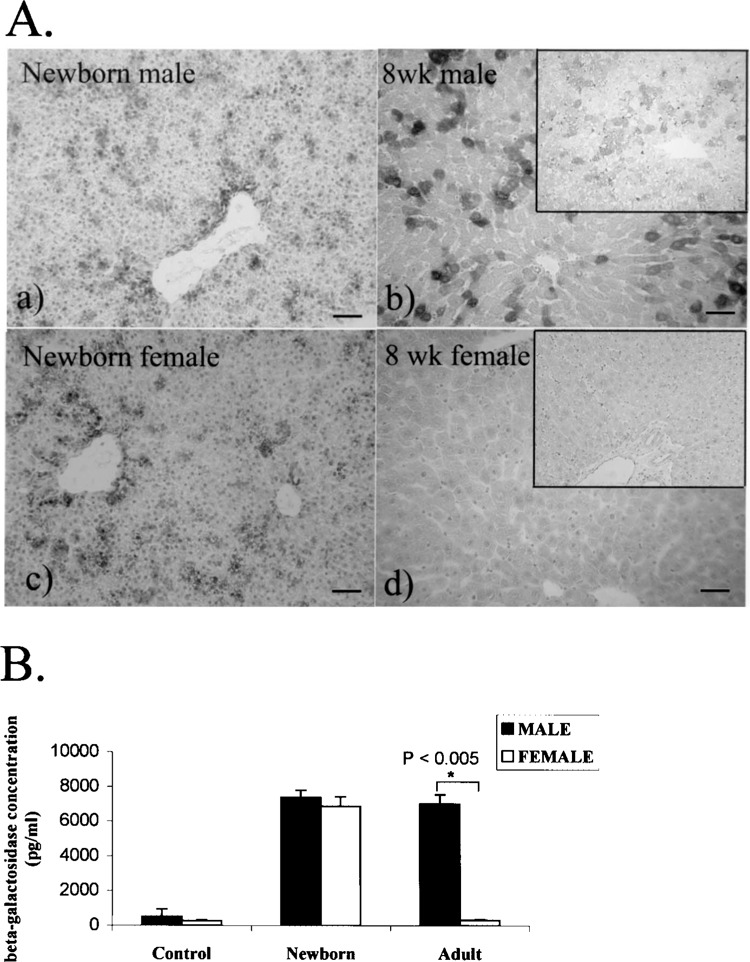
Transgene expression in the liver of CAG-lacZ Tg rats. (A) β-Gal protein was examined by X-gal staining and confirmed by immunohistochemistry (insets). In female neonates (c), the transgene expression is comparable to that in males (a). In 8-week-old adults, β-gal expression is maintained in male Tg rats (b), whereas it is lost in females (d). Scale bars: 100 μ. (B) Bars represent the mean ± SD of β-gal concentration. Liver lysates from adult non-Tg rats, neonates, and 8-week-old CAG-lacZ Tg rats of both sexes (n = 3 each) were quantitated using an ELISA method. Neonatal expression does not differ significantly between sexes. However, in adulthood, males have significantly higher (22-fold) β-gal expression than females (*p < 0.005).
TABLE 1.
β-GAL EXPRESSION IN CAG-lacZ Tg RATS AND TESTOSTERONE CONCENTRATION IN THE CIRCULATION
| Sex | Procedure | Liver | Brain | Heart | Muscle | Kidney | Testosterone Concentration (Mean ± SD ng/ml, n = 3 Each) |
|---|---|---|---|---|---|---|---|
| Male | none | ++ | − | +++ | +++ | ++ | 0.83 ± 0.2 |
| Male | castration | − | − | +++ | +++ | ++ | <0.01 |
| Female | none | − | − | +++ | ++ | ++ | <0.01 |
| Female | ovariectomy | − | − | +++ | ++ | ++ | not tested |
β-Gal and testosterone were measured 8 weeks after bilateral castration or ovariectomy. −, negative transgene expression; ++, strongly positive, not all cells; +++, strongly positive in all cells.
Figure 1B shows the results of quantitative measurement of β-gal protein by ELISA. The neonatal β-gal level was not significantly different between the male and female Tg rats (p = 0.38, n = 3 each). A striking difference was seen in adulthood when liver transgene expression in females did not differ significantly from the β-gal level of expression in nontransgenic littermates that served as negative controls (n = 3) but was 22-fold lower than male rats (p = 0.001, n = 3 each).
Decreased β-Gal Level in Adult Male Livers After Castration
Because sex-dependent differences in β-gal levels were found only in the liver and the difference was much higher in males than in females, we hypothesized that sex hormones, especially testosterone, regulate the transgene expression in the liver. According to this hypothesis, the rats were divided into four groups, as described previously, and bilateral gonadectomy was performed to alter the physiologic level of gonadal hormones and evaluate changes in β-gal. The β-gal level in the group of castrated male rats detected by X-gal decreased dramatically 2 weeks after castration (Fig. 2B) compared with before gonadectomy (Fig. 2A), and was undetectable after 4 weeks (Fig. 2C). In contrast, the expression in ovariectomized rats (Fig. 2D–F) remained undetectable in the liver throughout the period of evaluation.
Figure 2.
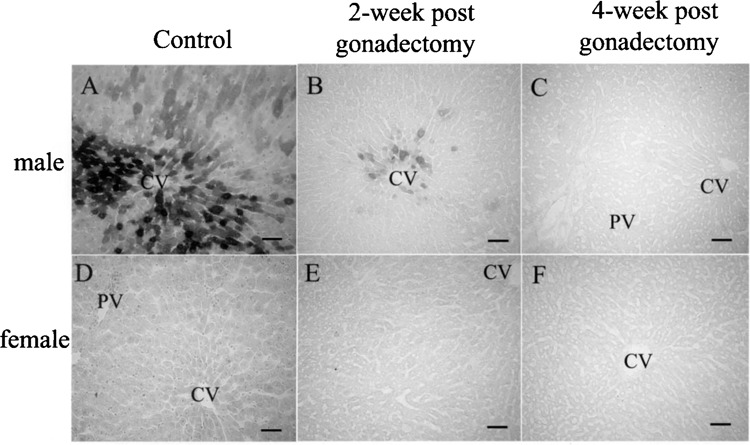
Histological changes of hepatic transgene expression after gonadectomy detected by X-gal. In male rats (A–C), compared with before gonadectomy (A), the β-gal level decreased significantly by the second week and disappeared by the fourth week (B and C, respectively) after castration. Ovariectomized rats after 2 and 4 weeks (E and F, respectively) remained negative as in the intact females (D). Bars: 100 μm.
We further quantified the change of β-gal expression in the liver. The β-gal concentration was analyzed in liver lysates from all experimental groups using an ELISA assay (Fig. 3). The β-gal in castrated rats was significantly lower after 2 weeks (p = 0.009) and was much lower after 4 weeks (p = 0.0008), but there was no significant change of expression in the ovariectomized group or in both the intact male and intact female rats. These quantitative data agree with the histological observation (Fig. 2) and suggest that testosterone perpetuates hepatic β-gal expression.
Figure 3.
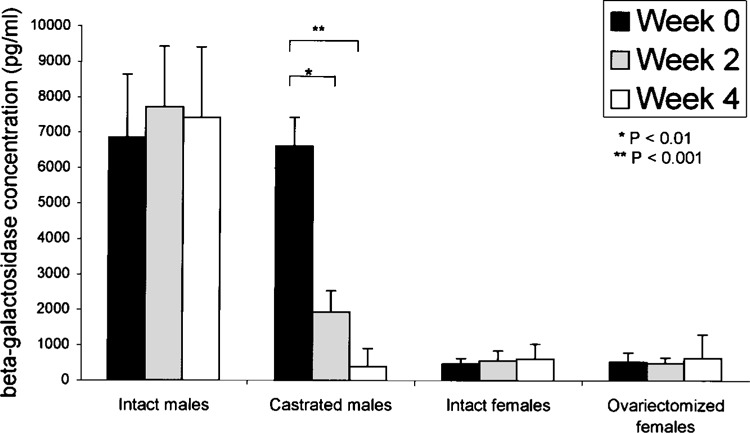
Quantitative analysis of transgene expressions in liver after gonadectomy. Liver lysates from four animal groups (n = 3 each): group 1, intact male rats; group 2, castrated rats; group 3, intact female rats; and group 4, ovariectomized rats. The animals were analyzed for β-gal concentration by ELISA before gonadectomy and 2 weeks and 4 weeks postgonadectomy, respectively. In castrated male rats, β-gal was significantly lower after 2 weeks (*p < 0.01) and was much lower after 4 weeks (**p < 0.001). There was no significant change at any time point in the ovariectomized rats and in both nongonadectomized groups (p > 0.05).
Changes of B-Gal After Sex Hormone Administration in Male Liver
Testosterone, rather than 5-α dihydrotestosterone, is believed to be a major determinant of extragenital sexual dimorphism (3). To examine the potential role of testosterone in reactivating the transgene-negative hepatocytes, we reintroduced a high dose of testosterone (10 mg/kg) daily for 1 week to the male rats that had been castrated longer than 4 weeks, in comparison to castrated animals injected with vehicle only (Fig. 4A, a). Reexistence of β-gal-positive hepatocytes was observed in castrated rats after testosterone injection for 7 days (Fig. 4A, b), and number of the positive cells was significantly higher than that of the control group (Fig. 4B) (p = 0.0012). Decrease in lacZ expression in castrated Tg rat liver could be reversed by testosterone administration. Additionally, we tested the effect of estrogen hormone by administrating a high dose of E2 (10 mg/kg) into intact males every day for 1 week. The number of β-gal-positive hepatocytes detected by X-gal staining was significantly declined (Fig. 4A, c, 4B) (p = 0.0018).
Figure 4.
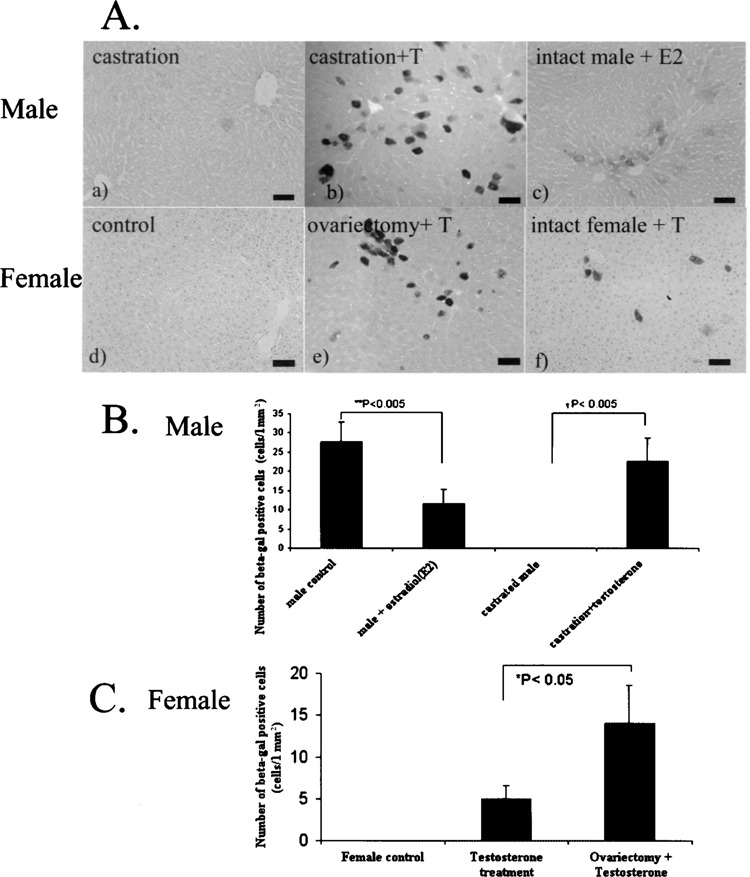
Sex hormones influence hepatic transgene expression in CAG-lacZ Tg rats. In male rats (4A, a–c), injection of testosterone (T) to the castrated animals upregulated β-gal expression (b), compare to the negative expression in the control castrated rats (a). Administration of 17β-estradiol (E2) to intact male rats downregulated the transgene expression (c). In female rats (4A, d–f), administration of testosterone displayed upregulation of β-gal expression, compared to the negative expression in the controlled intact females (d). The ovariectomized rats (e) show higher transgene expression than the intact rats (f) after testosterone injection. Bars: 100 μm. (B) Bar graph represents numbers of β-gal-positive hepatocyts indicated as mean ± SD. Male liver showed increase β-gal-positive cells after reintroduction of testosterone into castrated animals (†p < 0.001), and high-dose 17β-estradiol injection led to the decrease in β-gal-positive hepatocytes, compared to male control (**p < 0.001). (C) Bars represent that the β-gal-positive hepatocytes in intact female rats after testosterone injection was significantly lower than those from ovariectomy plus testosterone group (*p < 0.05).
Reactivation in Female Liver by Testosterone Administration
We also exploited the female rats in order to evaluate transgene expression in those silenced hepatocytes. Results 1 week after daily testosterone administration showed increased β-gal protein detected by X-gal. lacZ-expressing cells were identified in liver tissue from both ovariectomized rats (Fig. 4A, e) and nonovariectomized rats (Fig. 4A, f) in any randomly selected fields of liver sections. The number of β-gal-positive cells in the ovariectomized group was significantly higher than in the nonovariectomized rats (p = 0.032) (Fig. 4C). Because the transgene protein in control animals was undetectable (Fig. 4A, d), the presence of β-gal-positive cells in the liver of both experimental groups could be explained by the injected testosterone. These results clearly showed that testosterone could also upregulate transgene expression in the female liver.
Endogenous Male-Predominant Liver Gene Expressions in CAG-lacZ Tg Rats
In rodent liver, it has been known that CYP expression differs markedly between sexes (25). Here we selected well-characterized examples of male-predominant liver genes (1), CYP4A2 and CYP3A18, to determine whether the intrinsic hepatic gene expressions are altered after sex hormone change by gonadectomy in this Tg rat. Total RNA samples from every rat were evaluated for these genes’ expression semi-quantitatively by RT-PCR. As shown in Figure 5, lacZ mRNA expression was apparently decreased after 4 weeks of castration and unable to be seen in females. Because β-gal protein could not be detected after castration, limitation of detection by X-gal method is believed to be the major cause of difference in mRNA and protein expression levels. On the other hand, while CYP4A2 displayed no obvious change of mRNA level before and after castration, CYP3A18 gene’s mRNA was markedly decreased by gonadectomy. Results indicate the different expression patterns in male-predominant hepatic genes according to different sex hormone level.
Figure 5.
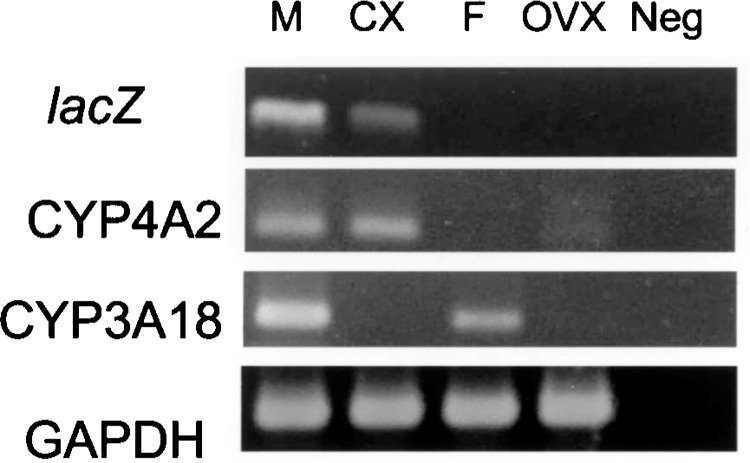
Comparison of lacZ and intrinsic liver gene expressions before and after gonadectomy. lacZ transgene, CYP4A2, and CYP3A18 genes from liver cytochrome P450 (CYPs) family, which are expressed in male-predominant manner, were evaluated semiquantitatively by RT-PCR. The extracted total mRNAs from liver tissues were amplified and PCR products of the above genes and GAPDH (internal control) were shown in an ethidium bromide-stained agarose gel. After castration, mRNAs from lacZ and CYP3A18 were markedly decreased whereas CYP4A2 mRNAs in pre- and postcastrated lanes were comparable. Lane M, adult male rat; lane Cx, 4-week-castrated male; lane F, adult female; lane Ovx, 4-week-ovariectomized female; lane Neg, negative control.
DISCUSSION
In this study we focused on the effect of gonadectomy and testosterone influence on transgene expressions in the livers of CAG-lacZ Tg rats. This rat strain demonstrated a sex-dependent β-gal level only in the liver, which could not be observed in other organs after sex hormone manipulation (data not shown), suggesting liver-specific regulation of sexually dimorphic transgene expression as well as that seen in endogenous male-predominant liver genes. The major observation made in this study was that the status of the transgene could be upregulated in accordance with the testosterone concentration. Liver-specific and testosterone-dependent β-gal expressions are notable phenotypes in this rat.
Furthermore, the results indicated the repression effect of estradiol. Although ovariectomy alone did not affect transgene expression in female rats, external testosterone administration demonstrated that ovariectomized rats significantly gained more β-gal-positive cells than nonovariectomized counterparts. And a high-dose estradiol injection to male Tg rats also led to the decrease of liver β-gal level.
In RT-PCR, we evaluated the expressivity of sex-specific intrinsic liver genes in the rat. After castration, the CYP4A2 gene expression was not decreased whereas mRNAs from lacZ transgene and CYP3A18 were downregulated. These gene expressions displayed their own property after the change of sex hormone level.
In previous studies, male-predominant transgene expression has been sporadically reported (2,6,13,14,16,24). Many investigators identified this phenomenon after generating Tg animals, mainly by using endogenous hepatic genes/promoter-related transgene construct. For example, Li et al. established Tg mice containing the rabbit cytochrome P450 5′-flanking region fused to a luciferase reporter gene, and the transgene was expressed in a male-specific pattern in the liver. Those investigators suggested that their findings were the consequence of a positional effect and/or cis-regulatory element (16). Davidoff et al. (8) and Mochizuki et al. (18) reported their observations that adeno-associated viral vector-5 (AAV5) less effectively transduced the livers of female mice than males. Davidoff and coworkers showed that sex did not have a significant effect on the transduction in nonhepatic organs, and there are tissue- and sex-specific differences in the mechanisms responsible for efficient transduction with this vector, irrespective of transgene factors. Together with this study, our findings support that hepatic transgene expression followed distinct male and female patterns.
Several expressions of endogenous hepatic genes have shown that mammalian livers exhibit sex-dependent patterns in numerous genes during puberty (1,21). These include various genes belonging to different genomic sites and with different functions. Early studies established that growth hormone, the secretion of which shows sex-dependent patterns, is the main cause of sexually dimorphic liver protein expression, and androgen acts indirectly to induce male-predominant gene expression (5,11,20,26). We believe that this endogenous pathway may involve sexual dimorphism of transgene in liver, together with other mechanisms to restrict expression of a foreign gene, leading to different expression of each transgene in each line. However, the regulatory mechanism of sex-different transgene expression pattern in the liver has not been clearly understood. We have been analyzing and proposing that specific positional effect and/or specific epigenetic regulation are responsible for this phenomenon. The promising underlying mechanism of this transgene expression should be further investigated.
Using our CAG-lacZ Tg rats, we showed that sexual dimorphism of transgene expression occurs specifically in the liver. Testosterone is a factor to sustain and mediate upregulation of the reporter gene in this Tg rat.
ACKNOWLEDGMENTS
This study was partly supported by a grant for research on Health Science focusing on Drug Innovation from Japan Health Science Foundation (to E.K.). For further information and any requests for this Tg rat embryo, readers should contact the Health Science Research Resources Bank at hsrrb@osa.jhsf.or.jp.
REFERENCES
- 1. Ahluwalia A.; Clodfelter K. H.; Waxman D. J. Sexual dimorphism of rat liver gene expression: Regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol. Endocrinol. 18:747–760; 2004. [DOI] [PubMed] [Google Scholar]
- 2. al-Shawi R.; Wallace H.; Harrison S., et al. Sexual dimorphism and growth hormone regulation of a hybrid gene in transgenic mice. Mol. Endocrinol. 6:181–190; 1992. [DOI] [PubMed] [Google Scholar]
- 3. Bardin C. W.; Catterall J. F. Testosterone: A major determinant of extragenital sexual dimorphism. Science 211:1285–1294; 1981. [DOI] [PubMed] [Google Scholar]
- 4. Biancifiori C. J. Hepatomas in CBA/cb/Se mice and liver lesions in golden hamsters induced by hydrazine sulfate. J. Natl. Cancer Inst. 44:943–945; 1970. [PubMed] [Google Scholar]
- 5. Carmignac D. F.; Gabrielsson B. G.; Robinson I. C. Growth hormone binding protein in the rat: Effects of gonadal steroids. Endocrinology 133:2445–2452; 1993. [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee B.; Song C. S.; Jung M. H.; et al. Targeted overexpression of androgen receptor with a liver-specific promoter in transgenic mice. Proc. Natl. Acad. Sci. USA 93:728–733; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colby H. D. Regulation of hepatic drug and steroid metabolism by androgens and estrogens. Adv. Sex Horm. Res. 4:27–71; 1980. [Google Scholar]
- 8. Davidoff A. M.; Ng C. Y.; Zhou J.; et al. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 102:480–488; 2003. [DOI] [PubMed] [Google Scholar]
- 9. Emery D. W.; Aker M.; Stamatoyannopoulos, G. Chromatin insulators and positional effects. In: Makrides S. C. ed. Gene transfer and expression in mammalian cells. Amsterdam: Elsevier Science B.V.; 2003:381–395. [Google Scholar]
- 10. Gustafsson J. A.; Mode A.; Norstedt G.; et al. Sex steroid induced changes in hepatic enzymes. Annu. Rev. Physiol. 45:51–60; 1983. [DOI] [PubMed] [Google Scholar]
- 11. Gustafsson J. A.; Mode A.; Norstedt G.; et al. Growth hormone: A regulator of the sexually differentiated steroid metabolism in rat liver. Prog. Clin. Biol. Res. 135:37–59; 1983. [PubMed] [Google Scholar]
- 12. Higginson J. The epidemiology of primary carcinoma of the liver. In: Pack G. T.; Islami A. H., eds. Tumors of the liver. Berlin and New York: Springer-Verlag; 1970:38–52. [Google Scholar]
- 13. Janne M.; Hogeveen K. N.; Deol H. K.; et al. Expression and regulation of human sex hormone-binding globulin transgenes in mice during development. Endocrinology 140:4166–4174; 1999. [DOI] [PubMed] [Google Scholar]
- 14. Keeney D. S.; Murry B. A.; Bartke A.; et al. Growth hormone transgenes regulate the expression of sex-specific isoforms of 3 beta-hydroxysteroid dehydrogenase/delta 5 → 4-isomerase in mouse liver and gonads. Endocrinology 133:1131–1138; 1993. [DOI] [PubMed] [Google Scholar]
- 15. Keller E. T.; Ershler W. B.; Chang C. The androgen receptor: A mediator of diverse responses. Front. Biosci. 1:59–71; 1996. [DOI] [PubMed] [Google Scholar]
- 16. Li H.; Liu S.; Kemper B. Sex- and tissue-specific expression of a cytochrome P450 2C2-luciferase transgene. Mol. Cell. Endocrinol. 120:77–83; 1996. [DOI] [PubMed] [Google Scholar]
- 17. Matsumoto T.; Takagi H.; Mori H. Androgen dependency of hepatocarcinogenesis in TGF transgenic mice. Liver 20:228–233; 2000. [DOI] [PubMed] [Google Scholar]
- 18. Mochizuki S.; Mizukami H.; Ogura T.; et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 11:1081–1086; 2004. [DOI] [PubMed] [Google Scholar]
- 19. Niwa H.; Yamamura K.; Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199; 1991. [DOI] [PubMed] [Google Scholar]
- 20. Norstedt G.; Palmiter R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell 36:805–812; 1984. [DOI] [PubMed] [Google Scholar]
- 21. Roy A. K.; Chatterjee B. Sexual dimorphism in the liver. Annu. Rev. Physiol. 45:37–50; 1983. [DOI] [PubMed] [Google Scholar]
- 22. Takahashi M.; Hakamata Y.; Murakami T.; et al. Establishment of lacZ-transgenic rats: A tool for regenerative research in myocardium. Biochem. Biophys. Res. Commun. 305:904–908; 2003. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi K.; Sereemaspun A.; Inagaki T.; et al. Morphologic characterization of green fluorescent protein in embryonic, neonatal, and adult transgenic rats. Anat. Rec. 274:883–836; 2003. [DOI] [PubMed] [Google Scholar]
- 24. Veniant M.; Menard J.; Bruneval P.; et al. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J. Clin. Invest. 98:1966–1970; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waxman D. J. Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem. Pharmacol. 37:71–84; 1988. [DOI] [PubMed] [Google Scholar]
- 26. Waxman D. J. Growth hormone pulse-activated STAT5 signaling: A unique regulatory mechanism governing sexual dimorphism of liver gene expression. Novartis Found. Symp. 227:61–74; 2000. [DOI] [PubMed] [Google Scholar]


