Abstract
Barrett esophagus (BE) is the precursor lesion to adenocarcinoma of the esophagus. The current surveillance strategy of 4-quadrant Seattle protocol biopsies has been associated with sampling error and missing higher-risk lesions, and there is often less adherence to endoscopic surveillance with long segments. Advancements in endoscopic imaging and sampling techniques allow for better surveillance of BE, particularly when assessing for dysplasia. This article highlights the key endoscopic imaging and sampling advancements in the evaluation of dysplasia in BE.
Keywords: Barrett esophagus, dysplasia, narrow-band imaging, chromoendoscopy, confocal laser endomicroscopy, volumetric laser endomicroscopy
Barrett esophagus (BE) is a premalignant condition in which abnormal columnar epithelium replaces the stratified squamous epithelium that normally lines the distal esophagus.1 This metaplastic process is thought to be due to reparative processes in the setting of acid or bile reflux that cause recurrent inflammation in the distal esophagus.2,3 Thus, having a diagnosis of chronic gastroesophageal reflux disease is a major risk factor for the development of BE. The prevalence of BE is estimated to be approximately 15% among patients with gastroesophageal reflux disease.1 Other risk factors for the development of BE include age older than 50 years, male sex, white race, presence of central obesity (men: waist circumference, >102 cm or waist-to-hip ratio, >0.9; women: waist circumference, >88 cm or waist-to-hip ratio, >0.8), current or history of smoking, confirmed family history of BE or esophageal adenocarcinoma (EAC) in a first-degree relative, and the presence of a hiatal hernia.4
Affecting more than an estimated 2% of the adult population, BE is a known precursor to EAC.2 The risk of developing EAC is 40 to 50 times greater among patients with BE than the general population.5 The oncogenesis from nondysplastic BE to adenocarcinoma in situ has been traditionally considered a stepwise progression, and population studies have shown that the incidence of high-grade dysplasia or EAC increases depending on the degree of dysplasia on the spectrum of malignant transformation.1,2 However, next-generation sequencing studies have suggested that BE progression may accelerate under a variety of potential mechanisms, which has led investigators to reconsider the concept of stepwise progression from nondysplastic BE to EAC.6 Ultimately, with EAC having a poor prognosis and 5-year survival rate of less than 15%,7 the goals of a screening and surveillance protocol for BE are early diagnosis and treatment of patients with the highest risk of EAC.8
Much controversy remains surrounding the efficacy of screening and surveillance for BE to decrease progression to and mortality from EAC.9 The vast majority of patients with EAC (>90%) do not have a history of BE, and 40% of patients with EAC do not report a history of gastroesophageal reflux disease.10 The gold standard and the most widely used approach to screening for BE is esophagogastroduodenoscopy with forceps biopsies. Several alternative methods, including transnasal endoscopy and esophageal capsule endoscopy, have been developed in more recent years with the hope of finding less-invasive strategies to screen patients.11 In both screening and surveillance protocols for BE, the ability to identify dysplasia is of great importance. This article reviews the various modalities that are currently being used to evaluate for dysplasia and the progression to malignancy in BE.
Diagnosis of Barrett Esophagus
Evaluation of the esophagus for BE on upper endoscopy begins with a macroscopic observation via standard white-light endoscopy (WLE). Typically, BE presents as pink- or salmon-colored mucosa, which visually differentiates it from normal esophageal squamous mucosa.12 BE is a histopathologic diagnosis defined as the extension of a metaplastic columnar epithelium into the esophagus extending at least 1 cm proximal to the gastroesophageal junction with biopsy-confirmed intestinal metaplasia.12 If BE is present, an endoscopist is recommended to describe the extent of metaplastic change using the Prague C&M classification (Figure 1) as well as to document the precise location of the diaphragmatic pinch, gastroesophageal junction, and squamocolumnar junction.13,14 Subsequently, the endoscopist should proceed with a systematic 4-quadrant biopsy sampling technique using the Seattle protocol, obtaining specimens at intervals of every 2 cm in patients without dysplasia and every 1 cm in patients with prior dysplasia.15 This technique is applicable for both short- (<3 cm) and long-segment (≥3 cm) BE.16,17
Figure 1.
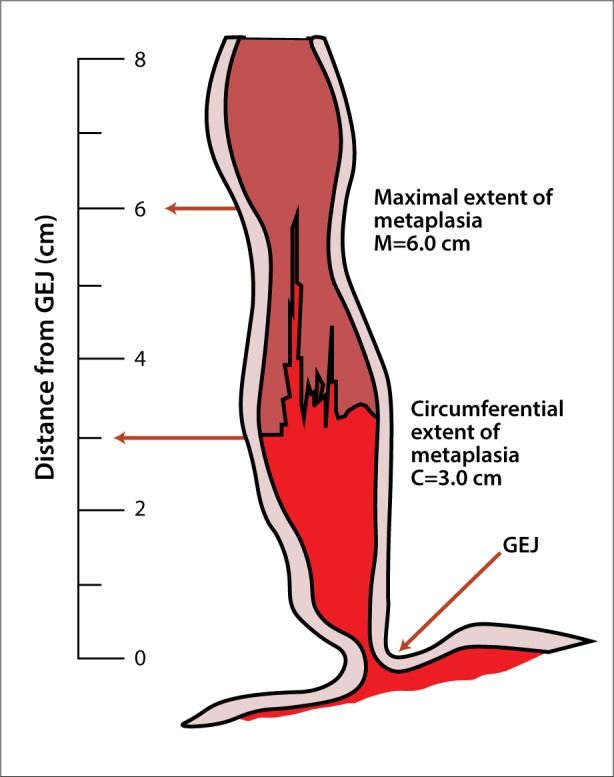
The Prague C&M classification is used to endoscopically grade Barrett esophagus. Prague C3 and M6 are shown in the figure, in which C represents the circumferential difference in endoscope insertion distance between the positions recorded for the gastroesophageal junction (GEJ) and the proximal margin of the circumferential Barrett epithelium, and M represents the difference in endoscope insertion distance between the positions recorded for the GEJ and the proximal margin of the longest tongue-like segment of Barrett epithelium.14
Histologically, the presence of dysplasia or further progression to EAC is graded using the following nomenclature: nondysplastic BE, indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia, intramucosal carcinoma, and invasive EAC. Nondysplastic BE is characterized by minimal cytologic atypia, normal architecture, abundant lamina propria between glands, and a low nucleus-to-cytoplasm ratio. Nuclei are regular, have smooth membranes, and are basally located. Indefinite for dysplasia is indicated by hyperchromasia, overlapping nuclei, irregular nuclear borders, and nuclear stratification in deep glands or on the sides of villiform structures, whereas the surface epithelium is free of atypia. Additionally, cytologic changes are not definitely reactive or neoplastic, and may be secondary to pronounced inflammation. Low-grade dysplasia is defined by epithelial cells with enlarged nuclei and a high nucleus-to-cytoplasm ratio, stratification of nuclei, mucin depletion, partial loss of nuclear polarity, and a lack of surface maturation. High-grade dysplasia is marked by an increased nucleus-to-cytoplasm ratio with enlarged nuclear pleomorphisms with prominent nuclei, full-thickness nuclear stratification, and loss of polarity.18,19
For dysplasia of any grade, biopsies should be reviewed by 2 pathologists, at least 1 of whom is a specialized gastrointestinal pathologist, due to interobserver variability in interpretation.18
Surveillance of Barrett Esophagus
Once a diagnosis of BE has been made, patients should be counseled regarding the significance of the diagnosis as well as the risks and benefits of undergoing endoscopic surveillance. Commitment to a surveillance program entails adherence to scheduled endoscopies at appropriate intervals, which may be a time-consuming and costly ordeal for patients. The age of the patient and comorbidities should also be taken into consideration, as these factors may affect the ability to tolerate therapeutic intervention (eg, endoscopic mucosal resection) if advanced disease is identified. Table 1 outlines the latest surveillance guidelines published by 4 major gastroenterology societies.1,3,20-23
Table 1.
Barrett Esophagus Surveillance Guideline Recommendations
| Grade of Dysplasia | Guideline Recommendations | |||
|---|---|---|---|---|
| ACG (2016)1 | ASGE (2012)3 | AGA (2011)20,21 | BSG (2014)22,23 | |
| Nondysplastic | EGD every 3-5 years | Consider no surveillance. Alternatively, EGD every 3-5 years | EGD every 3-5 years | If the maximum length is >3 cm, repeat EGD every 2-3 years. If the maximum length is ≤3 cm, repeat the EGD every 3-5 years. |
| IFD | Repeat EGD after 3-6 months of PPI use. If IFD is confirmed, use surveillance interval of 12 months. | Confirm presence and grade of dysplasia with an expert GI pathologist. Maximize PPI therapy and repeat EGD plus biopsy to confirm dysplasia. | No recommendations | Repeat EGD in 6 months with maximal acid suppression |
| LGD | Endoscopic therapy, or surveillance every 12 months | Confirm with an expert GI pathologist; repeat in 6 months. Surveillance every 12 months | EGD every 6-12 months. 2016 Clinical Practice Update: Repeat EGD in 8-12 weeks. If LGD is confirmed by an expert GI pathologist, treat with radiofrequency ablation or EGD every 6 months for 1 year, then surveillance annually |
EGD every 6 months. 2018 revision: Repeat EGD in 6 months. If LGD is confirmed by 2 independent pathologists, offer endoscopic ablation therapy (radiofrequency ablation preferred) |
| HGD | Endoscopic therapy unless the patient has a life-limiting comorbidity | Confirm with an expert GI pathologist. Surveillance every 3 months. Consider resection. | Eradication therapy or surveillance EGD every 3 months | Therapeutic intervention |
ACG, American College of Gastroenterology; AGA, American Gastroenterological Association; ASGE, American Society for Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; EGD, esophagogastroduodenoscopy; GI, gastrointestinal; HGD, high-grade dysplasia; IFD, indefinite for dysplasia; LGD, low-grade dysplasia; PPI, proton pump inhibitor.
Endoscopic Modalities for the Evaluation of Barrett Esophagus
The gold standard for detecting dysplasia in the surveillance of BE involves obtaining 4-quadrant biopsies following the Seattle protocol using WLE. Guidelines also suggest the use of high-definition or high-resolution WLE, as there is evidence that it is superior to standard-definition WLE for the detection of dysplastic lesions.1,24 However, it is possible that abnormal tissue may be missed between the discrete samples, leading to potential sampling errors and inaccurate diagnoses.25 Longer endoscopic inspection time during surveillance examinations has been shown to increase the detection of high-grade dysplasia or EAC.26 Adherence to the Seattle protocol decreases in the community setting as the BE segment gets longer.27 Wide-area transepithelial sampling (WATS) was recently introduced as a means of trying to overcome the sampling limitations of traditional forceps biopsies. Instead of collecting a discrete number of samples per quadrant, the WATS brush takes a circumferential sweep of the esophagus, which allows for the evaluation of more esophageal surface area as well as an assessment of deeper layers that are sampled by the abrasive brush. The 3-dimensional specimens are then scanned by a computer, which identifies the most abnormal cells that serve as the starting point for pathologists to analyze. There is an intraobserver agreement of 0.86 for all degrees of BE, including dysplasia.28 Johanson and colleagues29 reported a 39.8% increase in the detection of BE when WATS was used as an adjunctive tool to the standard Seattle protocol. A recent prospective, randomized trial demonstrated a 4-fold increase for diagnosing dysplasia when WATS was used as an adjunctive tool to forceps biopsy.30 The cohort was an enriched population from tertiary care centers rather than from a nondysplasia-rich community setting. However, a larger community-based study showed a similar increase in detecting BE that was not picked up by forceps biopsies, suggesting that WATS is a promising technology to decrease sampling error in BE.31
Advanced Imaging Modalities for the Detection of Early Neoplasia in Barrett Esophagus
In recent years, numerous imaging modalities have been developed to aid in the detection of early Barrett neoplasia, although in most cases, these modalities have not been evaluated for the detection of low-grade vs high-grade dysplasia. Advanced imaging modalities, including narrow-band imaging (NBI), chromoendoscopy (with indigo carmine or acetic acid), confocal laser endomicroscopy (CLE), and volumetric laser endomicroscopy (VLE), may help guide the endoscopic workup and treatment of BE, and in some cases allow for real-time diagnosis and decision-making during endoscopy (Table 2).32 Modalities such as optical coherence tomography (OCT) and methylene blue staining, which are no longer used to a significant extent, are not discussed in this paper. Recently, the Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) Committee of the American Society for Gastrointestinal Endoscopy (ASGE) established performance thresholds for adopting imaging-assisted targeted biopsies during surveillance of BE. The thresholds were a sensitivity of 90%, specificity of 80%, and negative predictive value of 98% for the detection of high-grade dysplasia or EAC.33 Of the technologies currently available, only NBI and acetic acid chromoendoscopy have met the ASGE performance thresholds.33
Table 2.
Summary of Advanced Imaging Modalities for the Assessment of Barrett Esophagus
| Modality | Definition | Equipment | Field |
|---|---|---|---|
| Narrow-band imaging | Uses blue-green spectrum of light (415-540 nm) to capitalize on the peak absorption of hemoglobin, accentuating visualization of the mucosal vasculature | Olympus | Field of view: 140° Depth of field: 3-100 mm |
| Chromoendoscopy | Absorptive and contrast stains applied to esophageal mucosa to highlight superficial mucosal architecture | Stains, white-light endoscope | NA |
| Confocal laser endomicroscopy | Fluorescence emission by a low-powered laser that can generate in vivo images of esophageal mucosa at histologic-level resolution | pCLE: Cellvizio, Mauna Kea Technologies eCLE: Pentax confocal laser endomicroscope |
pCLE field of view: 240-600 µm Special resolution: 1.0-3.5 µm eCLE field of view: 475 µm |
| Volumetric laser endomicroscopy | Second-generation optical coherence tomography device; generates wide-field, cross-sectional views of the esophagus, allowing for a comprehensive assessment of the esophageal mucosa and submucosa | NvisionVLE, NinePoint Medical | Lateral resolution: 30 µm |
eCLE, endoscope-based confocal laser endomicroscopy; NA, not applicable; pCLE, probe-based confocal laser endomicroscopy.
Narrow-Band Imaging
NBI, or optical chromoendoscopy, uses the blue-green spectrum of light (415-540 nm), which is absorbed by hemoglobin molecules, to highlight superficial and subepithelial vessels. Since the inception of this modality, several randomized trials have investigated its use in detecting advanced lesions in BE.25,34-36 In a randomized trial from 2013, Sharma and colleagues compared WLE to NBI in patients undergoing surveillance for BE.25 The detection of intestinal metaplasia was similar between the 2 modalities; however, NBI identified more dysplastic lesions with fewer biopsies (Figure 2). The authors concluded that NBI may reduce the cost of surveillance, as fewer biopsies can be taken.25 A meta-analysis of NBI studies shows superiority of NBI over WLE, with a pooled sensitivity of 0.96 (95% CI, 0.93-0.99) and specificity of 0.94 (95% CI, 0.84-1.00) for the detection of high-grade dysplasia or EAC.37 Additionally, a meta-analysis reported that NBI meets the recommended ASGE performance thresholds.33
Figure 2.
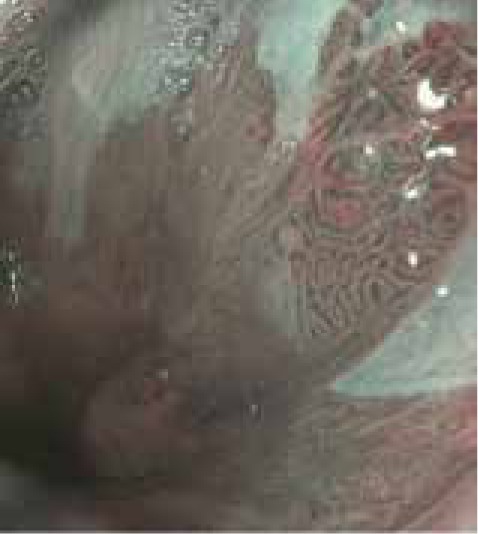
A narrow-band image of a distal esophagus demonstrates high-grade dysplasia in an area of Barrett esophagus.
The Barrett International NBI Group Classification was developed for the detection of dysplasia and cancer, and classifies lesions based on their mucosal and vascular patterns.38 The system can identify patients with dysplasia with an 80% sensitivity, 88% specificity, and 88% negative predictive value, with high interrater reliability. Using the classification system, Sharma and colleagues were able to demonstrate that when NBI images are assessed by expert endoscopists, the diagnostic accuracy of NBI exceeds 90%.38 A benefit of NBI is that the technology is a standard feature on most endoscopes, making it a cost-neutral investment.
Chromoendoscopy
Chromoendoscopy uses stains that are applied to the tissue of interest during endoscopy in order to accentuate superficial mucosal features. The equipment necessary for chromoendoscopy is widely available, and the stains are inexpensive and generally safe. The stains are categorized as absorptive, or vital, if they are absorbed by the esophageal epithelium (ie, methylene blue), or as contrast if they accumulate in the pits and grooves of the esophageal epithelium (ie, indigo carmine, acetic acid). Both absorptive and contrast stains highlight the superficial architecture of the esophageal mucosa.
Indigo Carmine Indigo carmine is a contrast stain that can be either ingested prior to endoscopy or sprayed on the esophagus during endoscopy. Its use in the detection of high-grade dysplasia or EAC revealed a limited sensitivity (83%), but a high negative predictive value (98%) and specificity (88%).39 Indigo carmine has not been studied extensively in the surveillance of BE and is not commonly used.
Acetic Acid Acetic acid is a contrast stain that is sprayed onto the esophageal mucosa during endoscopy and causes normal esophageal tissue to display a pale or white appearance as compared to the red appearance of gastric-type tissue. The effect is brief, so the stain must be reapplied frequently during endoscopy. Although no randomized trials of acetic acid chromoendoscopy have been performed comparing it to the standard Seattle protocol, several publications suggest its utility in BE surveillance.40 A meta-analysis of 4 studies shows a pooled sensitivity, negative predictive value, and specificity of 96.6% (95% CI, 95-98), 98.3% (95% CI, 94.8-99.4), and 84.6% (95% CI, 68.5-93.2), respectively, in the detection of high-grade dysplasia or EAC, which meets performance thresholds established by the ASGE.33 However, because acetic acid chromoendoscopy is not commonly used, training is required to accurately identify lesions to detect BE and dysplasia. Prospective, randomized trials are also needed to evaluate this contrast stain.
Confocal Laser Endomicroscopy
CLE employs fluorescence emission by a low-powered laser that can generate in vivo images of esophageal mucosa at histologic-level resolution (Figure 3).41 CLE can be performed using a probe inserted into the working channel (pCLE) or by using an integrated endoscope with CLE capability built into the tip of the endoscope (eCLE). The integrated endoscope is no longer in use despite meeting performance thresholds for detecting high-grade dysplasia or EAC based on the ASGE PIVI Committee. pCLE allows for concurrent use of high-definition WLE, but as the working channel is in use, biopsies cannot be obtained during imaging. Early studies suggest that the use of pCLE improves the early detection of neoplasia in BE when compared to WLE.32,42 Five pooled studies using CLE technology (2 pCLE and 3 eCLE) have also displayed a substantial pooled sensitivity (90.4%; 95% CI, 75.7-96.6), negative predictive value (96.2%; 95% CI, 93.1-97.9), and specificity (89.9%; 95% CI, 83.8-93.9), respectively.33 CLE is limited by a focused field of view, which can result in sampling error. Additionally, matching the CLE findings to forceps biopsies can be difficult. CLE is currently often limited to academic settings due to its capital cost and the training required to become proficient.
Figure 3.
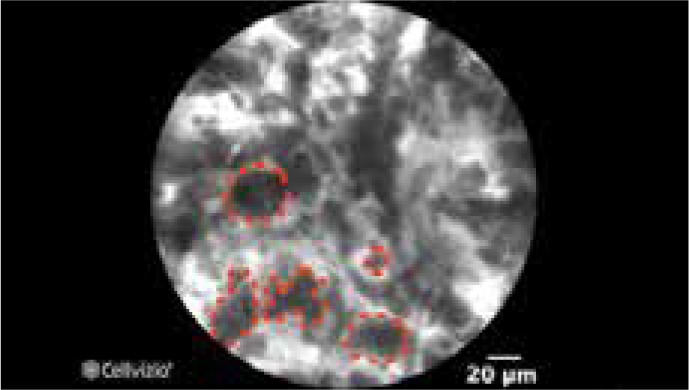
Confocal laser endomicroscopy image shows enlarged pleomorphic cells that are characteristic of Barrett esophagus with high-grade dysplasia.
Image provided by Cellvizio.
Volumetric Laser Endomicroscopy
VLE was developed to address the relatively slow processing time and limited scanning area of OCT. VLE utilizes a second-generation OCT device (termed optical frequency domain imaging) in a balloon-based system. The technology generates a circumferential scan of the esophagus to a depth of 3 mm. After a short processing time, VLE allows for the visualization of esophageal layers and submucosal vascular networks (Figure 4).43 A scoring system developed for the first-generation OCT device has been used in VLE, and early studies suggest that it also correlates with neoplasia.44,45 An ex vivo study compared the diagnostic performance of pCLE and VLE.46 Fifty specimens obtained from endoscopic mucosal resection of suspicious lesions in patients undergoing BE surveillance endoscopy were evaluated. The sensitivity of VLE for detecting BE dysplasia was 86% (95% CI, 69-96), specificity was 88% (95% CI, 60-99), and diagnostic accuracy was 87% (95% CI, 86-88). The diagnostic accuracy of VLE was significantly superior to that of pCLE.46
Figure 4.
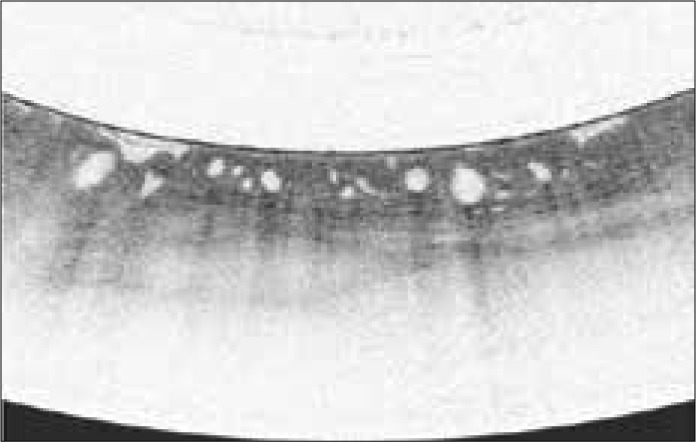
Volumetric laser endomicroscopy image shows large glands below the mucosal surface with loss of layering that is consistent with Barrett esophagus with high-grade dysplasia.
Image provided by NinePoint Medical.
Conclusion
BE is a premalignant condition that affects more than 2% of the adult population and confers a 40- to 50-fold increased risk of developing EAC over the general population. The goals of a screening and surveillance program for patients with BE are to identify early precursor lesions to EAC and to intervene prior to the development of advanced cancer. The gold standard for surveillance of BE is performing 4-quadrant biopsies every 1 to 2 cm of detected BE using high-definition WLE; however, sampling error may result in missing high-risk lesions. Careful inspection of the BE segment is paramount when evaluating a segment of BE, as a longer inspection time is associated with an increased detection of advanced lesions.
Over the last several years, tremendous advancements have been made in imaging technologies to better allow endoscopists to identify dysplasia and cancer in a BE segment. The clinical data have shown improved detection with these modalities. To date, only NBI and acetic acid chromoendoscopy have met the ASGE PIVI criteria to show benefit in improving the detection of dysplasia in BE. Mass adoption of CLE and VLE is hindered by additional training and costly equipment. The future holds promise to make the currently available technology easier to learn and more cost-conscious, leading to the ultimate goals of improved quality in the area of BE and better patient outcomes.
References
- 1.Shaheen NJ, Falk GW, Iyer PG, Gerson LB American College of Gastroenterology. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis-Yadley AH, Neill KG, Malafa MP, Pena LR. Advances in the endoscopic diagnosis of Barrett esophagus. Cancer Control. 2016;23(1):67–77. doi: 10.1177/107327481602300112. [DOI] [PubMed] [Google Scholar]
- 3.Evans JA, Early DS, Fukami N, et al. ASGE Standards of Practice Committee; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76(6):1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Ireland CJ, Thompson SK, Laws TA, Esterman A. Risk factors for Barrett’s esophagus: a scoping review. Cancer Causes Control. 2016;27(3):301–323. doi: 10.1007/s10552-015-0710-5. [DOI] [PubMed] [Google Scholar]
- 5.Macías-García F, Domínguez-Muñoz JE. Update on management of Barrett’s esophagus. World J Gastrointest Pharmacol Ther. 2016;7(2):227–234. doi: 10.4292/wjgpt.v7.i2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contino G, Vaughan TL, Whiteman D, Fitzgerald RC. The evolving genomic landscape of Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2017;153(3):657–673.e1. doi: 10.1053/j.gastro.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(3):235–244. doi: 10.1016/j.cgh.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Naveed M, Dunbar KB. Endoscopic imaging of Barrett’s esophagus. World J Gastrointest Endosc. 2016;8(5):259–266. doi: 10.4253/wjge.v8.i5.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena N, Inadomi JM. Effectiveness and cost-effectiveness of endoscopic screening and surveillance. Gastrointest Endosc Clin N Am. 2017;27(3):397–421. doi: 10.1016/j.giec.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64(1):20–25. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 11.Offman J, Fitzgerald RC. Alternatives to traditional per-oral endoscopy for screening. Gastrointest Endosc Clin N Am. 2017;27(3):379–396. doi: 10.1016/j.giec.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eluri S, Shaheen NJ. Barrett’s esophagus: diagnosis and management. Gastrointest Endosc. 2017;85(5):889–903. doi: 10.1016/j.gie.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong D. Review article: towards consistency in the endoscopic diagnosis of Barrett’s oesophagus and columnar metaplasia. Aliment Pharmacol Ther. 2004;20(suppl 5):40–47. doi: 10.1111/j.1365-2036.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392–1399. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Levine DS, Blount PL, Rudolph RE, Reid BJ. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am J Gastroenterol. 2000;95(5):1152–1157. doi: 10.1111/j.1572-0241.2000.02002.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102(6):1154–1161. doi: 10.1111/j.1572-0241.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus—the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93(7):1033–1036. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32(4):368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 19.Booth CL, Thompson KS. Barrett’s esophagus: a review of diagnostic criteria, clinical surveillance practices and new developments. J Gastrointest Oncol. 2012;3(3):232–242. doi: 10.3978/j.issn.2078-6891.2012.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ American Gastroenterological Association. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):e18–e52. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151(5):822–835. doi: 10.1053/j.gastro.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 23.di Pietro M, Fitzgerald RC BSG Barrett’s guidelines working group. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut. 2018;67(2):392–393. doi: 10.1136/gutjnl-2017-314135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sami SS, Subramanian V, Butt WM, et al. High definition versus standard definition white light endoscopy for detecting dysplasia in patients with Barrett’s esophagus. Dis Esophagus. 2015;28(8):742–749. doi: 10.1111/dote.12283. [DOI] [PubMed] [Google Scholar]
- 25.Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62(1):15–21. doi: 10.1136/gutjnl-2011-300962. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Gaddam S, Wani SB, Bansal A, Rastogi A, Sharma P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012;76(3):531–538. doi: 10.1016/j.gie.2012.04.470. [DOI] [PubMed] [Google Scholar]
- 27.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7(7):736–742. doi: 10.1016/j.cgh.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vennalaganti PR, Naag Kanakadandi V, Gross SA, et al. Inter-observer agreement among pathologists using wide-area transepithelial sampling with computer-assisted analysis in patients with Barrett’s esophagus. Am J Gastroenterol. 2015;110(9):1257–1260. doi: 10.1038/ajg.2015.116. [DOI] [PubMed] [Google Scholar]
- 29.Johanson JF, Frakes J, Eisen D EndoCDx Collaborative Group. Computer-assisted analysis of abrasive transepithelial brush biopsies increases the effectiveness of esophageal screening: a multicenter prospective clinical trial by the EndoCDx Collaborative Group. Dig Dis Sci. 2011;56(3):767–772. doi: 10.1007/s10620-010-1497-6. [DOI] [PubMed] [Google Scholar]
- 30.Vennalaganti PR, Kaul V, Wang KK, et al. Increased detection of Barrett’s esophagus-associated neoplasia using wide-area trans-epithelial sampling: a multicenter, prospective, randomized trial. Gastrointest Endosc. 2018;87(2):348–355. doi: 10.1016/j.gie.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Gross SA, Smith MS, Kaul V. Increased detection of Barrett’s esophagus and esophageal dysplasia with adjunctive use of wide-area transepithelial sample with three-dimensional computer-assisted analysis (WATS) [published online November 28, 2017] United European Gastroenterol J. doi: 10.1177/2050640617746298. doi:10.1177/2050640617746298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swager AF, Curvers WL, Bergman JJ. Diagnosis by endoscopy and advanced imaging of Barrett’s neoplasia. Adv Exp Med Biol. 2016;908:81–98. doi: 10.1007/978-3-319-41388-4_5. [DOI] [PubMed] [Google Scholar]
- 33.Thosani N, Abu Dayyeh BK, Sharma P, et al. ASGE Technology Committee. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc. 2016;83(4):684–698.e7. doi: 10.1016/j.gie.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology. 2008;135(1):24–31. doi: 10.1053/j.gastro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy. 2005;37(10):929–936. doi: 10.1055/s-2005-870433. [DOI] [PubMed] [Google Scholar]
- 36.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9(3):568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 37.Mannath J, Subramanian V, Hawkey CJ, Ragunath K. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Endoscopy. 2010;42(5):351–359. doi: 10.1055/s-0029-1243949. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, Bergman JJ, Goda K, et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus using narrow band imaging. Gastroenterology. 2016;150(3):591–598. doi: 10.1053/j.gastro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Marcon N, Wani S, et al. Non-biopsy detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a prospective multicenter study. Endoscopy. 2006;38(12):1206–1212. doi: 10.1055/s-2006-944974. [DOI] [PubMed] [Google Scholar]
- 40.Longcroft-Wheaton G, Duku M, Mead R, Poller D, Bhandari P. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2010;8(10):843–847. doi: 10.1016/j.cgh.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4(8):979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74(3):465–472. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vakoc BJ, Shishko M, Yun SH, et al. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video) Gastrointest Endosc. 2007;65(6):898–905. doi: 10.1016/j.gie.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suter MJ, Vakoc BJ, Yachimski PS, et al. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc. 2008;68(4):745–753. doi: 10.1016/j.gie.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trindade AJ, Vamadevan AS, Sejpal DV. Finding a needle in a haystack: use of volumetric laser endomicroscopy in targeting focal dysplasia in long-segment Barrett’s esophagus. Gastrointest Endosc. 2015;82(4):756–757. doi: 10.1016/j.gie.2015.03.1984. [DOI] [PubMed] [Google Scholar]
- 46.Leggett CL, Gorospe EC, Chan DK, et al. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest Endosc. 2016;83(5):880–888.e2. doi: 10.1016/j.gie.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


