Abstract
Treatment of depression often requires long-term management with medication. Practitioners should be aware of potentially significant drug interactions with the use of antidepressants in order to effectively prevent or manage adverse events while optimizing patient response to treatment. Most antidepressants are metabolized by the liver, primarily via the CYP450 system. Pharmacokinetic profiles of the most recently approved antidepressants are reviewed in addition to evidence supporting potentially significant interactions. In addition, pharmacokinetic interactions between multiple antidepressants and other drug classes, including opiates, antineoplastics, antiepileptics, and antipsychotics, are discussed. This article provides recommendations for the monitoring and management of drug interactions. In addition, limitations of the evidence are reviewed.
Keywords: pharmacokinetic, drug interaction, CYP enzyme, inducer, inhibitor antidepressant
Introduction
Major depression remains a prevalent disorder that has a significant impact on the mental health of our American population. According to results from the 2012 National Survey on Drug Use and Health, 9.1% of youth (ages 12-17 years) had a major depressive episode in the past year, which is an increase in prevalence compared with 2006-11 survey results (7.9%-8.3% of youth with a major depressive episode in past year).1 In the adult population, 6.9% had a major depressive episode in 2012, which is similar to survey results from the last 10 years. Approximately 20% of youth and 68% of these adults with depression sought treatment in 2012.1 In order to effectively manage depressive episodes, medications are often necessary as part of the treatment plan. Subsequently, practitioners should be aware of potential drug interactions when using antidepressants and how to effectively monitor for and manage these interactions.
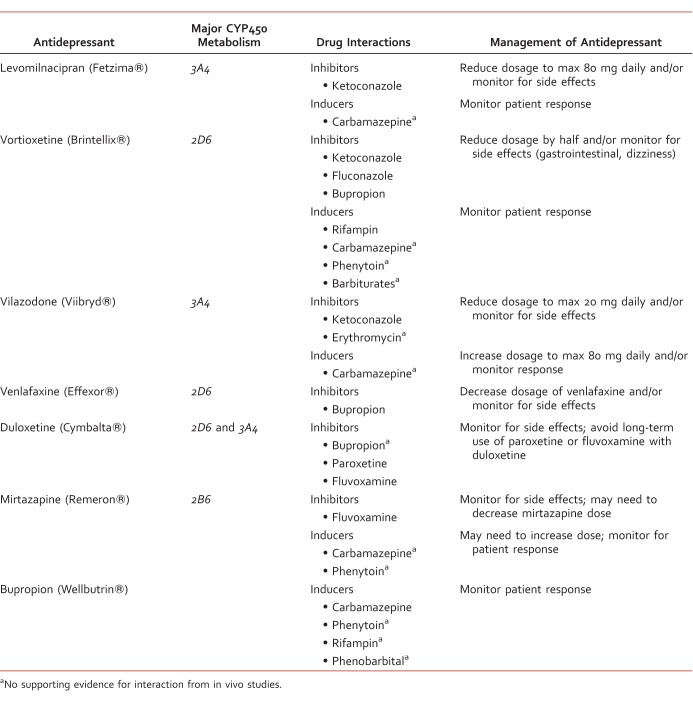
Drug interactions are described as either pharmacodynamic or pharmacokinetic. Pharmacodynamic drug interactions occur when medications act at similar receptors, resulting in additive, synergistic, or antagonistic effects. Pharmacokinetic drug interactions describe how one medication can affect the absorption, distribution, metabolism, or excretion of another medication.2 Antidepressants are primarily metabolized via the hepatic CYP450 system, making this class of medications a likely target for pharmacokinetic drug interactions when used with strong CYP450 inducers or inhibitors. This article focuses on pharmacokinetic drug interactions involving antidepressants and the CYP450 system. A list of antidepressants and their interactions can be found in the Table. Recent study results are reviewed and assessed for potential clinical significance. In addition, strategies for monitoring and management of potentially significant interactions are discussed.
TABLE:
Antidepressant CYP450 drug interactions and management
Examples of antidepressants that have more potential for significant pharmacokinetic interactions include moderate to strong inhibitors of metabolism via the CYP450 system and include fluoxetine and its metabolite norfluoxetine (strong inhibitors of CYP2D6, moderate inhibitors of CYP3A4 and CYP2C9), paroxetine (very strong CYP2D6 inhibitor), sertraline (dose-dependent inhibition of CYP2D6 at dosages of at least 150 mg daily), duloxetine (moderate CYP2D6 inhibitor), bupropion (moderate CYP2D6 inhibitor), and fluvoxamine (strong CYP1A2 and CYP2C19 inhibitor).3-5 There are recent studies involving pharmacokinetic drug interactions between two antidepressants and between antidepressants and other drug classes, including opiates, anticonvulsants, antineoplastics, and antipsychotics.
Pharmacokinetics of the Newest Antidepressants and Significant Drug Interactions
In recent years, 3 antidepressants have entered the US drug market. Vilazodone was approved by the Food and Drug Administration in January 2011 as the first selective-serotonin reuptake inhibitor (SSRI) with serotonin (5HT)-1A partial agonist activity.6 Levomilnacipran was approved in July 2013 as a selective-norepinephrine reuptake inhibitor with potent norepinephrine reuptake inhibition.7 Vortioxetine was approved in September 2013 with activity at several serotonin receptors, some of which are not yet understood.8 In vivo drug interaction studies for these new antidepressants are minimal.
Vilazodone is primarily metabolized by CYP3A4, with minor metabolism through CYP2C19 and CYP2D6.9,10 There is evidence that ketoconazole increases vilazodone concentrations by up to 50%. Subsequently, the daily dosage of vilazodone may need to be reduced when used concurrently with strong CYP3A4 inhibitors like ketoconazole. The manufacturer specifically recommends a vilazodone dosage of 20 mg daily or less.9 Although there is no supporting evidence, the manufacturer also recommends using a lower dosage of vilazodone, specifically 20 mg daily, if the patient is taking a moderate CYP3A4 inhibitor, such as erythromycin, and experiencing intolerable side effects from vilazodone.9 However, a short course of erythromycin or ketoconazole would likely not require a dosage reduction of vilazodone. A recent study using steady-state carbamazepine with vilazodone 40 mg daily resulted in a decrease in vilazodone exposure by up to 45%.10 If a CYP3A4 inducer is used for more than 14 days with vilazodone, it is recommended to increase the vilazodone dose by up to 2-fold, with a maximum dosage of 80 mg daily.9 However, the decision to increase the dosage of vilazodone should be patient specific and depends on whether or not the patient's depressive symptoms are being managed at the current dosage. Currently, there are no data to support significant pharmacokinetic interactions involving CYP2C19 or CYP2D6, theoretically because these enzymes are minor pathways of vilazodone's metabolism. Vilazodone does not significantly induce or inhibit any CYP enzymes.9,10
Levomilnacipran is primarily metabolized via the CYP3A4 isoenzyme. In vitro studies have observed interactions with strong CYP3A4 inhibitors, such as ketoconazole, clarithromycin, and ritonavir. A recent in vivo study showed a significant increase in levomilnacipran concentrations when coadministered with ketoconazole.11 However, the study did not show a significant decrease in levomilnacipran concentrations when coadministered with the strong CYP3A4 inducer carbamazepine.11 Although evidence is limited, the manufacturer advises the use of a lower dosage of levomilnacipran with strong CYP3A4 inhibitors, specifically 80 mg daily or less.12 In most cases, a dosage decrease would not be warranted if the CYP3A4 inhibitor is used short term. Symptoms of levomilnacipran toxicity, including tachycardia and hypertension, should be monitored.11,12 Although there is no supporting evidence, use of levomilnacipran with strong CYP3A4 inducers requires closer monitoring for adequate management of depressive symptoms at the current dosage. Levomilnacipran is not known to significantly induce or inhibit any CYP enzymes.11,12
Vortioxetine is primarily metabolized via CYP2D6 to an inactive metabolite. Several other CYP enzymes are minimally involved in vortioxetine's metabolism, including CYP3A4/5, CYP2C9, CYP2C19, CYP2A6, CYP2C8, and CYP2B6.13,14 A recent study evaluated potential drug interactions with vortioxetine and fluconazole, ketoconazole, rifampin, bupropion, omeprazole, and ethinyl estradiol/levonorgestrel.14 Vortioxetine concentrations did not change with use of ethinyl estradiol/levonorgestrel (CYP3A substrates). In addition, omeprazole (CYP2C19 substrate and inhibitor) did not change concentrations of vortioxetine.14 Fluconazole and ketoconazole increased vortioxetine's maximum concentration (Cmax) and area under the curve (AUC) but did not lead to any significant side effects.14 However, use of bupropion (strong CYP2D6 inhibitor) with vortioxetine significantly increased vortioxetine concentrations.14 The use of bupropion with vortioxetine resulted in an increase in vortioxetine's AUC and Cmax by more than double that of vortioxetine alone. This increase in vortioxetine concentrations increased the incidence of side effects, including nausea, vomiting, insomnia, and dizziness. A total of 9 of the 60 patients studied on bupropion and vortioxetine terminated the study early because of adverse events.14 This interaction supports the theory that CYP2D6 is the major pathway of vortioxetine metabolism. The manufacturer of vortioxetine recommends a dose decrease by half with use of bupropion. Study results show that rifampin decreases vortioxetine's Cmax by 51% and AUC by 75%.14 The clinical effects of this decrease in vortioxetine concentration are not fully understood. A dosage increase may be warranted if vortioxetine is given with CYP450 inducers, like carbamazepine, phenytoin, rifampin, and barbiturates.14 Theoretically, the use of a CYP3A4 inducer would not decrease vortioxetine concentrations as significantly as rifampin, which inhibits multiple CYP enzymes. Vortioxetine is not known to significantly induce or inhibit any CYP enzymes.13-15
Pharmacokinetic Drug Interactions Within the Class of Antidepressants
Bupropion has evidence for potentially significant drug interactions with other antidepressants. Venlafaxine and bupropion are used together as a multimodal approach for treatment-resistant depression. Venlafaxine is extensively metabolized via CYP2D6. Inhibition of CYP2D6 by bupropion may increase the risk for side effects.3-5,16 A recent case series shows a significant increase in venlafaxine concentrations with use of bupropion.16 This interaction may be used to increase concentrations of venlafaxine in extensive CYP2D6 metabolizers. However, this combination may increase anxiety and risk of serotonin syndrome in poor CYP2D6 metabolizers.16 As mentioned previously, bupropion also inhibits vortioxetine's metabolism.14 The use of bupropion with duloxetine, which is primarily metabolized by CYP2D6 and CYP1A2, may increase duloxetine levels.3-5 However, there is no evidence to support a significant interaction between these antidepressants.
There is evidence supporting a significant interaction between duloxetine and paroxetine, which increases the AUC of duloxetine by 60%, and duloxetine and fluvoxamine, which increases the AUC of duloxetine by 460%.3-5 However, SSRIs are not usually taken in combination with selective-norepinephrine reuptake inhibitors unless the patient is cross-titrating between these two classes of antidepressants.
Mirtazapine is primarily metabolized by CYP2D6 and CYP3A4, and to a lesser extent, CYP1A2. It does not significantly inhibit any CYP isoenzymes.3-5 There is no strong evidence supporting any significant drug interactions with mirtazapine. Limited evidence shows a minimal increase in the AUC of mirtazapine when used with paroxetine.3-5 A small trial studying the interaction between fluvoxamine and mirtazapine resulted in a 3-4 fold increase in plasma concentrations of mirtazapine.3-5 The use of carbamazepine and phenytoin with mirtazapine may decrease mirtazapine concentrations significantly.17,18 Use of a strong CYP2D6 and CYP3A4 inducer with mirtazapine warrants close monitoring of the patient's response to mirtazapine. A dose increase of mirtazapine may be necessary. There is limited information available for clinically significant pharmacokinetic interactions between other second-generation antidepressants.
Pharmacokinetic Drug Interactions Between Antidepressants and Opiates
It is estimated that at least 65% of patients with depression have pain complaints. Contrarily, patients with multiple pain complaints are three to five times more likely to be depressed than patients without pain.19 It is fairly common for patients to take both opiates and antidepressants, which carries a risk for significant pharmacokinetic interactions. This article will focus on recent studies evaluating drug interactions between antidepressants and oral opiates.
Oxycodone is metabolized in the liver via CYP2D6 to the active metabolite oxymorphone (10%), and via CYP3A4 to the inactive metabolite noroxycodone (80%).20-22 Theoretically, strong CYP2D6 inhibitors, like paroxetine, decrease the metabolism of oxycodone to its active metabolite oxymorphone. A few recent studies have examined the potential for a pharmacokinetic drug interaction between paroxetine, a strong CYP2D6 inhibitor, and oxycodone. These studies have not shown a significant change in the kinetics of oxycodone or its pharmacologic effects.20-22 However, use of the strong 3A4 inhibitor itraconazole, along with the strong CYP2D6 inhibitor paroxetine, did inhibit the metabolism of oxycodone to its inactive metabolite noroxycodone and increased oxycodone concentrations by 2- to 3-fold.20-22 However, the increase in oxycodone concentrations did not reflect a change in degree of pain control. The use of ketoconazole with oxycodone also has evidence supporting a significant increase in oxycodone concentrations, specifically an increase in Cmax by 77% and AUC by 146%.20-22 This increase in oxycodone levels did increase side effects, including nausea, itching, and drowsiness. More studies are needed to validate the results of these studies, including the use of other CYP2D6 inhibitors, like bupropion and fluoxetine. However, practitioners should consider avoiding the use of oxycodone with strong CYP3A4 inhibitors, especially in combination with a strong CYP2D6 inhibitor like paroxetine, particularly in patients that are more prone to side effects from opiates.
Hydrocodone is metabolized to its active form, hydromorphone, via CYP2D6. Prior to its metabolism, hydrocodone has limited affinity for opiate receptors. Its active metabolite, hydromorphone, is responsible for most of the analgesic effects.22,23 In theory, CYP2D6 inhibitors may decrease the efficacy of hydrocodone. However, studies examining the interaction between quinidine, a strong CYP2D6 inhibitor, and hydrocodone have mixed results.22,23 Studies examining this interaction with antidepressants that are strong CYP2D6 inhibitors are lacking. Until there is more evidence supporting this possible interaction, patients who use a strong CYP2D6 inhibitor along with hydrocodone should be monitored for adequate pain control.
Tramadol also depends on its CYP2D6 metabolism into two different active isomers for its analgesic efficacy. Tramadol is also metabolized by CYP3A4 into an inactive metabolite. There are a few recent studies showing reduced analgesic efficacy with use of tramadol and paroxetine.24,25 One of these studies resulted in a decrease in metabolism of tramadol into its active metabolites, which became more significant as the dose of paroxetine increased.24 Another study resulted in a 67% decrease in concentration of an active metabolite of tramadol. The decreased concentration of tramadol did reduce analgesic efficacy.25 A recent study examined whether itraconazole (a strong CYP3A4 inhibitor) increased tramadol concentrations. Results did not show a significant increase in AUC or Cmax for tramadol when used with itraconazole.26 It is recommended that pain control be monitored if a patient is using a strong CYP2D6 inhibitor along with tramadol, especially in poor 2D6 metabolizers. This interaction may necessitate a change in medication. According to the evidence available, use of a strong CYP3A4 inhibitor does not warrant any additional monitoring or dosage changes.
Codeine is metabolized by CYP2D6 into its active metabolite morphine.22,23 Theoretically, strong CYP2D6 inhibitors would decrease concentrations of the active metabolite and render codeine fairly ineffective. However, there is no recent evidence to support this theory. It would be prudent to monitor for adequate pain control when using codeine with a strong CYP2D6 inhibitor.
Methadone has a complex pharmacokinetic profile and thus has potential for several drug interactions. It is metabolized via CYP3A4 and CYP2B6, with some metabolism via CYP2D6, CYP2C9, and CYP2C19.22,23,27 Use with CYP2D6 inhibitors has resulted in increased methadone levels, which may result in toxicity.27 Methadone levels may also increase with inhibition of CYP3A4. However, a recent study showed that indinavir (a strong CYP3A4 inhibitor) had no effect on methadone levels.28 Reports of death from methadone toxicity have been described with use of fluoxetine.22,23 Paroxetine is a potent CYP2B6 inhibitor (in addition to having potent CYP2D6 inhibition), so this medication is not recommended for use with methadone.22,23 The evidence suggests that use of a strong CYP3A4 or CYP2D6 inhibitor with methadone should be avoided in order to prevent methadone toxicity.
Drug Interactions Between Antidepressants and Anticonvulsants
Anticonvulsants are used for mood stability, anxiety, neuropathic pain, and seizure control. The use of anticonvulsants with antidepressants has the potential for pharmacokinetic interactions. Anticonvulsants that are strong CYP inducers include carbamazepine, phenobarbital, phenytoin, and primidone. Use of carbamazepine with SSRIs, mirtazapine, and bupropion has been shown to decrease concentrations of these antidepressants by at least 25%, which may lead to decreased efficacy.17,18,29 There is a lack of data on the effects of these anticonvulsants with the newest antidepressants. Vilazodone may be affected by CYP3A4 inducers like carbamazepine.17,18,29
Antidepressant Interactions Between Antidepressants and Antineoplastics
Patients with cancer are at increased risk of adverse events from drug interactions due to the use of multiple medications, some of which have a narrow therapeutic index. It is estimated that approximately 20% to 30% of adverse drug reactions in cancer patients are due to drug interactions.30 Such interactions are in part from the use of antineoplastics with antidepressants, resulting in decreased efficacy of the patient's anticancer regimen or an increased risk of toxicity.30 One of these significant interactions involves the use of tamoxifen with SSRIs. Tamoxifen is metabolized into its active form via CYP2D6.31,32 Its metabolites are 100-fold more potent for the estrogen receptor than the parent compound. Studies have shown a significant decrease in active metabolites with the use of paroxetine and fluoxetine. Weak inhibitors of CYP2D6, like sertraline and venlafaxine, do not significantly decrease concentrations of tamoxifen's active metabolites.33 The risk of breast cancer recurrence is somewhat controversial because of mixed results from observational and case-control studies.31-33 However, a recent large, population-based cohort study did show that the use of paroxetine with tamoxifen increased risk of death from breast cancer and all-cause mortality. A longer duration of tamoxifen and paroxetine use increased the risk of breast cancer mortality. The study also showed no significant increase in risk of mortality from other SSRIs.31-33 It is recommended that patients on tamoxifen not take paroxetine or fluoxetine. It would also be advisable to avoid bupropion and duloxetine because of moderate CYP2D6 inhibition.32,33
Hypericum extracts (St John's Wort), a popular over-the-counter supplement with antidepressant properties, also carry risk for a pharmacokinetic interaction with antineoplastics by induction of CYP3A4, especially at hypericum dosages of at least 900 mg daily. Studies have shown the use of hypericum with imatinib increases imatinib clearance by 43% and decreases its half-life from 13 hours to 9 hours.30,34 This decrease in concentrations may have an impact on patient response to imatinib, but patient outcomes have not been studied. However, phenytoin, a CYP3A4 inducer, has been shown to reduce patient response to imatinib. A similar interaction can be seen with the concurrent use of hypericum and irinotecan or taxanes. There is evidence that patients have significantly less myelosuppression when hypericum is used with irinotecan. It is not advisable to use hypericum with these antineoplastics.30,34
Antidepressant Interactions Between Antidepressants and Antipsychotics
Antipsychotics can be used with antidepressants for treatment-resistant depression in addition to other indications. All second-generation antipsychotics, excluding paliperidone, are metabolized via the CYP system. Fluoxetine and paroxetine have been shown to significantly increase plasma concentrations of clozapine, risperidone, aripiprazole, and iloperidone by up to 70% from strong inhibition of CYP2D6. Sertraline dosages of 150 mg daily or more produce similar effects.35 Fluvoxamine has also been shown to increase plasma concentrations of clozapine by up to 10-fold, primarily because of inhibition of CYP1A2.35 The impact of these interactions on the patient is not fully understood. At this time, management may include dosage reduction of the antipsychotic and/or monitoring for adverse events. In some cases, this interaction may prove beneficial for the patient in management of his or her depression, especially if he or she rapidly metabolizes the antipsychotic. There are no known pharmacokinetic interactions between vortioxetine, vilazodone, levomilnacipran, and antipsychotics. Antipsychotics do not significantly induce or inhibit CYP enzymes.
Conclusion
Recent evidence does provide some insight into potentially significant drug interactions with antidepressants. It is important to understand the implications of these interactions to reduce the risk of adverse events and to optimize pharmacologic response. On the other hand, it is essential to recognize the limitations of studies when relating evidence to clinical practice. Several studies use prototypes for CYP induction and inhibition. This forces practitioners to extrapolate data to medications with similar pharmacokinetic profiles to help identify potential drug interactions. Furthermore, drug interactions vary for each patient because of individual genetic variability. Practitioners should be aware of potential drug interactions and use their clinical judgment to determine whether close monitoring is sufficient or if a medication change or dosage adjustment is necessary.
References
- 1. Center for Behavior Health Statistics and Quality. (2013). Results from the 2012 National Survey on Drug Use and Health: summary of national findings. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2013. HHS Publication No.: SMA 13 4795, NSDUH Series H-46.
- 2. Concepts in clinical pharmacokinetics: lesson 1: introduction to pharmacokinetics and pharmacodynamics [Internet]. Bethesda (MD): American Society of Health System Pharmacists 2014 April 10 [cited 2015 January 1]. Available from: http://www.ashp.org/doclibrary/bookstore/p2418-chapter1.aspx
- 3. Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008; 30 7: 1206- 27. DOI: 10.1016/j.clinthera.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4. Schellander R, Donnerer J. Antidepressants: clinically relevant drug interactions to be considered. Pharmacology. 2010; 86 4: 203- 15. DOI: 10.1159/000319744. [DOI] [PubMed] [Google Scholar]
- 5. Spina E, Trifirò G, Caraci F. Clinically significant drug interactions with newer antidepressants. CNS Drugs. 2012; 26 1: 39- 67. DOI: 10.2165/11594710-000000000-00000. PubMed PMID: 22171584. [DOI] [PubMed] [Google Scholar]
- 6. Croft HA, Pomara N, Gommoll C, Chen D, Nunez R, Mathews M. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014; 75 11: e1291- 8. DOI: 10.4088/JCP.14m08992. PubMed PMID: 25470094. [DOI] [PubMed] [Google Scholar]
- 7. Scott LJ. Levomilnacipran extended-release: a review of its use in adult patients with major depressive disorder. CNS Drugs. 2014; 28 11: 1071- 82. DOI: 10.1007/s40263-014-0203-1. [DOI] [PubMed] [Google Scholar]
- 8. Pae CU, Wang SM, Han C, Lee SJ, Patkar A, Masand P, et al. Vortioxetine: a meta-analysis of 12 short-term, randomized, placebo-controlled clinical trials for the treatment of major depressive disorder. J Psychiatry Neurosci. 2015; 40 3: 174- 86. DOI: 10.1503/jpn.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. VIIBRYD oral tablets, vilazodone hydrochloride tablets [package insert]. Forest Pharmaceuticals, Inc (per manufacturer), St Louis, MO, 2014.
- 10. Boinpally R, Gad N, Gupta S, Periclou A. Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects. Clin Ther. 2014; 36 11: 1638- 49. DOI: 10.1016/j.clinthera.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 11. Mago R, Forero G, Greenberg WM, Gommoll C, Safety Chen C. and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013; 33 10: 761- 71. DOI: 10.1007/s40261-013-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FETZIMA(TM) oral extended-release capsules, levomilnacipran oral extended-release capsules [package insert]. St Louis (MO): Forest Pharmaceuticals Inc; 2013. [Google Scholar]
- 13. BRINTELLIX oral tablets, vortioxetine oral tablets [package insert]. Deerfield (IL): Takeda Pharmaceuticals America Inc; 2014. [Google Scholar]
- 14. Chen G, Lee R, Højer AM, Buchbjerg JK, Serenko M, Zhao Z. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013; 33 10: 727- 36. DOI: 10.1007/s40261-013-0117-6. PubMed PMID: 23975654; PubMed Central PMCID: PMC3775155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Areberg J, Petersen KB, Chen G, Naik H. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol. 2014; 115 6: 552- 9. DOI: 10.1111/bcpt.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paslakis G, Gilles M, Deuschle M. Clinically relevant pharmacokinetic interaction between venlafaxine and bupropion: a case series. J Clin Psychopharmacol. 2010; 30 4: 473- 4. DOI: 10.1097/JCP.0b013e3181e5c0e4. PubMed PMID: 20631572. [DOI] [PubMed] [Google Scholar]
- 17. Mula M. Anticonvulsants - antidepressants pharmacokinetic drug interactions: the role of the CYP450 system in psychopharmacology. Curr Drug Metab. 2008; 9 8: 730- 7. PubMed PMID: 18855610. [DOI] [PubMed] [Google Scholar]
- 18. Italiano D, Spina E, de Leon J. Pharmacokinetic and pharmacodynamic interactions between antiepileptics and antidepressants. Expert Opin Drug Metab Toxicol. 2014; 10 11: 1457- 89. DOI: 10.1517/17425255.2014.956081. [DOI] [PubMed] [Google Scholar]
- 19. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003; 163 20: 2433- 45. DOI: 10.1001/archinte.163.20.2433. PubMed PMID: 14609780. [DOI] [PubMed] [Google Scholar]
- 20. Kummer O, Hammann F, Moser C, Schaller O, Drewe J, Krähenbühl S. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol. 2011; 67 1: 63- 71. DOI: 10.1007/s00228-010-0893-3. [DOI] [PubMed] [Google Scholar]
- 21. Grönlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Laine K, Olkkola KT. Effect of inhibition of cytochrome P450 enzymes 2D6 and 3A4 on the pharmacokinetics of intravenous oxycodone: a randomized, three-phase, crossover, placebo-controlled study. Clin Drug Investig. 2011; 31 3: 143- 53. DOI: 10.2165/11539950-000000000-00000. PubMed PMID: 21142269. [DOI] [PubMed] [Google Scholar]
- 22. Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care. 2011; 17 Suppl 11: S276- 87. PubMed PMID: 21999760. [PubMed] [Google Scholar]
- 23. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009; 50 2: 169- 76. DOI: 10.1176/appi.psy.50.2.169. PubMed PMID: 19377028. [DOI] [PubMed] [Google Scholar]
- 24. Nielsen AG, Pedersen RS, Noehr-Jensen L, Damkier P, Brosen K. Two separate dose-dependent effects of paroxetine: mydriasis and inhibition of tramadol's O-demethylation via CYP2D6. Eur J Clin Pharmacol. 2010; 66 7: 655- 60. DOI: 10.1007/s00228-010-0803-8. PubMed PMID: 20354688. [DOI] [PubMed] [Google Scholar]
- 25. Laugesen S, Enggaard TP, Pedersen RS, Sindrup SH, Brøsen K. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005; 77 4: 312- 23. PubMed PMID: 15903129. [DOI] [PubMed] [Google Scholar]
- 26. Hagelberg NM, Saarikoski T, Saari TI, Neuvonen M, Neuvonen PJ, Turpeinen M, et al. Ticlopidine inhibits both O-demethylation and renal clearance of tramadol, increasing the exposure to it, but itraconazole has no marked effect on the ticlopidine-tramadol interaction. Eur J Clin Pharmacol. 2013; 69 4: 867- 75. DOI: 10.1007/s00228-012-1433-0. [DOI] [PubMed] [Google Scholar]
- 27. Lee HY, Li JH, Wu LT, Wu JS, Yen CF, Tang HP. Survey of methadone-drug interactions among patients of methadone maintenance treatment program in Taiwan. Subst Abus Treat Prev Policy. 2012; 7 1: 11 DOI: 10.1186/1747-597X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kharasch ED, Bedynek PS, Hoffer C, Walker A, Whittington D. Lack of indinavir effects on methadone disposition despite inhibition of hepatic and intestinal cytochrome P450A (CYP3A). Anesthesiology. 2012; 116 2: 432- 47. DOI: 10.1097/ALN.Ob013e3182423478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol. 2003; 2 6: 347- 56. DOI: 10.1016/S1474-4422(03)00409-5. [DOI] [PubMed] [Google Scholar]
- 30. Caraci F, Crupi R, Drago F, Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: focus on selective serotonin reuptake inhibitors and hypericum extract. Curr Drug Metab. 2011; 12 6: 570- 7. DOI: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- 31. Lash TL, Cronin-Fenton D, Ahern TP, Rosenberg CL, Lunetta KL, Silliman RA, et al. Breast cancer recurrence risk related to concurrent use of SSRI antidepressants and tamoxifen. Acta Oncol. 2010; 49 3: 305- 12. DOI: 10.3109/02841860903575273. PubMed PMID: 20156115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahern TP, Pedersen L, Cronin-Fenton DP, Sorensen HT, Lash TL. No increase in breast cancer recurrence with concurrent use of tamoxifen and some CYP2D6-inhibiting medications. Cancer Epidemiol Biomarkers Prev. 2009; 18 9: 2562- 4. DOI: 10.1158/1055-9965.EPI-09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010; 340:c693. DOI: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed]
- 34. Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H. The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol. 2006; 62 5: 512- 26. DOI: 10.1111/j.1365-2125.2006.02755.x. PubMed PMID: 17010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spina E, Leon J. Clinically relevant interactions between new antidepressants and second-generation antipsychotics. Expert Opin Drug Metab. 2014; 10 5: 721- 46. [DOI] [PubMed] [Google Scholar]