Abstract
The symbiotic bacterium Wolbachia pipientis has been considered unique in its ability to cause multiple reproductive anomalies in its arthropod hosts. Here we report that an undescribed bacterium is vertically transmitted and associated with thelytokous parthenogenetic reproduction in Encarsia, a genus of parasitoid wasps. Although Wolbachia was found in only one of seven parthenogenetic Encarsia populations examined, the “Encarsia bacterium” (EB) was found in the other six. Among seven sexually reproducing populations screened, EB was present in one, and none harbored Wolbachia. Antibiotic treatment did not induce male production in Encarsia pergandiella but changed the oviposition behavior of females. Cured females accepted one host type at the same rate as control females but parasitized significantly fewer of the other host type. Phylogenetic analysis based on the 16S rDNA gene sequence places the EB in a unique clade within the Cytophaga-Flexibacter-Bacteroid group and shows EB is unrelated to the Proteobacteria, where Wolbachia and most other insect symbionts are found. These results imply evolution of the induction of parthenogenesis in a lineage other than Wolbachia. Importantly, these results also suggest that EB may modify the behavior of its wasp carrier in a way that enhances its transmission.
Some vertically transmitted bacteria increase in frequency by manipulating host reproduction in ways that enhance their own transmission, but do not necessarily benefit their host. The best known of these “reproductive parasites” is Wolbachia, a single bacterial lineage in the alpha-group of the Proteobacteria that has been implicated in all of the types of reproductive manipulations discovered to date, including cytoplasmic incompatibility, male killing, thelytokous parthenogenesis induction (PI), and feminization (1–3). Wolbachia has been found in between 16 and 76% of arthropods sampled (4, 5) and may effect reproductive isolation from other populations, fundamental changes in genetic structure, and evolution of the sex-determination system of infected populations (6–9).
It has been suggested that Wolbachia is unique in its ability to cause cytoplasmic incompatibility, PI, and feminization (3), because the delicate manipulations of chromosomes required are likely to have evolved only once (10). Recently, this argument was weakened by the finding of an undescribed bacterium unrelated to Wolbachia that causes feminization in the mite Brevipalpus phoenicis (11).
PI Wolbachia has been recorded in many parasitic wasps (12). In these haplodiploid species, PI Wolbachia causes the chromosome complement of haploid incipient male eggs to double, and the eggs to develop as females. Antibiotic treatment of infected females generally kills the bacteria, and unfertilized eggs then give rise to male offspring. These males may sometimes be fertile (12), but reproductive barriers are often observed in populations fixed for the infection, presumably because of relaxed selection on sexual traits (13–15).
Sex-specific oviposition behaviors are also likely to evolve in host populations infected with a PI bacterium. For example, one might expect selection on both bacterial and wasp genomes to act to prevent infected females from accepting hosts that may be suitable for male but not female development. In most cases, these behavioral refinements may be too subtle to measure, but they are likely to be very important in those parasitoids in which males and females generally develop in different host environments (16).
Most sexual parasitic wasps of the genus Encarsia (Hymenoptera: Aphelinidae) are autoparasitoids. They lay fertilized incipient female eggs in hemipteran nymphs (the “primary hosts”). In contrast, unfertilized eggs are laid in immature hemipteran parasitoids (the “secondary hosts”) and develop as male hyperparasitoids. Unmated females, capable of laying only unfertilized male eggs, usually refrain from ovipositing when they are confined with primary hosts suitable only for female development. Generally, when eggs of the “wrong” sex are laid in a host, they do not develop (16, 17).
Parthenogenetic Encarsia usually produce only daughters from primary hosts. However, the transition from sexual reproduction to parthenogenesis in this genus requires a change in the oviposition behavior of females. Although unmated sexual females refrain from laying unfertilized eggs in the primary host (unmated), females in populations fixed for parthenogenesis willingly do so. These unfertilized eggs then become diploid females (15, 18). Thus, after acquisition of a PI bacterium, the evolutionary interests of both wasp and bacterium are served by unmated females' acceptance of the primary host for oviposition.
Here we report that an undescribed bacterium is implicated in causing thelytokous parthenogenesis in six populations of Encarsia, a genus of parasitoid wasps. We characterize the bacterium found in Encarsia pergandiella by its 16S rDNA sequence, its relatedness to other bacteria, its ultrastructure, and location in host ovarian tissue. We show a statistically significant association of the bacterium with parthenogenetic reproduction in a survey of 14 Encarsia populations. Further, we find that a parthenogenetic population of Encarsia hispida, previously shown to produce males after antibiotic treatment, does not have Wolbachia but has the undescribed bacterium. Lastly, we compare the reproductive phenotypes of antibiotic-fed and control E. pergandiella females on both primary and secondary hosts.
Materials and Methods
Parasitoid Wasp Rearing.
E. pergandiella was reared on the greenhouse whitefly, Trialeurodes vaporariorum, on green beans under fluorescent and natural light at ≈25°C with a 14-h/10-h light/dark photoperiod.
Electron Microscopy.
The ovaries of adult E. pergandiella wasps were fixed in 4% glutaraldehyde in 0.05 M cacodylate overnight at 4°C. After postfixation in 2% OsO4 for 2 h, the samples were washed, en bloc-stained in 2% uranyl acetate, and dehydrated through an ethanol series (50, 70, 95, and 100%). Then the samples were placed in propylene oxide and embedded in Epon. Serial sections were cut by using an RMC (Tucson, AZ) MT7000 ultra microtome. The grids were stained with saturated uranyl acetate and lead citrate and viewed under a Philips Electronic Instruments (Hillsboro, OR) CM12 transmission electron microscope.
PCR Amplification and Sequencing.
DNA was extracted from 50 E. pergandiella by using cetyltrimethylammonium bromide (CTAB). The 16S ribosomal DNA gene (16S rDNA) was amplified by using PCR and general primers (27F and 1495R) that amplify this gene across all known Eubacteria (19). Samples that yielded amplicons of the expected size (≈1,500 bp) were cloned by using p-GEMT Easy Vector system (Promega). Plasmids were amplified, purified, and sequenced.
Sequence-specific primers for the most abundant bacterium, denoted here as the “Encarsia bacterium” (EB), were designed, based on the nucleotide sequence. Primers were (forward) EPS-f 5′-TACAATCTTTATTAACCCATGTT-3′ and (reverse) EPS-r 5′-TTCAAAGTAGCAAAATACATTC-3′. The specific EB primers were used in a PCR (parameters: denaturation for 2 min at 95°C, followed by 40 cycles of 30 sec at 92°C, 30 sec at 50°C, 30 sec at 72°C, and a 5-min final extension at 72°C) to screen other Encarsia populations for EB (see Table 1 for populations screened and their origins) and offspring of antibiotic-treated E. pergandiella (see Antibiotic Treatment for details). All but 2 of the 14 population samples were derived from cultures from 5 different laboratories and all had a well characterized reproductive mode. PCR primers specific for the 16S rDNA gene of Wolbachia (20) were also used to determine whether the different Encarsia populations were infected with that bacterium. As an internal control, primers known to amplify the mitochondrial cytochrome oxidase-I (COI) gene were also used for all samples.
Table 1.
The presence of the EB and Wolbachia in parthenogenetic and sexual populations of Encarsia species from various geographic localities
| Encarsia species | Repro. mode* | Host type† | EB‡ | Wolbachia§ | Collection site | Source for this study |
|---|---|---|---|---|---|---|
| E. berlesei | P | AS | + | − | Italy | ¶ |
| E. bimaculata | S | W | − | − | Sudan | ‖ |
| E. citrina | P | AS | + | − | Italy | ¶ |
| E. formosa | P | W | − | + | Ciba-Bunting Insectary, U.K. | ** |
| E. lutea | S | W | − | − | Italy | ¶ |
| E. luteola | S | W | − | − | CA | ** |
| E. hispida | P | W | + | − | Spain | ¶ |
| E. pergandiella | P | W | + | − | Brazil | ** |
| E. pergandiella | S | W | + | − | TX | ‡‡ |
| E. pergandiella | S | W | − | − | CA | ¶ |
| E. perniciosi | P | AS | + | − | CA | †† |
| E. protransvena | P | W | + | − | CA | §§ |
| E. smithi | S | W | − | − | HI | §§ |
| E. sophia | S | W | − | − | Murcia, Spain | ** |
P, parthenogenetic; S, sexual.
AS, armored scale; W, whitefly.
±, presence or absence of EB.
±, presence or absence of Wolbachia.
Laboratory culture, Universitá “Federico II,” Italy (P. Pedata).
Laboratory culture, University of Florida, Gainesville, FL (R. Nguyen). Obtained from J. Heraty, University of California, Riverside, CA.
Laboratory culture, University of Arizona, Tucson, AZ (M.H.).
Laboratory culture, U.S. Department of Agriculture-Animal and Plant Health Inspection Service, Mission, TX (M. Ciomperlik).
Laboratory culture, University of California, Riverside, CA (R. Luck). Obtained from J. Heraty, University of California, Riverside, CA.
Field collection. Obtained from J. Heraty, University of California, Riverside, CA.
It has been suggested recently that parts of a phage incorporated into the genome of Wolbachia may contribute to the various reproductive effects of that bacterium (21). Therefore the EB was screened for phage sequences recently found in Wolbachia (WO; ref. 21) and in the “T-type” secondary symbiont of the pea aphid Acyrthosiphon pisum (APSE- 1; ref. 22). The two primer pairs used in PCR were phgWOF 5′-CCCACATGAGCCAATGACGTCTG-3′ and phgWOR 5′-CGTTCGCTCTGCAAGTAACTCCATTAAAAC-3′, for phage WO (21), and APSE-2F 5′-GGACAATCAGGAAGAGT-3′ and APSE-2R 5′-GAGCCATCTTCGTTTTC-3′, for APSE-1, based on the published sequence (22). Encarsia formosa, known to be infected with Wolbachia (23), and A. pisum served as positive controls for these primers, respectively.
Phylogenetic Analysis.
When the EB 16S rDNA sequence was compared with other known sequences by using an advanced blast (National Center for Biotechnology Information) search, all closely matching bacteria identified were affiliated with the “Cytophaga-Flexibacter-Bacteroid” (CFB) group. Based on this result, two phylogenetic analyses were performed. In both, sequences were aligned by clustal; manually corrected by using macclade (Sinavc, Sunderland, MA) software; and subjected to parsimony, maximum likelihood, and distance analyses using PAUP*4.0b2. The robustness of the results was evaluated by 100 bootstrap replicates. An analogous sequence obtained for the primary symbiont of Bemisia tabaci was used as an out-group. The Ribosomal Database Project was used as a reference source of information regarding contemporary bacterial phylogeny (24). In the first analysis, the phylogenetic position of the EB was determined by comparing the nearly full-length 16S rDNA gene sequence (≈1,500 bp) of that bacterium [from E. pergandiella (Brazil)] with nucleotide sequences representing major groups within the CFB. A second phylogenetic analysis was performed on all of the sequences within the CFB that are known to belong to symbionts. This analysis included the EB from 5 Encarsia populations (about 830 bp), two bacteriocyte symbionts of cockroaches (25), male-killing agents of two coccinellid beetles (10, 26), a bacterium found in a tick (ref. 27; GenBank accession no. AB001518), a symbiont of an ectomycorrhizal mycelium (28), and an acanthamoeba symbiont (GenBank accession no. AF215634).
Antibiotic Treatment.
E. pergandiella females less than 24-h-old were collected in vials streaked with either honey only (control females) or 50 mg/ml rifampicin in honey (antibiotic-fed females), and held for 48 h. After this period, all females were placed on leaves in Petri dishes with primary hosts for 8 h to allow females to lay eggs matured before antibiotic treatment. Then adult females were collected in vials with honey and used in the experiment the following day.
Reproductive Phenotype of Infected and Antibiotic-Cured Parthenogenetic E. pergandiella.
In this experiment, antibiotic-fed and control females of the parthenogenetic E. pergandiella were provided with either primary hosts (T. vaporariorum fourth instar nymphs) or secondary hosts (E. formosa early pupae) shown to be suitable for development of E. pergandiella (29). Hosts in half of the arenas were dissected to determine oviposition patterns, and hosts in the other half of the arenas were reared until wasp pupation.
Primary hosts were presented on living cotton leaves in circular covered arenas (16-mm interior diameter). Excess nymphs were removed to leave 15 hosts. In arenas of secondary hosts, 15 E. formosa pupae were presented on leaf disks arranged on moistened filter paper within the same arenas.
Females were introduced individually to arenas for 6 h. In an additional treatment, sexual Encarsia luteola females were introduced individually to secondary host arenas to serve as a positive control for the adequacy of our rearing regime for parasitized secondary hosts. The arenas from which hosts were to be dissected were incubated for 24 h and then refrigerated until dissection, whereas the other arenas were held until pupation of wasp progeny or host death. In the case of the E. luteola treatment, all arenas were incubated until wasp pupation. Dissection of hosts was performed in a drop of insect Ringer's solution under a dissecting microscope.
Results
Electron Microscopy.
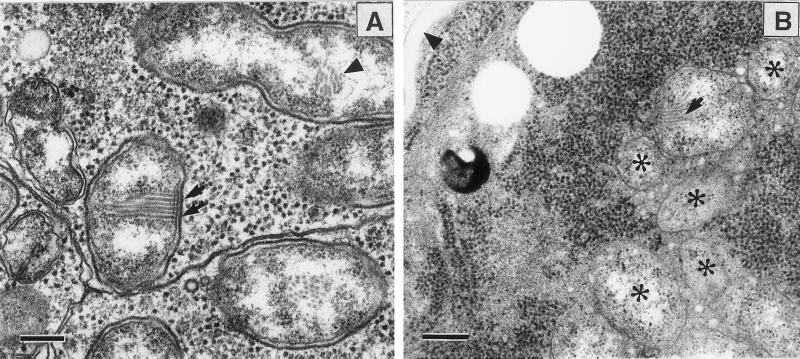
Electron microscopic examination of the ovaries of E. pergandiella showed numerous bacteria in virtually all cell types, including the nurse cells, follicle cells, and developing oocytes (Fig. 1). Analysis of the substructure revealed a two-layered envelope (an outer cell wall and an inner plasma membrane) and an array of filament-like structures attached to the inner membrane (Fig. 1). These filaments were not observed in all of the bacteria and were observed only in one of two daughter cells of a bacterium in the process of dividing (Fig. 1). This observation suggests that they are either not detectable in all sections or may not be inherited by both daughter cells after division. No evidence was found for more than one type of bacterium in any ovarian cells examined.
Figure 1.
Ovaries of E. pergandiella. (A) Nurse cell. Bacteria in the nurse cell show a striate array of microfilament-like structures (arrows) attached to the inner cell membrane. A dividing cell containing a portion of the array (arrowhead) is seen in one of the daughter cells. [Bar = 200 nm.] (B) Oocyte. The E. pergandiella oocyte is distinguished by its lack of yolk and tightly packed ribosomes. Note the presence of the egg chorion (arrowhead). Some of the densely packed bacteria (asterisks) can be seen. Some of the arrays seen in the nurse cells are apparent (arrow). (Bar = 300 nm.)
PCR Amplification and Sequencing.
PCR amplification, using general primers for eubacterial 16S rDNA genes, showed a product of the expected size of about 1,500 bp. Of 10 plasmid inserts, 7 were identical and showed 96% similarity to both the 16S rDNA sequence of an endosymbiont of the tick Ixodes scapularis and to the feminizing symbiont of Brevipalpus phoenicis (11). Each of the three other clone sequences was 100% identical to three different known bacteria (Comamonas, Achromobacter, and Pediococcus) and was assumed to be contaminants. Based on the dominant clones, the nearly full-length 1,491-bp 16S rDNA gene of the symbiont found in E. pergandiella was determined (GenBank accession no. AF319783).)
By using EB-specific primers, we screened 14 populations of Encarsia to determine the incidence of EB infection in this genus. Of the seven parthenogenetic populations of Encarsia screened, six were found to carry EB but not Wolbachia (Table 1). The EB was not detected in the progeny of antibiotic-treated parthenogenetic E. pergandiella. One population, E. formosa, was found to carry Wolbachia but not EB (Table 1); Wolbachia infection in this species confirms published findings (23). Of the seven sexual populations of Encarsia, none were found to carry Wolbachia (Table 1), but EB was found in the population of E. pergandiella from Texas (Table 1). The association of parthenogenesis with EB is highly statistically significant (Gadj,1 df = 7.17, P < 0.01). For all samples reported, the mitochondrial COI amplicon was also obtained by PCR, confirming that the preparations were of sufficient quality to permit the detection of EB if the bacterium was present. The EB 16S rDNA gene was cloned and sequenced from five of the seven populations in which EB was present [GenBank accession nos. AY026336 (E. berlesei), AY026333 (E. citrina), AY026334 (E. hispida), AF319783 (E. pergandiella, Brazil), and AY026335 (E. pergandiella, Texas)].
The possibility that the EB-infected population from Texas consisted of a mixture of uninfected sexual and infected parthenogenetic forms was investigated. Parthenogenetic females can produce female progeny without mating. In several separate trials (n = 8), none of 119 unmated females produced any female progeny. Because DNA preparations from single females consistently yielded amplification of the EB 16S rDNA gene, we concluded that the sexual forms were indeed infected.
Phylogenetic Analysis.
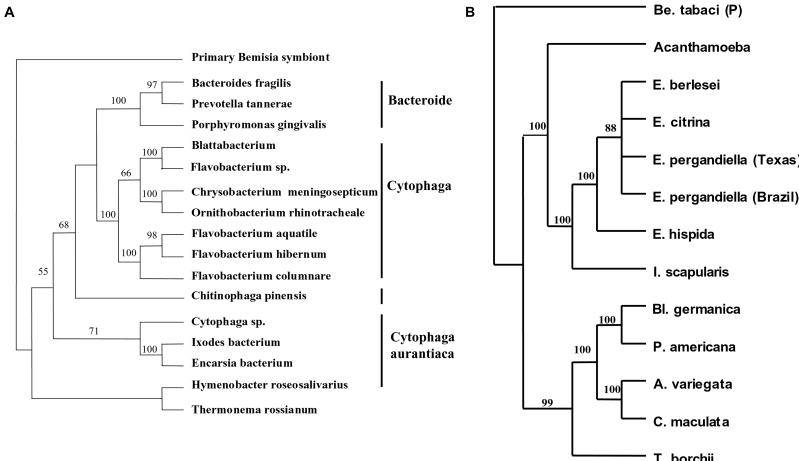
To determine the relationship between EB and other symbiotic bacteria, we performed a phylogenetic analysis of the 16S rDNA sequences. Each one of the methods used for placing EB in a phylogenetic context resulted in one tree, and all trees had the same basic topology. The tree resulting from a heuristic search (distance analyses-minimum evolution, general time-reversible distance measure) is presented in Fig. 2A. Based on the sequence of the 16S rDNA gene, the bacterium found in E. pergandiella belongs to the CFB group of the Eubacteria, with the I. scapularis symbiont as its closest relative (Fig. 2A). These two bacteria form a unique clade within the group designated as Cytophaga aurantiaca (ref. 24; Fig. 2A). When all of the sequences of the 16S rDNA gene within the CFB known to belong to various symbionts were analyzed, all phylogenetic analyses performed produced a single tree with all nodes highly supported by bootstraps. The tree presented in Fig. 2B is the result of a maximum parsimony analysis (branch and bound search, 100 bootstraps, all parameters set at default). This phylogenetic analysis showed two distinct clades, one of which consisted of the symbionts of Encarsia, the tick I. scapularis, and an undescribed acanthamoeba isolate, whereas the other included the symbionts of cockroaches, coccinellid beetles, and a mycorrhizal fungus (Fig. 2B).
Figure 2.
Phylogenetic analysis of the EB based on 16S rDNA gene sequences. (A) Analysis within the CFB group. The EB and representatives of the major CFB groups were analyzed by using the distance (minimum evolution) optimality criterion. (B) Analysis of the symbionts within the CFB group. The 16S rDNA of the EB was compared with those of other CFB bacteria with known symbiotic associations by using a maximum parsimony analysis (branch and bound search, all parameters set at default). Symbionts are referred to by the name of their hosts. Be, Bemisia; E, Encarsia; I, Ixodes; Bl, Blattella; P, Periplaneta; A, Adonia; C, Coleomegilla; T, Tuber. Both analyses were performed by using PAUP*4.0b2, with whitefly (B. tabaci) primary symbionts (Proteobacteria) as an outgroup. Bootstraps values are given for the nodes.
EB Changes the Oviposition Behavior of E. pergandiella Females.
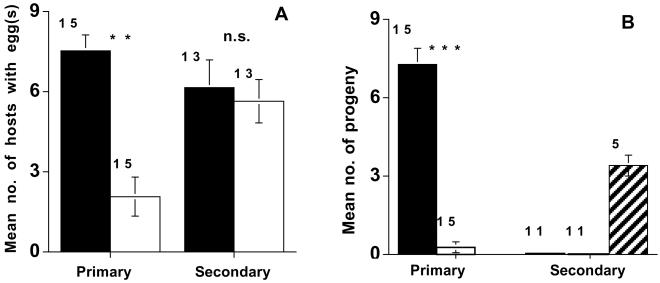
Control and antibiotic-fed females of the parthenogenetic E. pergandiella parasitized equivalent numbers of secondary hosts (F1,25 = 0.07, P > 0.05); Fig. 3A), suggesting that the antibiotic did not reduce the vigor of treated wasps relative to the controls. However, antibiotic treatment did affect the oviposition behavior of E. pergandiella on primary hosts (Fig. 3A); antibiotic-fed females laid significantly fewer eggs on primary hosts than did control wasps (F1,28 = 9.63, P < 0.01).
Figure 3.
Effects of antibiotic curing on oviposition patterns and progeny production of the parthenogenetic E. pergandiella population from Brazil. (A) The mean number of primary and secondary hosts dissected per arena that contained eggs. Black bars are arenas that contained control females and white bars are arenas with antibiotic-fed females. (B) The mean number of hosts that produced wasp progeny per arena incubated. Black bars are arenas with control females, white bars are arenas with antibiotic-fed females, and the hatched bar represents arenas with E. luteola females. Number of replicates is indicated by the number above each bar. NS, not significant. **, P < 0.01; ***, P < 0.001.
Arenas of primary hosts parasitized by control females produced female progeny (Fig. 3B). Of 15 antibiotic-fed females, only 1 produced progeny on the primary hosts, and the 3 progeny she produced were females. Antibiotic-fed females produced significantly fewer progeny than control females (F1, 28 = 23.30, P < 0.001), in large part as a result of reduced oviposition in these hosts. On secondary hosts, all of the positive control E. luteola females tested (n = 5) produced male progeny, suggesting that our rearing procedure was adequate for the development of wasp progeny (Fig. 3B). However, neither the antibiotic-fed nor the control E. pergandiella females produced any progeny on these hosts (Fig. 3B).
Discussion
Here we report on a parthenogenesis-associated bacterium that is not a member of the Proteobacteria. The 16S rDNA sequence of the EB places it in the CFB group. Based on the 16S rDNA sequence, the EB is only 81% similar to its closest described relative, thus EB is used as a provisional designation until this bacterium can be properly described.
The majority of described arthropod symbionts, including Wolbachia and most bacteriocyte endosymbionts, fall within the Proteobacteria (30), but the distantly related CFB has recently been emerging as a new source of endosymbiont diversity (10, 25, 26, 28). The phylogenetic analysis of symbionts within the CFB showed two clades (Fig. 2B) with the EB more closely allied with the tick and acanthamoeba symbionts than with the cockroach, mycorrhizal fungus, and coccinellid beetle symbionts (Fig. 2B). The analysis also showed that apparently close relatives, such as cockroach bacteriocyte symbionts and a male-killing bacterium in coccinellid beetles, may have remarkable differences in the way they interact with their hosts.
Ultrastructural analysis of E. pergandiella (Brazil) ovaries showed an abundance of the EB in the follicle cells, nurse cells, and oocytes, indicating vertical transmission. Many of these bacteria contained regular arrays of microfilament-like structures attached to the inner membrane (Fig. 1). This striate-like ultrastructure has been seen in other symbiotic bacteria (31–33), most recently in a symbiont of the tick I. scapularis (27) that is the closest relative of EB (Fig. 2A). The function of these arrays remains unknown.
Analysis of the different EB sequences from four infected parthenogenetic Encarsia populations and one infected sexual Encarsia population shows a very high degree of sequence identity (99.7%). Because the infected Encarsia populations are not themselves all close relatives (34), the similarity among sequences suggests that the bacterium can be horizontally transmitted. Horizontal transfer of Wolbachia has been demonstrated experimentally (35) and has similarly been implicated in the lack of concordance of the phylogenies of the bacteria and their hosts (e.g., ref. 23).
In this report we show that EB, but not Wolbachia, is associated with parthenogenetic reproduction in six species of Encarsia, including E. hispida. In a previous study, antibiotic treatment of the same population of E. hispida caused the production of male offspring (15). By using the same standard of evidence commonly used for establishing PI by bacterial symbionts such as Wolbachia (12), these results suggest EB is the cause of parthenogenesis in at least this population. The EB was also found in one sexual population of E. pergandiella. Although the effects of EB in this population are not yet known, the recent finding of a closely related bacterium causing feminization in a mite (11) suggests that like Wolbachia, EB may have multiple reproductive effects.
Antibiotic treatment of parthenogenetic E. pergandiella failed to cause females to produce sons; the very few progeny produced in this treatment were daughters. PCR analysis of a larger sample of female offspring of antibiotic-fed females showed that F1 females were not infected. In other studies, antibiotic treatment of some parasitoid wasps with PI Wolbachia has caused females to produce uninfected daughters that then produce sons (36). Although in the experiment reported here there were too few F1 females to test for progeny production, other preliminary data suggest that F1 females of E. pergandiella fail to produce any progeny despite their apparently normal ovarian development. A probable explanation for the absence of males observed here is that relaxed selection on male function, implicated in male sterility in species fixed for PI Wolbachia (15, 36), is responsible for male mortality. Lethal mutations in male-specific traits might be expected if EB has long been associated with this population of E. pergandiella.
Both antibiotic-fed and control females oviposited at similar rates in secondary hosts, but no progeny were produced on these hosts. Male inviability might explain the lack of progeny produced by antibiotic-treated females, and secondary hosts may be incapable of supporting the development of female eggs laid by control females. Although it seems maladaptive for females to oviposit on a host unsuitable for progeny development, this is normal behavior of the sexual ancestors of this population that seems to be retained by their parthenogenetic descendents.
Interestingly, antibiotic treatment of the parthenogenetic E. pergandiella females caused a change in their oviposition behavior. Although both antibiotic-fed and control females oviposited at similar rates in secondary hosts, antibiotic-fed females largely refrained from ovipositing in primary hosts. This behavior is similar to that of unmated, uninfected sexual E. pergandiella (15). This result suggests that EB is responsible for infected females' acceptance of primary hosts in this population, an effect reversible by treatment with antibiotics. Because meiosis and (in infected eggs) restoration of diploidy occurs after the egg is laid, it is unlikely that any cues from the egg are used as signals to the female to adopt the appropriate oviposition behavior. Further, although we cannot rule out the possibility that the ovipositional restraint we observe in primary hosts is caused by antibiotic treatment, it seems unlikely that antibiotics could influence ovipositional behavior toward one host type and not the other. It is in the interests of the bacterium, as well as the wasp, for unmated infected female wasps to accept primary hosts (because unfertilized eggs may develop as females in these hosts). Further, a rapid switch in behavior of its wasp host may be critical to the invasion of EB in an autoparasitoid population such as E. pergandiella. A PI bacterium will ordinarily spread in a population by converting unfertilized incipient male eggs to females, thus increasing the relative number of daughters produced by infected females. In an autoparasitoid population, however, unfertilized eggs are usually laid only in secondary hosts, and our results indicate that in E. pergandiella, the daughters of infected females do not develop in these hosts. Thus, in order for EB to spread, females must accept primary hosts as oviposition sites for unfertilized eggs. This restriction does not seem to occur in E. hispida, where daughters of infected females may develop on secondary hosts, and where there is no abrupt change in oviposition behavior of antibiotic-treated females (15), perhaps as a result of selection on the nuclear genome of infected females to accept these hosts.
Pathogen- or parasite-associated changes in host behavior that are adaptive for parasite fitness or transmission are well known (37, 38). In one example, a protozoan parasite of mosquitoes, Lambornella clarki, causes unmated mosquito females to act as though they are mated and gravid and “oviposit” protozoans in many bodies of water (39). We believe the example presented here, however, is the first report, to our knowledge, of host behavior modification by a reproductive parasite. Behavioral changes in populations infected with a reproductive parasite have been shown. In one example, the high frequency of a male-killing element in a population of butterflies has led to interfemale competition for mates (40), yet it is most reasonable to assume that this change is in response to selection on the genome of infected females rather than a change caused directly by the bacterium.
In conclusion, our findings suggest that the ability to induce thelytokous parthenogenesis is neither a unique property of Wolbachia nor even of the Proteobacteria, but has evolved in an unrelated bacterial group, the CFB. Whether this ability has evolved independently or was acquired by means of horizontal transfer, for example by infection with a common bacteriophage, is as yet unclear. It was discovered recently that all Wolbachia tested carry the phage WO, a bacteriophage-like genetic element incorporated into the bacterium genome (21). Among the genes encoded by bacteriophage WO are genes encoding for ankyrin-like protein that are considered to be involved in protein–protein interactions (21). Masui et al. (21) speculate that these genes may be important in the reproductive alteration of the host. Although the presence of a bacteriophage was not revealed in this study by using WO and APSE-1 primers, the possibility that a phage is present in EB, and may be involved in the reproductive manipulation of its host, cannot be ruled out until the entire genomes have been sequenced.
However the effects of Wolbachia and EB on host chromosomes have evolved, our discovery undermines the idea that they are a property of a single bacterial lineage and raises the possibility that there may be other as yet undiscovered bacteria with similar effects. The present study suggests that the relative simplicity of screening insects by using Wolbachia-specific primers may have channeled research in such a way that other potential reproductive parasites go undescribed (3). One intriguing example is the case of Galeopsomyia fausta, a parthenogenetic leaf miner parasitoid in the Eulophidae that produces males after antibiotic treatment but does not seem to be infected with Wolbachia (41) or EB (Y.G., unpublished data). Other examples of parthenogenetic parasitoids where Wolbachia involvement has been suggested solely on the evidence of male production after heat or antibiotic treatment (e.g., see table 4.1 of ref. 12) should perhaps be less explicitly categorized pending molecular analyses. Lastly, even in arthropods where Wolbachia has been found, the possibility of double infections of Wolbachia and an unrelated bacterium such as EB makes the assignment of particular effects on host reproduction to a particular bacterium somewhat less certain.
Acknowledgments
We thank P. Pedata and M. Ciomperlik for sending us Encarsia; J. Russell and N. Moran for primer sequences; J. Russell, K. Oliver, J. Jaenike, and M. Kidwell for comments on the manuscript; and D. Bentley for technical assistance. This research was partially supported by Vaadia–Binational Agricultural Research and Development (BARD) Postdoctoral Award No. FI-285-99 (to E.Z.-F.) a grant from the Binational Science Foundation (U.S. and Israel) (to T.L.K.), and by USDA National Research Initiative Grant 98-35302-6974 (to M.S.H.).
Abbreviations
- EB
Encarsia bacterium
- CFB
Cytophaga-Flexibacter-Bacteroid
- PI
parthenogenesis induction
Footnotes
References
- 1.Werren J H. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill S L, Hoffmann A A, Werren J H, editors. Influential Passengers: Inherited Microorganisms and Invertebrate Reproduction. New York: Oxford Univ. Press; 1997. p. 214. [Google Scholar]
- 3.Stouthamer R, Breeuwer J A J, Hurst G D D. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Werren J H, Guo L, Windsor D W. Proc R Soc London Ser B. 1995;262:147–204. [Google Scholar]
- 5.Jeyaprakash A, Hoy M A. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 6.Turelli M, Hoffmann A A, McKechnie S W. Genetics. 1992;132:713–723. doi: 10.1093/genetics/132.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigaud T. In: Influential Passengers: Inherited Microorganisms and Invertebrate Reproduction. O'Neill S L, Hoffmann A A, Werren J H, editors. Oxford: Oxford Univ. Press; 1997. pp. 81–101. [Google Scholar]
- 8.Shoemaker D D, Katju V, Jaenike J. Evolution (Lawrence, Kans) 1999;53:1157–1164. doi: 10.1111/j.1558-5646.1999.tb04529.x. [DOI] [PubMed] [Google Scholar]
- 9.Bordenstein S, O'Hara F, Werren J. Nature (London) 2001;409:707–710. doi: 10.1038/35055543. [DOI] [PubMed] [Google Scholar]
- 10.Hurst G D D, Hammarton T C, Bandi C, Majerus T M O, Bertrand D, Majerus M E N. Gen Res. 1997;70:1–6. [Google Scholar]
- 11.Weeks A R, Marec F, Breeuwer J A J. Science. 2001;292:2479–2482. doi: 10.1126/science.1060411. [DOI] [PubMed] [Google Scholar]
- 12.Stouthamer R. In: Influential Passengers: Inherited Microorganisms and Invertebrate Reproduction. O'Neill S L, Hoffmann A A, Werren J H, editors. Oxford: Oxford Univ. Press; 1997. pp. 102–124. [Google Scholar]
- 13.Zchori-Fein E, Faktor O, Zeidan M, Gottlieb Y, Czosnek H, Rosen D. Insect Mol Biol. 1995;4:173–178. doi: 10.1111/j.1365-2583.1995.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 14.Pijls J W A M, van Steenbergen J J, van Alphen J J M. Heredity. 1996;76:506–513. [Google Scholar]
- 15.Hunter M S. J Evol Biol. 1999;12:735–741. [Google Scholar]
- 16.Hunter M S, Woolley J B. Annu Rev Entomol. 2001;46:251–290. doi: 10.1146/annurev.ento.46.1.251. [DOI] [PubMed] [Google Scholar]
- 17.Walter G H. J Entomol Soc S Afr. 1983;46:261–282. [Google Scholar]
- 18.Hunter M S. In: Theoretical Approaches to Biological Control. Hawkins B A, Cornell H V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 231–258. [Google Scholar]
- 19.Weisburg W G, Barns S M, Pelletier D A, Lane D J. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neill S L, Giordano R, Colbert A M E, Karr T L. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masui S, Kamoda S, Sasaki T, Ishikawa H. J Mol Evol. 2000;51:491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- 22.Sandström J P, Russell J A, White J P, Moran N A. Mol Ecol. 2001;10:217–228. doi: 10.1046/j.1365-294x.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 23.van Meer M M M, Witteveldt J, Stouthamer R. Insect Mol Biol. 1999;8:399–408. doi: 10.1046/j.1365-2583.1999.83129.x. [DOI] [PubMed] [Google Scholar]
- 24.Maidak B L, Cole J R, Lilburn T G, Parker C T, Saxman P R, Stredwick J M, Garrity G M, Li B, Olsen G J, Paramanik S, et al. Nucleic Acids Res. 2000;28:173–174. doi: 10.1093/nar/28.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandi C, Damiani G, Magrassi L, Grigolo A, Fani R, Sacchi L. Proc R Soc London Ser B. 1994;257:43–48. doi: 10.1098/rspb.1994.0092. [DOI] [PubMed] [Google Scholar]
- 26.Hurst G D D, Hammarton T C, Bandi C, Majerus T M O, Bertrand D, Majerus M E N. Parasitology. 1999;118:125–134. doi: 10.1017/s0031182098003655. [DOI] [PubMed] [Google Scholar]
- 27.Kurtti T J, Munderloh U G, Andreadis T G, Magnarelli L A, Mather T N. J Invertebr Pathol. 1996;67:318–321. doi: 10.1006/jipa.1996.0050. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri E, Potenza L, Rossi I, Sisti D, Giomaro G, Rossetti S, Beimfohr C, Stocchi V. Appl Environ Microbiol. 2000;66:5035–5042. doi: 10.1128/aem.66.11.5035-5042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedata P A, Hunter M S. Entomol Exp Appl. 1996;81:207–214. [Google Scholar]
- 30.Moran N A, Telang A. Bioscience. 1998;48:295–304. [Google Scholar]
- 31.Chang K P, Musgrave A J. J Cell Sci. 1972;11:275–293. doi: 10.1242/jcs.11.1.275. [DOI] [PubMed] [Google Scholar]
- 32.Endo B Y. J Ultrastruct Res. 1979;67:1–14. doi: 10.1016/s0022-5320(79)80012-x. [DOI] [PubMed] [Google Scholar]
- 33.Costa H S, Westcot D M, Ullman D E, Rosell R, Brown J K, Johnson M W. Protoplasma. 1995;189:194–202. [Google Scholar]
- 34.Babcock C S, Heraty J M, DeBarro P J, Driver F, Schmidt S. Mol Phylogenet Evol. 2001;18:306–323. doi: 10.1006/mpev.2000.0875. [DOI] [PubMed] [Google Scholar]
- 35.Huigens M E, Luck R F, Klaassen R H G, Maas M F P M, Timmermans M J T N, Stouthamer R. Nature (London) 2000;405:178–179. doi: 10.1038/35012066. [DOI] [PubMed] [Google Scholar]
- 36.Zchori-Fein E, Gottlieb Y, Coll M. J Invertebr Pathol. 2000;75:267–272. doi: 10.1006/jipa.2000.4927. [DOI] [PubMed] [Google Scholar]
- 37.Dobson A P. Q Rev Biol. 1988;63:139–165. doi: 10.1086/415837. [DOI] [PubMed] [Google Scholar]
- 38.Moore J. Bioscience. 1995;45:89–96. [Google Scholar]
- 39.Egerter D E, Anderson J R, Washburn J O. Proc Natl Acad Sci USA. 1986;83:7335–7339. doi: 10.1073/pnas.83.19.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiggins F M, Hurst G D D, Majerus M E N. Proc R Soc London Ser B. 2000;267:69–73. doi: 10.1098/rspb.2000.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argov Y, Gottlieb Y, Amin-Spector S, Zchori-Fein E. Phytoparasitica. 2000;28:212–218. [Google Scholar]