Abstract
The capabilities of interventional gastrointestinal endoscopy have significantly increased over the past several decades. Improvements in devices and techniques have eased the transfer of novel concepts from bench to bedside. The concept of submucosal endoscopy with mucosal flap safety valve has enabled endoscopists to securely use submucosal space, or third space. Peroral endoscopic myotomy was the initial procedure performed utilizing submucosal space in patients with achalasia. Subsequently, this technique has been used successfully for removal of subepithelial tumors from the esophagus and the stomach. All third-space endoscopy procedures use a similar technique—a submucosal tunnel is created, and then a myotomy is performed or a subepithelial tumor is dissected away from the initial site of the mucosal incision. The other potential indications for third-space endoscopy include refractory gastroparesis, Zenker diverticulum, and restoration of completely obstructed esophageal lumen. Although the emerging data look promising for peroral endoscopic myotomy and pyloromyotomy, randomized studies with long-term follow-up are lacking. Submucosal endoscopy is largely safe, and the occurrence of major adverse events is uncommon. Therefore, the majority of third-space endoscopy procedures can be performed in an endoscopy suite. The most frequently encountered adverse events during submucosal endoscopy include those related to insufflation, bleeding, and perforations.
Keywords: Submucosal endoscopy, peroral endoscopic myotomy, gastric peroral endoscopic pyloromyotomy, submucosal tunneling endoscopic resection, achalasia, gastroparesis
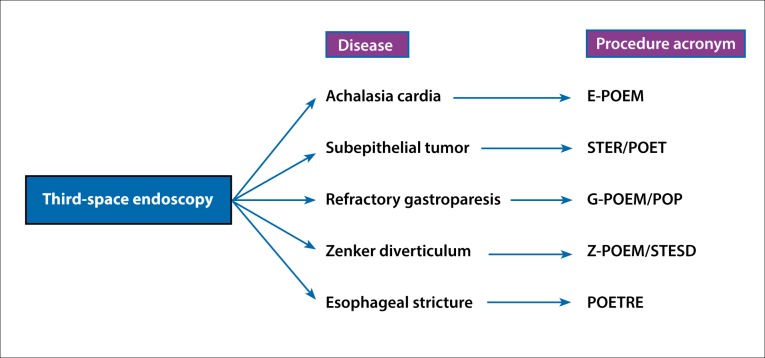
The scope of flexible endoscopy has increased with the introduction of natural orifice transluminal endoscopic surgery (NOTES). In the current era, endoscopists have gained access into the second space (peritoneal cavity) and third space (intramural or submucosal space). One of the major concerns with NOTES is the secure closure of the entry point into these spaces. This concern has largely been addressed with the introduction of the submucosal endoscopy with mucosal flap safety valve (SEMF) technique by Sumiyama and colleagues.1 With this technique, the authors demonstrated that the peritoneal cavity could be accessed successfully and the defect closed by using the mucosal flap. Subsequently, the SEMF technique was used successfully for endoscopic myotomy by Pasricha and colleagues in an animal study.2 The significance of submucosal space as an operating field was seen, and soon the first human results of peroral endoscopic myotomy (POEM) were published by Inoue and colleagues.3 Since then, third-space endoscopy, or submucosal endoscopy utilizing the SEMF technique, has been used for various gastrointestinal (GI) diseases, such as achalasia cardia, submucosal tumors, gastroparesis, and Zenker diverticulum (Figure 1).
Figure 1.
Third-space endoscopy procedures for diseases of the gastrointestinal tract.
E-POEM, esophageal peroral endoscopic myotomy; G-POEM, gastric peroral endoscopic myotomy; POET, peroral endoscopic tunneling; POETRE, peroral endoscopic tunneling for restoration of the esophagus; POP, peroral pyloromyotomy; STER, submucosal tunneling endoscopic resection; STESD, submucosal tunneling endoscopic septum division; Z-POEM, peroral endoscopic myotomy for Zenker diverticulum.
Equipment and Accessories for Third-Space Endoscopy
Advances in equipment and accessories have propelled the development of third-space endoscopy and its application in various GI diseases. Most of the equipment and accessories used in various procedures utilizing the third space are already being used for endoscopic submucosal dissection (Table 1). The main equipment includes an endoscope with a water jet, an electrosurgical generator, transparent caps, and a carbon dioxide insufflator. The various accessories include different types of electrosurgical knives, coagulation forceps, endoscopic flushing pumps, low- or extra low–flow gas tubes, and endoclips.
Table 1.
Equipment and Accessories Commonly Used During Third-Space Endoscopy (Partial List)
| Model Numbers and Manufacturers | |
|---|---|
| Endoscope | GIF-HQ190, Olympus (Outer diameter of 9.2 mm, integrated water channel) |
| Electrosurgical Generators | VIO 300 D, Erbe ESG-300, Olympus |
| Carbon Dioxide Insufflators | UCR, Olympus CO2MPACT, US Endoscopy |
| Low-Flow Gas Tube or Extra Low–Flow Gas Tube | MAJ-1742, Olympus or MAJ-1816, Olympus |
| Electrosurgical Knives | Triangle Tip Knife (KD-640L, Olympus) |
| HybridKnife (20150-060, Erbe) Triangle Tip Knife J (KD-645L, Olympus) | |
| HookKnife (KD-620LR, Olympus) ITknife2 (KD-611L, Olympus) | |
| Coagulation Forceps | Coagrasper (FD-410/411UR/412LR, Olympus) |
| Endoscopic Flushing Pumps | OFP-2, Olympus ERBEJET 2, Erbe |
The choice of some of the accessories, such as the electrosurgical knife and the distal attachment, is usually based on the operator’s preference. Electrosurgical knives with water jet function may be chosen to reduce the need for replacing accessories. Diluted indigo carmine or methylene blue solutions with or without epinephrine are used for submucosal injections. These dyes are preferentially taken up by the submucosa and, therefore, guide the dissection process.
Peroral Endoscopic Myotomy
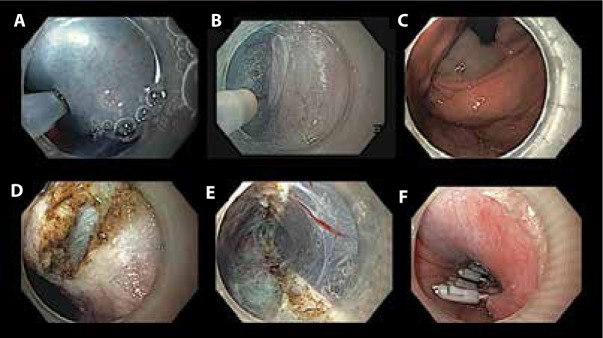
The endoscopic management of achalasia and nonachalasia spastic esophageal motility disorders has advanced with the introduction of POEM. Esophageal POEM (E-POEM) is the most studied of the third-space endoscopy procedures currently being performed. The technique involves the following steps: a submucosal bleb raising, a mucosal incision, tunneling in the submucosa, a myotomy, and closure of the mucosal incision (Figure 2).4 The length of the myotomy is decided by the type of achalasia on high-resolution manometry. A longer myotomy is required for type III achalasia and nonachalasia spastic esophageal motility disorders, such as diffuse esophageal spasm and jackhammer esophagus.5
Figure 2.
Peroral endoscopic myotomy in a patient with achalasia: (A) submucosal lifting injection with saline and indigo carmine, (B) submucosal tunneling with a triangular knife with integrated water jet (Triangle Tip Knife J, KD-645L, Olympus), (C) confirmation of gastric extension of submucosal tunnel by bluish discoloration of cardia, (D) selective circular myotomy in proximal tunnel, (E) completion of myotomy (note full-thickness myotomy toward the distal end of the tunnel), and (F) closure of the mucosal incision with clips.
The available evidence suggests excellent short- and mid-term results with E-POEM in treatment-naive cases of achalasia.4,6,7 However, the data are limited regarding the long-term efficacy of E-POEM.8-10 In 2 studies evaluating long-term response, the clinical success at 5-year follow-up was 83% and 87.1%, respectively.8,9 Emerging data suggest that E-POEM is equally effective in treatment-failure cases with achalasia.11-15 In a large study that included 502 patients, clinical success at 3 years was 87.1% and 76.3% in treatment-naive and treatment-failure cases, respectively.14
POEM has been found to be more effective than pneumatic dilatation at 1-year follow-up (92.2% vs 70%, respectively).16 The advantages of E-POEM over Heller myotomy include reduced postoperative pain, shorter hospitalization, shorter operating time, and less blood loss.17-20 Therefore, E-POEM has the potential to become the treatment of choice in a variety of spastic as well as nonspastic motility disorders of the esophagus (Table 2).
Table 2.
Outcomes of Peroral Endoscopic Myotomy in Achalasia Cardia (Select Large Studies)
| Study | Patients, N | Median Follow-Up, Months | Efficacy, % | Major Adverse Events, % | Reflux, %: 24-hr pH Study/Endoscopy |
|---|---|---|---|---|---|
| Inoue et al7 | 500 | ≥36 | 91.0 (1-2 years) 88.5 (≥3 years) | 3.2 (overall) | NA/59.2 |
| Nabi et al14 | 502 | 20 | 90.9 (1 year) | 1.6 | 28.9/21.5 |
| Zhang et al15 (HM and non-HM) |
318 | 28 and 23 | 95.7 and 95.1 | 0 and 2.9 | 50.0/46.2 and 47.9/34.0 |
| Kumbhari et al65 | 282 | 12 | 94.3 | NA | 57.8/23.2 |
| Ngamruengphong et al10 | 205 | 31 | 91.0 | 8.2 | 37.5/18.0 |
HM, Heller myotomy; NA, not available.
Submucosal Endoscopy Beyond Peroral Endoscopic Myotomy
Submucosal Tunneling Endoscopic Resection
Small submucosal lesions of the upper GI tract are usually benign and do not require resection. Some of these tumors, especially those larger in size (>3 cm) and arising from the muscularis propria, bear malignant potential. Traditionally, a thoracoscopic approach has been utilized to enucleate these submucosal tumors. With submucosal endoscopy, these tumors can be safely and efficiently resected endoscopically. This technique, also known as endoscopic submucosal tunneling dissection or submucosal tunneling endoscopic resection (STER), is based on principles similar to those of E-POEM (ie, the SEMF technique). Most research has evaluated STER for esophageal and gastric submucosal tumors,21-23 although the feasibility of STER for rectal submucosal tumors has also been demonstrated recently.24
Preprocedural imaging, including endoscopic ultrasonography and/or computed tomography, is essential to determine the depth of the tumor and its relationship with the surrounding structures. The factors that determine the feasibility and ease of STER include the size and location of the submucosal tumor. En-bloc removal of submucosal tumors greater than 3.5 cm and those irregular in shape is difficult. Likewise, submucosal tunneling is more challenging for submucosal tumors situated deep in the fundus or along the lesser curvature. Management in such cases should be individualized, and alternative treatment strategies, such as endoscopic full-thickness resection, should be considered when appropriate.
STER is performed under general anesthesia with the same tools as those described for E-POEM. The steps of the STER procedure are as follows: submucosal injection of saline mixed with indigo carmine dye 3 to 5 cm proximal to the tumor location, mucosal incision, submucosal tunneling that extends 1 to 2 cm distal to the tumor, dissection of the tumor from the surrounding tissue and muscularis propria, en-bloc removal of the tumor with a snare, and finally closure of the incision (Figure 3).
Figure 3.
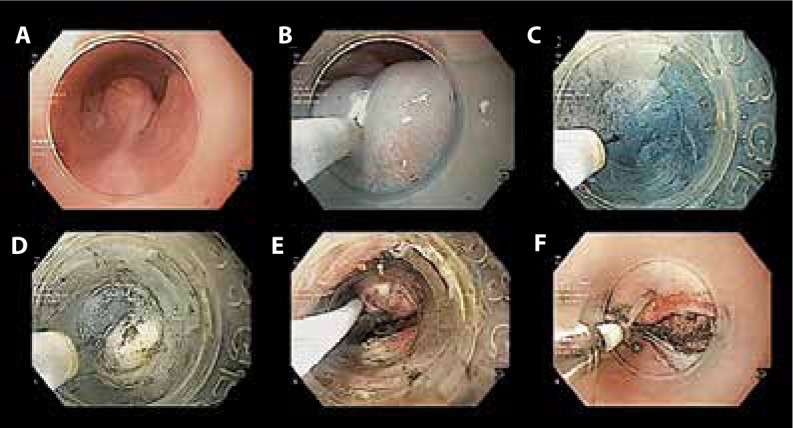
Submucosal tunneling endoscopic resection in a patient with an esophageal subepithelial tumor: (A) a subepithelial tumor in the esophagus, (B) submucosal lifting injection and mucosal incision at approximately 3 cm from the lesion, (C) submucosal tunneling extending until approximately 2 cm distal to the tumor, (D) dissection of the tumor from the surrounding tissue, (E) grasping of the dissected tumor with a snare, and (F) closure of the incision with clips.
The efficacy and safety of STER have been demonstrated in multiple small to large studies (Table 3).25-29 In a large retrospective study, STER was performed in 290 patients with subepithelial tumors localized in the esophagus, esophagogastric junction, and stomach. The median tumor size was 21 mm (range, 10-70 mm). Overall, en-bloc resection was achieved in 89.3% of patients.25 High rates of en-bloc resection have also been confirmed in a recent systematic review and meta-analysis that included 28 studies. The en-bloc resection rate in this review was 94.6% (95% CI, 91.5%-96.7%).21 The main factors that preclude en-bloc resection are large size and irregular shape of the tumor.22,30,31 In studies comparing STER with thoracoscopic enucleation, the 2 modalities were found to be equally effective. However, STER was associated with shorter operating time, reduced postoperative chest pain, and shorter hospitalization.31,32
Table 3.
Safety and Efficacy of Submucosal Tunneling Endoscopic Resection in Subepithelial Tumors
| Study | Patients, N | Location of Subepithelial Tumors | Size, Cm (Range) | En-Bloc Resection, % | Complications, % | Recurrence, %/Median Follow-Up, Months |
|---|---|---|---|---|---|---|
| Chen et al25 | 290 | Esophagus: 199 Esophagogastric junction: 68 Stomach: 23 |
2.1 (1.0-7.0) | 89.3 | 23.4 | NA |
| Ye et al26 | 85 | Esophagus: 60 Cardia: 16 Stomach: 9 |
1.9 (1.0-3.0) | 100 | 9.4 | 0/8 |
| Wang et al27 | 80 (total tumors: 83) | Esophagus: 67 Cardia: 16 |
2.3 (1.0-5.5) | 97.6 | 8.75 | 0/10.2 (mean) |
| Li et al28 | 74 | Esophagus: 74 | 1.89 ± 0.72 (mean) | 98.6 | 5.4 | 2.7/19.5 |
| Mao et al29 | 56 | Esophagus: 18 Stomach: 38 |
1.8 (1.0-3.2) | 100 | 15.3 | 0/25 |
NA, not available.
Gastric Peroral Endoscopic Pyloromyotomy
Gastroparesis is defined as delayed gastric emptying in the absence of a mechanical obstruction. A subset of patients do not respond to dietary interventions and medications. The endoscopic armamentarium in patients with refractory gastroparesis is limited. Endoscopic injection of botulinum toxin and transpyloric stenting have been performed in these patients with the aim of decreasing gastric outflow resistance due to the pyloric sphincter. However, injection of botulinum toxin did not prove effective in randomized trials,33,34 and transpyloric stenting was associated with high stent migration rates.35
Among surgical options, laparoscopic pyloroplasty has been shown to be safe and effective in patients with refractory gastroparesis.36 It seems logical that endoscopic myotomy of the pyloric sphincter may reproduce the results of surgical pyloroplasty. Endoscopic pyloromyotomy was initially described in patients with postoperative pyloric stenosis and later in pediatric patients with congenital pyloric stenosis.37,38 However, the technique described in these studies did not appear to be risk-free, as it involved freehand incision with a needle knife directly over the pyloric ring. After the establishment of E-POEM for achalasia, initial animal studies revealed the feasibility of performing endoscopic pyloromyotomy with the submucosal tunneling technique.39,40 The technique and accessories used for gastric peroral endoscopic myotomy (G-POEM, also known as peroral pyloromyotomy or POP) are essentially similar to those of POEM for esophageal achalasia. In brief, the steps include submucosal injection at 7 o’clock along the greater curvature of the stomach and approximately 4 to 5 cm proximal to the pyloric ring, mucosal incision, submucosal tunneling, myotomy involving the pyloric muscle, and finally closure of the incision using endoclips. It is important to repeatedly confirm the path of the submucosal tunnel by looking at the bluish hue from the injected dye near the pyloric ring. Alternatively, fluoroscopy can be utilized to guide the direction of submucosal tunneling. In one study, endoclips were placed at the pylorus and fluoroscopy was utilized to guide the orientation of the tunneling. The procedure time was significantly shorter in fluoroscopy-guided G-POEM compared to conventional G-POEM.41
Initial studies evaluating G-POEM have shown encouraging results, with significant improvements in subjective as well as objective parameters, such as the Gastroparesis Cardinal Symptom Index and the gastric emptying study (GES) (Table 4).41-47 Khashab and colleagues evaluated the outcome of G-POEM in 30 patients with refractory gastroparesis due to various etiologies (idiopathic, diabetic, and postsurgical).42 The procedure was successfully completed in all of the patients, and clinical improvement was observed in 26 patients (86%) during a median follow-up of 5.5 months.42 In the same study, complete resolution of nausea, vomiting, and abdominal pain was observed in 47%, 53%, and 53% of patients, respectively. In another study, G-POEM resulted in significant improvements in overall symptoms, objective gastric emptying, and quality of life.44 Mean 4-hour gastric retention on GES decreased from 62.9% ± 24.3% to 17.6% ± 16.7% (P=.007) after G-POEM.44
Table 4.
Studies Evaluating the Outcome of G-POEM in Refractory Gastroparesis
| Study | Patients, N | Etiology of Gastroparesis | Mean Procedure Time, Minutes | Outcome Measures, Pre–G-POEM/Post–G-POEM: GCSI GES Retention at 4 Hours |
Follow-Up, Months |
|---|---|---|---|---|---|
| Gonzalez et al43 | 12 | Diabetic: 5 Idiopathic: 6 Postsurgical: 1 |
51 (range, 32-105) | 3.5 ± 0.8/1.1 ± 1.5 40%/19% |
3 |
| Khashab et al42 | 30 | Diabetic: 11 Idiopathic: 7 Postsurgical: 12 |
72 ± 42 | Clinical response, -86% GES improvement, -82% |
5.5 |
| Rodriguez et al47 | 47 | Diabetic: 12 Idiopathic: 27 Postsurgical: 8 |
41.2 ± 28.5 | 4.6 ± 0.9/3.3 ± 1.4 37%/20% |
3 |
| Xue et al41 | 14 | Diabetic: 6 Idiopathic: 6 Postsurgical: 1 Postinfectious: 1 |
36 ± 13 (with fluoroscopy guidance) 56 ± 13 (without fluoroscopy guidance) |
3.42 ± 0.48/1.33 ± 0.6 (pylorus identified) 3.00 ± 0.5/1.88 ± 1.74 (pylorus not identified) 83%/33%a |
NA |
| Dacha et al44 | 16 | Diabetic: 9 Idiopathic: 5 Postsurgical: 1 Postinfectious: 1 |
49.7 ± 22.1 | 3.40 ± 0.50/1.46 ± 1.4 62.9%/17.6% |
12 |
Decrease in GES.
GCSI, Gastroparesis Cardinal Symptom Index; GES, gastric emptying study; G-POEM, gastric peroral endoscopic myotomy; NA, not available.
It is important to note that none of these studies were randomized and that they included small numbers of patients with short follow-up durations (Table 4). Therefore, it would be premature to draw conclusions from the early results of G-POEM for the management of refractory gastroparesis.
Other Indications for Third-Space Endoscopy
Submucosal tunneling has also been explored for other indications, such as Zenker diverticulum and complete occlusion of esophageal lumen after chemoradiation. Other potential applications of third-space endoscopy using the submucosal tunneling technique include perrectal endoscopic myotomy for Hirschsprung disease or internal anal sphincter achalasia and transesophageal mediastinoscopy.48,49 The use of submucosal endoscopy for these indications has been restricted to isolated case reports, small case series, and animal studies.48,50-52 Therefore, further evaluation is required prior to wider clinical application.
Zenker Diverticulum
Zenker diverticulum is an uncommon condition and is usually managed by endoscopic division of the septum between the esophageal and diverticular lumen. An endoscopic approach is preferred over surgical treatment, as the former is associated with fewer complications, shorter procedure duration, and shorter length of hospital stay.53 However, symptoms recur in approximately 11% of patients and may be higher than with surgery.53,54 Symptom recurrence after flexible endoscopic treatment is mainly due to incomplete division of the septum. Recently, the submucosal tunneling technique has been utilized to safely divide the septum.52,55,56 Because the mucosa is preserved—unlike with conventional endoscopic myotomy, where both the mucosa and muscle are divided—the risk of perforation is reduced. This technique has been termed submucosal tunneling endoscopic septum division (abbreviated as either STESD or Z-POEM [POEM for Zenker diverticulum]).52,55-57 The accessories and initial steps of this technique, including mucosal incision and submucosal tunneling, are similar to those of the POEM procedure. The tunnel is fashioned on both sides of the septum and extended 1 to 2 cm distal to the base of the diverticulum.56 This ensures complete division of the septum.
Complete Esophageal Occlusion
Complete obliteration of the esophageal lumen is rare and usually occurs after chemoradiation for esophageal malignancies. A combined endoscopic approach (antegrade plus retrograde) has been successfully utilized for restoration of the esophageal lumen in such cases.58 However, there is a risk of perforation, as this is a semi-blind procedure. In addition, lumen restoration may not be possible in cases where the length of obstruction is long (>3-4 cm).59 Endoscopic submucosal tunneling for restoration of the esophageal lumen is a novel approach that has been described recently.51,60-62 This technique, known as peroral endoscopic tunneling for restoration of the esophagus (POETRE), has been described in detail by Wagh and colleagues.51 In POETRE, an antegrade or retrograde submucosal tunnel is created depending on the location of the esophageal stricture. The fibrotic tissue encountered during submucosal tunneling is carefully dissected. The second endoscope (ie, retrograde endoscope) is passed through the gastrostomy site to guide dissection through the first endoscope. Once the endoscopes are in close proximity, the retrograde endoscope is passed into the tunnel to grasp a guidewire passed through the antegrade endoscope. Subsequently, a self-expandable metal stent is placed through the tunnel to maintain the patency of the new esophageal lumen that has been created. Wagh and colleagues described POETRE in 4 patients with complete esophageal obstruction of at least 3 cm in length.51 The procedure was successful in all of the patients, with significant improvement in mean dysphagia score. However, the patients required endoscopic dilations after the POETRE procedure.
Adverse Events of Third-Space Endoscopy
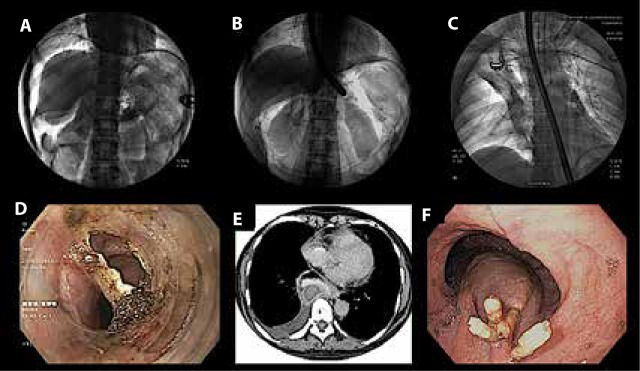
Because the submucosal plane is close to the mediastinum or peritoneum, the consequences of insufflation are not infrequent and are more or less similar across the plethora of procedures performed under the umbrella of third-space endoscopy. Besides adverse events associated with insufflation, the other commonly encountered adverse events include intraoperative bleeding and inadvertent mucosotomy or perforation (Figure 4).
Figure 4.
Adverse events encountered during third-space endoscopy procedures: (A) capnoperitoneum (note air below the diaphragm on the right side), (B) retroperitoneal carbon dioxide outlining the kidneys on both sides, (C) right-sided capnothorax, (D) mucosal perforation during peroral endoscopic myotomy, (E) computed tomography of the thorax revealing bleeding within the tunnel (note the hyperdensity within the esophageal wall), and (F) endoscopic view of the case in Figure 4E showing a submucosal bulge due to delayed submucosal bleeding.
The occurrence of major adverse events is uncommon with POEM. In a large multicenter study that included 1826 patients, the overall prevalence of bleeding (intraoperative and delayed) and mucosotomy was 0.9% and 2.8%, respectively.63 Severe adverse events, including intraoperative bleeding, perforation, cardiac arrhythmia, esophageal leak, capnomediastinum, and pulmonary events (pneumonia and empyema), occurred in 0.5% of patients.63 Gastroesophageal reflux disease (GERD) is an adverse event that is particular to POEM in contrast to other submucosal tunneling procedures. POEM is not accompanied by an antireflux procedure—unlike Heller myotomy, in which a partial fundoplication is performed to prevent postoperative GERD. Therefore, the prevalence of GERD is likely higher after POEM as compared to Heller myotomy with fundoplication.64 However, although the occurrence of GERD is common after POEM, it is usually asymptomatic in the majority of cases. In a multicenter study, GERD was detected by 24-hour pH analysis in 57.8% of patients, whereas erosive esophagitis was present in 23.2% of patients.65
The overall incidence of complications with STER ranges from 5% to 25%.66 Similar to POEM, insufflation-related adverse events are the most common. However, the majority of these do not require an intervention. In a large study that included 290 patients, the overall incidence of complications was 23.4 %. These complications included mucosal injury (1.0%), major bleeding (1.7%), pneumothorax (3.1%), and thoracic effusions (3.8%). Of these complications, only 10.0 % required an intervention.25 Predictors of complications include tumors of large size and irregular shape, tumors arising from the deep muscularis propria layer, resection of synchronous lesions, use of air for insufflation, and long operative time.22,25,66
It is important to acknowledge that the majority of insufflation-related events do not require any intervention and, therefore, are not adverse events in a true sense. Likewise, intraprocedural bleeding and perforation can be managed in most cases without any untoward consequences.67 Variability in the reported occurrence of adverse events in different studies is partly due to differences in the definitions used to describe them. In addition, the use of air instead of carbon dioxide for insufflation is associated with a high occurrence of adverse events.68,69 A standardized classification system is required for uniform reporting of adverse events associated with third-space endoscopy procedures.67
Summary
Third space, or submucosal space, is a novel operating field and has been utilized in clinical practice for approximately a decade now. In submucosal endoscopy, the integrity of the mucosa is preserved, and a mucosal flap safety valve is fashioned for enhanced safety. Improved devices and techniques have reduced procedure-related complexities and have allowed the endoscopist to perform these procedures in an endoscopy suite. Initially utilized for achalasia, third-space endoscopy procedures are now being used for other indications, such as subepithelial tumors, refractory gastroparesis, and Zenker diverticulum. As our understanding of this space improves, the future of third-space endoscopy holds promise in diagnostic as well as therapeutic GI endoscopy.
References
- 1.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Marler RJ. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007;65(4):688–694. doi: 10.1016/j.gie.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39(9):761–764. doi: 10.1055/s-2007-966764. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42(4):265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 4.Nabi Z, Ramchandani M, Chavan R, et al. Peroral endoscopic myotomy for achalasia cardia: outcomes in over 400 consecutive patients. Endosc Int Open. 2017;5(5):E331–E339. doi: 10.1055/s-0043-105517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video) Gastrointest Endosc. 2015;81(5):1170–1177. doi: 10.1016/j.gie.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Hungness ES, Sternbach JM, Teitelbaum EN, Kahrilas PJ, Pandolfino JE, Soper NJ. Peroral endoscopic myotomy (POEM) after the learning curve: durable long-term results with a low complication rate. Ann Surg. 2016;264(3):508–517. doi: 10.1097/SLA.0000000000001870. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Sato H, Ikeda H, et al. Peroral endoscopic myotomy: a series of 500 patients. J Am Coll Surg. 2015;221(2):256–264. doi: 10.1016/j.jamcollsurg.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Teitelbaum EN, Dunst CM, Reavis KM, et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2018;32(1):421–427. doi: 10.1007/s00464-017-5699-2. [DOI] [PubMed] [Google Scholar]
- 9.Li QL, Wu QN, Zhang XC, et al. Outcomes of peroral endoscopic myotomy for treatment of esophageal achalasia with a median follow-up of 49 months. Gastrointest Endosc. published online November 3, 2017. doi:10.1016/j. gie.2017.10.031.
- 10.Ngamruengphong S, Inoue H, Chiu PW, et al. Long-term outcomes of peroral endoscopic myotomy in patients with achalasia with a minimum follow-up of 2 years: an international multicenter study. Gastrointest Endosc. 2017;85(5):927–933.e2. doi: 10.1016/j.gie.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Jones EL, Meara MP, Pittman MR, Hazey JW, Perry KA. Prior treatment does not influence the performance or early outcome of peroral endoscopic myotomy for achalasia. Surg Endosc. 2016;30(4):1282–1286. doi: 10.1007/s00464-015-4339-y. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen HO, Kirkegård J, Kjær DW, Mortensen FV, Kunda R, Bjerregaard NC. Long-term outcome of peroral endoscopic myotomy for esophageal achalasia in patients with previous Heller myotomy. Surg Endosc. 2017;31(6):2596–2601. doi: 10.1007/s00464-016-5267-1. [DOI] [PubMed] [Google Scholar]
- 13.Louie BE, Schneider AM, Schembre DB, Aye RW. Impact of prior interventions on outcomes during per oral endoscopic myotomy. Surg Endosc. 2017;31(4):1841–1848. doi: 10.1007/s00464-016-5182-5. [DOI] [PubMed] [Google Scholar]
- 14.Nabi Z, Ramchandani M, Chavan R, et al. Peroral endoscopic myotomy in treatment-naïve achalasia patients versus prior treatment failure cases [published online November 23, 2017] Endoscopy. doi:10.1055/s-0043-121632. [DOI] [PubMed]
- 15.Zhang X, Modayil RJ, Friedel D, et al. Peroral endoscopic myotomy in patients with or without prior Heller’s myotomy: comparing long-term outcomes in a large U.S. single-center cohort (with videos) [published online November 6, 2017] Gastrointest Endosc. doi: 10.1016/j.gie.2017.10.039. [DOI] [PubMed]
- 16.Ponds FA, Fockens P, Neuhaus H, et al. Peroral endoscopic myotomy (POEM) versus pneumatic dilatation in therapy-naive patients with achalasia: results of a randomized controlled trial. Gastroenterology. 2017;152(5) suppl 1:S139. [Google Scholar]
- 17.Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with peroral endoscopic myotomy (POEM) for achalasia. Ann Surg. 2014;259(6):1098–1103. doi: 10.1097/SLA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 18.Ujiki MB, Yetasook AK, Zapf M, Linn JG, Carbray JM, Denham W. Peroral endoscopic myotomy: a short-term comparison with the standard laparoscopic approach. Surgery. 2013;154(4):893–897. doi: 10.1016/j.surg.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Hungness ES, Teitelbaum EN, Santos BF, et al. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17(2):228–235. doi: 10.1007/s11605-012-2030-3. [DOI] [PubMed] [Google Scholar]
- 20.Docimo S, Jr, Mathew A, Shope AJ, Winder JS, Haluck RS, Pauli EM. Reduced postoperative pain scores and narcotic use favor peroral endoscopic myotomy over laparoscopic Heller myotomy. Surg Endosc. 2017;31(2):795–800. doi: 10.1007/s00464-016-5034-3. [DOI] [PubMed] [Google Scholar]
- 21.Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017;31(1):49–63. doi: 10.1007/s00464-016-4978-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen T, Zhou PH, Chu Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. 2017;265(2):363–369. doi: 10.1097/SLA.0000000000001650. [DOI] [PubMed] [Google Scholar]
- 23.Li QL, Chen WF, Zhang C, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video) Surg Endosc. 2015;29(12):3640–3646. doi: 10.1007/s00464-015-4120-2. [DOI] [PubMed] [Google Scholar]
- 24.Hu JW, Zhang C, Chen T, et al. Submucosal tunneling endoscopic resection for the treatment of rectal submucosal tumors originating from the muscular propria layer. J Cancer Res Ther. 2014;10(suppl):281–286. doi: 10.4103/0973-1482.151533. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy. 2016;48(2):149–155. doi: 10.1055/s-0034-1393244. [DOI] [PubMed] [Google Scholar]
- 26.Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28(2):524–530. doi: 10.1007/s00464-013-3197-8. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Tan Y, Zhou Y, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol. 2015;27(7):776–780. doi: 10.1097/MEG.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 28.Li QY, Meng Y, Xu YY, et al. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc. 2017;86(3):485–491. doi: 10.1016/j.gie.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Mao XL, Ye LP, Zheng HH, et al. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus. 2017;30(4):1–7. doi: 10.1093/dote/dow023. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Gao Y, Chai N, et al. Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc. 2018;32(3):1326–1335. doi: 10.1007/s00464-017-5810-8. [DOI] [PubMed] [Google Scholar]
- 31.Chen T, Lin ZW, Zhang YQ, et al. Submucosal tunneling endoscopic resection vs thoracoscopic enucleation for large submucosal tumors in the esophagus and the esophagogastric junction. J Am Coll Surg. 2017;225(6):806–816. doi: 10.1016/j.jamcollsurg.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Du C, Linghu E. Treatment of esophageal submucosal tumors: endoscopic submucosal tunneling dissection versus thoracoscopic enucleation. Gastrointest Endosc. 2017;86(5):925. doi: 10.1016/j.gie.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103(2):416–423. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 34.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26(9):1251–1258. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 35.Khashab MA, Besharati S, Ngamruengphong S, et al. Refractory gastroparesis can be successfully managed with endoscopic transpyloric stent placement and fixation (with video) Gastrointest Endosc. 2015;82(6):1106–1109. doi: 10.1016/j.gie.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 36.Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30(4):1326–1332. doi: 10.1007/s00464-015-4385-5. [DOI] [PubMed] [Google Scholar]
- 37.Hagiwara A, Sonoyama Y, Togawa T, Yamasaki J, Sakakura C, Yamagishi H. Combined use of electrosurgical incisions and balloon dilatation for the treatment of refractory postoperative pyloric stenosis. Gastrointest Endosc. 2001;53(4):504–508. doi: 10.1067/mge.2001.113281. [DOI] [PubMed] [Google Scholar]
- 38.Ibarguen-Secchia E. Endoscopic pyloromyotomy for congenital pyloric stenosis. Gastrointest Endosc. 2005;61(4):598–600. doi: 10.1016/s0016-5107(05)00075-1. [DOI] [PubMed] [Google Scholar]
- 39.Chaves DM, Gusmon CC, Mestieri LH, et al. A new technique for performing endoscopic pyloromyotomy by gastric submucosal tunnel dissection. Surg Laparosc Endosc Percutan Tech. 2014;24(3):e92–e94. doi: 10.1097/SLE.0b013e31829cec0e. [DOI] [PubMed] [Google Scholar]
- 40.Kawai M, Peretta S, Burckhardt O, Dallemagne B, Marescaux J, Tanigawa N. Endoscopic pyloromyotomy: a new concept of minimally invasive surgery for pyloric stenosis. Endoscopy. 2012;44(2):169–173. doi: 10.1055/s-0031-1291475. [DOI] [PubMed] [Google Scholar]
- 41.Xue HB, Fan HZ, Meng XM, et al. Fluoroscopy-guided gastric peroral endoscopic pyloromyotomy (G-POEM): a more reliable and efficient method for treatment of refractory gastroparesis. Surg Endosc. 2017;31(11):4617–4624. doi: 10.1007/s00464-017-5524-y. [DOI] [PubMed] [Google Scholar]
- 42.Khashab MA, Ngamruengphong S, Carr-Locke D, et al. Gastric peroral endoscopic myotomy for refractory gastroparesis: results from the first multicenter study on endoscopic pyloromyotomy (with video) Gastrointest Endosc. 2017;85(1):123–128. doi: 10.1016/j.gie.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez JM, Lestelle V, Benezech A, et al. Gastric peroral endoscopic myotomy with antropyloromyotomy in the treatment of refractory gastroparesis: clinical experience with follow-up and scintigraphic evaluation (with video) Gastrointest Endosc. 2017;85(1):132–139. doi: 10.1016/j.gie.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 44.Dacha S, Mekaroonkamol P, Li L, et al. Outcomes and quality-of-life assessment after gastric peroral endoscopic pyloromyotomy (with video) Gastrointest Endosc. 2017;86(2):282–289. doi: 10.1016/j.gie.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 45.Mekaroonkamol P, Li LY, Dacha S, et al. Gastric peroral endoscopic pyloromyotomy (G-POEM) as a salvage therapy for refractory gastroparesis: a case series of different subtypes. Neurogastroenterol Motil. 2016;28(8):1272–1277. doi: 10.1111/nmo.12854. [DOI] [PubMed] [Google Scholar]
- 46.Shlomovitz E, Pescarus R, Cassera MA, et al. Early human experience with peroral endoscopic pyloromyotomy (POP) Surg Endosc. 2015;29(3):543–551. doi: 10.1007/s00464-014-3720-6. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez JH, Haskins IN, Strong AT, et al. Per oral endoscopic pyloromyotomy for refractory gastroparesis: initial results from a single institution. Surg Endosc. 2017;31(12):5381–5388. doi: 10.1007/s00464-017-5619-5. [DOI] [PubMed] [Google Scholar]
- 48.Bapaye A, Wagholikar G, Jog S, et al. Per rectal endoscopic myotomy for the treatment of adult Hirschsprung’s disease: first human case (with video) Dig Endosc. 2016;28(6):680–684. doi: 10.1111/den.12689. [DOI] [PubMed] [Google Scholar]
- 49.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA. Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve technique. Gastrointest Endosc. 2007;65(4):679–683. doi: 10.1016/j.gie.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Turner BG, Gee DW, Cizginer S, et al. Endoscopic transesophageal mediastinal lymph node dissection and en bloc resection by using mediastinal and thoracic approaches (with video) Gastrointest Endosc. 2010;72(4):831–835. doi: 10.1016/j.gie.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagh MS, Draganov PV. Peroral endoscopic tunneling for restoration of the esophagus: a novel endoscopic submucosal dissection technique for therapy of complete esophageal obstruction. Gastrointest Endosc. 2017;85(4):722–727. doi: 10.1016/j.gie.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 52.Hernández Mondragón OV, Solórzano Pineda MO, Blancas Valencia JM. Zenker’s diverticulum: submucosal tunneling endoscopic septum division (Z-POEM) Dig Endosc. 2018;30(1):124. doi: 10.1111/den.12958. [DOI] [PubMed] [Google Scholar]
- 53.Albers DV, Kondo A, Bernardo WM, et al. Endoscopic versus surgical approach in the treatment of Zenker’s diverticulum: systematic review and meta-analysis. Endosc Int Open. 2016;4(6):E678–E686. doi: 10.1055/s-0042-106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishaq S, Hassan C, Antonello A, et al. Flexible endoscopic treatment for Zenker’s diverticulum: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83(6):1076–1089.e5. doi: 10.1016/j.gie.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 55.Brieau B, Leblanc S, Bordacahar B, et al. Submucosal tunneling endoscopic septum division for Zenker’s diverticulum: a reproducible procedure for endoscopists who perform peroral endoscopic myotomy. Endoscopy. 2017;49(6):613–614. doi: 10.1055/s-0043-105574. [DOI] [PubMed] [Google Scholar]
- 56.Li QL, Chen WF, Zhang XC, et al. Submucosal tunneling endoscopic septum division: a novel technique for treating Zenker’s diverticulum. Gastroenterology. 2016;151(6):1071–1074. doi: 10.1053/j.gastro.2016.08.064. [DOI] [PubMed] [Google Scholar]
- 57.Ishaq S, Sultan H, Siau K, Kuwai T, Mulder CJ, Neumann H. New and emerging techniques for endoscopic treatment of Zenker’s diverticulum: state-of-the-art review [published online February 9, 2018]. Dig Endosc. doi:10.1111/den.13035. [DOI] [PubMed]
- 58.Dellon ES, Cullen NR, Madanick RD, et al. Outcomes of a combined antegrade and retrograde approach for dilatation of radiation-induced esophageal strictures (with video) Gastrointest Endosc. 2010;71(7):1122–1129. doi: 10.1016/j.gie.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 59.Goguen LA, Norris CM, Jaklitsch MT, et al. Combined antegrade and retrograde esophageal dilation for head and neck cancer-related complete esophageal stenosis. Laryngoscope. 2010;120(2):261–266. doi: 10.1002/lary.20727. [DOI] [PubMed] [Google Scholar]
- 60.Perbtani Y, Suarez AL, Wagh MS. Emerging techniques and efficacy of endoscopic esophageal reconstruction and lumen restoration for complete esophageal obstruction. Endosc Int Open. 2016;4(2):E136–E142. doi: 10.1055/s-0041-107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagh MS, Yang D, Chavalitdhamrong D, Draganov PV. Peroral endoscopic tunneling for restoration of the esophagus (POETRE) Gastrointest Endosc. 2014;80(2):330. doi: 10.1016/j.gie.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 62.Babich JP, Diehl DL, Entrup MH. Retrograde submucosal tunneling technique for management of complete esophageal obstruction. Surg Laparosc Endosc Percutan Tech. 2012;22(4):e232–e235. doi: 10.1097/SLE.0b013e318257c9e5. [DOI] [PubMed] [Google Scholar]
- 63.Haito-Chavez Y, Inoue H, Beard KW, et al. Comprehensive analysis of adverse events associated with per oral endoscopic myotomy in 1826 patients: an international multicenter study. Am J Gastroenterol. 2017;112(8):1267–1276. doi: 10.1038/ajg.2017.139. [DOI] [PubMed] [Google Scholar]
- 64.Repici A, Fuccio L, Maselli R, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy as compared with Heller’s myotomy with fundoplication: a systematic review with meta-analysis [published online November 1, 2017] Gastrointest Endosc. doi:10.1016/j.gie.2017.10.022. [DOI] [PubMed]
- 65.Kumbhari V, Familiari P, Bjerregaard NC, et al. Gastroesophageal reflux after peroral endoscopic myotomy: a multicenter case-control study. Endoscopy. 2017;49(7):634–642. doi: 10.1055/s-0043-105485. [DOI] [PubMed] [Google Scholar]
- 66.Du C, Linghu E. Submucosal tunneling endoscopic resection for the treatment of gastrointestinal submucosal tumors originating from the muscularis propria layer. J Gastrointest Surg. 2017;21(12):2100–2109. doi: 10.1007/s11605-017-3579-7. [DOI] [PubMed] [Google Scholar]
- 67.Nabi Z, Reddy DN, Ramchandani M. Adverse events during and after peroral endoscopic myotomy: prevention, diagnosis, and management. Gastrointest Endosc. 2018;87(1):4–17. doi: 10.1016/j.gie.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 68.Wang XY, Xu MD, Yao LQ, et al. Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos) Surg Endosc. 2014;28(6):1971–1977. doi: 10.1007/s00464-014-3420-2. [DOI] [PubMed] [Google Scholar]
- 69.Zhang XC, Li QL, Xu MD, et al. Major perioperative adverse events of peroral endoscopic myotomy: a systematic 5-year analysis. Endoscopy. 2016;48(11):967–978. doi: 10.1055/s-0042-110397. [DOI] [PubMed] [Google Scholar]