Abstract
Context:
Platelet-rich fibrin (PRF), platelet concentrate composed of cytokines and structural glycoproteins trapped within polymerized fibrin meshwork, has the potential to accelerate healing of periodontal tissues. Amnion membrane has also attracted a lot of attention in periodontal regeneration due to the presence of variety of growth factors.
Aims:
The aim of the study is to evaluate and compare the regenerative potential of autologous PRF with and without amnion membrane in the treatment of Grade II furcation defects.
Settings and Design:
This was a double-masked randomized, split-mouth design study.
Materials and Methods:
Fifteen patients with thirty mandibular degree II furcation defects were randomly allotted into Group I (PRF and amnion membrane) and Group II (PRF). Clinical parameters such as plaque index and gingival index-at defect site along with probing pocket depth, and relative attachment level and furcation defect depth were recorded at baseline, 3 months, and 6 months postoperatively. Assessment of radiographic parameters was done at baseline and 6 months postoperatively aided by computer-assisted tomography (Dentascan).
Statistical Analysis Used:
For intragroup variations, Wilcoxon signed-rank test, and for comparison between the two groups/intergroup variations, Independent t-test and Mann–Whitney test was performed.
Results:
All clinical and radiographic parameters showed statistically significant improvement at the sites treated with PRF and amnion membrane compared to those with PRF alone.
Conclusions:
Within the limitation of this study, there was greater pocket reduction, attachment level gain, and bone fill at sites treated with PRF and amnion membrane as compared to PRF alone.
Key words: Amnion membrane, autologous platelet-rich fibrin, Grade II furcation defects
INTRODUCTION
Furcation defects present one of the greatest challenges to the success of periodontal tissue regeneration because of its plaque retentive nature, anatomical factors, and difficulty in instrumentation.[1] Review literature shows that molars are more susceptible to attachment loss and are liable to extraction than other teeth.[2] The application of a specific treatment for furcation defect requires a thorough understanding of furcation anatomy, etiologic factors, and the rationale behind different treatment procedures. Several treatment modalities such as tunneling,[3] furcation plasty,[4] odontoplasty,[5] guided tissue regeneration (GTR) using various barrier membranes,[6] bone grafts,[7] and growth factors [8] have been anticipated based on the grade of furcation defect.
Over the past four decades, extensive research has been carried out in evaluating the efficacy of various treatment modalities employed for managing Grade II furcation defects. The improvement in the clinical parameters such as probing pocket depth, clinical attachment levels, and bone defect fill leads to the consideration of GTR better than bone replacement grafts alone as the treatment of choice for Grade II furcation defects.[9] The local application of biological modifiers like enamel matrix derivative [10] and growth factors such as bone morphogenetic protein,[11] platelet-derived growth factor,[12] platelet-rich plasma,[13] and platelet-rich fibrin (PRF)[14] have been widely investigated to be used in the process of regeneration and healing of periodontal tissues in the Grade II furcation defects.
The preparation and use of concentrated suspension of growth factors found in platelets is the latest innovation in regeneration that shows promising results. Choukroun's PRF contains platelets and growth factors (platelet-derived growth factor [PDGF] and transforming growth factor-α [TGF-α]). PRF is autologous platelet concentrate which has all the essential factors for maturation of the soft tissue and regeneration of the bone.[15,16]
The literature on PRF has revealed significant bone gain when used in implant surgeries,[17] sinus-lift procedure [18] and gingival recessions,[19] and facial plastic-surgical treatment procedures.[20] PRF in vitro has shown to act as an appropriate scaffold for breeding human periosteal cells, which may be suitable for applications of bone tissue engineering.[21] Thus, an attempt to use of PRF as a grafting material in the management of Grade II furcation defects was evaluated.
The amniotic membrane is a fetal tissue which consists of a variety of proteins that provide a bioactive matrix to facilitate wound healing, including various collagen types, PDGF, fibroblast growth factor, and TGF-β.[22,23] Amnion-derived cells with multipotent differentiating ability have involved lot of attention in periodontal regeneration and tissue engineering because of their role in each phase of the wound-healing process: Inflammatory, proliferative, and remodeling.[24] Amnion barrier membrane intimately adapts to contours around roots and over furcation defects due to self-adherent property and minimal thickness.[25]
For the treatment of furcation defects, no single regenerative material is considered as the gold standard. PRF and amnion membrane combination was used to enhance the healing potential of bone and soft tissues. Thus, the present study was designed to evaluate and compare clinically and radiographically, the regenerative potential of autologous PRF with and without amnion membrane in the treatment of Grade II furcation defects.
MATERIALS AND METHODS
This 6-month longitudinal interventional study was carried out in the Department of Periodontology. For this study, a total of 15 patients (8 males and 7 females; mean age: 36.1 years) were selected from the outpatient department. The study was conducted from December 2014 to July 2015. After ethical approval from the Review Board and Institutional Ethical Committee of the University, each patient was given detailed verbal and written descriptions of risks and benefits of treatment with the consent to treatment agreement.
The inclusion criteria were the patients exhibiting clinical buccal Grade II furcation defects [26] on contralateral sides of each arch in asymptomatic mandibular molars with probing depth (PD) ≥5 mm and horizontal PD ≥3 mm [Figure 1] and radiographically radiolucency in the furcation area on an intraoral periapical radiograph.
Figure 1.
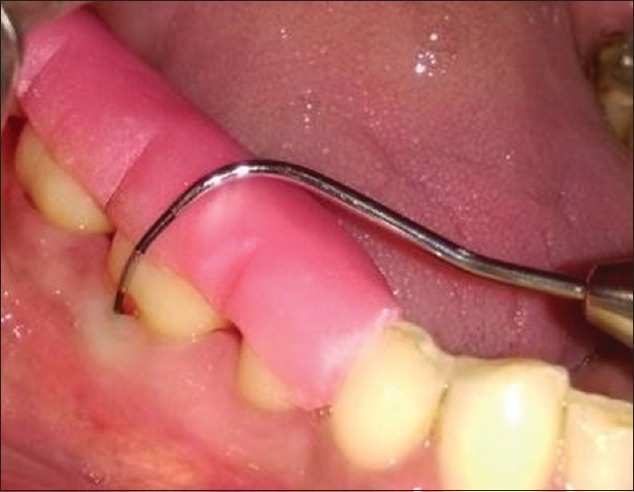
Furcation defect with Nabers probe at baseline
The exclusion criteria for the study were the patients on long-term systemic therapy (anti-inflammatory, bisphosphonates, hormonal replacement therapy) that could influence the bone density, patients with any systemic illness, smokers, alcoholics, and patients with other adverse habits.
Fifteen patients with thirty Grade II furcation defects were selected for the study. Using split-mouth study design, these furcation defect sites were divided randomly into two groups as Group I sites and Group II sites by tossing a coin. Group I sites were treated with autologous PRF and amnion membrane; Group II sites were treated with autologous PRF. Patients were camouflaged for allocation to a particular group and treatment. All surgeries were performed by one operator (S), whereas all clinical and radiographic measurements by another operator (X) without knowledge of the groups. Selected patients of both the groups were subjected to the evaluation of presurgical, clinical, and radiographic parameters aided by computed tomography (CT) Dentascan imaging by the operator (X).
Presurgical therapy
In all selected patients, phase I therapy, that is, full-mouth supra and subgingival scaling and root planing were performed. After 4 to 6 weeks, periodontal evaluation was performed in each selected patient to confirm the desired sites for the study.
Platelet-rich fibrin preparation
Just before surgery, from the patient's antecubital vein, approximately 10 ml of intravenous blood was drawn with a sterilized disposable syringe and was collected in a sterile tubes, without anticoagulant. The blood tubes were centrifuged at 2700 rpm, for 10 min in centrifugation machine at room temperature only.[15] Following centrifugation, the blood was separated into distinct layers. The resultant product could be seen into three divided fractions, namely, acellular platelet poor plasma (PPP) at the topmost, PRF in middle, and red blood corpuscles at the bottom. Just after the removal of PPP, PRF was separated from the red blood corpuscles using sterilize tweezers [Figure 2] and scissors. PRF was then transferred onto a sterile gauze piece.
Figure 2.
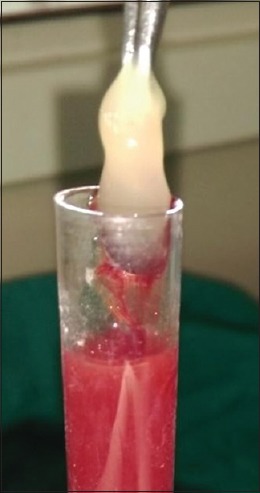
Platelet-rich fibrin retrieved with forceps
Surgical procedure
The selected patients were subjected to surgical procedure under aseptic conditions by operator (S). The selected surgical site was anaesthetized with 2% xylocaine HCL with adrenaline (1:80,000). Buccal and lingual intracrevicular incisions were given at least one tooth mesial and distal to the furcation defect using Bard–Parker knife with blade no. 12. Crevicular incisions were given to allow better coverage of the membrane interproximally and prevent exposure of PRF and membrane. Using periosteal elevator, full thickness mucoperiosteal flaps were reflected on both the buccal and lingual sides. A thorough furcation defect debridement and root planing were done using furcation ultrasonic tips and mini curettes.
PRF was tightly packed and flushed into the furcation defect to the most apical area of the furcation and to the roof of the furcation defect in both group sites [Figure 3] with the help of amalgam condensers. In Group I sites only, required size of amnion membrane was placed over PRF filled furcation defect [Figure 4]. The full thickness mucoperiosteal flaps were repositioned and sutured with interrupted sutures using 3-0 nonabsorbable black silk suture [Figure 5]. Periodontal dressing was placed over sutured surgical site.
Figure 3.
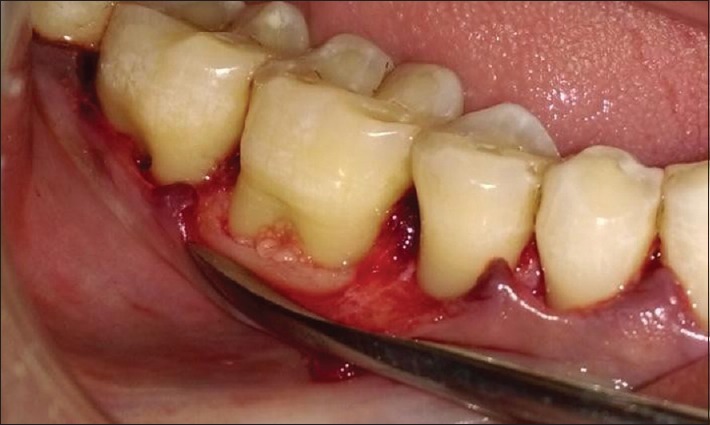
Platelet-rich fibrin placed in furcation defect
Figure 4.
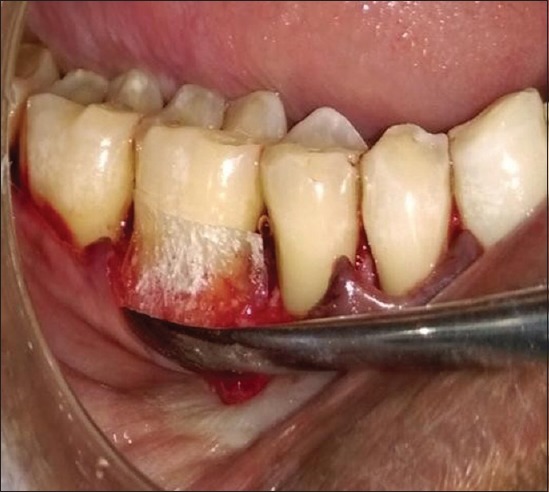
Amnion membrane placed over platelet-rich fibrin filled furcation defect
Figure 5.
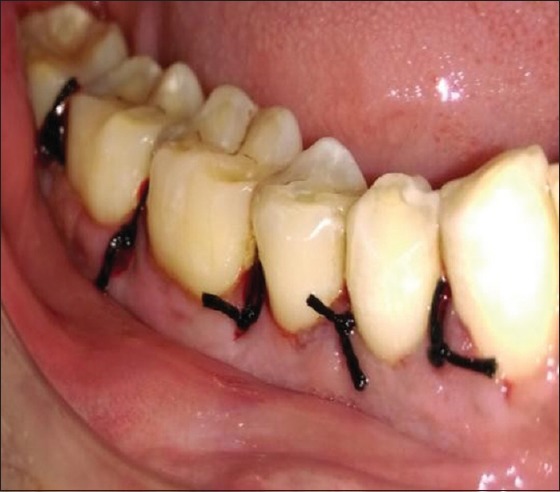
Surgical site sutured
Postoperative care
All patients were prescribed systemic antibiotic capsule amoxicillin 500 mg, four times per day for 5 days and analgesic tablet Ibuprofen 600 mg, three times per day for 3 days. Patients were instructed to rinse with 0.2% Chlorhexidine digluconate mouthwash twice daily for 14 days and other postoperative instructions were also given. Seven days postoperatively, periodontal dressing and sutures were removed. Patients were given proper oral hygiene instructions and gentle brushing with a soft toothbrush thereafter. All the operated patients of both the groups were subjected to the evaluation of clinical parameters at 3 and 6 months [Figures 6 and 7] and radiographic parameters aided by CT Dentascan imaging after 6 months.
Figure 6.
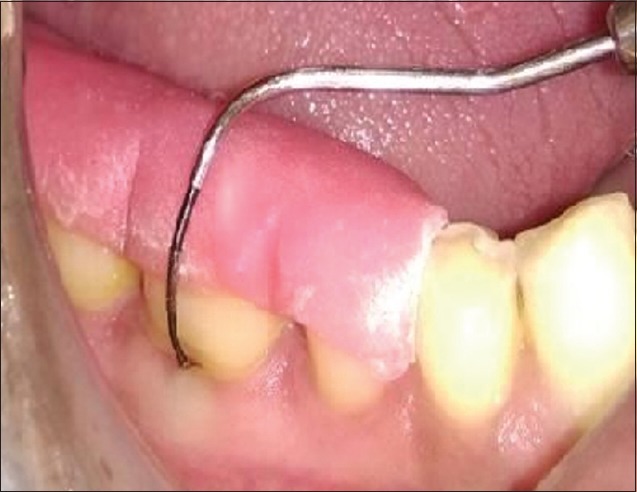
Postoperative furcation defect with Nabers probe after 3 months
Figure 7.
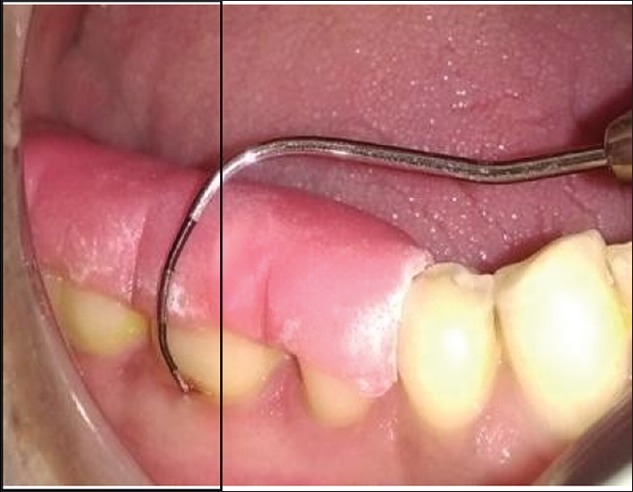
Postoperative furcation defect with Nabers probe after 6 months
For intragroup variations, Wilcoxon Signed Rank test, and for comparison between the two groups/intergroup variations, Independent t-test and Mann–Whitney test were performed.
RESULTS
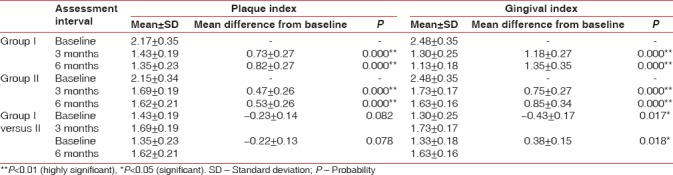
Both groups showed a statistically highly significant reduction in gingival index (GI) and plaque index (PI) score at 3 months and 6 months from baseline. On comparison between Group I and Group II, there was statistically significant reduction in GI scores [Table 1], but in plaque scores [Table 1], reduction was statistically nonsignificant at 3 months and 6 months from baseline.
Table 1.
Mean and mean differences in plaque index, gingival index of Group I, Group II, and Group I versus Group II at different intervals
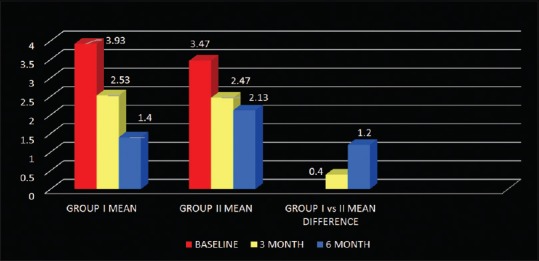
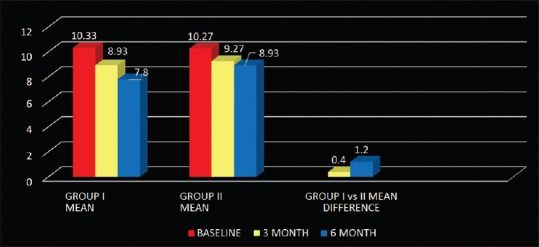
On comparison, between probing pocket depth of Group I and Group II, the mean difference at 3 months was statistically significant and at 6 months highly significant with Group I showing more reduction in probing pocket depth [Table 2 and Figure 8]. The mean difference of relative attachment level of both the groups at 3 and 6 months was statistically highly significant. On comparison, between relative attachment level of Group I and Group II, Group I showed more gain in attachment levels. The mean difference at 3 months was statistically significant and at 6 months highly significant [Table 2 and Figure 9]. Hard tissue parameters including furcation defect depth, defect volume, linear bone growth along with percentage change, and percentage defect fill volume were measured at baseline and 6 months on images acquired through CT scan. Mean difference of furcation defect depth of Group I at 3 and 6 months was statistically highly significant. Mean difference of furcation defect depth of Group II at 3 and 6 months was statistically highly significant. On comparison, between the groups, Group I showed more reduction in furcation defect depth. The mean difference at 3 months was nonsignificant and at 6 months statistically significant [Table 2 and Figure 10].
Table 2.
Mean and mean differences in probing pocket depth, relative attachment level, and furcation defect depth of Group I, Group II, and Group I versus Group II at different intervals
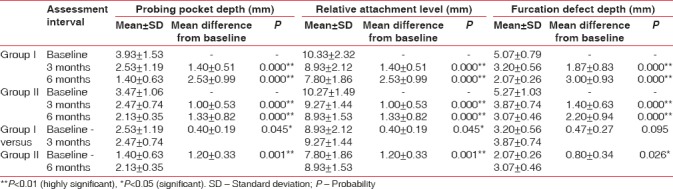
Figure 8.
Mean and mean differences in probing pocket depth of Group I, Group II, and Group I versus Group II
Figure 9.
Mean and mean differences in relative attachment level of Group I, Group II, and Group I versus Group II
Figure 10.
Mean and mean differences in Furcation defect depth of Group I, Group II, and Group I versus Group II
The percentage radiographic horizontal linear bone growth of Group I at 6 months was 46.07 ± 20.97 and of Group II was 31.00 ± 13.33. On comparison between the groups, Group I showed more bone growth. The mean difference of percentage change in the amount of radiographic linear bone growth at 6 months was 15.08 ± 6.41 with t-value 2.349 and P value 0.026 which was statistically significant [Table 3 and Figure 11].
Table 3.
Mean and mean differences in percentage of linear bone growth - horizontal (Dentascan) of Group I, Group II, and Group I versus Group II at different intervals
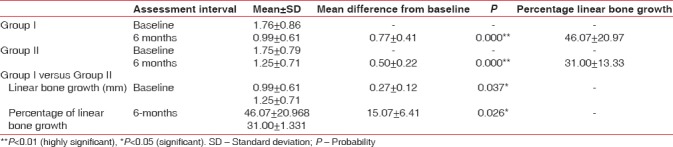
Figure 11.
Mean and mean differences in linear bone growth (horizontal) of Group I, Group II, and Group I versus Group II
In the present study, Group I and Group II showed statistically highly significant mean volume gain from baseline to 6 months (3.73 ± 2.10 and 1.98 ± 0.65 resp.) (P = 0.000). On comparison between volumetric bone gain of both the groups, mean difference at 6 months was 1.75 ± 0.57 (P = 0.005) which was statistically highly significant [Table 4 and Figure 12], showing more volumetric bone gain in Group I.
Table 4.
Mean and mean differences in percentage of volumetric bone gain (Dentascan) of Group I, Group II, and Group I versus Group II at different intervals
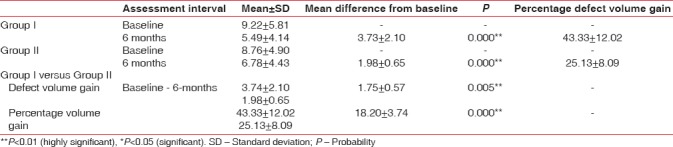
Figure 12.
Mean and mean differences in volumetric bone gain of Group I, Group II, and Group I versus Group II
DISCUSSION
The principal research tool design in periodontal clinical trials is the split-mouth study design. Advantages of using this design study over other designs are – patients serve as their own controls, there is reduction in biasing and number of patients. Both groups were compared under the same treatment protocol and recall visits.[27] The patients being studied were randomly allocated by tossing a coin.[28] The study was double-blinded as the participants were concealed for allocation to a particular group and thus the treatment. That is the reason why all surgeries were performed by one operator (S), whereas all clinical and radiographic parameters were taken by another operator (X) without the knowledge of the groups.
The knowledge of furcation entrance architecture is of utmost importance for successful periodontal regenerative therapy, as it influences the viability of gaining access to the inter-radicular area with mechanical instruments. Bower did a study on maxillary and mandibular first permanent molars and showed that in 58% furcation entrance diameter was 0.75 mm or less.[29] Comparison of the furcation entrance diameter and blade face width of some of the more commonly used periodontal curettes (approximately 0.75 mm) revealed a size disparity thus suggesting that curettes used alone will not achieve adequate preparation of complex furcation defect. That is the reason for using furcation ultrasonic tips and mini curettes in this study.
Radiographic measurements using CT was done to measure the linear horizontal and vertical components and volume gain in the defect. Furthermore, defect mapping with the help of Dentascan software was done in three sections, that is, axial, sagittal, and coronal to obtain volumetric analysis of the defect site [Figures 13–15]. Using above measurements at baseline and 6 months postoperatively, the values of linear bone growth both horizontal and vertical components and bone volume gain at defect site were calculated [Figures 16 and 17]. This is the first study dealing with a combined treatment approach consisting of PRF and amnion membrane in assessing bone fill using Dentascan in Grade-II furcation defects and showing favorable results. Juneja and Bharti [30] also showed a significant bone fill in intrabony defects with platelet-rich fibrin and porous hydroxyapatite bone graft and assessed with volumetric CT scan analysis.
Figure 13.
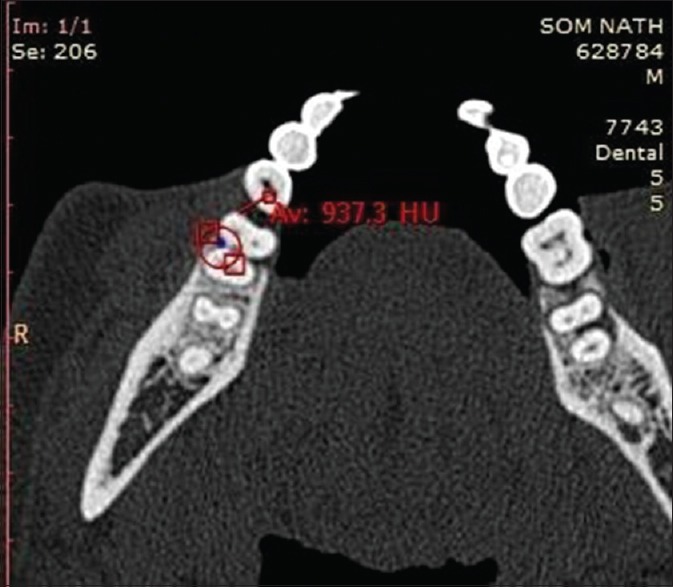
Computed tomography Dentascan image of the axial view
Figure 15.
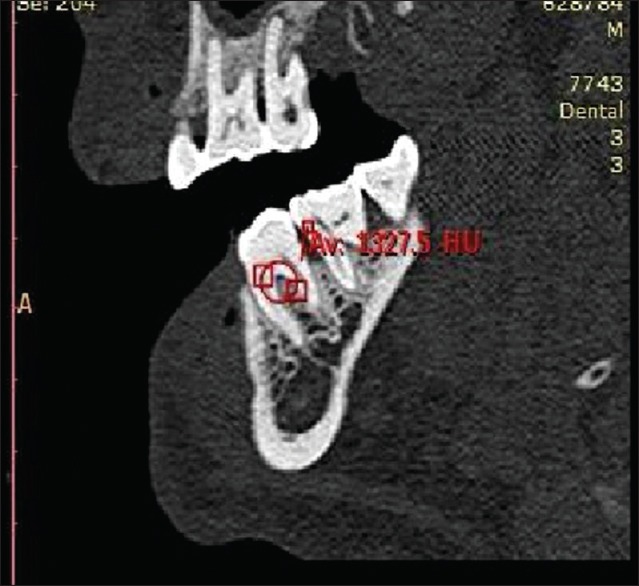
Computed tomography Dentascan image of the coronal view
Figure 16.
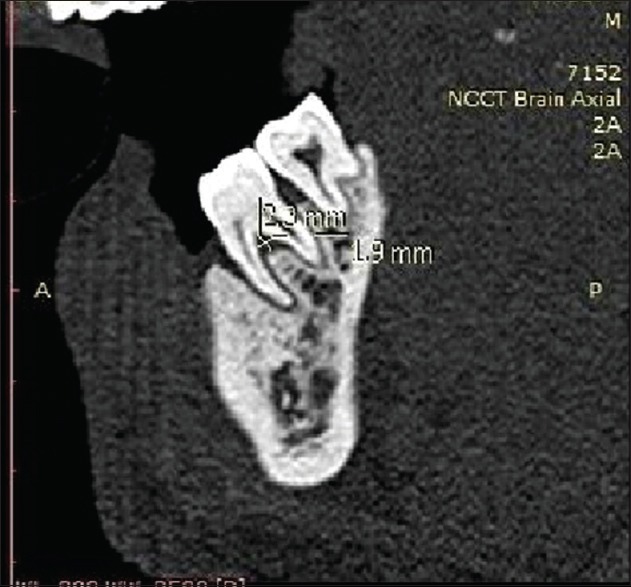
Computed tomography Dentascan image of linear defect depth at baseline
Figure 17.
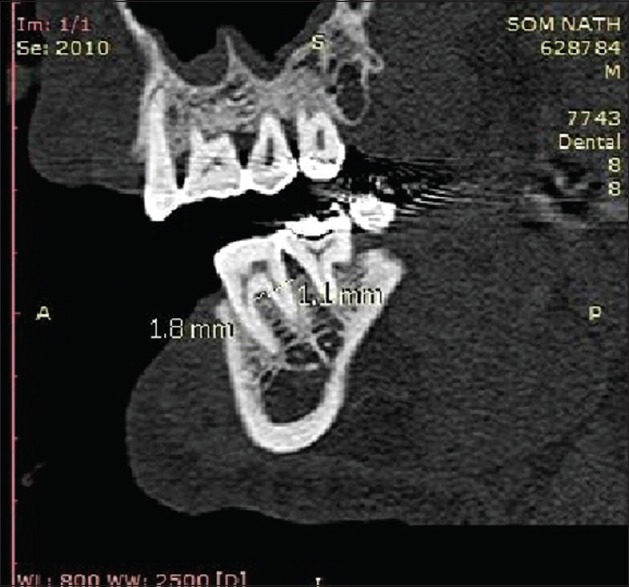
Computed tomography Dentascan image of linear defect depth after 6 months
Figure 14.
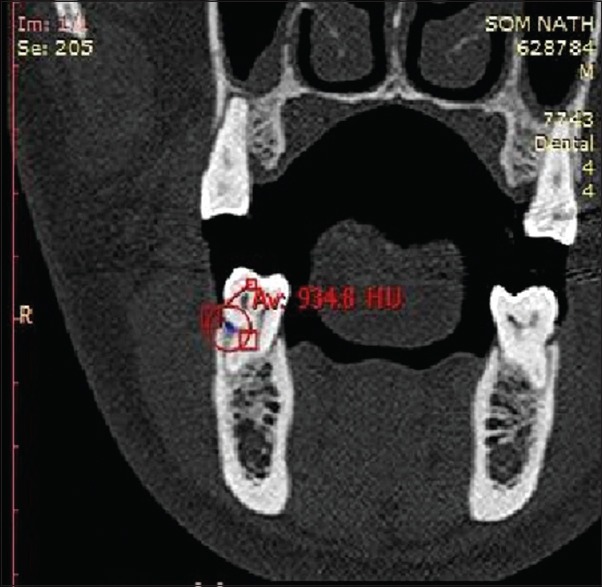
Computed tomography Dentascan image of the sagittal view
PRF preparation has certain other advantages also such as single-stage centrifugation and the absence of bovine thrombin. There is no risk of human to human disease transmission, as it requires patient's own blood, making it a safer treatment modality. Platelet-rich fibrin has a very dense meshwork of fibrin which protect the growth factors from proteolysis and keep them active for longer period of time[16,31] to stimulate regeneration effectively. Platelet-rich fibrin was used as a bone grafting material in Grade II furcation defects in both group sites. It upregulates the expression of phosphorylated extracellular protein kinase and suppresses the osteoclastogenesis by supporting secretion of osteoprotegrin. PRF act as a suitable framework for human periosteal cells and induces the proliferation of osteoblasts.[32] Various healing cytokines such as interleukin-4 and vascular endothelial growth factor present in PRF clot inhibit inflammatory signal pathways and support and coordinate neovascularisation.[33] This explains the uneventful wound healing seen in this study.
Amnion membrane was placed over PRF-filled furcation defect in Group I sites. Amnion membrane consists of pluripotent cellular element embedded in a semi-permeable membranous structure. It maintains the structural and anatomical configuration of the regenerated tissues due to the presence of various pluripotent stem cells having the ability to transdifferentiate to other cellular elements of periodontium. Due to its self-adhering property, sutures are avoided making the procedure simpler and less time-consuming. Amnion membrane intimately molds easily according to the defect anatomy and root surfaces because of it being extremely thin (approximately 300 nm in cross-sectional).[34]
To the best of our knowledge, no study reported the use of autologous PRF with amnion membrane in the treatment of Grade II furcation defects. Thus, a direct comparison with other studies was not possible. The findings in the present study were in accordance with findings of the study carried out by Sharma and Pradeep [14] in the treatment of Grade II furcation defect and those of Aroca et al.[19] in the treatment of gingival recession. Kothiwale et al.[35] observed a statistically significant gain in attachment level 9-month postoperatively when used amnion membrane with DFDBA and bovine-derived xenograft in furcation defects. Combination of PRF and amnion membrane showed synergistic effect and enhanced the clinical outcome.
CONCLUSIONS
The overall observations of the present study were encouraging and showed promising results with significant reduction in clinical parameters with the use of autologous PRF with amnion membrane in the treatment of furcation defects. However, extensive study period needs to be carried out to assess the long-term stability of the results on bone regeneration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Martin M, Gantes B, Garrett S, Egelberg J. Treatment of periodontal furcation defects. (I). Review of the literature and description of a regenerative surgical technique. J Clin Periodontol. 1988;15:227–31. doi: 10.1111/j.1600-051x.1988.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 2.Chace R, Sr, Low SB. Survival characteristics of periodontally-involved teeth: A 40-year study. J Periodontol. 1993;64:701–5. doi: 10.1902/jop.1993.64.8.701. [DOI] [PubMed] [Google Scholar]
- 3.Shah UP, Shah M, Shukla T, Chandran S. Tunnel preparation: Management of class III furcation involving mandibular molars. J Adv Med Dent Sci Res. 2015;3:152–5. [Google Scholar]
- 4.Bathla S. Textbook of Periodontics. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; 2017. Furcation; p. 549.p. 60. [Google Scholar]
- 5.Needleman I. How long do multirooted teeth with furcation involvement survive with treatment? Evid Based Dent. 2010;11:38–9. doi: 10.1038/sj.ebd.6400714. [DOI] [PubMed] [Google Scholar]
- 6.Prathibha PK, Faizuddin M, Pradeep AR. Clinical evaluation of guided tissue regeneration procedure in the treatment of grade II mandibular molar furcations. Indian J Dent Res. 2002;13:37–47. [PubMed] [Google Scholar]
- 7.Yukna RA. Clinical evaluation of HTR polymer bone replacement grafts in human mandibular class II molar furcations. J Periodontol. 1994;65:342–9. doi: 10.1902/jop.1994.65.4.342. [DOI] [PubMed] [Google Scholar]
- 8.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74:1282–92. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 9.Machtei EE, Schallhorn RG. Successful regeneration of mandibular class II furcation defects: An evidence-based treatment approach. Int J Periodontics Restorative Dent. 1995;15:146–67. [PubMed] [Google Scholar]
- 10.Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8:193–204. doi: 10.1902/annals.2003.8.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Jung RE, Glauser R, Schärer P, Hämmerle CH, Sailer HF, Weber FE, et al. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14:556–68. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 12.Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, Hinrichs JE, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: Results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–15. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 13.Pradeep AR, Pai S, Garg G, Devi P, Shetty SK. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J Clin Periodontol. 2009;36:581–8. doi: 10.1111/j.1600-051X.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Pradeep AR. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J Periodontol. 2011;82:1396–403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 15.Choukroun J, Adda F, Schoeffer C, Vervelle A. PRF: An opportunity in perio-implantology. Implantodontie. 2000;42:55–62. [Google Scholar]
- 16.Tsai CH, Shen SY, Zhao JH, Chang YC. Platelet-rich fibrin modulates cell proliferation of human periodontally related cells in vitro. J Dent Sci. 2009;4:130–5. [Google Scholar]
- 17.Diss A, Dohan DM, Mouhyi J, Mahler P. Osteotome sinus floor elevation using choukroun's platelet-rich fibrin as grafting material: A 1-year prospective pilot study with microthreaded implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:572–9. doi: 10.1016/j.tripleo.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, Dohan Ehrenfest DM, et al. Sinus floor augmentation with simultaneous implant placement using choukroun's platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J Periodontol. 2009;80:2056–64. doi: 10.1902/jop.2009.090252. [DOI] [PubMed] [Google Scholar]
- 19.Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 20.Sclafani AP. Applications of platelet-rich fibrin matrix in facial plastic surgery. Facial Plast Surg. 2009;25:270–6. doi: 10.1055/s-0029-1242033. [DOI] [PubMed] [Google Scholar]
- 21.Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J, Becker ST, et al. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543–9. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 22.Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8:295–308. doi: 10.1089/107632702753725058. [DOI] [PubMed] [Google Scholar]
- 23.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 24.Chopra A, Thomas BS. Amniotic membrane: A novel material for regeneration and repair. J Biomim Biomater Tissue Eng. 2013;18:1–8. [Google Scholar]
- 25.Holtzclaw DJ, Toscano NJ. Amnion-chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: A retrospective observational report. Clin Adv Periodontics. 2013;3:131–7. [Google Scholar]
- 26.Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–35. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 27.Hujoel PP, Loesche WJ. Efficiency of split-mouth designs. J Clin Periodontol. 1990;17:722–8. doi: 10.1111/j.1600-051x.1990.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 28.Ranjith G. Interferon-alpha-induced depression: When a randomized trial is not a randomized controlled trial. Psychother Psychosom. 2005;74:387. doi: 10.1159/000087787. [DOI] [PubMed] [Google Scholar]
- 29.Bower RC. Furcation morphology relative to periodontal treatment. Furcation entrance architecture. J Periodontol. 1979;50:23–7. doi: 10.1902/jop.1979.50.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Juneja G, Bharti V. Treatment of periodontal intrabony defects with platelet-rich fibrin and porous hydroxyapatite bone graft: A comparative clinical and radiographic study using dentascan. Saint Int Dent J. 2015;1:22–7. [Google Scholar]
- 31.Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16:356–63. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang IC, Tsai CH, Chang YC. Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J Biomed Mater Res A. 2010;95:327–32. doi: 10.1002/jbm.a.32839. [DOI] [PubMed] [Google Scholar]
- 33.Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in choukroun's platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27:63–9. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- 34.Shetty SS, Chatterjee A, Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18:102–6. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kothiwale SV, Anuroopa P, Gajiwala AL. A clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]


