Abstract
Purpose
To describe different patterns of macular pigment (MP) seen in fluorescence lifetime imaging ophthalmoscopy (FLIO) and to analyze ex vivo fluorescence characteristics of carotenoids.
Methods
A total of 31 eyes of young healthy subjects, 4 eyes from patients with albinism, 36 eyes with macular telangiectasia type 2 (MacTel), 24 eyes with retinitis pigmentosa, and 1 eye with a macular hole were included in this clinic-based, cross-sectional study. All subjects underwent Heidelberg Engineering FLIO and MP measurements (dual-wavelength autofluorescence). Fundus autofluorescence (FAF) lifetimes of a 30° retinal field were detected in two spectral channels (SSC: 498–560 nm; LSC: 560–720 nm), and amplitude-weighted mean fluorescence lifetimes (τm) were calculated. Additionally, autofluorescence lifetimes of known dilutions of lutein and zeaxanthin were measured in a cuvette in free- and protein-associated states.
Results
MP shows a significant inverse correlation to foveal FAF lifetimes measured with FLIO (SSC: r = −0.608; P < 0.001). Different distribution patterns can be assigned to specific disease-related changes. Two patients with albinism, who did not have MP, were found to be missing short FAF lifetimes. In solvent, lutein and zeaxanthin show very short autofluorescence lifetimes (∼50–60 ps; SSC), as do their respective binding proteins (∼40–50 ps; SSC). When combining carotenoids with their specific binding proteins, the decay times shift to longer means (∼70–90 ps; SSC).
Conclusions
This study expands upon previous findings of an impact of MP on short FAF lifetimes by describing ex vivo autofluorescence lifetimes of carotenoids and different in vivo autofluorescence patterns that can be associated with certain diseases.
Keywords: FLIO, fluorescence lifetime imaging, macular pigment, macular telangiectasia type 2, albinism
Fluorescence lifetime imaging ophthalmoscopy (FLIO) is a novel method to investigate and monitor changes in the retina.1,2 By measuring the fundus autofluorescence (FAF) lifetimes, which are independent of the quantum efficiency and concentration of retinal fluorophores, FLIO provides an additional dimension to information obtained from standard FAF intensity images.3,4 FLIO is a helpful tool to assess changes at the human fundus in vivo, especially in the diagnosis of macular telangiectasia type 2 (MacTel).5 In AMD, FLIO also seems to show specific changes in early disease stages.6 Moreover, FLIO may be beneficial in monitoring many other diseases, such as the closure of macular holes after surgery, recessive Stargardt disease, central retinal artery occlusion, central serous chorioretinopathy, and diabetes mellitus.7–12
Many retinal diseases are associated with changes in the macular pigment (MP).13–19 Therefore, characterizing patterns and changes of MP in retinal diseases may be beneficial in monitoring the progression or severity of a disease. Imaging of MP with FLIO has previously been described in normal subjects and in patients with macular holes; the MP levels and distributions were compared with single-wavelength reflectometry.20 However, MP reflectometry results may deviate from other MP measurement methods.21 Here, we expand the spectrum of diseases in which FLIO can image MP to include MacTel, albinism, and retinitis pigmentosa (RP), and we compare these levels and distributions in diseased and normal maculas with MP images obtained by dual-wavelength autofluorescence imaging (AFI), an MP imaging method that often works well even in the presence of significant macular pathology.22 Additionally, we compare in vivo MP fluorescence lifetimes with ex vivo fluorescence lifetimes of lutein (L) and zeaxanthin (Z) dissolved in detergent and associated with their specific binding proteins to gain insights into the physical state of the macular carotenoids in the living human's fovea.
Methods
This cross-sectional study was approved by the University of Utah's Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained prior to all investigations. All patients and healthy subjects were examined between March and June 2017 at the Moran Eye Center. Each underwent a FLIO and a dual wavelength AFI MP measurement after pupil dilation. No topical fluorescein was used to check intraocular pressure, and all study procedures were completed prior to any fluorescein angiography.
Macular Pigment Imaging with AFI
The total amount of macular pigment (macular pigment volume [MPV]) was measured by dual wavelength AFI (Heidelberg MultiColor Spectralis, Heidelberg Engineering, Heidelberg, Germany), which has been previously described.21 A recent study showed this method to be highly reproducible and only minimally influenced by pathology within the eye and macula, such as cataracts, diabetes, and intermediate AMD, especially when measuring macular pigment volumes with a zero reference point set at 9° of eccentricity from the fovea.22 Although FLIO images are not restricted to these 9°, it is helpful to compare both measurements to fully understand the benefits of FLIO. ImageJ was used to show the three-dimensional distribution of MP measured with the dual-wavelength AFI method. An optical coherence tomography (OCT) scan (Heidelberg Spectralis, HRA+OCT; Heidelberg Engineering) was used to obtain cross-sectional images of the macular region when macular pathology was suspected.
FLIO-Setup and Image Acquisition
Based on an OCT imaging platform (Heidelberg Engineering), FLIO records FAF lifetimes from a 30° retinal field in vivo relying on the principle of time-correlated single photon counting.23,24 The detailed setup and safety of FLIO have been described previously.20,23,25 Briefly, FAF is excited by a pulsed diode laser (473 nm, 80 MHz). Two hybrid photo-multipliers (HPM-100-40, Becker & Hickl GmbH, Berlin, Germany) detect fluorescence photons, resulting in two separate spectral channels: the short spectral channel (SSC; 498–560 nm) and the long spectral channel (LSC; 560–720 nm). A high-contrast confocal infrared reflectance (IR) image for eye tracking is included. A photon arrival histogram, representing the probability density function of the decay process, is based on the detection of photons in 1024 time channels. To ensure reliable image quality, at least 1000 photons were recorded for each pixel as a minimal signal threshold. Typically, 2 minutes of acquisition time were required for each eye.
The fluorescence data were analyzed using commercial software (SPCImage 4.4.2; Becker & Hickl GmbH, Berlin, Germany). By calculating the least-square fit of a series of three exponential functions, the fluorescence decay was approximated, and 3 × 3 pixel binning was used for the purpose of noise reduction. The amplitude weighted mean fluorescence decay time (τm), was used for further analysis, representing the average of the 3 time constants from the fit, weighted by their amplitude. Further details have been described elsewhere.20,24
The FLIMX software was used for all FAF lifetime analyses over certain regions and to illustrate the FAF lifetimes.26 This software is documented and freely available for download online under the open source BSD-license (http://www.flimx.de). Of special interest was a region 1 mm in diameter centered at the fovea, which we refer to as the “central area” or “Area C”.
Carotenoid Measurements Ex Vivo
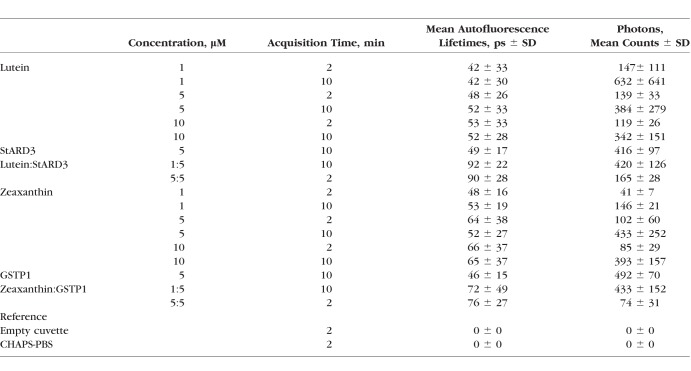
For ex vivo carotenoid measurements, crystals of zeaxanthin (DSM, Kaiseraugust, Switzerland) and lutein (Kemin Health, Des Moines, IA, USA) were first dissolved in methanol, and their stock concentrations were determined by spectrophotometry using a commercial device (SmartSpec 3000; Bio-Rad Laboratories, Hercules, CA, USA) and adjusted to be ∼100 μM. Next, the carotenoid stock solutions were diluted into 1X PBS containing 8 mM of 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate (CHAPS) detergent. Zeaxanthin's specific binding protein, glutathione S-transferase Pi isoform (GSTP1), and lutein's specific binding protein, steroidogenic acute regulatory domain protein 3 (StARD3), were dissolved in the same CHAPS-PBS buffer with a final concentration of 1 mg/mL (∼40 μM).27,28 The relevant protein-carotenoid complexes were prepared by mixing carotenoid and protein at a 1:5 ratio by a 1:100 dilution of the 100 μM carotenoid stock solution in methanol into 5 μM protein solutions in 8 mM CHAPS-PBS and at a 1:1 ratio by a 1:20 dilution of the carotenoid stock solution. For each ex vivo FLIO measurement, the solutions were placed in 1-2 mm path-length quartz cuvettes, which were then placed in a special FLIO cuvette holder attachment. For the investigations of ex vivo carotenoid fluorescence, a bi-exponential approach resulted in the most accurate fit and was therefore utilized here for data analysis. Acquisition times of 2 and 10 minutes were used, and average photon counts were recorded. In order to protect carotenoids from light exposure, measurements were performed in darkness. Mean autofluorescence lifetimes from each measurement were obtained from a rectangular region of the cuvette.
Statistical Analysis
For all statistical analyses, statistical software (SPSS 21; SPSS, Inc., Chicago, IL, USA) was employed. A Pearson correlation was used to correlate FAF lifetime means to MP volume. Data were checked to confirm normal distributions. All reported results are mean ± SD.
Results
Subjects
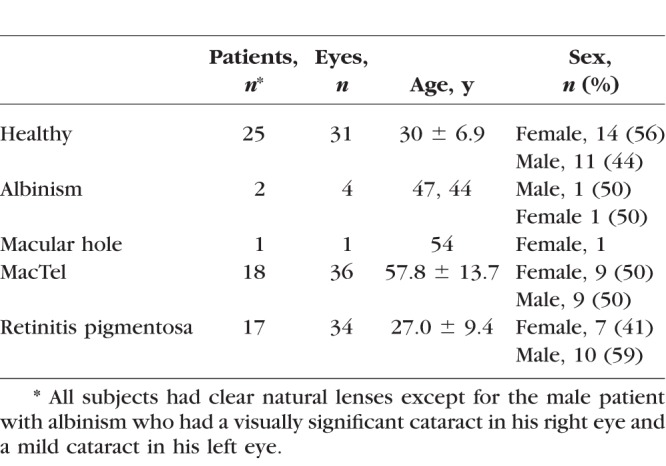
We included 31 eyes of 25 healthy, young subjects (mean age 30.0 ± 6.9 years; range, 19–40 years) in this study. None of these subjects reported supplementation with lutein or zeaxanthin. We also included 4 eyes of 2 patients with albinism, 1 patient with a full-thickness macular hole, 18 patients with MacTel, and 12 patients with RP. Neither the two patients with albinism, nor the patient with the macular hole were taking any carotenoid supplementation. Of the MacTel patients, two took 10 mg zeaxanthin daily, and five reported taking the AREDS2 formula containing 10 mg lutein + 2 mg zeaxanthin daily for more than 1 year. Two of the RP patients supplemented with 10 mg lutein + 2 mg zeaxanthin daily. All subjects had natural lenses with no significant cataracts except one patient with albinism. Table 1 provides further information about each group of patients.
Table 1.
Investigated Subjects
Different Macular Pigment Patterns
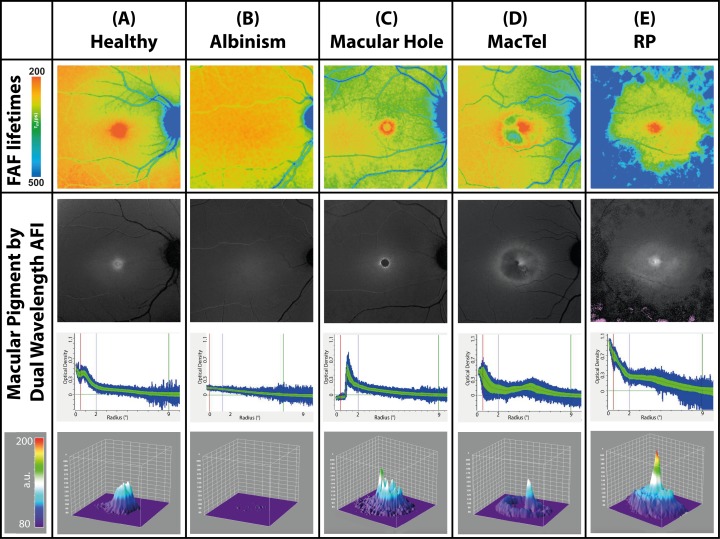
At an excitation of 488 nm, the fluorescence of carotenoids shows an emission peak between 500 and 550 nm.29 Our excitation at 473 nm is similar to the previously described wavelength and should have comparable fluorescence emission properties. Based on this described emission spectrum of carotenoids and previous FLIO investigations, the short wavelength spectral channel (SSC, 498–560 nm) was used to investigate patterns of MP.20,29 Various retinal diseases show specific aberrant localizations of MP relative to controls. Figure 1 gives an overview over the investigated pathologies: one FLIO image (SSC, representing τm) as well as three different images to present MP obtained with AFI are presented for each of these five subjects.
Figure 1.
Mean FAF lifetimes of the short spectral channel (SSC: 498–560 nm; top row) and dual wavelength AFI-MP images in a healthy eye and different retinal diseases (bottom three rows). Row 2: En face image of macular pigment distributions obtained by dual wavelength AFI. Row 3: Decremental slope of MP decline as a function of distance from foveal center. Row 4: Three-dimensional projection of MP. Only the MacTel patient shown in this figure was on supplementation (10 mg zeaxanthin daily).
FLIO and Macular Pigment in Healthy Eyes
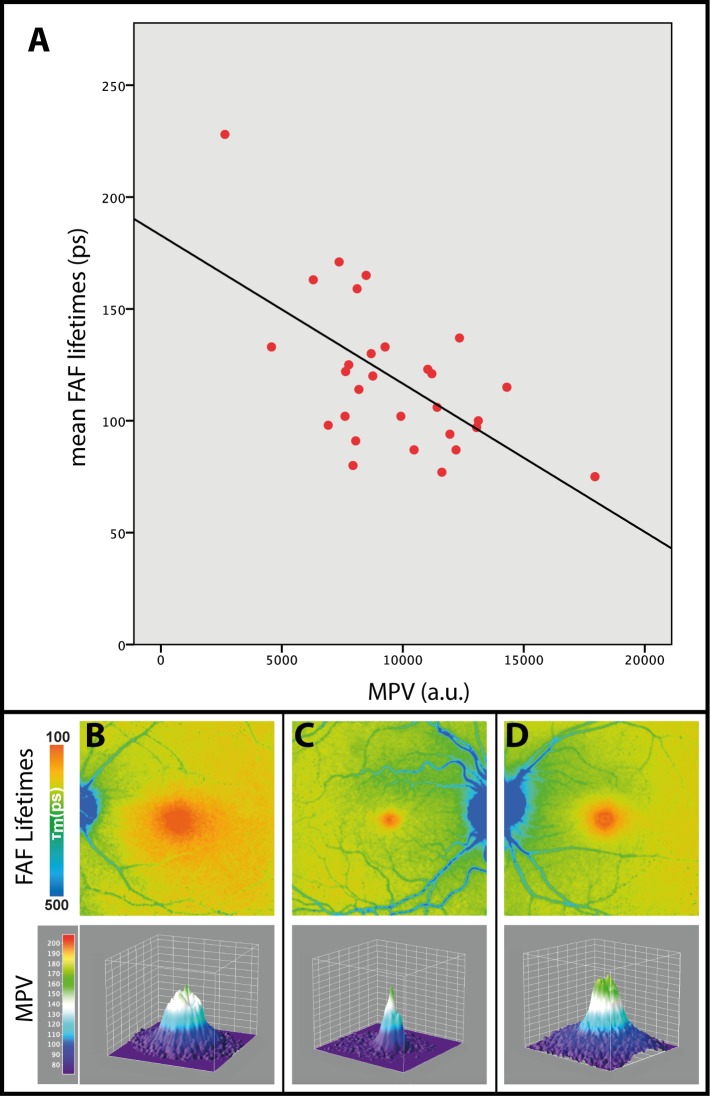
In healthy eyes, MP can be assigned to the central spot of short mean FAF lifetimes at the fovea, which is depicted in red color. A representative image is presented in Figure 1A. Mean FAF lifetimes of area C in healthy eyes ranged from 75 to 228 ps in the SSC (mean 120 ± 32 ps) and from 145 to 241 ps in the LSC (mean 183 ± 22 ps). We focused on the SSC, as MP has a stronger impact within the 498 to 560 nm wavelength range. The MPV ranged from 2647 to 17942 a.u. (mean 9615 a.u. ± 3006 a.u.) and showed a significant inverse correlation to FAF lifetimes of the SSC with r = −0.608 and P < 0.001. The correlation is shown in Figure 2A.
Figure 2.
Pearson correlation of mean FAF lifetimes within the short spectral channel (498–560 nm; SSC) with MPV: r.0608, P < 0.001 (A) as well as three different MP distribution patterns in three healthy subjects: B, broad; C, cone; and D, ring-like. En face FAF lifetime images as well as three-dimensional projection of MPV are shown.
Comparing 31 healthy eyes, different patterns can be found within the short autofluorescence lifetimes at the macular region. For the six subjects who had both eyes measured, the left and the right eye were very similar in pattern and MP level. MP can be located in a narrow cone, a broad plateau, or a ring-like shape, resulting in mean FAF lifetimes of the same shape. These patterns are more apparent in the SSC. In our 31 investigated normal eyes, 9 showed a cone, 16 a broad plateau, and 6 a ring-like shape. One example of each with its corresponding MP is shown in Figures 2B through 2D.
FLIO and Macular Pigment in Albinism
Patients with albinism often show a reduced foveal pit.30,31 This typically results in reduced or no detectable macular pigment.32,33 Our patients with albinism showed corresponding reduced or no detectable short FAF lifetimes within the central area. A representative image is depicted in Figure 1B.
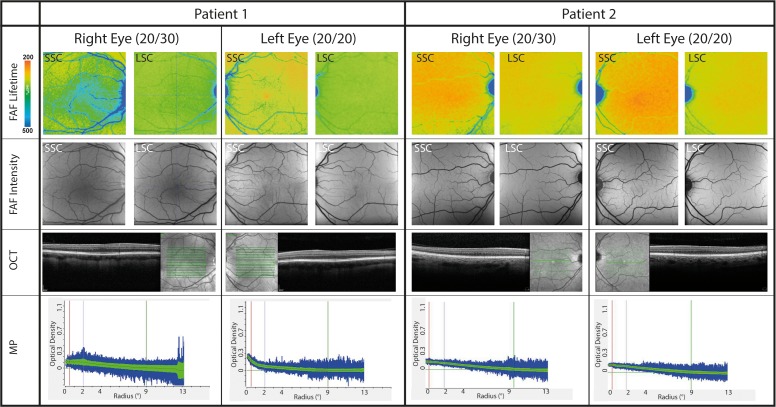
Figure 3 focuses on the findings of our two patients with albinism, both of whom showed absence of a foveal pit. Both patients were only mildly affected, with photophobia and lighter skin tone than the rest of their family members, and FAF intensity imaging shows vascular intrusion through the avascular zone. Both had good visual acuity, and neither of them had nystagmus. Neither of the patients has been genotyped, but even without the genotype, we were able to clinically confirm albinism in both patients electrophysiologically because pattern onset visual evoked potentials (VEP) across the occipital scalp showed reversal of VEP components, which is indicative of optic misrouting associated with albinism. In addition to FLIO images, we show OCT images, FAF intensity images, and the AFI MPV measurements for both eyes in Figure 3. The visual acuities of both patients were 20/30 in the right eye and 20/20 in the left eye. On examination, the first patient was found to have a 1+ nuclear sclerotic cataract in his right eye and a trace nuclear sclerotic cataract in his left eye, while the second patient had clear lenses.
Figure 3.
Mean FAF lifetime and intensity images of two spectral channels (SSC: 498–560 nm; LSC: 560–720 nm) as well as OCT and macular pigment optical density of two patients with albinism. Both eyes are shown. patient 1: male, 47 years old; patient 2: female, 42 years old.
FLIO and Macular Pigment in Macular Hole
Macular holes were intensively studied in a previous study.11 We only included one eye to present in Figure 1C. No further statistical analysis was performed. Short FAF lifetimes are not found inside the macular hole but in a ring-shaped pattern immediately around the macular hole. Macular pigment distribution follows similar patterns, consistent with the distribution of inner retinal layers (especially Henle fiber layer) beside the macular hole.
FLIO and Macular Pigment in MacTel
In eyes affected with MacTel, the pattern of MP distribution is changed significantly. Whereas MP accumulates within the foveal center in healthy eyes and in early stages of the disease, in advanced cases of MacTel MP accumulates in a ring-like manner around the so-called “MacTel area,” an oval shaped region of 5° vertically and 6° horizontally centered at the fovea. The ring is enhanced in AFI images from patients regularly using lutein and zeaxanthin supplementation.5,33,34 In SSC FLIO images, we see short FAF lifetimes surrounding the MacTel area. This is depicted in Figure 1D.
This ring is enhanced in patients who supplement with lutein or zeaxanthin. A paired sample t-test was used to confirm this by comparing two areas of interest: the ring of approximately 6° to 10° outside the fovea (ring) and the retina between this ring and the large vessel arcade (surrounding retina). The ring FAF lifetime was 318 ± 79 ps, significantly (P < 0.001) longer than the retina outside of this area: 340 ± 77 ps (mean difference: 22 ± 30 ps). Investigating MacTel patients according to their supplementation status revealed further differences. In non-supplemented patients, there was only a non-significant trend (P = 0.19) of FAF lifetimes being shorter in the ring (ring: 332 ± 82 ps; surrounding retina: 338 ± 76 ps, mean difference: 6 ± 20 ps) However, in the supplemented group, the ring showed significantly (P < 0.001) shorter FAF lifetimes which in mean were 49 ± 26 ps shorter than the surrounding retina (ring: 293 ± 72 ps; surrounding retina: 342 ± 82 ps).
FLIO and Macular Pigment in Retinitis Pigmentosa
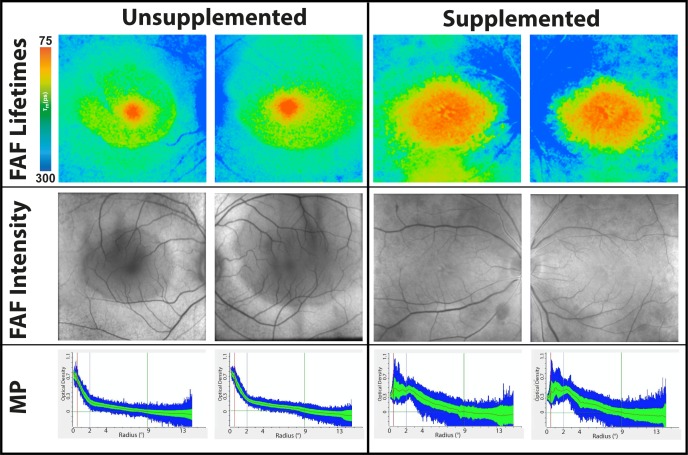
In RP, the macula is relatively unaffected by the disease process until very late in the course of the disorder. This results in a constricted, tunnel-like visual field with preserved central visual acuity. The MP seems to be fairly unaffected with a steep central concentration of MP observed by FLIO. As presented in Figure 1E, FLIO demonstrates the concentration peak of short FAF lifetimes in the fovea. However, mean autofluorescence lifetimes from area C were slightly longer than those of the age-matched healthy eyes (SSC: 170 ± 49 ps). Two of the investigated patients took a supplement containing 10 mg of lutein and 2 mg of zeaxanthin daily, which appeared to accumulate in the center of the fovea (Fig. 4).
Figure 4.
Mean FAF lifetime and intensity images of the short spectral channel (498–560 nm) as well as MP optical density of two patients with retinitis pigmentosa. Both eyes are shown. Both patients are female. The unsupplemented patient is 26 years old, and the carotenoid supplemented patient (on 10 mg lutein for 2 years) is 14 years old.
FLIO in Ex Vivo Solutions
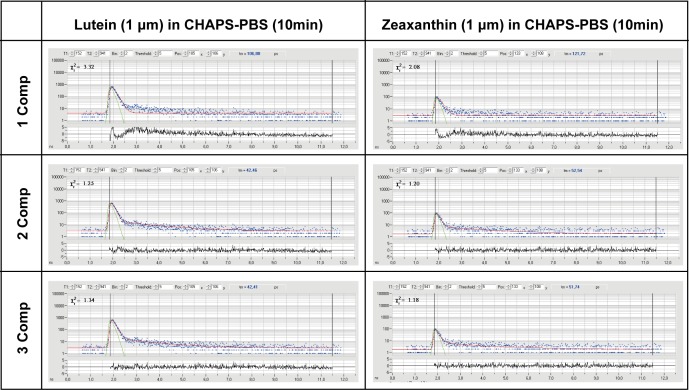
Ex vivo solutions of lutein (L) and zeaxanthin (Z) dissolved in CHAPS-PBS and measured in a quartz cuvette at various concentrations and durations consistently show very short mean autofluorescence (AF) lifetimes in the SSC of around 50 ps (L) and 60 ps (Z; Table 2), while the cuvette and the solvent (CHAPS-PBS) have no measurable fluorescence. When these carotenoids were complexed at a 1:5 ratio as well as a 1:1 ratio with their respective specific binding proteins in 1%–5% methanol, mean autofluorescence lifetimes prolong relative to either of the carotenoids or the proteins dissolved in CHAPS detergent separately. Although StARD3 (lutein-binding protein) apoprotein shows autofluorescence decays of approximately 50 ps, the mean autofluorescence lifetime shifts to approximately 90 ps when L is bound to it at a 1:5 or 1:1 ratio. GSTP1 (zeaxanthin-binding protein) apoprotein shows autofluorescence lifetimes of 46 ps, but when zeaxanthin binds to it at 1:5 or 1:1 ratios, these lifetimes prolong to approximately 75 ps. The long spectral channel's carotenoid fluorescence was extremely weak and therefore not investigated, and we did not test the third foveal carotenoid, meso-zeaxanthin, because its FLIO behavior should be indistinguishable from dietary zeaxanthin. Figure 5 shows individual FAF lifetime decay curves from both L and Z.
Table 2.
Autofluorescence Lifetimes of Ex Vivo Carotenoids in CHAPS-PBS
Figure 5.
FAF lifetime decay curves of ex vivo carotenoids. Possible fit-mechanisms with different components (Comp) are presented.
Discussion
FLIO is a novel noninvasive imaging method that is useful for detecting anatomic and metabolic changes of the human retina in vivo.1,9,11,20,25,35,36 In order to distinguish disease-related changes precisely, it is important to understand factors that influence the FLIO measurements. Previous studies described the influence of MP on fundus autofluorescence lifetimes using single-wavelength reflectometry to measure MP.11,20,37 The results presented in our study are in accordance with these previously published findings. Our study aimed to replicate parts of the study, with the difference of using dual wavelength AFI to measure MP.38–40 Previously, both strong37 as well as weak21,41 correlations were reported between single-wavelength reflectometry and dual wavelength AFI, and both methods show advantages and disadvantages.20 However, this study clearly shows that MP has a strong impact on FLIO measurements, regardless of the measurement method for MP. Additional to previous findings, we also focus on studying a wider variety of pathologies, including albinism, a macular hole, MacTel, and retinitis pigmentosa. The following paragraphs will describe our findings for each of these diseases.
This study is the first to describe FAF lifetime characteristics in two patients with albinism. Patients with albinism often show a reduced foveal depression and typically have little to no MP.30–33,42 In our study, the two investigated patients seemed to have a normal background autofluorescence based on FAF intensity images. Including the FLIO images in the investigation of albinism is complementary to the evaluation of MP measurements by dual-wavelength AFI. A previous report by Wolfson et al.43 focused on the investigation of MP in albinism, and with dual-wavelength AFI the authors describe that they may found evidence for MP accumulation in patients with albinism. Other reports found MP to be unmeasurable in these patients.32,44 As most MP measurements use a reference region, MP might be miscalculated in dual wavelength AFI due to the absence of melanin, potentially manifesting by the sloping baselines seen in Figure 3. In FLIO such miscalculation would not occur, as no reference region is necessary for the calculation. We believe that in the MP measurements with dual-wavelength AFI, the linear slope toward the fovea might be a calculation error of the dual-wavelength AFI software. We currently plan to investigate more patients with albinism to confirm this finding.
We are aware that the number of eyes with albinism presented in this study is very small; however, we believe that we can confirm the presence of MP in albinism for one of these eyes. One of our patients with albinism showed a small amount of MP in FLIO and AFI, which appeared to accumulate in the foveal region in his left eye. This is interesting, as his left eye did not show a foveal depression. No MP was detected in his right eye. Additionally, he had developed a visually significant cataract in this eye. Compared to the series of healthy eyes, the amount of MP in the left eye is very small. The patient had no history of supplementation with lutein or zeaxanthin; we cannot say whether a higher intake of carotenoids would lead to a different accumulation of MP. Our findings suggest that albinism can present with variable macular pigment amounts from little to none, although certainly with only two patients, more patients with albinism still need to be assessed with FLIO to gain a better understanding of the variability in presentation.
As macular holes were described in a previous study, we included only one eye in Figure 1 and abstained from extensive statistics, which would be redundant.11 Briefly, due to a disruption of retinal layers, the macular pigment is distributed immediately adjacent to the defect, resulting in a ring-like distribution of short FAF lifetimes around the hole as MP merely stays within its original layers.45–48 Previous studies have suggested that FLIO may have utility in monitoring how effectively MP-containing cells migrate back to the fovea after successful surgery.11
Early changes in MP may be characteristic in the development of MacTel.14,49,50 Whereas MP accumulates at the fovea in healthy eyes and in early stages of MacTel, advanced disease states show an abnormal distribution of MP at 6° to 10° outside of the fovea. In FLIO images, short FAF lifetimes can also be found in this area of MP accumulation. We found that this accumulation is significantly stronger in patients with supplementation, underlining the effect of MP.
The MP seems to be relatively unaffected in patients with RP when measured by resonance Raman spectroscopy.51 This is in accordance with our current findings, which suggest normal to slightly lower levels and normal distributions of MP in RP. Although lutein supplementation increases MP amounts in some RP patients, studies have failed to show that increased carotenoid intake slows the disease progression.52 FLIO measurements in the present study demonstrate a greatly enlarged area of MP in a young RP patient who had been taking lutein supplements for 2 years when compared to another young RP patient who had not supplemented with lutein (Fig. 4). Thus, FLIO confirms increased deposition of MP in supplemented RP patients and normal MP levels in RP patients who are not taking carotenoid supplementation. As the edge of the macula is often affected by paracentral atrophy in RP, MP measurements in RP can be challenging with the AFI method, especially when the reference region is affected. As can be seen in Figure 1, the MP levels are calculated to be very high. This could be an artifact due to the atrophy in the reference region for MP calculation. Looking at FLIO images, they provide images that are comparable to other diseases, indicating a relatively normal to slightly lower amounts of MP. We think that FLIO could enhance the investigation of MP in RP by showing more reliable calculations. A recent study investigates characteristics of FLIO in RP more thoroughly, showing characteristic patterns and also comparing RP patients to age-matched controls.53
This study also addresses the measurements of lutein and zeaxanthin in solution and associated with their binding proteins, allowing us to characterize the fluorescence lifetime of ex vivo carotenoids. This is the first study describing mean autofluorescence lifetimes of ex vivo carotenoids measured with FLIO in detail. As macular carotenoids block the fluorescence in FAF intensity images, they were long believed to show no fluorescence at all. A direct fluorescence of carotenoids has previously been described in one resonance Raman-based study, as well as FLIO-based measurements.11,20,54 Up to this point, the autofluorescence lifetimes of L and Z were not characterized in detail with FLIO. The results presented here suggest a very short mean autofluorescence lifetime of ex vivo carotenoids in FLIO measurements, especially when in detergent solution which should simulate carotenoids dissolved in biological membranes. Both types of carotenoids show very similar changes within the series of measurements. Based on fluorescence emission spectra of carotenoids, the majority of the fluorescence from L and Z is found within the SSC.29 This is in accordance with our findings, as the carotenoids in CHAPS-PBS show a greater fluorescence intensity in the SSC. Therefore, we focus on describing the SSC here. The mean autofluorescence lifetimes for carotenoids are very short and found at the previously described resolution limit of the camera.20 The MP-binding proteins had very short mean autofluorescence lifetimes in the SSC (around 50 ps). The measurements of lutein and zeaxanthin combined with their respective binding proteins reveal that mean autofluorescence lifetimes shift to longer means by approximately 40 ps for lutein and 20 ps for zeaxanthin. This knowledge allows for a better understanding of how the physical state of carotenoids in the macula may impact the FLIO measurements.
The greatest limitations of this study lie within the relatively small group sizes of some of the patients. As macular holes were previously intensively studied, this justifies the inclusion of only one eye. Our study is the first to describe FAF lifetimes in patients with albinism. As this is a rare disease, we were able to include only two patients, so the results of this study should be verified by the investigation of a larger number of patients with albinism. Our albinism patients refused genetic characterization, and neither of them had a family history of albinism. Future investigations should span the full genotypic and phenotypic disease spectrum that has been reported in albinism. Although we were able to statistically show an effect of carotenoid supplementation in patients with MacTel, our number of two RP patients on supplements was too small to do comparative studies. Therefore, we think that also for RP, the supplementation changes should be re-investigated including a larger patient population.
The findings in this study demonstrate the value of FLIO as an additional tool to image and characterize MP and also confirm previous findings regarding the direct impact of MP on the short FAF lifetimes at the fovea in FLIO images. At the current point of research, we believe that FLIO could serve as a complementary method to investigate MP, but we do not believe that it is designed to replace dual-wavelength AFI. FLIO gives information on the MP state in the eye, and this information may be of importance in retinal diseases where reference regions for MP calculation are affected. By monitoring the FAF lifetimes at each individual pixel-point of a 30° retinal field within a frame of 256 × 256 pixels, there is no need to identify an unaffected reference region to obtain MP measurements. FLIO may be a useful clinical tool when investigating MP in several advanced retinal diseases, such as retinitis pigmentosa, where reference regions for the MP calculation (psychophysical or autofluorescence) are affected by the disease. It may also be helpful in the confirmation of diseases like albinism, especially if patients refuse genetic testing. Moreover, FLIO also provides novel ways to look at the human eye, and we believe that the range of novel findings goes beyond simply detecting the presence and absence of macular pigment. FLIO may be of special interest in the early detection of diseases, such as MacTel and AMD,5,6 and also when investigating disease progression as in Stargardt disease.9
We suggest that FLIO provides a different way to approach the investigation of MP. As FLIO correlates with both dual-wavelength AFI as well as single-wavelength reflectometry, it is suitable as an additional tool to investigate MP. Moreover, FLIO seems useful for the early diagnosis of a variety of retinal diseases and could enhance the clinical diagnosis of many different retinal diseases. Other recent FLIO studies5,6,9 in combination with the here presented findings of MP fluorescence may lead to better understanding and detection of retinal diseases.
Acknowledgments
The authors thank Heidelberg Engineering for providing the FLIO as well as for their technical assistance, especially Yoshihiko Katayama, PhD; Donnell J. Creel, PhD, for providing detailed data on the electrophysiology of our patients with albinism; the Lowy family and the Lowy Medical Research Institute (LMRI) for their support; and all colleagues from the John A. Moran Eye Center who helped recruit and image patients.
Supported by National Institutes of Health Grants EY11600 and EY14800, Research to Prevent Blindness, and the Lowy Medical Research Institute.
Disclosure: L. Sauer, None; K.M. Andersen, None; B. Li, None; R.H. Gensure, None; M. Hammer, None; P.S. Bernstein, None
References
- 1. Schweitzer D, Schenke S, Hammer M,et al. Toward metabolic mapping of the human retina. Microsc Res Tech. 2007; 70: 410– 419. [DOI] [PubMed] [Google Scholar]
- 2. Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS. . Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res. 2017; 60: 120– 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakowicz JR. . Principles of Fluorescence Spectroscopy: Springer; 2007. [Google Scholar]
- 4. Becker W. . Fluorescence lifetime imaging--techniques and applications. J Microsc. 2012; 247: 119– 136. [DOI] [PubMed] [Google Scholar]
- 5. Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy (FLIO): a novel way to assess macular telangiectasia type 2 (MacTel). Ophthalmology Retina. 2017; 2: 587– 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sauer L, Gensure RH, Anderson KM,et al. Patterns of fundus autofluorescence lifetimes in eyes of individuals with non-exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018; 59: AMD65– AMD77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dysli C, Wolf S, Zinkernagel MS. . Fluorescence lifetime imaging in retinal artery occlusion. Invest Ophthalmol Vis Sci. 2015; 56: 3329– 3336. [DOI] [PubMed] [Google Scholar]
- 8. Schweitzer D, Deutsch L, Klemm M,et al. Fluorescence lifetime imaging ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic retinopathy. J Biomed Opt. 2015; 20: 61106. [DOI] [PubMed] [Google Scholar]
- 9. Dysli C, Wolf S, Hatz K, Zinkernagel MS. . Fluorescence lifetime imaging in stargardt disease: potential marker for disease progression. Invest Ophthalmol Vis Sci. 2016; 57: 832– 841. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt J, Peters S, Sauer L,et al. Fundus autofluorescence lifetimes are increased in non-proliferative diabetic retinopathy. Acta Ophthalmol. 2017; 95: 33– 40. [DOI] [PubMed] [Google Scholar]
- 11. Sauer L, Peters S, Schmidt J,et al. Monitoring macular pigment changes in macular holes using fluorescence lifetime imaging ophthalmoscopy (FLIO). Acta Ophthalmol. 2017; 95: 481– 492. [DOI] [PubMed] [Google Scholar]
- 12. Dysli C, Berger L, Wolf S, Zinkernagel MS. . Fundus autofluorescence lifetimes and central serous chorioretinopathy. Retina. 2017; 37: 2151– 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. . Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001; 42: 439– 446. [PubMed] [Google Scholar]
- 14. Helb HM, Charbel Issa P, Van der Veen RL, Berendschot TT, Scholl HP, Holz FG. . Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008; 28: 808– 816. [DOI] [PubMed] [Google Scholar]
- 15. Lima VC, Rosen RB, Maia M,et al. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Invest Ophthalmol Vis Sci. 2010; 51: 5840– 5845. [DOI] [PubMed] [Google Scholar]
- 16. Nolan JM, Loskutova E, Howard AN,et al. Macular pigment, visual function, and macular disease among subjects with Alzheimer's disease: an exploratory study. J Alzheimers Dis. 2014; 42: 1191– 1202. [DOI] [PubMed] [Google Scholar]
- 17. Nolan JM, Loskutova E, Howard A,et al. The impact of supplemental macular carotenoids in Alzheimer's disease: a randomized clinical trial. J Alzheimers Dis. 2015; 44: 1157– 1169. [DOI] [PubMed] [Google Scholar]
- 18. Scanlon G, Connell P, Ratzlaff M,et al. Macular pigment optical density is lower in type 2 diabetes, compared with type 1 diabetes and normal controls. Retina. 2015; 35: 1808– 1816. [DOI] [PubMed] [Google Scholar]
- 19. Akuffo KO, Nolan JM, Peto T,et al. Relationship between macular pigment and visual function in subjects with early age-related macular degeneration. Br J Ophthalmol. 2016; 101: 190– 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sauer L, Schweitzer D, Ramm L, Augsten R, Hammer M, Peters S. . Impact of macular pigment on fundus autofluorescence lifetimes. Invest Ophthalmol Vis Sci. 2015; 56: 4668– 4679. [DOI] [PubMed] [Google Scholar]
- 21. Dennison JL, Stack J, Beatty S, Nolan JM. . Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp Eye Res. 2013; 116: 190– 198. [DOI] [PubMed] [Google Scholar]
- 22. Conrady CD, Bell JP, Besch BM,et al. Correlations between macular, skin, and serum carotenoids. Invest Ophthalmol Vis Sci. 2017; 58: 3616– 3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schweitzer D, Hammer M, Schweitzer F,et al. In vivo measurement of time-resolved autofluorescence at the human fundus. J Biomed Opt. 2004; 9: 1214– 1222. [DOI] [PubMed] [Google Scholar]
- 24. Becker W. . The bh TCSPC Handbook. 6th ed. Berlin: Becker & Hickl GmbH; 2014. [Google Scholar]
- 25. Dysli C, Quellec G, Abegg M,et al. Quantitative analysis of fluorescence lifetime measurements of the macula using the fluorescence lifetime imaging ophthalmoscope in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 2106– 2113. [DOI] [PubMed] [Google Scholar]
- 26. Klemm M, Schweitzer D, Peters S, Sauer L, Hammer M, Haueisen J. . FLIMX: a software package to determine and analyze the fluorescence lifetime in time-resolved fluorescence data from the human eye. PLoS One. 2015; 10: e0131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. . Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004; 279: 49447– 49454. [DOI] [PubMed] [Google Scholar]
- 28. Li B, Vachali P, Frederick JM, Bernstein PS. . Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011; 50: 2541– 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharifzadeh M, Bernstein PS, Gellermann W. . Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 2373– 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harvey PS, King RA, Summers CG. . Spectrum of foveal development in albinism detected with optical coherence tomography. J AAPOS. 2006; 10: 237– 242. [DOI] [PubMed] [Google Scholar]
- 31. Gregor Z. . The perifoveal vasculature in albinism. Br J Ophthalmol. 1978; 62: 554– 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abadi RV, Cox MJ. . The distribution of macular pigment in human albinos. Invest Ophthalmol Vis Sci. 1992; 33: 494– 497. [PubMed] [Google Scholar]
- 33. Bernstein PS, Li B, Vachali PP,et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016; 50: 34– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi RY, Gorusupudi A, Wegner K, Sharifzadeh M, Gellermann W, Bernstein PS. . Macular pigment distribution responses to high-dose zeaxanthin supplementation in patients with macular telangiectasia type 2. Retina. 2017; 37: 223– 2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schweitzer D. . Metabolic mapping. : Holz F, Spaide R, . Medical Retina. Berlin: Springer Berlin Heidelberg; 2010: 107– 123. [Google Scholar]
- 36. Klemm M, Dietzel A, Haueisen J, Nagel E, Hammer M, Schweitzer D. . Repeatability of autofluorescence lifetime imaging at the human fundus in healthy volunteers. Curr Eye Res. 2013; 38: 793– 801. [DOI] [PubMed] [Google Scholar]
- 37. Schweitzer D, Jentsch S, Dawczynski J, Hammer M, Wolf-Schnurrbusch UE, Simple Wolf S. . and objective method for routine detection of the macular pigment xanthophyll. J Biomed Opt. 2010; 15: 061714. [DOI] [PubMed] [Google Scholar]
- 38. Trieschmann M, Spital G, Lommatzsch A,et al. Macular pigment: quantitative analysis on autofluorescence images. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 1006– 1012. [DOI] [PubMed] [Google Scholar]
- 39. Canovas R, Lima VC, Garcia P, Morini C, Prata TS, Rosen RB. . Comparison between macular pigment optical density measurements using two-wavelength autofluorescence and heterochromatic flicker photometry techniques. Invest Ophthalmol Vis Sci. 2010; 51: 3152– 3156. [DOI] [PubMed] [Google Scholar]
- 40. Wustemeyer H, Moessner A, Jahn C, Wolf S. . Macular pigment density in healthy subjects quantified with a modified confocal scanning laser ophthalmoscope. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 647– 651. [DOI] [PubMed] [Google Scholar]
- 41. Creuzot-Garcher C, Koehrer P, Picot C, Aho S, Bron AM. . Comparison of two methods to measure macular pigment optical density in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 2941– 2946. [DOI] [PubMed] [Google Scholar]
- 42. Sparrow JR, Hicks D, Hamel CP. . The retinal pigment epithelium in health and disease. Curr Mol Med. 2010; 10: 802– 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolfson Y, Fletcher E, Strauss RW, Scholl HPN. . Evidence of macular pigment in the central macula in albinism. Exp Eye Res. 2016; 145: 468– 471. [DOI] [PubMed] [Google Scholar]
- 44. Putnam CM, Bland PJ. . Macular pigment optical density spatial distribution measured in a subject with oculocutaneous albinism. J Optom. 2014; 7: 241– 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snodderly DM, Brown PK, Delori FC, Auran JD. . The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984; 25: 660– 673. [PubMed] [Google Scholar]
- 46. Bernstein PS, Balashov NA, Tsong ED, Rando RR. . Retinal tubulin binds macular carotenoids. Invest Ophthalmol Vis Sci. 1997; 38: 167– 175. [PubMed] [Google Scholar]
- 47. Gass JD, Van Newkirk M. . Xanthic scotoma and yellow foveolar shadow caused by a pseudo-operculum after vitreofoveal separation. Retina. 1992; 12: 242– 244. [DOI] [PubMed] [Google Scholar]
- 48. Ezra E, Munro PM, Charteris DG, Aylward WG, Luthert PJ, Gregor ZJ. . Macular hole opercula. Ultrastructural features and clinicopathological correlation. Arch Ophthalmol. 1997; 115: 1381– 1387. [DOI] [PubMed] [Google Scholar]
- 49. Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. . The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010; 50: 716– 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Degli Esposti S, Egan C, Bunce C, Moreland JD, Bird AC, Robson AG. . Macular pigment parameters in patients with macular telangiectasia (MacTel) and normal subjects: implications of a novel analysis. Invest Ophthalmol Vis Sci. 2012; 53: 6568– 6575. [DOI] [PubMed] [Google Scholar]
- 51. Zhao DY, Wintch SW, Ermakov IV, Gellermann W, Bernstein PS. . Resonance Raman measurement of macular carotenoids in retinal, choroidal, and macular dystrophies. Arch Ophthalmol. 2003; 121: 967– 972. [DOI] [PubMed] [Google Scholar]
- 52. Aleman TS, Duncan JL, Bieber ML,et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2001; 42: 1873– 1881. [PubMed] [Google Scholar]
- 53. Andersen KM, Sauer L, Gensure RH, Hammer M, Bernstein PS. . Characterization of retinitis pigmentosa using fluorescence lifetime imaging ophthalmoscopy (FLIO). TVST 2018. In press. [DOI] [PMC free article] [PubMed]
- 54. Ermakov IV, Ermakova MR, Gellermann W. . Simple Raman instrument for in vivo detection of macular pigments. Appl Spectrosc. 2005; 59: 861– 867. [DOI] [PMC free article] [PubMed] [Google Scholar]