Abstract
Aim:
To evaluate the outcome of newly diagnosed anaplastic glioma patients treated in our institution in relation to the 2016 WHO classification suggestions.
Methods:
This retrospective study included patients who underwent surgery plus adjuvant chemotherapy alone or concomitant and adjuvant chemoradiotherapy. Response was recorded using the Response Assessment in Neuro-Oncology criteria.
Results:
123 patients were analyzed. The median progression-free survival time and the 2, 3 and 5 years progression-free survival rate were 27 months, 65.5, 21.2 and 21.2%; the 2, 3 and 5 years overall survival rate were 89.7, 83.0 and 58.4%. From the univariate/multivariate analysis, the factors conditioning survival were Karnofsky performance scale, extent of resection, IDH1 mutation status and presence of 1p/19q codeletion.
Conclusion:
The choice of adjuvant treatment have to consider molecular assessment and, in our experience, the extent of surgical resection.
KEYWORDS : 2016 WHO classification, anaplastic glioma, chemotherapy, multimodal approach, radiotherapy, surgery
Anaplastic gliomas represent less than 10% of all newly diagnosed primary brain tumors. The 2007 WHO classification defined three different subtypes based on their histopathological characteristics: the anaplastic oligodendroglioma (AO), the mixed anaplastic oligoastrocytoma (AOA) and the anaplastic astrocytoma (AA) [1]. In the last few years, comprehensive and integrative genomic and molecular analysis led to identify biologic classes with clinically distinct behavior and to determine whether gliomas are more accurately defined by their molecular status rather than by the histological features [2]. Before 2008, the molecular markers identified as characterizing the tumor behavior were O6-methilguanine-DNA-methyltransferase (MGMT) promoter and 1p/19q codeletion. In anaplastic gliomas, methylated-MGMT promoter has been generally associated with prolonged progression-free survival (PFS) [3,4]. The cytogenetic aberration of 1p/19q codeletion, strongly associated with oligodendroglial feature and detected in up to 70% of AO/AOA, has been identified as a predictive factor of response to chemotherapy (CHT) with either procarbazine-lomustine-vincristine (PCV) or temozolomide (TMZ), and as an important prognostic biomarker correlated with longer survival [5,6]. On the other hand, a survival improvement was recorded also in those not-codeleted tumor patients who received adjuvant TMZ. This was demonstrated by an interim analysis of the European Organization for Research and Treatment of Cancer (EORTC) randomized Phase III CATNON trial, presented at American Society of Clinical Oncology meeting 2016. More recent discoveries indicated that IDH-mutation status is a parameter stronger than 1p/19q codeletion and MGMT-methylation in defining biological behavior [3,7,8]. Based on comprehensive and integrative genomic analysis, three cohesive tumor classes were identified: class I including IDH-mutated/1p/19q codeleted tumors, class II IDH-mutated/1p/19q no-codeleted tumors and class III IDH-wild-type tumors [2,5,6]. The findings summarized above were considered so relevant to urge a revision and an update of the CNS WHO classification, in which different glioma entities were introduced relaying to molecular parameters [9]. The definition of these new entities is going to change the treatment strategies. So far, the choice of treatment modality was mainly conditioned by the histological subtypes. After surgery, radiation therapy (RT) alone or associated to concurrent and adjuvant TMZ has been advocated as the recommended treatment for the astrocytic tumors, although the potential benefit of adding CHT in these patients is still unclear. Some retrospective analysis evaluating the outcome and toxicity of postoperative concurrent radiochemotherapy with TMZ compared with RT alone, showed no significant differences in overall survival (OS) or PFS [10,11]. Concerning the oligodendroglial tumors, some randomized studies showed that the addition of PCV to radiotherapy increases PFS and OS, and that upfront CHT with either PCV or TMZ is not different for survival [7,12,13]. In the light of the new 2016 WHO classification, the treatment choice shall be redefined as low as the molecular assessment [14]. Based on the recent update of 2016 WHO classification we wanted to evaluate whether molecular parameters were more prognostic and predictive of outcome compared with histopathological categorization in a clinical series. We evaluated the clinical outcome of newly diagnosed anaplastic glioma patients treated in our institution before 2015 in terms of PFS and OS, comparing, although retrospectively, the two classification system.
Materials & methods
• Patients & procedures
All the patients analyzed were treated in agreement with the Helsinki declaration. This study is a summary of a retrospective analysis to the treatment charts. To define the appropriate therapy, each patient was evaluated by a multidisciplinary team including a neurosurgeon, a neuro-oncologist and a radiation oncologist. Surgery was performed in all patients with the aim to maximally remove the tumor according with functional boundaries. The extent of the resection (EOR) was assessed on volumetric MRI studies obtained within 48 h after surgery by segmentation analysis (BrainLab, Heimstemen, Germany). Complete resection (CR) was defined as residual tumor volume lower than 1.0 cm3, subtotal resection as residual tumor volume between 1.0 and 10.0 cm3 and partial resection as residual tumor volume greater than 10.0 cm3 [15]. The adjuvant treatment proposed after surgery was CHT in the case of young patients with oligodendroglial tumors, IDH-mutated and 1p/19q codeleted, underwent CR. All the other cases were treated with RT associated to concurrent and adjuvant TMZ. All patients received only TMZ CHT based on studies showing that upfront CHT with either PCV or TMZ is not different for survival. PCV CHT has been used at disease progression [3,11–13]. To precisely delineate the RT target volume, volumetric CT scans and MRI were acquired, and all images were coregistered. The clinical target volume included the surgical cavity and the residual tumor, if present. The planning target volume was defined as an isotropic expansion from clinical target volume of 3 mm. Plans were processed using three dimensional conformal radiotherapy (3DCRT) techniques and more recently volumetric modulated arc therapy (RapidArc, Varian Medical System, CA, USA) to ensure maximal dose conformity and rapid dose fall off toward critical structures. The total dose prescribed was 60 Gy in 30 fractions. We reviewed the entire cohort considering the indications of the revised 2016 WHO classification in which histological subtypes are better related to the molecular markers. Tumor molecular profile was analyzed for all cases. Immunohistochemical staining for IDH1 was done on BenchMark XT automated tissue staining systems (Ventana Medical Systems, Inc., AZ, USA) using validated protocols. MGMT-promoter methylation status was determined by pyrosequencing (MGMT plus, Diatech Pharmacogenetics, Jesi, Italy) [16]. The standard method of dual-color FISH performed on 4-m-thick paraffin sections was used to determine the 1p/19q deletion status (Vysis probe 1p36/1q25 e 19q13/19p13) [17].
From a methodological point of view, being unable to modify the histopathological diagnosis we defined three classes regardless of the histological characterization but considering the molecular parameters, as follows: class I IDH-mutated/1p/19q codeleted tumors; class II IDH-mutated/1p/19q no-codeleted tumors; and class III IDH-wild-type tumors. At the time in which patients were treated, the prognostic and predictive role of TP53, telomerase reverse transcriptase promoter mutations and alpha-thalassemia/mental retardation syndrome X-linked (ATRX) status was unknown, and therefore they are not available in this analysis.
• Outcome evaluation
The clinical outcome was evaluated by neurological examination and brain MRI imaging performed 1 month after RT and then every 3 months. Response was recorded using the Response Assessment in Neuro-Oncology criteria [18]. The tumor progression was described as local, if it occurred in/or within 2.0 cm from primary site, and distant for new and noncontiguous enhancing or nonenhancing lesions. Hematologic and nonhematologic toxicities were graded according to Common Terminology Criteria for Adverse Events version 4.0.
• Statistical analysis
Standard descriptive statistics (e.g., median, mean, standard deviation and cross-tabulation analysis) were used to describe the data general behavior. Survival and recurrence time observations were evaluated according to the method of Kaplan and Meier, starting from the date of diagnosis. In order to assess the prognostic role of the different individual variables, the log-rank test or univariate Cox regression were used for categorical and continuous variables, respectively. Multivariate backward stepwise Cox regression was used as a method to estimate the independent association of a variable set with overall and PFS. Variables considered were: gender, age, Karnofsky performance scale (KPS), EOR, MGMT methylated status, IDH status, 1p19q codeletion and molecular classes. Neurophysiological assessment parameters were preliminarily evaluated for a difference before and after RT. Statistical analysis was performed by the use of the Stata software, version 13.1 (StataCorp LP, TX, USA).
Results
• Patients, tumor & treatments characteristics
From January 2008 to May 2014, 123 consecutive patients were treated in our institution and included in this analysis. Fifty-seven (46.3%) were female and 66 (53.7%) male with a median age of 43 years (range 19–66 years). All patients had a KPS > 80. According with histopathological features, 54 (43.9%) patients had diagnosis of AO, 36 (29.3%) of AOA and 33 (26.8%) of AA. Considering molecular parameters, 69 (56.1%) patients were in class I, 39 (31.7%) in class II and 15 (12.2%) in class III. All patients underwent surgical resection and no patient had a biopsy. Only adjuvant CHT alone with TMZ was performed in 51 (41.5%) patients and RT with concurrent and adjuvant TMZ in 72 (58.5%). Details are shown in Table 1.
Table 1. . Patients, tumor and treatment characteristics of the entire cohort.
| Parameter/factor | Number | % |
|---|---|---|
| Patients | 123 | 100 |
| Median age years (range years) | 43 (19–78) | |
| Gender: | ||
| – Male | 66 | 53.7 |
| – Female | 57 | 46.3 |
| KPS: | ||
| – 100 | 48 | 39.0 |
| – 90 | 63 | 51.2 |
| – 80 | 12 | 9.8 |
| Histopathological diagnosis: | ||
| – AO | 54 | 43.9 |
| – AOA | 36 | 29.3 |
| – AA | 29 | 26.8 |
| Molecular diagnosis: | ||
| – Class I | 69 | 56.1 |
| – Class II | 39 | 31.7 |
| – Class III | 15 | 12.2 |
| MGMT methylation status: | ||
| – Methylated | 105 | 85.3 |
| – Un-methylated | 18 | 14.7 |
| EOR: | ||
| – CR | 72 | 58.5 |
| – SR | 27 | 22.0 |
| – PR | 24 | 19.5 |
| Adjuvant chemotherapy alone (TMZ) | 51 | 41.5 |
| Concurrent/adjuvant chemoradiotherapy (TMZ) | 72 | 58.5 |
AA: Anaplastic oligoastrocytoma; AO: Anaplastic oligodendroglioma; AOA: Anaplastic oligoastrocytoma; Class I: IDH-mutated/1p19q codeleted tumor; Class II: IDH-mutated/1p19q no-codeleted tumor; Class III: IDH-wild-type tumor; CR: Complete resection; EOR: Extent of the resection; KPS: Karnofsky performance scale; MGMT: O6-methilguanine-DNA-methyltransferase; PR: Partial resection; SR: Subtotal resection; TMZ: Temozolomide.
• Progression-free survival & overall survival analysis
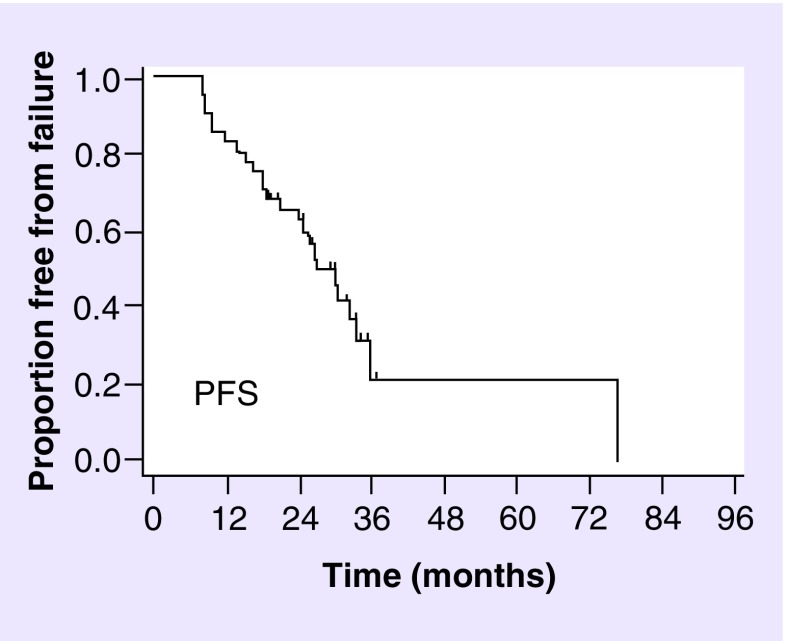
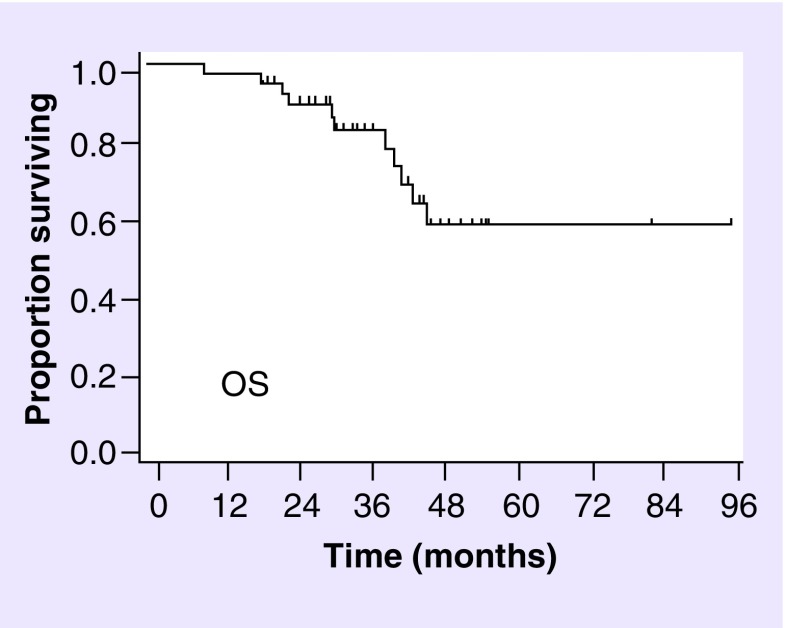
The median follow-up time for the whole cohort was 35.1 months (range 9.3–95.7 months) and 35.6 months (range 19.2–95.7 months) for the alive patients. The median PFS time and the 2, 3 and 5 years PFS rate were 27 months, 65.5% (95% CI: 56.2–73.2), 21.2% (95% CI: 10.7–34.0) and 21.2% (95% CI: 10.7–34.0), as shown in Figure 1. No progression occurred within 3 and 5 years. Details about PFS considering histopathological diagnosis or molecular classes are shown in Table 2. The 2, 3 and 5 years OS rate were 89.7% (95% CI: 82.5–94.0), 83.0% (95% CI:74.3–89.0) and 58.4% (95% CI: 45.8–69.1), as shown in Figure 2. Details about OS analysis for patients considering the histopathological diagnosis, including AO/AOA/AA tumors or molecular classes, including IDH-mutated/1p/19q codeleted, IDH-mutated/1p/19q no-codeleted and IDH-wild-type tumors, are shown In Table 3. PFS and OS by linking molecular classes and histology are shown in Tables 2 & 3, respectively. At the last observation time 90 patients (73.2%) were alive and 33 (26.8%) dead.
Figure 1. . Progression-free survival of the whole cohort.
Table 2. . Progression-free survival according to histopathological diagnosis and molecular classes and by linking histology and molecular classes.
| Parameter/factor | Median (months) | 2 years % (95% CI) | 3 years % (95% CI) | 5 years % (95% CI) |
|---|---|---|---|---|
| Histology | ||||
| AO | 32.5 | 70.7 (56.0–81.3) | 39.3 (22.2–56) | 39.3 (22.2–56) |
| AOA | 24.4 | 58.3 (40.7–72.4) | 36.5 (19.9–53.3) | 36.5 (19.9–53.3) |
| AA | 27.1 | 63.6 (45.0–77.5) | 0 | 0 |
| Molecular classes | ||||
| Class I | 30.4 | 72.7 (60.0–81.9) | 49.3 (33.7–63.2) | 49.3 (33.7–63.2) |
| Class II | 27.1 | 69.2 (52.2–81.2) | 0 | 0 |
| Class III | 9.9 | 20.0 (4.9–42.4) | 0 | 0 |
| Histology and molecular classes linked | ||||
| AO IDH-mutated/1p19q co-codeleted | Not reached | 73.1 (57.3–84.0) | 51.2 (32.6–67.1) | 51.2 (32.6–67.1) |
| AO IDH-mutated/1p19q no-codeleted | 9.9 | 50.0 (11.1–80.4) | 0 | 0 |
| AOA IDH-mutated/1p19q codeleted | 77.0 | 60.0 (31.8–79.7) | 60.0 (31.8–79.7) | 60 (31.8–79.7) |
| AOA IDH-mutated/1p19q no-codeleted | 27.1 | 80.0 (50.0–93.1) | 0 | 0 |
| AOA IDH-wild-type | 9.8 | 0 | 0 | 0 |
| AA IDH-mutated/1p19q codeleted | 27.1 | 100.0 | 0 | 0 |
| AA IDH-mutated/1p19q no-codeleted | 25.9 | 66.6 (40.4–83.4) | 0 | 0 |
| AA IDH-wild-type | 8.7 | 3.3 (7.8–62.3) | 0 | 0 |
AA: Anaplastic astrocytoma; AO: Anaplastic oligodendroglioma; AOA: Anaplastic oligoastrocytoma; Class I: IDH-mutated/1p19q codeleted tumor; Class II: IDH-mutated/1p19q no-codeleted tumor; Class III: IDH-wild-type tumor.
Figure 2. . Overall survival of the whole cohort.
Table 3. . Overall Survival according to histopathological diagnosis and molecular classes and by linking histology and molecular classes.
| Parameter/factor | Median (months) | 2 years % (95% CI) | 3 years % (95% CI) | 5 years % (95% CI) |
|---|---|---|---|---|
| Histology | ||||
| AO | Not reached | 93.3 (56.0–81.3) | 93.3 (80.7–97.8) | 80.0 (59.1–91.0) |
| AOA | 40.5 | 82.5 (65.1–91.8) | 82.5 (65.1–91.8) | 49.5 (26.8–68.7) |
| AA | 46 | 90.9 (74.4–97) | 70.7 (51.2–83.6) | 47.1 (27.9–64.2) |
| Molecular classes | ||||
| Class I | Not reached | 94.7 (84.6–98.3) | 94.7 (84.6–98.3) | 56.8 (35.1–73.7) |
| Class II | Not reached | 92.3 (52.2–81.2) | 75.5 (58.2–86.5) | 62.9 (43.1–77.5) |
| Class III | 40.5 | 60.0 (31.8–79.7) | 60.0 (31.8–79.7) | 40.0 (16.5–62.8) |
| Histology and molecular classes linked | ||||
| AO IDH-mutated/1p19q co-codeleted | Not reached | 92.3 (78.0–97.5) | 92.3 (78.0–97.5) | 61.0 (42.0–71.0) |
| AO IDH-mutated/1p19q no-codeleted | Not reached | 100.0 | 100.0 | 55.0 (31.0–67.0) |
| AOA IDH-mutated/1p19q codeleted | 39.2 | 100.0 | 100.0 | 50.0 (11.1–80.4) |
| AOA IDH-mutated/1p19q no-codeleted | 23.2 | 80.0 (50.0–93.1) | 80.0 (50.0–93.1) | 0 |
| AOA IDH-wild-type | 18.7 | 50.0 (11.1–80.4) | 50.0 (11.1–80.4) | 0 |
| AA IDH-mutated/1p19q codeleted | 45.9 | 100.0 | 100.0 | 0 |
| AA IDH-mutated/1p19q no-codeleted | 43.5 | 100.0 | 66.7 (40.4–83.4) | 0 |
| AA IDH-wild-type | 9.3 | 66.7 (28.2–87.8) | 66.7 (28.2–87.8) | 66.7 (28.2–87.8) |
AA: Anaplastic astrocytoma; AO: Anaplastic oligodendroglioma; AOA: Anaplastic oligoastrocytoma; Class I: IDH-mutated/1p19q codeleted tumor; Class II: IDH-mutated/1p19q no-codeleted tumor; Class III: IDH-wild-type tumor.
• Impact of demographic, clinical & molecular variables on outcome
Gender, age and MGMT-promoter methylation status were not predictive of survival. On univariate and multivariate analysis, the factors conditioning PFS were the IDH-mutation status, the presence of 1p/19q codeletion and the amount of tumor removal. Particularly, the patients with IDH-mutated and 1p/19q codeleted tumors (class I), underwent CR and had the better outcome. Regarding OS, the prognostic factors recorded as significantly impacting on survival were the higher KPS, the oligodendroglial features compared with the astrocytic ones and the IDH-mutated tumors compared with the wild-type ones, both on univariate and multivariate analysis. Details about factors analyzed and their statistical relevance are shown in Table 4.
Table 4. . Factors evaluated as conditioning progression-free survival and overall survival in univariate and multivariate analysis.
| Factors analyzed | PFS p-value | OS p-value | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | HR | Univariate | Multivariate | HR | |
| Gender | 0.4 | – | – | 0.8 | – | – |
| Age | 0.07 | 0.2 | 0.9 | 0.2 | – | – |
| KPS | 0.8 | 0.5 | 0.9 | <0.01 | <0.01 | 0.9 |
| MGMT promoter | 0.1 | – | – | 0.2 | <0.01 | 4.9 |
| Histology | 0.1 | 0.07 | 0.7 | 0.03 | 0.04 | 1.6 |
| IDH status | <<0.01 | <<0.01 | 3.3 | 0.01 | – | – |
| 1p19q codeletion | <<0.01 | 0.03 | 1.2 | 0.1 | – | – |
| EOR | <0.01 | 0.05 | 1.3 | 0.6 | – | – |
| Classes | <<0.01 | <<0.01 | 4.0 | 0.04 | <<0.01 | 3.6 |
Class I: IDH-mutated/1p19q codeleted tumor; Class II: IDH-mutated/1p19q no-codeleted tumor; Class III: IDH-wild-type tumor; EOR: Extent of the resection; GTR: Gross total resection; HR: Hazard ratio; KPS: Karnofsky performance scale; OS: Overall survival; PFS: Progression-free survival.
• Toxicity
Perioperative complications occurred in 11 (9%) patients. Immediate neurological deficits were found in eight (6.5%) patients, in four cases recovered within 1 month. No perioperative mortality occurred. All patients were evaluated for toxicity during adjuvant treatment time. No severe hematologic or neurologic toxicity was recorded during concurrent radiochemotherapy treatment and neurological examination scores remained stable. Grades I–II radionecrosis was recorded in six (4.9%) patients, no grades III–IV occurred. During adjuvant CHT, three (2.4%) patients had grade III thrombocytopenia, seven (5.7%) patients had grade III neutropenia and two (1.6%) patients had grade III anemia. No hematologic grade IV toxicity occurred. CHT was interrupted in one patient, and delayed or reduced in ten (8.1%) patients. A moderate to severe fatigue occurred in 22 (17.9%) patients. Two patients had a deep venous thrombosis and three had a severe lung infection resolved with medical therapy.
• Treatment at progression
Fifty-four (43.9%) patients relapsed: 42 had a local progression and 12 had a distant progression, at a median time of 16 months (range 8–65 months). Six patients did not received treatment at progression for rapid clinical deterioration and died within 3 months. Salvage treatment was performed in 48 (88.9%) patients and consisted in surgery alone in three, RT alone in three and second-line CHT alone in 36 patients. A combined treatment was performed in six patients; CHT plus RT in three and surgery plus RT followed by CHT (TMZ) in three patients. Patients with distant progression compared with those with local progression had the worse outcome: the median OS was 10 months (range 3–34 months) and 18 months (range 8–65 months), respectively. At the last observation time, 33/54 (61.1%) patients are dead and 21/54 (38.9%) are alive. Among alive patients, 18 had local progression and underwent second-line treatments.
Discussion
Treatment of anaplastic gliomas is a strongly debated topic in neuro-oncology, due to their heterogeneity, variable biological behavior and tendency to diffusely infiltrate the surrounding brain parenchyma. Until recently, the classification of brain tumors was primarily based on their histological appearance and the 2007 WHO classification system divided anaplastic gliomas into two major subtypes: the astrocytic and the oligodendroglial tumors including pure oligodendrogliomas and mixed oligoastrocytomas [1]. The main limits of this approach were the high intraobserver and interobserver variability, the poor predictive and prognostic value and the limited information provided about tumor biology. Several studies over the past two decades clarified the genetic basis of tumorigenesis showing a more robust prognostic value of the tumor molecular profile compared with the histological features [19–21]. In 2014, a meeting under the auspices of the International Society of Neuropathology established guidelines to incorporate molecular findings into brain tumor diagnoses, setting the stage for a major revision of the 2007 CNS WHO classification [22]. The revised 2016 WHO classification combined biology-guided molecular marker diagnosis with classical histological cancer diagnosis. The objective was to achieve a greater diagnostic accuracy, to improve patient management, and to determine more accurately prognosis and treatment response [9]. IDH was identified as the biomolecular marker with a greater predictive and prognostic value on outcome of glioma patients, much to define distinct entities with completely different behavior [23–27]. The finding that molecular profile could better define the tumor biology and clinical outcome will probably change the criteria guiding the therapeutic choice. Considering the anaplastic glioma patients as potential long-term survivors, the choice of the most appropriate adjuvant treatment results crucial to obtain the most effective impact on outcome. Among the first, Weller and Wick showed a ‘paradigm of treatment’ moving from the distinction between IDH-mutant and IDH-wild-type tumors, regardless of histological diagnosis [14]. The present analysis included newly diagnosed anaplastic glioma patients treated with surgery followed by CHT alone or concurrent and adjuvant chemoradiotherapy. By use of these approaches, survival rates were highly satisfactory, with an OS of 80 and 60% at 3 and 5 years, respectively. These results compare favorably with previous report [7,8,28]. The following issues require comment. Our retrospective study included patients treated before the new classification era, when the therapeutic strategies were mainly determined by histological subtypes. The treatment choice has taken into account the molecular profile and the extent of surgical resection with the aim to delay RT at progression in case of favorable assessment such as the oligodendroglial feature (IDH mutated and codeleted tumor), in patients underwent CR. The delivery of less aggressive treatments in these selected patients did not deteriorate the outcome. Instead, no difference in survival was recorded in relation to the various adjuvant treatment performed. The factors detected as impacting on outcome were the KPS, the EOR and exactly the IDH-mutated status, above all in association with 1p19q codeletion. KPS was recorded as influencing OS both in univariate and multivariate analysis (p < 0.01). The amount of surgical resection has shown to be a factor impacting on PFS both in univariate and multivariate analysis (p < 0.01), with a favorable trend on OS although without statistically significant value. About patients’ age, the absence of statistically significant relevance could be related to the young age of this series with only six patients older than 60 years, and about 80% younger than 50 years.
Reviewing our series, considering the suggestion of 2016 WHO, patients with tumors in classes I and II (IDH-mutated and codeleted/IDH-mutated and no-codeleted) had the better outcome with more than half of patients alive at 5 years compared with 40% in case of IDH-wild type (p = 0.03). In addition, matching histological subtypes and classes, the p-value, on multivariate analysis, was 0.4 vs <<0.01, respectively, confirming the greater prognostic and predictive value of molecular parameters. Redefining the whole series in relation to molecular characteristics, it led to an increase number of tumor in classes with a more favorable behavior (classes I and II). This data confirmed the need to identify tumors with worst prognosis requiring a different therapeutic approach. Finally, we are aware that our analysis has many limitations that included its retrospective nature, in which patients were yet evaluated basing on histological subtype. Another lacking point is represented by the absence of information about telomerase reverse transcriptase and ATRX status, not available at the time in which patients were treated. This later has probably led to overestimate the histological diagnosis of AOA. In different series, this diagnosis ranges from very frequent to rare, confirming an high interobserver discordance and to date it is strongly discouraged. The addition of ATRX status allowed to eliminate the mixed gliomas, dichotomizing the IDH-mutant AOA with loss of ATRX without 1p/19q codeletion into the group of AA and the IDH-mutant AOA with ATRX expressed and 1p/19q codeletion into the group of AO [29–31]. Recently, the French POLA cohort study suggested to consider true mixed glioma only the very rare cases (0.5%) of coexistence of loss of nuclear ATRX expression and 1p19q codeletion even after repeating immunostaining and comparative genomic hybridization analysis [32]. However, despite the several bias, to our knowledge, this is one of the few studies in which results were reviewed in relation to the upgrade of 2016 WHO classification.
Conclusion
Our results confirmed that a stratification of patients by molecular features could better define prognosis and guide the adjuvant therapeutic approaches, following surgical resection. In a more favorable setting, defined by oligodendroglial feature, IDH mutation, 1p19q codeletion and CR performed, adjuvant RT might be delayed to disease progression without worsening outcome, as recorded in our series. Well-designed collaborative trials, including stratification of patients by histology, molecular profile and amount of surgical resection are recommended to provide robust evidences in this field.
SUMMARY POINTS.
The aim of this study was to appraise the impact of 2016 WHO classification.
123 patients included in the study.
Univariate and multivariate analysis revealed performance status as conditioning survival.
Univariate and multivariate analysis revealed extent of resection as conditioning survival.
Univariate and multivariate analysis revealed molecular factors as conditioning survival.
The extent of surgical resection is important to be considered.
The treatment of choice shell includes molecolar assessment.
Footnotes
Availability of data & materials
Datasets can be retrieved from authors upon formal request from interested readers. Datasets cannot be directly shared on public repositories due to the national personal data protection act.
Authors’ contributions
P Navarria and L Cozzi drafted the manuscript. All authors read and approved it.
Financial & competing interests disclosure
L Cozzi acts as a scientific advisor to Varian Medical Systems and is a clinical research scientist at Humanitas Cancer Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved. For this type of prospective observational study formal consent is not required.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brat DJ, Verhaak RG, Aldape KD, et al. Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized Phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J. Clin. Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 4.Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin. Cancer Res. 2005;11:5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- 5.Chahlavi A, Kanner A, Peereboom D, et al. Impact of chromosome 1p status in response of oligodendroglioma to temozolomide: preliminary results. J. Neurooncol. 2003;61:267–273. doi: 10.1023/a:1022580610598. [DOI] [PubMed] [Google Scholar]
- 6.Thiessen B, Maguire J, McNeil K, Huntsman D, Martin MA, Horsman D. Loss of heterozygosity for loci on chromosome arms 1p and 10q in oligodendroglial tumors: relationship to outcome and chemosensitivity. J. Neurooncol. 2003;64:271–278. doi: 10.1023/a:1025689004046. [DOI] [PubMed] [Google Scholar]
- 7.van den Bent M, Brandes A, Taphoorn M, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013;31:344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 8.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J. Clin. Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 10.Scoccianti S, Magrini SM, Ricardi U, et al. Radiotherapy and temozolomide in anaplastic astrocytoma: a retrospective multi center study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology) Neuro Oncol. 2012;14:798–807. doi: 10.1093/neuonc/nos081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandes AA, Nicolardi L, Tosoni A, et al. Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro Oncol. 2006;8:253–260. doi: 10.1215/15228517-2006-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen T, Doyle T, Anderson J, et al. Temozolomide single-agent chemotherapy for newly diagnosed anaplastic oligodendroglioma. J. Neurooncol. 2009;92:57–63. doi: 10.1007/s11060-008-9735-x. [DOI] [PubMed] [Google Scholar]
- 13.Gan HK, Rosenthal MA, Dowling A, et al. A Phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors. Neuro Oncol. 2010;12:500–507. doi: 10.1093/neuonc/nop065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller M, Wick W. Neuro-oncology in 2013: improving outcome in newly diagnosed malignant glioma. Nat. Rev. Neurol. 2014;10:68–70. doi: 10.1038/nrneurol.2013.268. [DOI] [PubMed] [Google Scholar]
- 15.Castellano A, Bello L, Michelozzi C, et al. Role of diffusion tensor magnetic resonance tractography in predicting the extent of resection in glioma surgery. Neuro Oncol. 2013;14:192–202. doi: 10.1093/neuonc/nor188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wick W, Weller M, van den Bent M, et al. MGMT testing the challenges for biomarker-based glioma treatment. Nat. Rev. Neurol. 2014;10(7):372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 17.Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW. Ancillary FISH analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Front. Biosci. 2003;8:a1–a9. doi: 10.2741/896. [DOI] [PubMed] [Google Scholar]
- 18.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Ren X, Cui X, et al. 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol. 2013;15:775–782. doi: 10.1093/neuonc/not027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frenel JS, Leux C, Loussouarn D, et al. Combining two biomarkers, IDH1/2 mutations and 1p/19q codeletion, to stratify anaplastic oligodendroglioma in three groups: a single-center experience. J. Neurooncol. 2013;114:85–91. doi: 10.1007/s11060-013-1152-0. [DOI] [PubMed] [Google Scholar]
- 21.Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 2015;125:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology-Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24:429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiestler B, Capper D, Sill M, et al. Integrated DNA methylation and copynumber profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128:561–571. doi: 10.1007/s00401-014-1315-x. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015;47:458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 25.Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129:679–693. doi: 10.1007/s00401-015-1409-0. [DOI] [PubMed] [Google Scholar]
- 26.Weller M, Wick W, Aldape K, et al. Glioma. Nat. Rev. Dis. Primers. 2015;1:15017. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 27.Sahm F, Reuss D, Koelsche C, et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128:551–559. doi: 10.1007/s00401-014-1326-7. [DOI] [PubMed] [Google Scholar]
- 28.Wick W, Roth P, Hartmann C, et al. Long-term analysis of the NOA-04 randomized Phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18:1529–1537. doi: 10.1093/neuonc/now133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120:297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 31.Tabouret E, Nguyen AT, Dehais C, et al. Prognostic impact of the 2016 WHO classification of diffuse gliomas in the French POLA cohort. Acta Neuropathol. 2016;132:625–634. doi: 10.1007/s00401-016-1611-8. [DOI] [PubMed] [Google Scholar]
- 32.Huse JT, Diamond EL, Wang L, et al. Mixed glioma with molecular features of composite oligodendroglioma and astrocytoma: a true “oligoastrocytoma”? Acta Neuropathol. 2015;129:151–153. doi: 10.1007/s00401-014-1359-y. [DOI] [PubMed] [Google Scholar]