Abstract
Density-dependent territorial interactions have been suggested to cause immunosuppression and thereby decrease fitness, but empirical support from natural populations is lacking. Data from a natural lizard population (Uta stansburiana) showed that breeding females surrounded by many territorial neighbors had suppressed immune function. Furthermore, variation in immunological condition had different effects on the fitness of the two heritable female throat-color morphs in this population. These interactive fitness effects caused correlational selection between female throat color and immune responsiveness. Population genetic theory predicts that this should have lead to the buildup and preservation of a genetic correlation between female morphotype and immunological condition. Accordingly, the throat color of a female was genetically correlated (rA = −1.36; SE = 0.55) with her daughter's immune responsiveness.
Major fitness components, such as survival and fecundity, are strongly affected by density-dependent competition (1, 2). The ecological and physiological mechanisms causing reduced fitness at high densities remain unclear in most species, but density-dependent social interactions have been suggested to suppress immune function and thereby have a deleterious effect on fitness (3, 4). Despite recent and growing awareness of the importance of individual variation in immune function in ecological and evolutionary studies (5, 6), data from natural, free-living populations are lacking. Moreover, our knowledge of the fitness consequences of individual variation in immunological condition as well as the environmental and genetic factors influencing this variation in nature is very limited.
We studied variation in immune responsiveness, survival, and density-dependent competition in a free-living Californian population of side-blotched lizards, Uta stansburiana (7–9). This is an annual species throughout most of its range, and it is characterized by very high densities of both adults and juveniles. These high densities lead to territorial interactions between neighbors and have negative effects on progeny and adult survival (10, 11). In several lizard species, including side-blotched lizards, males and females occur in discrete and heritable color morphs that differ markedly with respect to behavior, physiology, and life-history traits (12–16). Although a simple genetic mechanism may control throat color in side-blotched lizards, color is also correlated with differences in steroid hormone levels, which, in turn, have pleiotropic effects on other behavioral, physiological, and life history traits (13–15). Moreover, throat color and other traits are under strong natural and sexual selection that favors particular combinations of life history traits in the different morphs (14).
Females in our study population occur in two heritable throat-color morphs: “orange” and “yellow” (14). Both total population size and the frequency of these two female morphs fluctuate rapidly across years, because of negative frequency-dependent selection in which each morph is selectively favored when rare (14). Orange females produce large clutches of small eggs and are favored at low density, whereas yellow females produce small clutches of large eggs and are favored at high density (14). The net result of these density- and frequency-dependent oscillating selection pressures is an intrinsic cycle in population density that requires only 2 years to complete one oscillation (14). We studied whether density has different effects on immune function of the two female morphs, and the effects on female survival. In addition, we estimated the heritability and genetic correlations for throat color and immune function in free-ranging lizards.
Materials and Methods
Study Organism, General Field, and Laboratory Work.
Side-blotched lizards, U. stansburiana, are small (7–10 g) lizards that mature 1 year after hatching, and most adults survive only 1 season of reproduction. In our study area in the Coast Range of central California, adult population densities in certain years may approach 800 per hectare and juvenile densities 2,600 per hectare, which is probably among the highest for terrestrial vertebrate populations (11). As part of our long-term population studies (14), we captured and observed lizards during the reproductive seasons (late February to August) of 1998 and 1999.
In the beginning of the season (late February and early March), all animals were individually marked (toe clips), and their subsequent behavioral activities were closely monitored for the rest of the season. Home range boundaries, home range sizes, and the number of neighbors for each focal female were calculated from mapped locations of individual females during the vitellogenesis of their first clutch (March to April). Home range areas were computed from the minimum convex polygon, circumscribing all mapped locations for each individual female (minimum of 5 female observations; refs. 9, 10, and 15). The females that had polygons that overlapped with a focal female were considered to be neighbors of that female.
Females were captured before they laid their eggs and were brought into the laboratory to obtain eggs. After oviposition, all females were released again on the site of capture. Females that survived in the field to lay later clutches were recaptured, hence, we could assess survival between clutches. Mean survival between the first and the second clutch was 0.588 (SE = ± 0.086) in 1998 and 0.527 (SE = ± 0.068) in 1999. The survival of females that remained in the field on a control outcrop was similar or equal to the survival of the females that experienced the brief laboratory stay that was required to obtain progeny and hence calculate heritability of immune and throat-color traits (10, 14).
Eggs obtained from the dams were incubated in a common laboratory environment with temperature and hydric conditions carefully controlled until hatching (7, 11). Incubation in this common laboratory environment controlled for maternal effects in the field because of differences in oviposition sites between dams (7). In addition, the sizes of some eggs were manipulated upwards (follicle ablations) or downwards (yolk removals), whereas others served as unmanipulated controls, as part of our long-term, ongoing experimental field study (7, 11, 14). These experimental manipulations controlled for maternal effects attributable to differences in the amount of yolk deposited in the eggs (7). Finally, hatchlings were released in the field shortly after hatching (1–3 days), and releases were randomized with respect to the dam's home range (11, 14), to control for maternal effects because of habitat differences of dam's home ranges (7). Hatchlings that survived in the field to maturity were recaptured at the beginning of next year's reproductive season and, because of high recruitment rates, we were able quantify maternal effects, heritabilities, and genetic correlations by comparing traits between parental and offspring generations (14).
Measuring Immune Function.
We performed immunization experiments in the lizards to asses immune function. The rationale behind these experiments is that by challenging the immune system with a novel antigen (typically a protein), one can get an estimate of an individual's ability to elicit an immune response toward a novel pathogen (17–19). Strength of individual immune responses (e.g., antibody responses) reflects the ability to mount a response against novel antigens from microorganisms and is therefore correlated with general resistance to diseases and parasites (18, 19).
Early in March during 1998 and 1999, we immunized female lizards with 50 μl of a vaccine containing a novel antigen (tetanus toxoid) that induced their primary antibody responses (vaccine provided by Statens Serum Institut, Copenhagen). Females were then revaccinated (boosted) in the field, 5–56 days after the primary immunization (mean = 29.69 days; SE = ± 1.391). Later in the season, we caught the gravid females and brought them into the laboratory to obtain eggs (see Study Organism, General Field, and Laboratory Work). Females were bled in conjunction with these captures (3–35 days after boosting; mean = 12.55; SE = ±1.139). Blood samples were taken from the postorbital sinus by using two to three 50-μl hematocrit tubes that were kept on ice for 4–6 h before centrifugation. Samples were centrifuged and plasma was extracted and frozen at −20°C until later analysis.
Antibody responses toward the tetanus antigen in the plasma samples were analyzed by using a standard ELISA (with a rabbit-anti-lizard immunoglobulin antibody as secondary antibody), with a modified laboratory protocol that was originally developed for birds (see ref. 17 for methodological details). Antibody titers were expressed in units/ml, by using a standard pool of plasma as reference (17). Antibody responses were not significantly influenced by time from immunization to boosting (F1,34 = 1.894; P = 0.18) or by time from boosting to bleeding (F1,34 = 0.079; P = 0.78). Before analysis, we removed the effects of year and laying date on antibody responses, and hence residual antibody responsiveness is shown (e.g., Fig. 1).
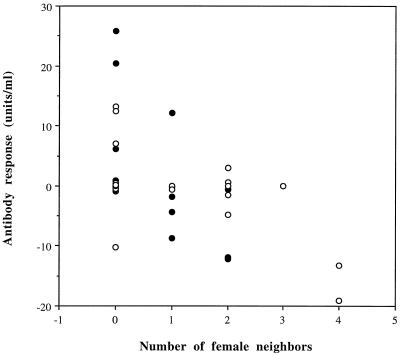
Figure 1.
Antibody responsiveness as a function of number of female neighbors surrounding female lizards. Effects of year and laying date of the focal female were removed in separate regression analyses before this model (residual variation is shown). ●, Orange females (n = 19); ○, yellow females (n = 29). The decline in antibody responsiveness was significant for both female morphs (see Results).
Cell-mediated immune responsiveness was measured by using delayed-type hypersensitivity (DTH) tests, a standard assay of cell-mediated immune responsiveness in veterinary medicine (18, 19). We injected the lizard's right and left front foot pads with phytohemagglutinin solution (PHA-P, Sigma) and sterile PBS, respectively, and measured the swellings with digital calipers (to the nearest 0.01 mm) 20–24 h after the injections. DTH index was calculated as the difference in swellings between the left foot pad (control: PBS only) and the right foot pad (50 mg of PHA-P dissolved in 5 ml PBS).
Statistical Analysis.
We used parametric statistics (ANOVAs and multiple regression) to analyze the effects of female morphotype and number of neighbors on immune function and survival. In parametric statistics it is assumed that residuals are normally distributed and that treatment effects are randomized (20). These assumptions may be violated, for instance, when the dependent variable (survival) is bimodal or when “treatments” (e.g., number of female neighbors) may not be completely randomized between the two morphs. We therefore checked and confirmed the significance of some of the P values we obtained from the parametric analyses by using the software RESAMPLING STATS (21). The original data sets were resampled (with replacement) in 1,000 bootstrap replications, and confidence limits (CLs) of parameters (regression slopes) were estimated (21). Results from the parametric analyses and the resampling procedure were identical, unless otherwise stated. In addition, we visualized the fitness functions between survival and immune responsiveness by using the nonparametric cubic-spline regression technique (22).
We calculated heritability of immune responsiveness and the genetic correlation between antibody responsiveness and female throat color by using parent–offspring regression analysis and by calculating the reciprocal cross-covariances of trait values measured in free-living dams and their free-living daughters (14, 23, 24). When estimating the genetic correlations, we scored yellow females as “0” and orange females as “1,” following our previous approach (14). Although the quantitative genetic estimates obtained from comparisons of mothers and their daughters may have been somewhat inflated by various nongenetic maternal effects (23–24), the design of our experimental field study (e.g., egg size manipulations and randomized releases of hatchlings) controlled for many of these confounding sources of variation (see Study Organism, General Field, and Laboratory Work).
Results
Antibody responsiveness declined significantly with increased number of neighbors, among both yellow and orange females (Fig. 1; F1,46 = 11.505, P = 0.0014, n = 48). Solitary females (no neighbors) had stronger antibody responses than females with 1, 2, 3, or 4 neighbors, indicating that the presence of many neighbors leads to immunosuppression through density-dependent territorial interactions and social crowding. Although antibody responsiveness declined with increased number of neighbors among both female morphs, the effect of local crowding was stronger in orange females (Fig. 1).
The stronger effects of crowding on fitness in orange than yellow females in our population (14) and the fact that orange females may be hyperdispersed relative to each other (unpublished observations), may have created spatial bias in the social environment that each morph encountered in nature (see Fig. 1). Because this may also have affected the results from the parametric statistical analyses, we resampled CLs of regression coefficients (21) when comparing how the two morphs responded to changes in the density environment.
Antibody responsiveness declined significantly with increased number of neighbors both among yellow females (99.9% CLs for regression slopes (units·ml−1·neighbors−1): −4.7606 to −0.1490, n = 29, P < 0.001) and among orange females (99% CLs: −11.8310 to −0.9287, n = 19, P < 0.01). The decline in antibody responsiveness with increased number of neighbors was significantly steeper among orange than among yellow females (Morph × Number of female neighbors: 95% CLs: −7.4679 to −0.5723, n = 48, P < 0.05).
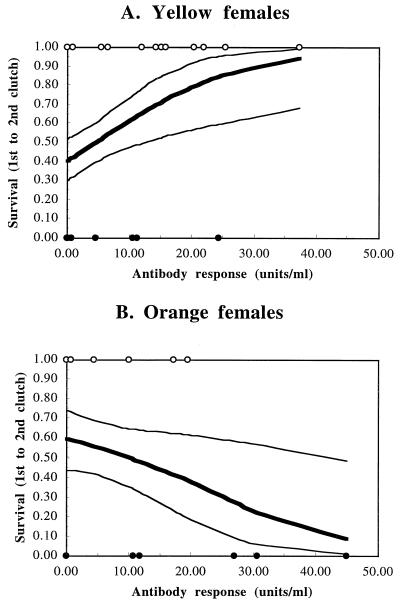
We recorded female survival from the first to the second clutch in the field and investigated the effects of female morphotype, number of neighbors, and antibody responsiveness. The fitness functions (22) linking survival to variation in immune responsiveness were different in sign for the two female color morphs: survival increased with increasing immune responsiveness among yellow females, whereas there was a negative relationship among orange females (Fig. 2). The relationship between antibody responsiveness and survival was positive in yellow females, and the effect was not confounded by any density effects unrelated to immune function (multiple regression using randomization procedure; Antibody responsiveness: 99% CLs, 0.0024 to 0.0346, n = 31, P < 0.01; Number of female neighbors: 95% CLs, −0.1133 to 0.0930, n = 31, P > 0.05). The negative relationship between survival and antibody responsiveness in orange females approached, but did not reach, significance, perhaps because of lower sample sizes of oranges (Antibody responsiveness: 95% CLs, −0.0308 to 0.0476, n = 18, P = 0.09; Number of female neighbors: 95% CLs, −0.3128 to 0.3007, n = 18, P > 0.05).
Figure 2.
Fitness functions (survival from first to second clutch) for yellow (A) and orange (B) female lizards as a function of antibody responsiveness. Fitness functions show probability of survival (died = 0; survived = 1) and were visualized by using the nonparametric cubic-spline regression technique (22). Thick line: mean survival probability. Upper and lower thin lines: ± SEs, calculated from 1,000 bootstrap replications. ○, Survivors; ●, nonsurvivors. The slopes of the fitness functions differ significantly between the two female morphs (see Results), reflecting correlational selection (25–28) between female morphotype and antibody responsiveness.
The slopes of the two fitness functions differed significantly between orange and yellow females (Morph × Antibody response: 99% CLs, −0.0597 to −0.0016; P < 0.01), but the number of neighbors had no effect (95% CLs: −0.1076 to 0.0922, P > 0.05). This finding suggests that variation in immune function affected survival in both morphs, albeit in different ways, and that these effects were not confounded by other density-dependent effects unrelated to immune function. More generally, the significantly different slopes of the two fitness functions is an example of morph-specific selection (“correlational selection”) between female morphotype and antibody responsiveness (see also refs. 14 and 25–27 for other examples of correlational selection). The correlational selection gradient (γ; ref. 26) between female throat color and antibody responsiveness was −0.365 (28).
Correlational selection of the kind we have demonstrated will build up a genetic correlation between two heritable characters, even if these traits are governed by separate loci (23–27). Both throat coloration (12, 14, 16) and variation in immune responsiveness are highly heritable traits in our population (heritability for antibody responsiveness: h2 = 0.88; SE = ±0.291; F1,40 = 9.223; P = 0.0042). In the heritability analysis of dams' and their daughters' antibody responsiveness, there was no significant effect of egg mass from which offspring hatched or maternal mean egg mass (P = 0.30 and P = 0.69, respectively). Because the local density suppressed immune function (Fig. 1), heritability may be inflated if parental and offspring generations would occupy similar density environments. However, there was no evidence for any such effect; if anything, the relationship between a daughter's local density and her mother's density was slightly negative, although not significantly (β = −0.35, F1,19 = 2.677; P = 0.12).
Even if female morphotype and immune responsiveness are likely to be under control by different sets of loci, correlational selection can lead to linkage disequilibrium of the alleles between these different loci, similar to that reported for antipredator and coloration traits in previous studies of color-polymorphic snakes (25–27). Hence, a genetic correlation between female morphotype and immune responsiveness would be formed with yellow dams producing daughters with relatively high immune responsiveness, and orange dams producing daughters with relatively low immune responsiveness.
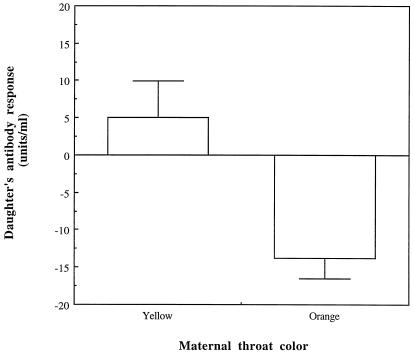
Indeed, we found evidence for such a genetic correlation when we compared the immune responsiveness between the daughters of orange and yellow females (Fig. 3). The average genetic correlation (±SE), based on reciprocal cross-covariances (23, 24), between female throat color and antibody responsiveness was −1.36 (± 0.55). In addition to the effects of maternal throat coloration, antibody responsiveness was also significantly affected by mean egg mass of the dam and cell-mediated immune response of the dam (dam's mean egg mass: F1,26 = 5.386; P = 0.028; dam's DTH: F1,26 = 8.943; P = 0.0060). Genetic correlations between humoral (antibody responsiveness) and cell-mediated immune responses (DTH) have previously also been found in quantitative genetic studies and laboratory selection experiments on immunological traits in domestic vertebrates such as poultry (29, 30). The reciprocal relationship between a daughter's throat score and maternal antibody responsiveness was also significant (dam's antibody responsiveness: F1,16 = 11.731; P = 0.0035; dam's mean egg mass: F1,16 = 6.896; P = 0.018; dam's DTH: F1,16 = 0.003; P = 0.95).
Figure 3.
Antibody responsiveness of daughters in relation to throat color of dams (maternal morphotype). Data show antibody responsiveness of daughters that recruited to the breeding population (1999) in relation to the throat color of their mothers breeding in the preceding year (1998). Yellow females produced daughters that had significantly higher antibody responses than the daughters of orange female (F1,28 = 4.880; P = 0.035). The effects of maternal mean egg mass and DTH response were removed before this analysis (residual variation is shown in the figure).
Discussion
The results in this study indicate that density-dependent social interactions from many neighbors had a negative effect on immune responsiveness of side-blotched lizards. The effect of crowding also differed between two heritable color morphs, and variation in immune function seemed to affect fitness in different ways in the two color morphs. This difference in sign of the fitness functions promotes correlational selection, which presumably is responsible for the genetic correlation between female throat color and antibody responsiveness. The results indicate that immune function is density-dependent, and that immune function and female morphotype jointly influence fitness. Genetic differences in sensitivity to density fluctuations have been recently suggested to play an important role in population ecology, and could even maintain genetic polymorphisms for immunological traits (3, 4). To verify the causality of these relationships, one would need to experimentally manipulate both variation in local densities and individual variation in immune function.
The proximate causes behind the negative relationship between local density and immune function (Fig. 1) may be due to increased levels of corticosteroids in high-density environments and concomitant immunosuppressive effects of these hormones (refs. 3 and 4; T.C., B.S., E.S., and J. Wingfield, unpublished data). Alternatively, both exposure and transfer of natural pathogens may increase in crowded neighborhoods. Such natural pathogens may divert the attention of the immune system, resulting in reduced antibody responses toward the novel antigen that we used in this study. These two scenarios are not mutually exclusive, and both the corticosteroid hypothesis and the infection hypothesis predict immunosuppression and increased risks of acquiring novel pathogens or parasites in high-density environments.
The positive relationship observed between immune responsiveness and survival in yellow females (Fig. 2A) suggests that there is selection for enhancement of antibody responsiveness in this morph. Preliminary field observations indicate that side-blotched lizards suffer from both ectoparasites, such as mites, and blood-borne parasites such as Plasmodium (E.S. and T. Mappes, unpublished observations). Ecto- and endoparasites may prove to be the factor that selects for enhanced immune responsiveness in yellow females (Fig. 2A).
Models of host–parasite co-evolution suggest that host resistance mechanisms, like immune defense, not only are beneficial but also are costly to maintain (31). The benefits of resistance in the presence of pathogens and parasites will make such costs difficult to detect. However, in the absence of parasites, immunity is expected to result in net fitness costs, particularly in competitive and harsh environments (32–33). Recent studies on invertebrates have indeed demonstrated not only that immune responses and other forms of host resistance are costly, but also that costs are accelerated during high workloads (5) and in competitive and crowded environments (32). The negative relationship we detected between survival and immunity among orange females (Fig. 2B) and orange female's higher sensitivity to social crowding (Fig. 1) are consistent with such costs of immunity. Orange females had markedly reduced fitness when surrounded by other orange females (14) and appear to be hyperdispersed relative to other orange females in our study area (unpublished observations). These results indicate that orange females are highly sensitive to social crowding, either because of elevated aggression from territorial neighbors or because they suffer from increased risks of parasites or pathogen transfer in high-density environments. Interestingly, antibody responses among orange females decreased more steeply with increased crowding than among yellow females (Fig. 1). This response could be part of an adaptive strategy among orange females to avoid eliciting costly immune responses in harsh high-density environments. Finally, morphs may also differ in their preferences for certain habitats or density environments, and such morph-specific environment preferences could in turn have led to differential exposure and pressure from parasites and pathogens in the two morphs.
The high heritability and genetic correlations found in this study and other studies of our population (14, 16) do not seem to be inflated by nongenetic maternal effects, because our experimental manipulations controlled for many confounding environmental variables (see Materials and Methods). Furthermore, in our studies we have found that sire–son and sire–daughter regressions using molecular paternity data gave similar results as dam–offspring regressions, which is of interest because sire–offspring comparisons are free from maternal effects (16, 23–24). The high heritability for throat color and the strong genetic correlations between color and other traits is consistent with the existence of a gene of major effect that both controls throat color and has pleiotropic effects on suites of other life history traits (14). Endocrine genes are good candidates in this regard, because hormones typically have multiple effects on several different traits, including throat coloration (13–15).
However, it is also likely that female morphotype and antibody responsiveness are at least partly governed by separate genes, given the large number of loci that contribute to immune function (34). Even if this is the case, correlational selection (26, 28) between these traits will, in the long run, lead to the buildup of adaptive linkage disequilibrium that will result in a genetic correlation between female throat color and antibody responsiveness (Fig. 3). Genetic correlations that are built up and preserved by natural or sexual selection may be eroded by recombination, but if selection is strong enough relative to recombination, the correlation can be maintained over a considerable time span (23–27). Because the female morphs are involved in an intrinsic genetic cycle driven by frequency-dependent selection (14), correlational selection on the morphs will continually be reinforced and chronic in the long run.
Correlational selection and its effects on genetic correlations have rarely been demonstrated in natural populations, but could be a general phenomenon that explains striking differences in morphology, physiology, and behavior of heritable morphs in this and other species (12–16, 25–27). Although our data are from female lizards, similar genetic correlations that are built up and preserved by correlational selection play an important role in theoretical models of sexual selection in males (35, 36). Recent empirical studies have demonstrated such genetic correlations between condition, male attractiveness, and ornamental characters (37, 38). Our results indicate that a genetic correlation between a signaling trait (throat coloration) and a condition trait (immunological condition) need not be restricted to the male sex and hence could be a more general phenomenon in natural populations. The genetic correlation arises because correlational selection (14, 26, 28) couples genetic variation in both throat color and immune function through these two trait's interactive effects on fitness (cf. “pure epistasis models”; ref. 36). Throat color plays an important role in sexual selection and the maintenance of alternative reproductive strategies in both male and female side-blotched lizards (12, 14, 16), and our results suggest that it could reliably provide information about immunological condition. The genetic correlations between throat color and other traits are likely to be the result of selection for different optimal character combinations in the morphs. Our future goal will be to further investigate the selectively driven buildup and maintenance of genetic correlations between throat color and other fitness-related characters (e.g., clutch size and egg mass, see ref. 14; for immune function, see above).
Acknowledgments
We are grateful to Jep Agrell, Thomas F. Hansen, Per Lundberg, David Richardson, and three anonymous reviewers for discussions and constructive criticisms on early drafts of the manuscript, and to Gwynne Corrigan, Ivy Gluesenkamp, John Lindgren, Melissa Mark, Tim Martin, and Yoni Brandt for assistance in the laboratory and field. We also wish to thank Leo Ortiz and Martha Zuniga for access to plate readers, Rudy Ortiz and Gerry Zeghers for technical advice regarding ELISAs, and Claus Koch for providing secondary antibody and reagents. Research was funded by National Science Foundation Grant DEB 9629793 (to B.S. and R. Repasky), postdoctoral research grants from The Swedish Foundation for International Cooperation in Research and Higher Education (Sweden) and the Fulbright Commission (to E.S.), and subsequently regular research grants from Swedish Forestry and Agricultural Research Council and the Swedish National Science Research Council (to E.S.).
Abbreviations
- DTH
delayed-type hypersensitivity
- CL
confidence limit
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lack D. The Natural Regulation of Animal Numbers. Oxford: Clarendon; 1954. [Google Scholar]
- 2.Sutherland J. From Individual Behaviour to Population Ecology. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 3.Lochmiller R L, Dabbert C B. Trends Comp Biochem Physiol. 1993;1:823–855. [Google Scholar]
- 4.Lochmiller R L. Oikos. 1996;76:594–602. [Google Scholar]
- 5.Moret Y, Schmid-Hempel P. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 6.Nunn C L, Gittleman J L, Antonovics J. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. [DOI] [PubMed] [Google Scholar]
- 7.Sinervo B. In: Maternal Effects as Adaptations. Mousseau T A, Fox C W, editors. Oxford: Oxford Univ. Press; 1998. pp. 288–306. [Google Scholar]
- 8.DeNardo D, Sinervo B. Horm Behav. 1994;28:53–65. doi: 10.1006/hbeh.1994.1005. [DOI] [PubMed] [Google Scholar]
- 9.DeNardo D, Sinervo B. Horm Behav. 1994;28:273–287. doi: 10.1006/hbeh.1994.1023. [DOI] [PubMed] [Google Scholar]
- 10.Sinervo B, DeNardo D. Evolution (Lawrence, Kans) 1996;50:1299–1313. doi: 10.1111/j.1558-5646.1996.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 11.Svensson E, Sinervo B. Evolution (Lawrence, Kans) 2000;54:1396–1403. doi: 10.1111/j.0014-3820.2000.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 12.Sinervo B, Lively C M. Nature (London) 1996;380:240–243. [Google Scholar]
- 13.Moore M C, Hews D K, Knapp R. Am Zool. 1998;38:133–151. [Google Scholar]
- 14.Sinervo B, Svensson E, Comendant T. Nature (London) 2000;406:985–988. doi: 10.1038/35023149. [DOI] [PubMed] [Google Scholar]
- 15.Sinervo B, Miles D B, Frankino W A, Klukowski M, DeNardo D F. Horm Behav. 2000;38:222–233. doi: 10.1006/hbeh.2000.1622. [DOI] [PubMed] [Google Scholar]
- 16.Zamudio K, Sinervo B. Proc Natl Acad Sci USA. 2000;97:14427–14432. doi: 10.1073/pnas.011544998. . (First Published December 5, 2000; 10.1073/pnas.011544998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svensson E, Råberg L, Koch C, Hasselquist D. Funct Ecol. 1998;12:912–919. [Google Scholar]
- 18.Luster M I, Portier C, Pait G, Rosenthal G J, Germolec D R, Corsini E, Blaylock B L, Pollock P, Kouchi Y, Craig W, et al. Fundam Appl Toxicol. 1993;21:71–82. doi: 10.1006/faat.1993.1074. [DOI] [PubMed] [Google Scholar]
- 19.Lochmiller R L, Vestey M R, Boren J C. Auk. 1993;110:503–510. [Google Scholar]
- 20.Sokal R R, Rohlf F J. Biometry. New York: Freeman; 1995. [Google Scholar]
- 21.Simon J L. Resampling Stats Software and User's Guide, 1973–2000. Arlington, VA: Resampling Stats; 2000. [Google Scholar]
- 22.Schluter D. Evolution (Lawrence, Kans) 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 23.Falconer D S, Mackay T F C. Introduction to Quantitative Genetics. Essex, U.K.: Longman; 1996. [Google Scholar]
- 24.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 25.Brodie E D., III Nature (London) 1989;342:542–543. doi: 10.1038/342542a0. [DOI] [PubMed] [Google Scholar]
- 26.Brodie E D., III Evolution (Lawrence, Kans) 1992;46:1284–1298. doi: 10.1111/j.1558-5646.1992.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 27.Brodie E D., III Evolution (Lawrence, Kans) 1993;47:844–854. doi: 10.1111/j.1558-5646.1993.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 28.Svensson, E., Sinervo, B., Comendant, T. (2001) Evolution (Lawrence, Kans.), in press.
- 29.Cheng S, Lamont S J. Poultry Sci. 1988;67:989–995. doi: 10.3382/ps.0670989. [DOI] [PubMed] [Google Scholar]
- 30.Cheng S, Rothschild M F, Lamont S J. Poultry Sci. 1991;70:2023–2027. doi: 10.3382/ps.0702023. [DOI] [PubMed] [Google Scholar]
- 31.Frank S A. Evol Ecol. 1991;8:74–94. [Google Scholar]
- 32.Kraaijeveld M, Godfray H C J. Nature (London) 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- 33.Råberg L, Grahn M, Hasselquist D, Svensson E. Proc R Soc London Ser B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roitt I, Brostoff J, Male D. Immunology. London: Mosby; 1998. [Google Scholar]
- 35.Iwasa I, Pomiankowski A, Nee S. Evolution (Lawrence, Kans) 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 36.Kirkpatrick M, Barton N H. Proc Natl Acad Sci USA. 1997;94:1282–1286. doi: 10.1073/pnas.94.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldon B C, Merilä J, Qvarnström A, Gustafsson L, Ellegren H. Proc R Soc London Ser B. 1997;264:297–302. [Google Scholar]
- 38.Johnsen A, Andersen V, Sunding C, Lifjeld J T. Nature (London) 2000;406:296–299. doi: 10.1038/35018556. [DOI] [PubMed] [Google Scholar]