Abstract
Background:
Photodynamic therapy (PDT) has developed as an alternative treatment modality in periodontitis patients. Different photosensitizers used over the years have shown contradictory results. Thus, recently indocyanine green (ICG)-mediated photothermal therapy has emerged for the treatment of chronic periodontitis.
Aim:
The present study aimed at comparing and evaluating the effects of photothermal therapy using ICG in the treatment of chronic periodontitis with scaling and root planing (SRP).
Materials and Methods:
This was a randomized, controlled, clinical trial where fifty participants were equally divided into two groups, i.e., control group (SRP) and test group (SRP + photothermal therapy). Clinical parameters were evaluated at baseline and 6-month follow-up. These were plaque index (PI), bleeding on probing (BOP), probing depth (PD), and clinical attachment level (CAL). Microbiological analysis of plaque sample was also done to check for anaerobic mixed flora.
Results:
Significant reduction was seen in PD, CAL, and BOP in the test group as compared to control group after 6 months (P < 0.05). However, intergroup comparison of PI showed nonsignificant results (P > 0.05). Anaerobic culture of plaque samples of test group also revealed a significant reduction of microorganisms in comparison with control group.
Conclusion:
ICG-mediated photothermal therapy can act as an alternative to antimicrobial PDT as an adjunct to SRP in the treatment of chronic periodontitis.
Key words: Chronic periodontitis, indocyanine green, photodynamic therapy, photothermal therapy, scaling and root planing
INTRODUCTION
Periodontitis is a multifactorial disease that is associated with loss of the periodontal ligament and alveolar bone around the tooth which may lead to tooth loss.[1] Primary objective of periodontal therapy is complete removal of supragingival and subgingival deposits present on the root surface which helps to stop disease progression.[2] The mechanical debridement of the root surface can be achieved either with the help of hand instruments or power-driven instruments (sonic and ultrasonic). In majority of cases, this gold standard nonsurgical therapy brings about the improvements in clinical parameters.[3] However, mechanical nonsurgical has some limitations such as incomplete removal of subgingival bacteria, particularly those invaded into periodontal tissues or in deep periodontal pockets.[4] As a result, recolonization of bacteria occurs resulting in a delay of periodontal healing process.[5]
Various antimicrobials or antiseptic agents (local or systemic) are used as an adjunct to mechanical nonsurgical therapy to overcome their limitations. However, their efficacy is limited due to the development of bacterial resistance.[6] As a result, search is on constantly for alternative medicine in periodontal treatment approaches.
Photodynamic therapy (PDT), a novel noninvasive photochemical approach for infection control, has emerged as an adjunctive to scaling and root planing (SRP).[7] It is based on the use of lasers (a light source) and photosensitizers, which gets activated in the presence of oxygen by light of specific wavelength. This leads to release of free radicals and singlet oxygen causing the destruction of bacteria.[8,9] This bactericidal action of PDT is unlikely to develop bacterial resistance as their activity is due to release of singlet oxygen which targets cellular contents.[10,11]
However, to date, there is no conclusive evidence present regarding the efficacy of PDT as an adjunct to therapy as suggested by recent systematic reviews.[12,13] Although majority of studies have reported improvements in probing depth (PD) reduction and clinical attachment level (CAL) gain, this effect is said to be moderate as compared to SRP.[14,15] In addition, no benefits were seen in the microbiological profile of the periodontitis patients.[16,17] Possible reasons for this might be the heterogeneity of photosensitizers and lasers' systems used. Thus, authors have concluded to conduct more clinical trials with longer follow-up.[12,13,14,15,16,17] Action of PDT is based on the activation of photosensitizers on irradiation with light in the presence of oxygen.[8,9] One thing to be taken into consideration in periodontal conditions is the lack of the presence of oxygen in subgingival area which might lead the PDT with the help of conventional photosensitizers (ex., toluidine blue O and methylene blue) to be ineffective.
Therefore, a new photosensitizer called indocyanine green (ICG) has been developed which gets activated upon absorbing light of specific wavelength.[18] ICG was granted approval by the United States Food and Drug Administration in 1959. It is widely used clinically for diagnostic purposes such as angiography in ophthalmology, monitoring of liver function and splanchnic perfusion, perfusion-related analysis of tissues and organs, sentinel lymph node biopsy, and diagnosis of rheumatic diseases.[19] Apart from its diagnostic applications, it is also used in PDT because of its ability to absorb light around 810 nm wavelength which is closer to the absorption peak of most diode lasers. ICG-mediated PDT is known to act without oxygen for its activation and releasing free radicals and singlet oxygen.[19] This ICG-mediated therapy is thus called as photothermal therapy. The fact that ICG does not require oxygen can make it even more effective in anaerobic subgingival area as compared to other photosensitizers and used for the treatment of periodontitis. Although its use in medical field is well known, it has been recently explored in periodontal research. In a study done by Srikanth et al.,[18] it was suggested that ICG-mediated PDT can provide potential therapeutic benefit in the treatment of chronic periodontitis patients. Similar studies conducted by Monzavi et al.,[11] Shingnapurkar et al.[20] have also evaluated the effect of ICG as a photosensitizer and has found positive results.
Due to the contradictory results among the studies regarding additional benefit of conventional PDT as an adjunctive to SRP, this study was designed to evaluate the additional clinical and microbiological effect of ICG-mediated photothermal therapy in the treatment of chronic periodontitis. Thus, the aim of the current clinical trial was to compare and evaluate the clinical and microbiological effect of ICG-mediated photothermal therapy as an adjunct to SRP in the treatment of chronic periodontitis. The null hypothesis was that the use of ICG might result in added benefit in treatment of chronic periodontitis.
MATERIALS AND METHODS
Sample size calculation
The sample size calculation determined that 44 participants for each treatment group would provide 90% power that would detect a true difference of 1 mm between test and control group. PD reduction was used as primary outcome variable. However, to compensate for possible dropout during the follow-up period, a convenient sample size of 25 patients per group was included in the study. Thus, a total of 50 subjects (28 males, 22 females age range 30–55 years) diagnosed with chronic periodontitis based on the criteria given by Armitage in 1999[21] were selected for the study from outpatient Department of Periodontology.[21,22] Ethical clearance was obtained from the Institutional Ethical Committee and written informed consent was signed by the patients.
Intraexaminer calibration
All the periodontal evaluation and sample collection were performed by an experienced calibrated examiner. The examination was performed at six sites per tooth and accepted if >90% of measurements were reproduced with 1 mm of difference with respect to per tooth 48 h apart. The examiner who performed measurements were blinded to the type of treatment given to the subjects and other examiner performed all treatment procedures.
Selection criteria
Inclusion Criteria for the study was (i) chronic periodontitis patients with probing pocket depth (PPD) >5 mm and CAL >4 mm, (ii) systemically healthy patients, and (iii) patients not receiving any antibiotic therapy or had not received any periodontal therapy 12 months before the study. Exclusion criteria were patients with aggressive periodontists, pregnant and lactating females, smokers and tobacco chewers, and previous history of allergic reaction to the use of any kind of dye.
Study design and parameters evaluated
The present study was a randomized, single-blind, controlled clinical trial. Randomization was performed by the lottery method, and patients were equally divided into two groups; the control group: (SRP only) and the test Group (PDT + SRP). Randomization process was done after the examiner was calibrated and before SRP. Clinical and microbial analysis was done at baseline and after 6 months.
Clinical parameters which were evaluated at baseline and 6-month follow-up were plaque index (PI),[23] bleeding on probing (BOP), PPD, and CAL. PPD was primary outcome variable and recorded using a University of North Carolina no. 15 periodontal probe (Hu-Friedy®) and a custom-made acrylic stent was used to standardize the measurement of site-specific PPD. CAL was calculated as the distance between the cementoenamel junction and base of the periodontal pocket. BOP was examined based on the presence or absence of bleeding after 30 s.[24] After examination, all the patients received through instrumentation of the root surface using both hand instruments (Gracey Curettes; Hu-Friedy) and ultrasonic device.
Laser parameters
The laser system used in the present study was diode laser (GaAlAs) with wavelength of 810 nm. Laser was applied in a continuous mode with a power of 0.8 Watts and irradiation time of 60 s. Total energy produced was 5.4 J/cm2.
Preparation of 5 mg/mL indocyanine green solution
Under sterile conditions, ICG aqueous solutions are unstable and should be used within 24 h. Hence whenever required, a fresh 5 mg/mL solution was prepared as follows:
ICG (Aurogreen®, Aurolabs, Madurai, India) was dissolved in 5 mL of sterile water to prepare an initial 5 mg/mL ICG stock solution. The stock ICG solution was further diluted in saline solution at the ratio of 1:5 to achieve the final ICG concentration of 5 mg/mL.[18]
Microbial sampling
Microbial sampling was done after SRP. The selected teeth were isolated with cotton rolls. A sterile paper point was inserted slowly with a sterile dental Tweezer into the pocket until the tissue resistance was felt. The paper point was left for 30 s and then it was carefully removed without touching the adjacent unrelated tissues and placed into a special sterile test tube containing Robertson's cooked meat broth medium. The medium was either freshly prepared. To prevent the contamination of the media with the aerobic bacteria, the test tube was filled with 2 mL of paraffin oil, a cotton plug was placed. Samples were inoculated on blood agar plates. Plates were incubated in an anaerobic condition at 37°C for 48 h.[25]
Treatment procedure
In test group, thorough instrumentation of the root was done and exposed to photothermal therapy. For photothermal therapy, 0.5–1 ml ICG was delivered into the pocket with the help of blunt needle. After a period of 60 s, pocket was thoroughly rinsed using saline solution to remove excess photosensitizer from the pocket.[18] Immediately after rinsing, the diode laser, with 810 nm wavelength and 0.8 W of power output, equipped with a probe tip, was placed at the depth of the pocket, and moved circumferentially around the tooth for 1 min. In the control group, SRP was performed and received off diode laser with physiological serum into the pocket.
Statistical analysis
Statistical analysis of the results was done using SPSS software version 20.1 (Chicago, IL, USA.) The unpaired Student's t-test was used for continuous variables after confirming normality of the data distribution. The method of Kolmogorov and Smirnov was used to confirm that the data were sampled from a Gaussian distribution. Fisher's exact tests were used for the comparison of BOP in both the groups. Statistical significance was defined as P < 0.05.
RESULTS
Forty-five patients (n = 5: failed to follow-up) completed 6-month study. Healing took place without any complications [Figure 1]. No side effects like staining of adjacent mucosa or teeth were reported from the subjects enrolled in the test group. These findings are in agreement with in vitro study conducted by Boehm and Ciancio 2011[26] where they found that application of ICG caused no staining of teeth surface apart from staining of calculus.
Figure 1.
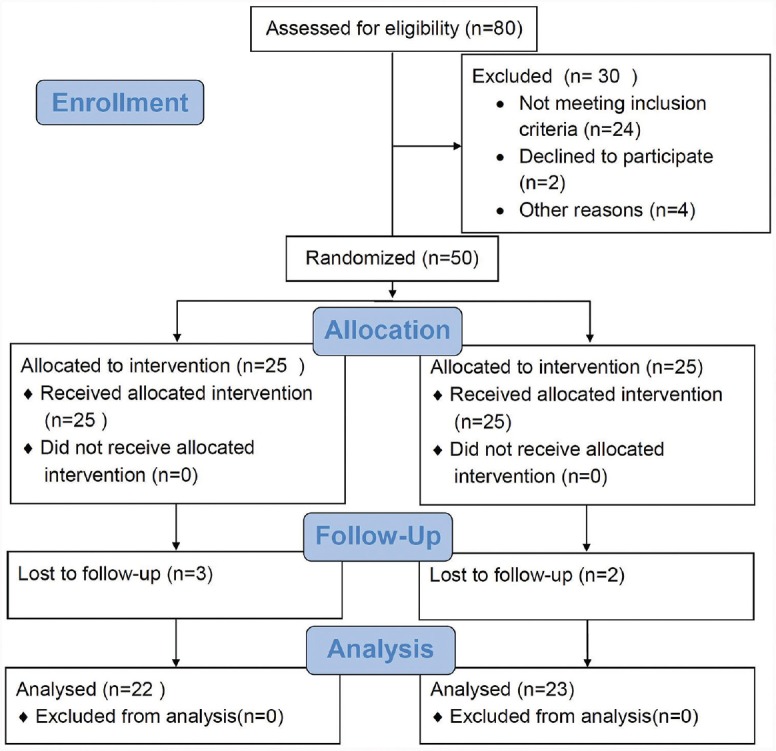
Consort flowchart
Demographic characteristics of the test and the control groups are shown in Table 1.
Table 1.
Demographic characteristics of the subjects at baseline

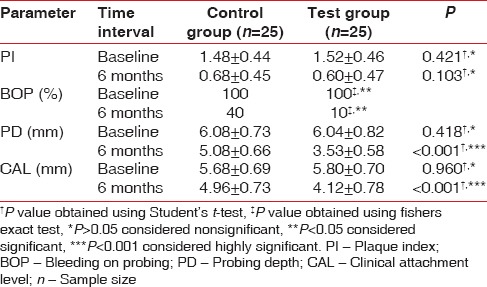
At baseline, there was no difference between the test and the control group with respect to all the clinical parameters (PI, BOP, PD, and CAL). On intergroup comparison, it was found that after 6 months, there were only 10% and 40% sites presenting BOP in test and control group, respectively. This difference was statistically significant [Table 2]. On intragroup comparison, both groups showed statistically significant improvement with respect to BOP sites after 6 months. On comparing PI scores, it was found that there was no difference between two groups at any point of time (P > 0.05) [Table 2]. However, there was a statistically significant reduction in PD and gain in CAL after 6 months in test group as compared to control group [Table 2].
Table 2.
Intergroup comparison of mean±standard deviation of all clinical parameters at baseline and 6 months
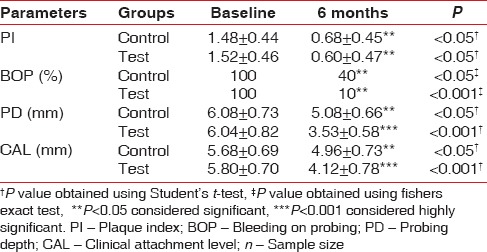
On intragroup comparison, it was found that there was a significant improvement in all parameters assessed after 6 months in both the test and the control group.(P < 0.05) [Table 3].
Table 3.
Intragroup comparison mean±standard deviation of clinical parameters at baseline, and 6 months
Results of the bacterial culture and microscopic examination are shown in figure [Figures 2 and 3]. There was marked reduction in a number of anaerobic bacterial colonies evident in both the cultured plates and microscopically [Figures 2 and 3]. At baseline, the CFU/ml was 6.11 × 106 CFU/ml in test site and 6.00 × 106 CFU/ml in control site. No significant difference was present between the two groups at baseline. The CFU/ml was reduced to 3.57 × 105 CFU/ml control site, whereas in test site to 2.15 × 105. Statistically significant reduction was also observed in the CFU from baseline to 6 months at test and control site with P < 0.05 [Graph 1]. However, mean reduction was greater in test group compared to control group at 6 months.
Figure 2.
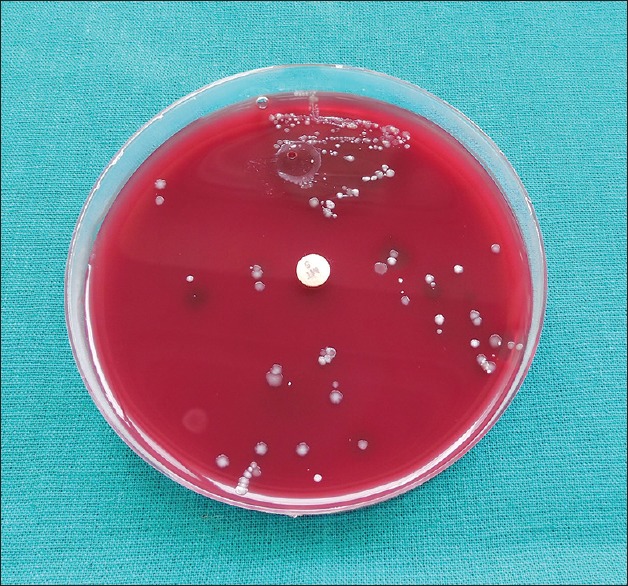
Colony-forming units in control site after 6 months
Figure 3.
Colony-forming units in test site after 6 months
Graph 1.
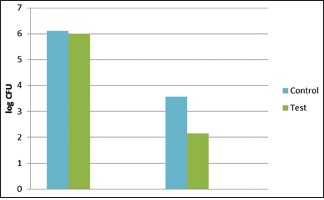
Changes in colony-forming units
DISCUSSION
Antimicrobial PDT (aPDT) was first utilized in medicine as a method for the treatment of cancer over a century ago.[27] PDT involves the application of a photosensitizer to a target area; it gets activated on absorbing light of specific wavelength. This produces reactive oxygen species which are cytotoxic effect to the cells. Different photosensitizer has specific absorption spectrum, for example, methylene blue – 675 nm, toluidine blue O – 675 nm, and ICG – 810 nm. Recently, many studies have been conducted where they have evaluated the efficacy of aPDT with different photosensitizing dyes most commonly methylene blue and toluidine blue O in periodontal treatment.[28,29,30] Although studies have been conducted using conventional photosensitizers, results are mostly contradictory and no conclusive evidence has been provided regarding their efficacy. Thus, new photosensitizers have been developed such as ICG, which is known to get activated in the absence of oxygen. There are very few studies investigating the effects of ICG photosensitizer as an adjunct to SRP in the treatment of chronic periodontitis.[11,18,20]
The present study was a single-blind randomized clinical trial, which evaluated the effects of ICG-mediated photothermal therapy in chronic periodontitis patients clinically and microbiologically. Results of the present study suggest that there was a significant improvement in BOP, PPD, and CAL in test group as compared to the control group after 6-month posttreatment. On comparing the PI scores, it was found that both the test and the control group showed significant improvement after 6 months from baseline.
After 6-month evaluation, there were 10% and 40% sites present with BOP in test and control group, respectively. This suggests that there was 90% reduction of bleeding sites in test group, whereas in control group, there was only 60% reduction. Improvement in BOP scores in test group suggests that combination of photothermal therapy and SRP results in better resolution of inflammation as compared to SRP alone. These results are comparable to the previous study done by Monzavi et al., 2007,[11] where they found that PDT and SRP resulted in a significantly greater reduction in bleeding scores compared with SRP alone over a period of 6 months. Several other studies have also concluded that PDT and SRP have an added advantage over SRP alone in reduction of BOP and thus providing the evidence that combination of PDT + SRP results in better resolution of inflammation.[29,30]
A significant reduction was also noted in relation to PPD in the test group as compared to control group. These results are comparable to the previous studies where they found that there was a significant reduction in PD after PDT + SRP.[5,29,30] Alwaeli et al., 2015,[5] concluded that there was a significant reduction in pocket depth of 1.51 + 1.54 (mm) in test group as compared to control group, where it was around 0.66 + 1.66. However, some studies contradict the changes in PPD reduction where they have concluded that PDT and SRP do not have any added advantage over SRP.[31,32] These variations in the results obtained are difficult to interpret because of heterogeneity in study designs and variety of photosensitizers with different wavelength of laser used.
Considering the CAL scores, there was a significant gain in CAL in test group in the present study as compared to control group. This resultant gain in attachment must be due to resultant decrease in PD as there was clinically irrelevant gingival recession. Although there was a decrease in PD and gain in CAL, we cannot comment upon the type of attachment occurred. However, it is most likely to be due to long epithelium formation apart from other contributing factors such as removal of local factors and resultant reduction in inflammation.[29,33] In a recently conducted randomized clinical trial by Segarra-Vidal et al., 2017,[29] significant gain in CAL was found in SRP and PDT group. Although SRP also showed significant CAL gain, mean CAL gain was more in PDT + SRP group. The resultant gain in CAL in the present study is consistent with findings of some other studies.[34] However, there is still some controversy regarding the attachment gain after the application of PDT. This has been concluded in various studies where they have said that PDT does not have any effect on the attachment gain.[17]
Considering PI scores, there was no additional benefit found from aPDT as an adjunctive treatment for patients with chronic periodontitis. There was a significant improvement in PI scores in both the groups after 6-month period. However, it needs to be kept in mind that changes in PI scores are dependent on patients' compliance and the fact that the PI scores were improved significantly in both groups in the present study suggests that patients had properly maintained the oral hygiene. It can also be suggested that enhanced oral hygiene maintenance might have occurred due to Hawthorne effect.[35]
In the present study, microbiological analysis for the anaerobic bacteria present in the periodontal pocket was also done. Marked reduction was noted in the colony-forming units after both SRP and PDT and SRP. However, a mean reduction of CFU/ml was greater in test group. These results are in agreement with the previous studies done by Srikanth et al.[18] where they found that there was a significant reduction in the amount of anaerobic pathogens in PDT group and laser group. This suggests that there is a reduction in total number of anaerobic flora. Although this was quantitative estimation, still an important factor as a reduction in total anaerobic bacterial load is a major determinant of periodontal health. This might be possible due to potential photothermal effect of ICG enhancing the effects of lasers. Although few studies conducted, evaluating the effect of PDT on specific pathogen has demonstrated contradictory results.[16,17] One possible reason for this may be the inability of other photosensitizers (methylene blue and toluidine blue) to get activated in anaerobic environment subgingivally as compared to ICG which can get activated without oxygen also. Thus, further studies using ICG should be conducted to strengthen the evidence regarding the same.
To the best of our knowledge until now, only three studies have been conducted evaluating the effect of ICG as a photosensitizer in treatment of chronic periodontitis. The results of the present study are consistent with previous studies using ICG as a photosensitizer.[11,18,20] Regarding the mechanism of action of ICG, the effects are mainly thought to be photothermal and photochemical. ICG is known to enhance the photothermal effects of high penetration 810 nm Diode lasers, thus potentiating the benefits of lasers. Therefore, 810 nm diode laser along with ICG can act as a potential photosensitizer in the treatment of chronic periodontitis.
However, it should be noted that the results of the present study are difficult to compare with previous studies as there is a lack of standardization regarding the use of PDT in the treatment of chronic periodontitis. This is because of different types of photosensitizers used, different types of wavelengths of lasers with different power output and irradiation time. Thus, there is a need for further studies with standardized treatment protocol using lasers.
The present study has some limitations that need to be taken into consideration before interpreting the results. These are limited sample size and lack of evidence on the effect of PDT against specific bacterial strains as only total anaerobic flora was detected. In addition, limited standardized data are available regarding the effective concentration of ICG.
Thus, further randomized controlled clinical trial needs to be conducted evaluating the effect of ICG as a photosensitizer with a larger sample size and longer study period. In addition, its effects also can be evaluated in conditions such as aggressive periodontitis and peri-implantitis.
CONCLUSION
Within the limitations of the study, it can be concluded that ICG-mediated photothermal therapy combined with 810 nm diode laser may be used as an adjunct to SRP in the treatment of chronic periodontitis and thus can act as an alternative to conventional photosensitizers which have provided only moderate benefits as an adjunct.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontology 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 2.Cobb CM. Non-surgical pocket therapy: Mechanical. Ann Periodontol. 1996;1:443–90. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 3.Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontology 2000. 2004;36:121–45. doi: 10.1111/j.1600-0757.2004.03676.x. [DOI] [PubMed] [Google Scholar]
- 4.Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, et al. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 2011;38:612–20. doi: 10.1111/j.1600-051X.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alwaeli HA, Al-Khateeb SN, Al-Sadi A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med Sci. 2015;30:801–7. doi: 10.1007/s10103-013-1426-y. [DOI] [PubMed] [Google Scholar]
- 6.Ready D, Roberts AP, Pratten J, Spratt DA, Wilson M, Mullany P, et al. Composition and antibiotic resistance profile of microcosm dental plaques before and after exposure to tetracycline. J Antimicrob Chemother. 2002;49:769–75. doi: 10.1093/jac/dkf005. [DOI] [PubMed] [Google Scholar]
- 7.Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontology 2000. 2011;55:143–66. doi: 10.1111/j.1600-0757.2010.00346.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson M. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci. 2004;3:412–8. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 9.Kosarieh E, Khavas SS, Rahimi A, Chiniforush N, Gutknecht N. The comparison of penetration depth of two different photosensitizers in root canals with and without smear layer: An in vitro study. Photodiagnosis Photodyn Ther. 2016;13:10–4. doi: 10.1016/j.pdpdt.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M, et al. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–40. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monzavi A, Chinipardaz Z, Mousavi M, Fekrazad R, Moslemi N, Azaripour A, et al. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn Ther. 2016;14:93–7. doi: 10.1016/j.pdpdt.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Azarpazhooh A, Shah PS, Tenenbaum HC, Goldberg MB. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J Periodontol. 2010;81:4–14. doi: 10.1902/jop.2009.090285. [DOI] [PubMed] [Google Scholar]
- 13.Akram Z, Hyder T, Al-Hamoudi N, Binshabaib MS, Alharthi SS, Hanif A, et al. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2017;19:86–92. doi: 10.1016/j.pdpdt.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Betsy J, Prasanth CS, Baiju KV, Prasanthila J, Subhash N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J Clin Periodontol. 2014;41:573–81. doi: 10.1111/jcpe.12249. [DOI] [PubMed] [Google Scholar]
- 15.Franco EJ, Pogue RE, Sakamoto LH, Cavalcante LL, Carvalho DR, de Andrade RV, et al. Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagnosis Photodyn Ther. 2014;11:41–7. doi: 10.1016/j.pdpdt.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho VF, Andrade PV, Rodrigues MF, Hirata MH, Hirata RD, Pannuti CM, et al. Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. J Clin Periodontol. 2015;42:440–7. doi: 10.1111/jcpe.12393. [DOI] [PubMed] [Google Scholar]
- 17.Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rössler R, et al. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J Periodontol. 2008;79:1638–44. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 18.Srikanth K, Chandra RV, Reddy AA, Reddy BH, Reddy C, Naveen A, et al. Effect of a single session of antimicrobial photodynamic therapy using indocyanine green in the treatment of chronic periodontitis: A randomized controlled pilot trial. Quintessence Int. 2015;46:391–400. doi: 10.3290/j.qi.a33532. [DOI] [PubMed] [Google Scholar]
- 19.Yoon HJ, Lee HS, Lim JY, Park JH. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl Mater Interfaces. 2017;9:5683–91. doi: 10.1021/acsami.6b16801. [DOI] [PubMed] [Google Scholar]
- 20.Shingnapurkar SH, Mitra DK, Kadav MS, Shah RA, Rodrigues SV, Prithyani SS, et al. The effect of indocyanine green-mediated photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A comparative split-mouth randomized clinical trial. Indian J Dent Res. 2016;27:609–17. doi: 10.4103/0970-9290.199598. [DOI] [PubMed] [Google Scholar]
- 21.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Schätzle M, Löe H, Lang NP, Heitz-Mayfield LJ, Bürgin W, Anerud A, et al. Clinical course of chronic periodontitis. III. Patterns, variations and risks of attachment loss. J Clin Periodontol. 2003;30:909–18. doi: 10.1034/j.1600-051x.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- 23.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Carranza FA, Takei HH. Clinical diagnosis. In: Carranza FA, editor. Carranza's Clinical Periodontology. 10th ed. Philadelphia: WB Saunders Co; 2006. pp. 540–60. [Google Scholar]
- 25.Spiegel CA, Minah GE, Krywolap GN. Improved procedure for transport of dental plaque samples and other clinical specimens containing anaerobic bacteria. J Clin Microbiol. 1979;9:637–9. doi: 10.1128/jcm.9.5.637-639.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm TK, Ciancio SG. Diode laser activated indocyanine green selectively kills bacteria. J Int Acad Periodontol. 2011;13:58–63. [PubMed] [Google Scholar]
- 27.Allison RR, Bagnato VS, Sibata CH. Future of oncologic photodynamic therapy. Future Oncol. 2010;6:929–40. doi: 10.2217/fon.10.51. [DOI] [PubMed] [Google Scholar]
- 28.Al-Zahrani MS, Austah ON. Photodynamic therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis in smokers. Saudi Med J. 2011;32:1183–8. [PubMed] [Google Scholar]
- 29.Segarra-Vidal M, Guerra-Ojeda S, Vallés LS, López-Roldán A, Mauricio MD, Aldasoro M, et al. Effects of photodynamic therapy in periodontal treatment: A randomized, controlled clinical trial. J Clin Periodontol. 2017;44:915–25. doi: 10.1111/jcpe.12768. [DOI] [PubMed] [Google Scholar]
- 30.Braun A, Dehn C, Krause F, Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J Clin Periodontol. 2008;35:877–84. doi: 10.1111/j.1600-051X.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 31.Pourabbas R, Kashefimehr A, Rahmanpour N, Babaloo Z, Kishen A, Tenenbaum HC, et al. Effects of photodynamic therapy on clinical and gingival crevicular fluid inflammatory biomarkers in chronic periodontitis: A split-mouth randomized clinical trial. J Periodontol. 2014;85:1222–9. doi: 10.1902/jop.2014.130464. [DOI] [PubMed] [Google Scholar]
- 32.Dilsiz A, Canakci V, Aydin T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2013;84:278–86. doi: 10.1902/jop.2012.120096. [DOI] [PubMed] [Google Scholar]
- 33.Van der Weijden GA, Timmerman MF. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29(Suppl 3):55–71. doi: 10.1034/j.1600-051x.29.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 34.Andersen R, Loebel N, Hammond D, Wilson M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent. 2007;18:34–8. [PubMed] [Google Scholar]
- 35.Feil PH, Grauer JS, Gadbury-Amyot CC, Kula K, McCunniff MD. Intentional use of the Hawthorne effect to improve oral hygiene compliance in orthodontic patients. J Dent Educ. 2002;66:1129–35. [PubMed] [Google Scholar]


