Abstract
Antiepileptic drugs (AEDs) are routinely prescribed for the management of a variety of neurologic and psychiatric conditions, including epilepsy and epilepsy syndromes. Physiologic changes due to aging, pregnancy, nutritional status, drug interactions, and diseases (ie, those involving liver and kidney function) can affect pharmacokinetics of AEDs. This review discusses foundational pharmacokinetic characteristics of AEDs currently available in the United States, including clobazam but excluding the other benzodiazepines. Commonalities of pharmacokinetic properties of AEDs are discussed in detail. Important differences among AEDs and clinically relevant pharmacokinetic interactions in absorption, distribution, metabolism, and/or elimination associated with AEDs are highlighted. In general, newer AEDs have more predictable kinetics and lower risks for drug interactions. This is because many are minimally or not bound to serum proteins, are primarily renally cleared or metabolized by non–cytochrome P450 isoenzymes, and/or have lower potential to induce/inhibit various hepatic enzyme systems. A clear understanding of the pharmacokinetic properties of individual AEDs is essential in creating a safe and effective treatment plan for a patient.
Keywords: antiepileptic drugs, pharmacokinetic interactions, CYP450, UGT, hepatic metabolism, protein binding
Introduction
Antiepileptic drugs (AEDs) decrease seizure frequency and severity in patients with seizure disorders, epilepsy, and epilepsy syndromes.1-28 These AEDs can be divided into older medications (ie, first generation)—carbamazepine, ethosuximide, methsuximide, phenobarbital, phenytoin, primidone, and valproic acid/divalproex sodium/valproate sodium—and newer medications (ie, second or third generation) (Table 1). Over the past 3 decades, AEDs (eg, carbamazepine, gabapentin, lamotrigine, oxcarbazepine, pregabalin, topiramate, valproic acid/divalproex sodium/valproate sodium, and zonisamide)3,15,17,23,24,28-31 were also used for management of non-epileptic neurologic conditions and psychiatric disorders (Table 1).32
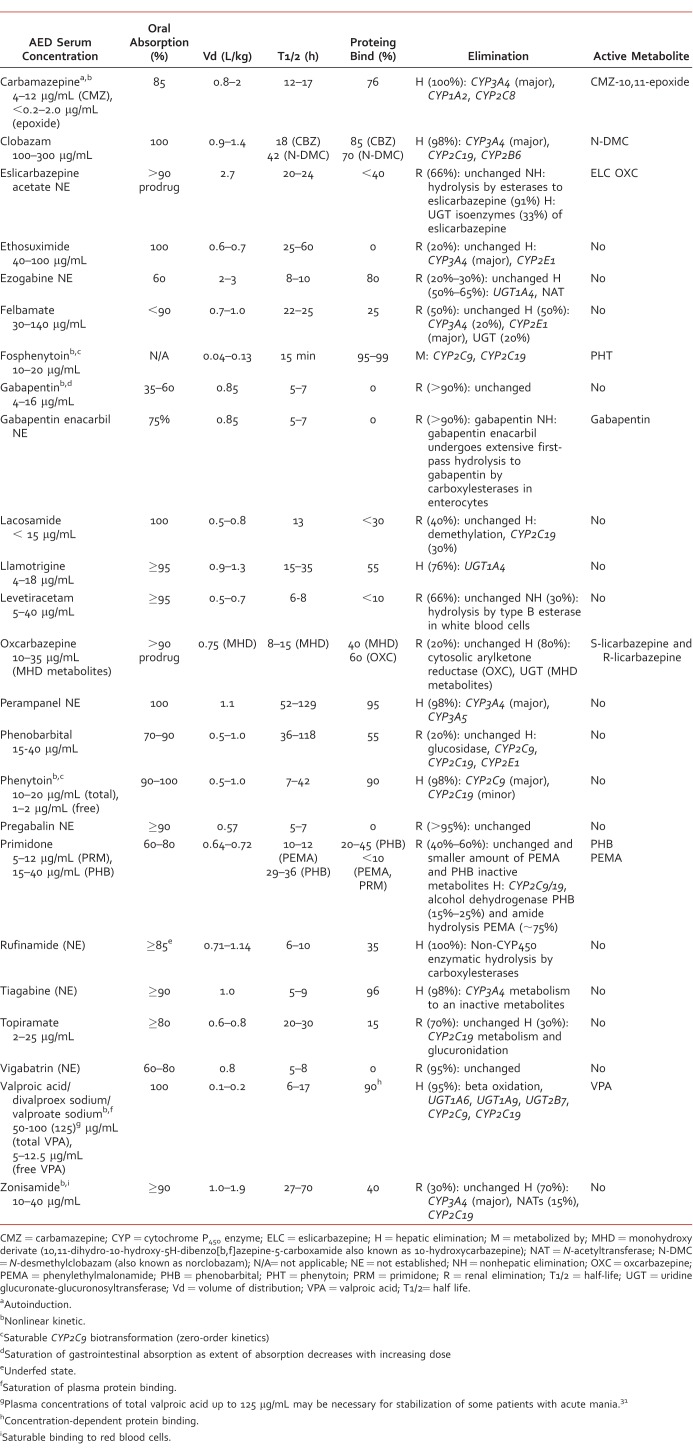
TABLE 1:
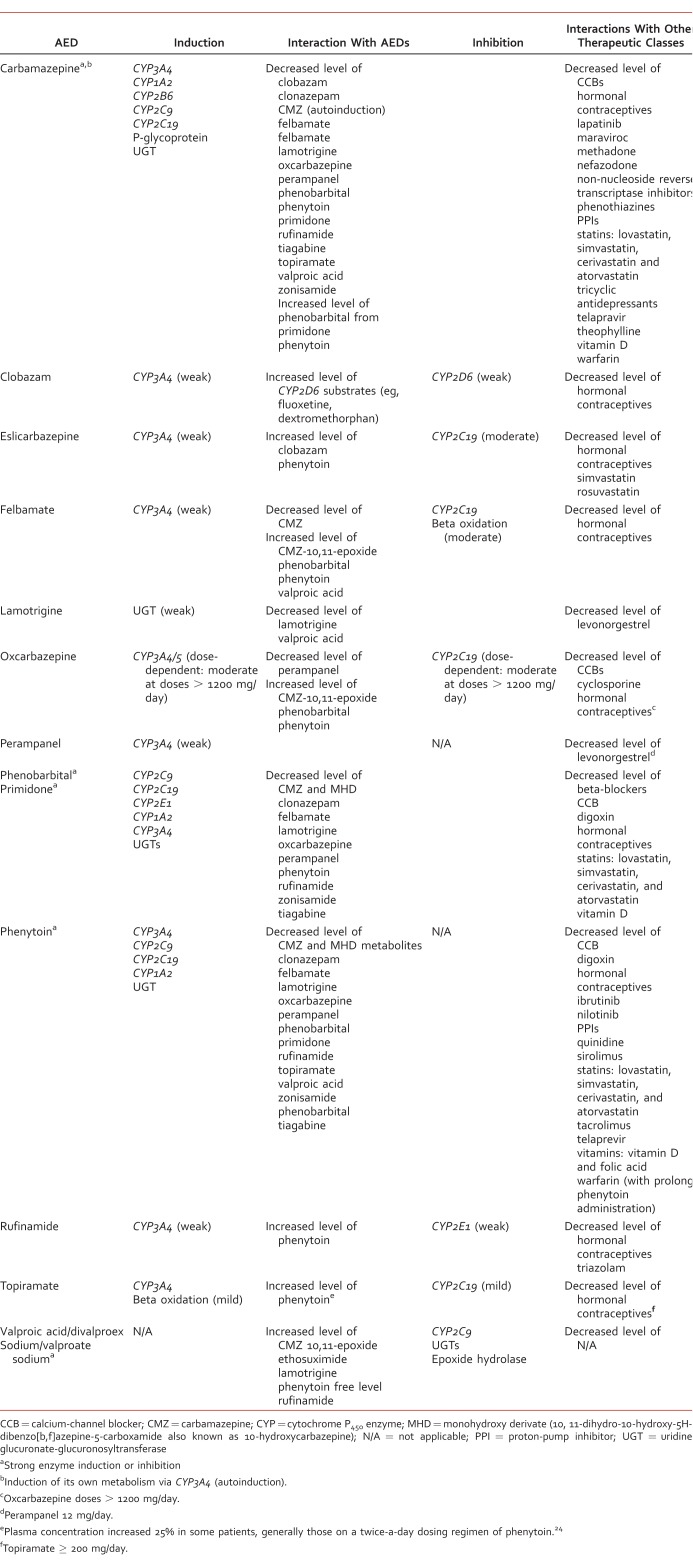
This article reviews foundational pharmacokinetic characteristics of AEDs currently available in the United States. including clobazam but excluding the other benzodiazepines. Pharmacokinetic properties of AEDs will be discussed in detail. Important differences among AEDs as well as clinically significant pharmacokinetic interactions are highlighted so health care professionals can provide safe and effective patient-centered care for neurologic and psychiatric disorders. Select pharmacokinetic characteristics of AEDs are summarized in Tables 2 and 3 and discussed in the text.
TABLE 2:
TABLE 3:
Absorption
All AEDs except fosphenytoin are available in oral formulation(s) with various dosing frequencies.33 Only a few AEDs (eg, phenytoin, fosphenytoin, phenobarbital, lacosamide, levetiracetam, and valproic acid) are available in intravenous (IV) formulation(s)33; however, IV administration is typically reserved for use during medical emergencies (eg, status epilepticus) when oral dosing is inappropriate or impossible or when the drug therapeutic level needs to be achieved quickly using an IV loading dose. In addition, fosphenytoin and phenobarbital are the only AEDs that may be administered intramuscularly, usually when IV access cannot be established and immediate action of medication is needed.33
Immediate-release (IR) oral formulations of AEDs with short half-lives (eg, carbamazepine, phenytoin, valproic acid/divalproex sodium/valproate sodium) are typically administered 3 or 4 times a day. Typically, AEDs with long half-lives (eg, eslicarbazepine acetate, lamotrigine, oxcarbazepine, perampanel, topiramate, and zonisamide) are administered once or twice a day. Prolonged-release oral formulations, such as extended-release (ER) or delayed-release tablets or capsules (Table 1), were designed to prolong absorption of AEDs with short half-lives (ie, carbamazepine, valproic acid/divalproex sodium/valproate sodium, phenytoin, and levetiracetam). The ER and delayed-release formulations allow for less frequent administration while minimizing fluctuations of drug serum concentration over the course of the day.34 Improved outcomes associated with use of ER formulations of AEDs have, however, been only clearly documented for carbamazepine. Compared with carbamazepine IR, the carbamazepine ER oral formulation, typically given twice a day, was shown to provide more consistent drug delivery with less variable absorption while minimizing transient adverse effects associated with peak dose.3,23,35 It has been suggested that fluctuations in the levels of select AEDs in cerebrospinal fluid may be less evident than in serum. This calls into question the additional benefits of ER formulations of other AEDs above and beyond benefits of increasing dosing interval for adherence.34
Current AEDs have different oral bioavailabilities (Table 2). Most AEDs are absorbed by passive diffusion in the gastrointestinal (GI) tract.1-16,18-29,31 Gabapentin absorption is dependent on a saturable low-capacity L-amino acid transporter in the proximal portion of the small bowel.2,17,36 Bioavailability of gabapentin IR decreases from 60% of a 300-mg dose to 35% of a 1600-mg dose given in 3 divided doses.17,37 Strategies to improve or optimize gabapentin bioavailability involve administration of smaller doses at more frequent intervals. Gabapentin enacarbil,30 a recently develop prodrug of gabapentin, is transported via high-capacity nutrient transport systems found along the length of the GI tract and has improved bioavailability compared with gabapentin (74.5% versus 36.6%) in the fed state.30,38
For most AEDs, coadministration with food can slow the absorption rate, but this does not have a clinically relevant effect on the extent of absorption (area under the curve [AUC]).1,3-29,31 Therefore, most AEDs can be administered with or without food. Coadministration with food can be a helpful strategy to reduce GI irritation and dose-dependent adverse effects associated with excessive drug peak blood levels, and thus can improve drug tolerability of such AEDs as tiagabine.12 Conversely, intake of rufinamide with food increases peak exposure (Cmax) by >50% and AUC by 30%–40%; therefore, administration of rufinamide with food is recommended.2,39 Gabapentin enacarbil should also be taken with food as the rate and extent of absorption increases when administered with a high-fat meal.30 When phenytoin is administered via enteral tube feeding, its bioavailability is reduced due to adhesion of phenytoin to the plastic tubing and drug-nutrition interactions.40 It is recommended that administration of phenytoin be separated from feeding by 1–2 hours (before or after) and to ensure adequate tube flush after each dose.10,40,41 In general, to achieve a therapeutic serum concentration of phenytoin, the daily maintenance dose of phenytoin should be increased by 50% when phenytoin is changed from oral administration to enteral tube feeding.42 It is imperative that phenytoin serum concentration be closely monitored and the phenytoin dose adjusted as needed.
Administration of phenytoin suspension10 or gabapentin17 with antacids containing aluminum, magnesium, and calcium has been associated with reduced bioavailability. In an open-label study by Yagi et al,43 13 healthy volunteers received gabapentin IR 200 mg orally, either alone or with 1 g magnesium oxide. The study found that the AUC of gabapentin when coadministered with magnesium oxide was decreased by 43% compared with gabapentin alone. The authors suggest that this is due to a reduction in the extent and rate of gabapentin intestinal absorption.43 It is recommended that gabapentin, as well as phenytoin, should be taken at least 2 hours apart from any antacid.10,17 This will ensure adequate absorption and minimize serum concentration fluctuation. Phenytoin ER capsules should also be administered at least 2 hours before or after any antacids containing calcium.9
Distribution
Distribution of AEDs in the body varies due to individual volume of distribution (Vd) (Table 2), which is influenced by drug plasma-protein binding and drug lipophilicity.44 The Vd information is useful for loading dose (LD) calculation: LD = (concentration desired – concentration at baseline) × weight (kg) × Vd (L/kg).45
Gabapentin, vigabatrin, and pregabalin are not serum protein bound, whereas other AEDs are bound to serum proteins, mainly albumin, to varying degrees (Table 2).1-28 For most AEDs, protein binding is linear, and the percent free fraction is a constant within serum concentration. Valproic acid is the single exception. Its free fraction is concentration dependent as protein binding is decreased markedly at high serum concentration due to protein-binding site saturation.5-7 For adults on monotherapy, the average of free fraction of valproic acid is between 10% at 40 μg/mL and 18.5% at 130 μg/mL.5-7
In general, only AEDs with a high protein binding, that is, ≥90%, are associated with clinically relevant protein-binding interactions that result in clinically significant changes in drug effect due to an increased or decreased drug-free fraction. Phenytoin, valproic acid, tiagabine, and perampanel have the greatest plasma protein binding (Table 2). Phenytoin has the highest risk for protein-binding interactions among all AEDs as it is a narrow therapeutic index agent with nonlinear pharmacokinetics that is extensively bound to serum albumin.9,10 Despite being highly protein bound, perampanel and tiagabine do not seem to have high risk for protein-binding interactions, though the relationships between tiagabine and perampanel plasma concentrations and clinical response are not currently understood.12,19
Protein binding of valproic acid and phenytoin can be reduced in the presence of decreased serum albumin levels or hypoalbuminemia associated with pregnancy (especially during the second and third trimesters), malnutrition, nephrotic/uremic states, or liver disease or when antiepileptic medication is coadministered with other highly protein-bound medications.46-48 This leads to a disproportionate increase in free fraction of phenytoin or valproic acid and increased risk for dose-dependent adverse effects and toxicity.5-10 Salicylates and certain nonsteroidal anti-inflammatory drugs (ie, naproxen) can significantly displace valproic acid from albumin-binding sites and thus increase free fraction of valproic acid.5-8 Phenytoin has a complex interaction with warfarin. This interaction results from changes in protein binding and increased metabolic clearance. In a patient taking warfarin, phenytoin can immediately displace warfarin from its protein binding sites, thereby causing a rapid increase in the international normalized ratio (INR). After prolonged administration, phenytoin may actually reduce the effectiveness of warfarin by inducing cytoprotein (CYP) 450–dependent metabolism of warfarin.9,10 Administration of valproic acid/divalproex sodium/valproate sodium was shown to increase the unbound fraction of warfarin by 33%. The therapeutic relevance of this interaction is unclear; however, monitoring INR more closely during concomitant therapy of warfarin with valproic acid/divalproex sodium/valproate sodium is advised.5-8 Perampanel and tiagabine have not been associated with significant interaction with warfarin or other highly protein-bound medications.12,19 In people with renal impairment, including those requiring hemodialysis, total and unbound tiagabine levels were unaffected.12
To decrease the risk for adverse effects and complications resulting from the aforementioned physiologic changes and drug interactions, clinicians should closely monitor for dose-dependent adverse effects and toxicities, adjusting the medication dose for altered or unpredictable protein-binding capacity of AEDs. Free fraction and total serum concentrations of phenytoin or valproic acid should be monitored closely when decreased protein binding may be indicated.5-10,49 When warfarin is coadministered with valproic acid/divalproex/valproate sodium sodium or phenytoin, INR should also be monitored closely, especially during antiepileptic drug initiation, dose increase, and discontinuation.5-10
Metabolism and Elimination/Excretion
Most AEDs undergo metabolic conversion to active or inactive metabolites in the liver. This is primarily through hydroxylation via the CYP450 enzyme system and/or conjugation with glucuronide metabolites by uridine glucuronate-glucuronyltranferase (UGT) (Table 2).3-12,14,16,18-21,23-29,31 A large proportion of AEDs are substrates for major CYP450 isoenzymes (including CYP1A2, CYP3A4, CYP2C9, and CYP2C19) and UGT isoenzymes. This makes them more susceptible to drug interaction with agents with induction (eg, rifampin, phenytoin, carbamazepine, phenobarbital, primidone, and St John's wort) or inhibition (eg, cimetidine, azole antifungals, macrolide antibiotics, nondihydropyridine calcium channel blockers, and grapefruit juice) properties of CYP450 and UGT isoenzymes (eg, valproic acid/divalproex sodium/valproate sodium).3-12,14,16,18-21,23-29,31 In order to predict or identify drug-drug interactions, understanding of CYP450 isoenzymes and other major enzyme systems involved in metabolism of individual AEDs is important.
Carbamazepine,23 oxcarbazepine,25 and eslicarbazepine acetate1 are structurally related medications. They do, however, differ significantly in terms of metabolism. Carbamazepine is chiefly converted to carbamazepine-10,11-epoxide (an active metabolite with anticonvulsant activity) by CYP3A4 in the liver and is later metabolized by epoxide hydrolase to inactive carbamazepine-10,11-trans-diol derivative metabolite.23 It is believed that carbamazepine-10,11-epoxide is responsible for increased risks for hepatotoxicity, congenital abnormalities observed during pregnancy (pregnancy class D), and autoinduction properties. Increased epoxide levels can be especially problematic for children, causing vomiting, tiredness, and increased seizure frequency.50 Carbamazepine autoinduction is usually initiated on day 3 and is fully completed within a month.23,51 Oxcarbazepine is a prodrug that is rapidly reduced in the liver to its 2 enantiomeric monohydroxy derivatives, namely (R)-licarbazepine (20%) and (S)-licarbazepine (80%), also known as eslicarbazepine.52,53 Eslicarbazepine is believed to be a significant active metabolite with anticonvulsant activity, and oxcarbazepine and (R)-licarbazepine are believed to be responsible for additional adverse effects.25,54 In addition, oxcarbazepine and monohydroxy derivative metabolites create dose-related inhibition of CYP2C19 and induction of CYP3A4/5.25 Eslicarbazepine acetate, a prodrug, is almost completely metabolized to eslicarbazepine (95%) by hydrolytic first-pass metabolism. It is an active metabolite with anticonvulsant activity and minor metabolites, including (R)-licarbazepine (4.5%) and oxcarbazepine (0.5%) formed by non-CYP450-mediated metabolism.1,52 Eslicarbazepine acetate has a lower risk for drug interactions due to lack of CYP450-dependent metabolism. It is also thought to have more favorable tolerability than carbamazepine and oxcarbazepine due to lack of epoxide formation and only minor presence of metabolites, oxcarbazepine, and (R)-licarbazepine.1,55
Gabapentin,17 pregabalin,15 and vigabatrin22 are completely renally excreted in unchanged form. Levetiracetam (66%)13 and topiramate (70%)24 are renally eliminated, predominantly in unchanged form (Table 2). Clinically significant interactions with other drugs through UGT or CYP450 hepatic enzyme systems are unlikely for all of the aforementioned medications except for topiramate (Table 3).56-59 Pharmacokinetic variability of these medications is less pronounced and more predictable under various physiologic conditions (eg, pregnancy and kidney impairment/disease) as renal function can be predicted by measuring creatinine clearance, which allows for adjustment in medication dose or frequency.13,15,17,22,24
The AEDs have varying half-lives (Table 2). In general, the half-life of a medication can be used to determine the time needed for a medication to reach steady-state plasma concentration (almost complete after 5 half-lives when it is ~97%). It is also used to determine dosing frequency required to maintain steady-state plasma concentration. The AEDs with the strongest evidence/justification for drug serum-level monitoring are carbamazepine, valproic acid, lamotrigine, oxcarbazepine, phenobarbital, and zonisamide, especially in patients with epilepsy.60 Available serum-level monitoring of AEDs can be particularly important (1) when changes in a patient occur that can significantly alter AED pharmacokinetics (eg, pregnancy, impaired kidney or liver function, concomitant therapy with enzyme inducer or enzyme inhibitor, and displacement from protein binding sites), (2) in assessing medication adherence, (3) in managing breakthrough seizures and status epilepticus, (4) for considering generic substitution, or (5) for changng dosage form.61
Most AEDs display first-order kinetics (drug serum concentration increases linearly with the total daily dose). This leads to a predictable increase or decrease in plasma drug concentration in response to a change in daily dose. Carbamazepine (autoinduction), gabapentin (saturable GI tract transport), valproic acid/divalproex sodium/valproate sodium (saturable albumin binding), zonisamide (saturable erythrocyte binding), and phenytoin (saturable CYP2C9 biotransformation) exhibit nonlinear kinetics, as shown in Table 2.62 Zonisamide is extensively bound to erythrocytes, and dose-proportional pharmacokinetics is reported at 200–400 mg/day. An increased AUC and a nonlinear relationship between zonisamide serum level and dose were reported at doses ≥800 mg/day, possibly due to saturable erythrocyte binding.28 Phenytoin dosing can be complicated because it exhibits zero-order kinetics due to metabolism saturation.10 Small changes in phenytoin dose can lead to disproportionate changes in phenytoin serum concentration.63 Careful phenytoin dose adjustment and close serum concentration monitoring are warranted to decrease patients' risk for toxicity from excessive dosing or risk for therapeutic ineffectiveness.9-10,63
Select AEDs carry risks for hepatic enzyme induction and/or inhibition, which can result in altered serum drug concentrations of concomitant medication(s).57,64 Table 3 lists AEDs and their effects on various hepatic metabolic enzyme systems and provides examples of outcomes of such interactions. Overall, first-generation AEDs have the greatest risk for drug interactions (Table 3).57,59 Phenytoin,10 carbamazepine,23 phenobarbital,20 and primidone16 are potent, broad-spectrum inducers of CYP450 and UGT isoenzymes, whereas valproic acid/divalproex sodium/valproate sodium5-8 strongly inhibit UGT isoenzymes, CYP2C19, and epoxide hydrolase. Coadministration of any of them can lead to altered metabolism of concomitant medications (Table 3). There is a complex interaction between carbamazepine and valproic acid when administered together. Carbamazepine induces hepatic clearance of valproic acid and decreases valproic acid serum concentration. Valproic acid decreases clearance of carbamazepine-10,11-epoxide by inhibiting epoxide hydrolase, leading to up to a 45% increase in the level of carbamazepine-10,11-epoxide and increased toxicity even at normal carbamazepine serum concentrations.6,23 When these 2 drugs are administered together, careful monitoring of free and total serum levels of valproic acid and carbamazepine as well as carbamazepine-10,11-epoxide serum concentrations is warranted.6,23
Newer AEDs, topiramate24 and oxcarbazepine,25 are dose-dependent inhibitors of CYP2C19, and topiramate,24 oxcarbazepine,25 perampanel,19 eslicarbazepine acetate,1 rufinamide,2 and felbamate11 are dose-dependent inducers of CYP3A4, usually causing drug interactions with select medications at high doses.
Lamotrigine is devoid of any significant enzyme-inducing or enzyme-inhibiting properties. However, its metabolism is dependent on extensive glucuronidation, primarily via UGT1A4, making it prone to several clinically significant interactions with some AEDs (Table 3).14,46,65 Coadministration of lamotrigine with valproic acid/divalproex sodium/valproate sodium leads to decreased elimination of lamotrigine, increased half-life from 30 to 60 hours, and increased lamotrigine serum concentration by >200%.6,14 Concurrent administration of lamotrigine with medications inducing UGT leads, in general, to decreased plasma concentration and shortened half-life from 30 to 15 hours.14 Lamotrigine steady-state serum concentrations have been reported to decrease by as much as 70% after the addition of methsuximide; 50% by phenytoin; 40% by carbamazepine, phenobarbital, and primidone; and 25% by oxcarbazepine.14,58 In addition, non-epileptic medications (ie, rifampin) inducing UGT can also decrease the effectiveness of lamotrigine.14
One of the important drug interactions for AEDs is with hormonal contraception (Table 3). They can stimulate CYP3A4-dependent metabolism of estrogen and/or progesterone components. Women of reproductive age should be counseled on possible hormonal contraceptive failure while on these medications.48 Clinicians should monitor for breakthrough bleeding, and alternative or supplemental forms of contraception should be considered while a patient is on an AED inducer.1-4,9-11,16,18-20,23-33,48 Conversely, estrogen-containing hormonal contraceptives and hormonal replacement therapies may increase lamotrigine clearance and reduce its serum concentration by 50%.14,67 Lamotrigine clearance is also increased during pregnancy and increases progressively until the 32nd gestational week with a mean increase from baseline to 365% during the third trimester. The return to pre-pregnancy clearance has been observed as early as 2 weeks after delivery.68 Clinicians should consider more frequent monitoring of lamotrigine serum concentration during pregnancy, initiation or discontinuation of estrogen-containing medications, and coadministration of medications with the potential to inhibit or increase hepatic glucuronidation.
Summary
A variety of pharmacokinetic interactions are associated with AEDs in absorption, distribution, metabolism, and elimination. Compared with older AEDs, newer AEDs have more predictable kinetics and fewer risks for drug interactions. This is because many are minimally or not bound to serum proteins, are primarily renally cleared or metabolized by non-CYP450 isoenzymes, and/or have less potential to induce/inhibit various hepatic enzyme systems. As all AEDs are frequently used for management of neurologic and psychiatric conditions, it is important to understand the pharmacokinetic characteristics of individual AEDs in order to create safe, effective, and patient-specific pharmacotherapeutic plans.
References
- 1. Aption.com [Internet]. Aptiom (eslicarbazepine acetate) package insert. Marlborough, (MA): Sunovion Pharmaceuticals Inc; c2013 [cited 2014 Dec 18] Available from: http://www.aptiom.com/Aptiom-Prescribing-Information.pdf [Google Scholar]
- 2. Banzel.com [Internet]. Banzel (rufinamide) package insert. Woodcliff Lake (NJ): Eisai Co, Ltd; c2015 [updated 2015 May; cited 2014 Dec 18] Available from: http://www.banzel.com/hcp/pdfs/BanzelPI.pdf [Google Scholar]
- 3. Food and Drug Administration [Internet]. Carbatrol® (carbamazepine) package insert. Silver Spring (MD): Food and Drug Administration; c.2009 [updated 2009 April; cited 2014 Dec 18] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020712s030lbl.pdf. [Google Scholar]
- 4. Pfizer.com [Internet]. Cerebyx (fosphenytoin sodium) package insert. New York: Pfizer Labs; [updated 2015 April; accessed 2014 Dec 18] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=749 [Google Scholar]
- 5. AbbVie [Internet]. Depacon. (valproate sodium), prescribing information. North Chicago (IL): AbbVie Inc; [updated 2015 Nov; cited 2014 Dec 18] Available from: http://www.rxabbvie.com/pdf/depacon.pdf [Google Scholar]
- 6. AbbVie [Internet]. Depakene (valproic acid) prescribing information. North Chicago (IL): AbbVie Inc; [updated 2015 Nov; cited 2014 Dec 18] Available from: http://www.rxabbvie.com/pdf/depakene.pdf [Google Scholar]
- 7. AbbVie [Internet]. Depakote® (Divalproex Sodium Delayed-Release tablets) package insert. North Chicago (IL): AbbVie Inc; [updated 2015 Nov; cited 2014 Dec 18] Available from: http://www.rxabbvie.com/pdf/depakote.pdf [Google Scholar]
- 8. AbbVie [Internet]. Depakote® ER (Divalproex Sodium Extended-Release tablets) package insert. North Chicago (IL): AbbVie Inc.; [updated 2014 Nov; cited 2015 Dec 18] Available from: http://www.rxabbvie.com/pdf/dep3.pdf [Google Scholar]
- 9. Pfizer [Internet]. Dilantin (phenytoin sodium) ER capsule package insert. New York: Parke-Davis Division of Pfizer Inc; [updated 2014 Nov; cited 2014 Dec 18] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=546 [Google Scholar]
- 10. Pfizer [Internet]. Dilantin (phenytoin sodium) oral suspension package insert. New York: Parke-Davis Division of Pfizer Inc; [updated 2015 March; cited 2014 Dec 18] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=560 [Google Scholar]
- 11. Felbatol.com [Internet]. Felbatol® (felbamate) package insert. Somerset (NJ): MedPointe Pharmaceuticals; [updated 2011 Jul; cited 2014 Dec 18] Available from: http://felbatol.com/FelbatolPI.pdf [Google Scholar]
- 12. Gabitril.com [Internet]/ Gabitril (tiagabine) package insert. West Chester (PA): Cephalon; [updated 2010 Sep; cited 2014 Dec 18] Available from: http://gabitril.com/pdf/Gabitril_PI_GAB-012.pdf [Google Scholar]
- 13. UCB [Internet]. Keppra (levetiracetam tablet and oral solution) package insert. Smyrna (GA): UCB, Inc; [updated 2013 Sep; cited 2014 Dec 18] Available from: http://www.ucb.com/_up/ucb_com_products/documents/Keppra_IR_Current_COL_06_2014.pdf [Google Scholar]
- 14. GlaxoSmithKline [Internet]. Lamictal® (lamotrigine) package insert. Research Triangle Park (NC): GlaxoSmithKline; [updated 2015 Nov; cited 2014 Dec 18] Available from: https://www.gsksource.com/gskprm/htdocs/documents/LAMICTAL-PI-MG.PDF [Google Scholar]
- 15. Pfizer [Internet]. Lyrica (pregabalin) package insert. New York: Pfizer; [updated 2013 Dec; cited 2014 Dec 18] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=561 [Google Scholar]
- 16. Food and Drug Administration [Internet]. Mysoline® (primidone) package insert. Silver Spring (MD): Food and Drug Administration; [updated 2009 March; cited 2014 Dec 18] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/009170s036lbl.pdf [Google Scholar]
- 17. Pfizer [Internet]. Neurontin (gabapentin) package insert. New York: Pfizer; [updated 2015 Sep; cited 2014 Dec 18] Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=630 [Google Scholar]
- 18. Food and Drug Administration [Internet]. Onfi (clobazam tablets) package insert. Silver Spring (MD): Food and Drug Administration; [updated 2013 November; cited 2014 Dec 18] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202067s001lbl.pdf [Google Scholar]
- 19. Fycompa.com [Internet]/ Perampanel (Fycompa) tablets. Woodcliff Lake (NJ): Eisai Inc; [updated 2014 September; cited 2014 Dec 18] Available from: http://www.fycompa.com/sites/all/themes/fycompa/pdf/Fycompa_Prescribing_Information.pdf [Google Scholar]
- 20. Drugs.com [Internet]/ Phenobarbital Tablets, USP package insert. Auckland (New Zealand): Drugsite Trust; [updated 2015 Aug; cited 2014 Dec 18] Available from: http://www.drugs.com/cdi/luminal.html [Google Scholar]
- 21. GlaxoSmithKline [Internet]. Potiga (ezogabine) package insert. Research Triangle Park (NC): GlaxoSmithKline; [updated 2015 Sep; cited 2014 Dec 18] Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Potiga/pdf/POTIGA-PI-MG.PDF [Google Scholar]
- 22. Lundbeck [Internet]/ Sabril (vigabatrin) tablets for oral use/powder for oral solution package insert. Deerfield (IL): Lundbeck Inc; [updated 2013 Oct; cited 2014 Dec 18] Available from: http://www.lundbeck.com/upload/us/files/pdf/Products/Sabril_PI_US_EN.pdf [Google Scholar]
- 23. Novartis Pharmaceuticals [Internet]. Tegretol (carbamazepine) package insert. East Hanover (NJ): Novartis Pharmaceuticals Corporation; [updated 2014 Jan; cited 2014 Dec 18] Available from: http://www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf [Google Scholar]
- 24. Topamax.com [Internet]/ Topamax (topiramate) package insert. Titusville (NJ): Janssen Pharmaceuticals, Inc; [updated 2009 Mar; cited 2014 Dec 18] Available from: http://www.topamax.com/sites/default/files/topamax.pdf [Google Scholar]
- 25. Novartis Pharmaceuticals [Internet]. Trileptal (oxcarbazepine) package insert. East Hanover (NJ): Novartis Pharmaceuticals Corporation; [updated 2014 Jul; cited 2014 Dec 18] Available from: http://www.pharma.us.novartis.com/product/pi/pdf/trileptal.pdf [Google Scholar]
- 26. UCB [Internet]. Vimpat (lacosamide) package insert. Smyrna (GA): UCB, Inc; [updated 2014 Aug; cited 2014 Dec 18] Available from: http://www.ucb.com/_up/ucb_com_products/documents/VIMPAT%20PRESCRIBING%20%20INFORMATION.pdf [Google Scholar]
- 27. DailyMed [Internet]. Zarontin® (ethosuximide) package insert. Bethesda (MD): National Library of Medicine; [updated 2014 Jul; cited 2014 Dec 18] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e008f33-70a1-4bc6-b3a0-d45214418ab6 [Google Scholar]
- 28. DailyMed [Internet]. Zonegran (zonisamide) package insert. Bethesda (MD): National Library of Medicine; [updated 2011 Sep; cited 2014 Dec 18] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e008f33-70a1-4bc6-b3a0-d45214418ab6 [Google Scholar]
- 29. DailyMed [Internet]. Equetro (carbamazepine extended release capsules) package insert. Bethesda (MD): National Library of Medicine; [updated 2012 Nov; cited 2014 Dec 18] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be478f3c-40f6-47cc-8ab9-f420a9372b1c [Google Scholar]
- 30. DailyMed [Internet]. Horizant (gabapentin enacarbil) extended-release tablets. Bethesda (MD): National Library of Medicine; [updated 2013 Jul; cited 2014 Dec 18] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c486fc7-c8c4-4c6c-b30c-366dabaeaadd [Google Scholar]
- 31. DailyMed [Internet]. Stavzor (valproic acid delayed-release capsules) package insert. Bethesda (MD): National Library of Medicine; [updated 2014 Aug; cited 2014 Dec 18] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95a4ddd8-b76a-4128-b2ed-8a36cd5738f9 [Google Scholar]
- 32. Spina E, Perugi G. Antiepileptic drugs: indications other than epilepsy. Epileptic Disord. 2004; 6 2: 57- 75. PubMed PMID: 15246950. [PubMed] [Google Scholar]
- 33. Clinical Pharmacology [Internet]. Tampa, FL: Gold Standard, Inc; [updated 2014; cited 2014 Dec 15] Available from: http://clinicalpharmacology-ip.com.ezproxy.galter.northwestern.edu/default.aspx [Google Scholar]
- 34. Anderson GD, Hakimian S. Pharmacokinetic of antiepileptic drugs in patients with hepatic or renal impairment. Clin Pharmacokinet. 2014; 53 1: 29- 49. DOI: 10.1007/s40262-013-0107-0. PubMed PMID: 24122696. [DOI] [PubMed] [Google Scholar]
- 35. Italiano D, Perucca E. Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update. Clin Pharmacokinet. 2013; 52 8: 627- 45. DOI: 10.1007/s40262-013-0067-4. PubMed PMID: 23640503. [DOI] [PubMed] [Google Scholar]
- 36. Patsalos PN. Drug interactions with the newer antiepileptic drugs (AEDs)—part 1: pharmacokinetic and pharmacodynamic interactions between AEDs. Clin Pharmacokinet. 2013; 52 11: 927- 66. DOI: 10.1007/s40262-013-0087-0. PubMed PMID: 23784470. [DOI] [PubMed] [Google Scholar]
- 37. McAuley JW, Anderson GD. Treatment of epilepsy in women of reproductive age: pharmacokinetic considerations. Clin Pharmacokinet. 2002; 41 8: 559- 79. DOI: 10.2165/00003088-200241080-00002. PubMed PMID: 12102641. [DOI] [PubMed] [Google Scholar]
- 38. Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008; 48 12: 1378- 88. DOI: 10.1177/0091270008322909. PubMed PMID: 18827074. [DOI] [PubMed] [Google Scholar]
- 39. Perucca E, Cloyd J, Critchley D, Fuseau E. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008; 49 7: 1123- 41. DOI: 10.1111/j.1528-1167.2008.01665.x. PubMed PMID: 18503564. [DOI] [PubMed] [Google Scholar]
- 40. Au Yeung SC, Ensom MH. Phenytoin and enteral feedings: does evidence support an interaction? Ann Pharmacother. 2000; 34 7-8: 896- 905. PubMed PMID: 10928402. [DOI] [PubMed] [Google Scholar]
- 41. Gilbert S, Hatton J, Magnuson B. How to minimize interaction between phenytoin and enteral feedings: two approaches. Nutr Clin Pract. 1996; 11 1: 28- 31. PubMed PMID: 8700059. [DOI] [PubMed] [Google Scholar]
- 42. Phelps N. Management of phenytoin with enteral tube feeding. Ment Health Clin. 2012; 2 5: 108- 9. DOI: 10.9740/mhc.n126907. [Google Scholar]
- 43. Yagi T, Naito T, Mino Y, Umemura K, Kawakami J. Impact of concomitant antacid administration on gabapentin plasma exposure and oral bioavailability in healthy adult subjects. Drug Metab Pharmacokinet. 2012; 27 2: 248- 54. PubMed PMID: 22240839. [DOI] [PubMed] [Google Scholar]
- 44. Jusko WJ, Gretch M. Plasma and tissue protein binding of drugs in pharmacokinetics. Drug Metab Rev. 1976; 5 1: 43- 140. PubMed PMID: 829788. [DOI] [PubMed] [Google Scholar]
- 45. Bauer L. Clinical pharmacokinetics and pharmacodynamics. : DiPiro JT, RL Talbert, Yee GC, Matzke GR, Wells BG. Pharmacotherapy: a pathophysiologic approach. 9th ed [Internet]. New York: McGraw-Hill; c2014 [cited 19 Dec 2014] Available from: http://accesspharmacy.mhmedical.com/content.aspx?bookid=689§ionid=48811430. [Google Scholar]
- 46. Perucca E. Extended-release formulations of antiepileptic drugs: rationale and comparative value. Epilepsy Curr. 2009; 9 6: 153- 7. DOI: 10.1111/j.1535-7511.2009.01326.x. PubMed PMID: 19936129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bazil CW, Pedley TA. Clinical pharmacology of antiepileptic drugs. Clin Neuropharmacol. 2003; 26 1: 38- 52. PubMed PMID: 12567163. [DOI] [PubMed] [Google Scholar]
- 48. Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002; 61 4: 729- 37. PubMed PMID: 11901210. [DOI] [PubMed] [Google Scholar]
- 49. von Winckelmann SL, Spriet I, Willems L. Therapeutic drug monitoring of phenytoin in critically ill patients. Pharmacotherapy. 2008; 28 11: 1391-400 DOI: 10.1592/phco.28.11.1391. PubMed PMID: 18956999. [DOI] [PubMed] [Google Scholar]
- 50. Johnson SD, Johns DW. Carbamazepine epoxide toxicity in children receiving carbamazepine and valproate. Ann Neurol. 1991; 30 3: 491- 3. [Google Scholar]
- 51. Kerr BM, Thummel KE, Wurden CJ, Klein SM, Kroetz DL, Gonzalez FJ, et al. Human liver carbamazepine metabolism. Role of CYP3A4 and CYP2C8 in 10,11-epoxide formation. Biochem Pharmacol. 1994; 47 11: 1969- 79. PubMed PMID: 8010982. [DOI] [PubMed] [Google Scholar]
- 52. Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012; 53 6: 935- 46. DOI: 10.1111/j.1528-1167.2012.03519.x. PubMed PMID: 22612290. [DOI] [PubMed] [Google Scholar]
- 53. Volosov A, Xiaodong S, Perucca E, Yagen B, Sintov A, Bialer M. Enantioselective pharmacokinetics of 10-hydroxycarbazepine after oral administration of oxcarbazepine to healthy Chinese subjects. Clin Pharmacol Ther. 1999; 66 6: 547- 53. DOI: 10.1053/cp.1999.v66.103170001. PubMed PMID: 10613609. [DOI] [PubMed] [Google Scholar]
- 54. Nunes T, Rocha JF, Falcão A, Almeida L, Soares-da-Silva P. Steady-state plasma and cerebrospinal fluid pharmacokinetics and tolerability of eslicarbazepine acetate and oxcarbazepine in healthy volunteers. Epilepsia. 2013; 54 1: 108- 16. DOI: 10.1111/j.1528-1167.2012.03595.x. PubMed PMID: 22812691. [DOI] [PubMed] [Google Scholar]
- 55. Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013; 22 7: 528- 36. DOI: 10.1016/j.seizure.2013.03.016. PubMed PMID: 23623245. [DOI] [PubMed] [Google Scholar]
- 56. Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand. 2004; 109 6: 374- 7. DOI: 10.1111/j.1600-0404.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- 57. Johannessen SI, Landmark CJ. Antiepileptic drug interactions— principles and clinical implications. Curr Neuropharmacol. 2010; 8 3: 254- 67. DOI: 10.2174/157015910792246254. PubMed PMID: 21358975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stewart BH, Kugler AR, Thompson PR, Bockbrader HN. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993; 10 2: 276- 81. PubMed PMID: 8456077. [DOI] [PubMed] [Google Scholar]
- 59. Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006; 61 3: 246- 55. DOI: 10.1111/j.1365-2125.2005.02529.x. PubMed PMID: 16487217; PubMed Central PMCID: PMC1885026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krasowski MD. Therapeutic drug monitoring of the newer anti-epilepsy medications. Pharmaceuticals. 2010; 3 6: 1909- 35. PubMed PMID: 20640233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stepanova D, Beran RG. The benefits of antiepileptic drug (AED) blood level monitoring to complement clinical management of people with epilepsy. Epilepsy Behav. 2015; 42: 7- 9. DOI: 10.1016/j.yebeh.2014.09.069. [DOI] [PubMed] [Google Scholar]
- 62. Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed?. Clin Pharmacokinet. 2006; 45 11: 1061- 75. DOI: 10.2165/00003088-200645110-00002. PubMed PMID: 17048972. [DOI] [PubMed] [Google Scholar]
- 63. Sander JW. The use of antiepileptic drugs—principles and practice. Epilepsia. 2004; 45 Suppl 6: 28- 34. DOI: 10.1111/j.0013-9580.2004.455005.x. PubMed PMID: 15315513. [DOI] [PubMed] [Google Scholar]
- 64. Benedetti MS. Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol. 2000; 14 4: 301- 19. PubMed PMID: 11030437. [DOI] [PubMed] [Google Scholar]
- 65. Gidal BE, Sheth R, Parnell J, Maloney K, Sale M. Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics: implications for conversion to lamotrigine monotherapy. Epilepsy Res. 2003; 57 2-3: 85- 93. DOI: 10.1016/j.eplepsyres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 66. May TW, Rambeck B, Jürgens U. Influence of oxcarbazepine and methsuximide on lamotrigine concentrations in epileptic patients with and without valproic acid comedication: results of a retrospective study. Ther Drug Monit. 1999; 21 2: 175- 81. PubMed PMID: 10217337. [DOI] [PubMed] [Google Scholar]
- 67. Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003; 61 4: 570- 1. DOI: 10.1212/01.WNL.0000076485.09353.7A. [DOI] [PubMed] [Google Scholar]
- 68. Tran TA, Leppik IE, Blesi K, Sathanandan ST, Remmel R. Lamotrigine clearance during pregnancy. Neurology. 2002; 59 2: 251- 5. PubMed PMID: 12136066. [DOI] [PubMed] [Google Scholar]